Abstract
Ethnopharmacological relevance
With the spread of Coronavirus Disease (2019) (COVID-19), combination with traditional Chinese medicine (TCM) has been widely used as a prevention and therapy strategy in China. Xin guan No.1 (XG-1) prescription is a preventive formula recommended by the Hunan Provincial Administration of TCM to prevent the pandemic of COVID-19.
Aim of the study
To explore the potential preventive mechanisms of XG-1 against COVID-19 in the combination of network pharmacology approach, single-cell RNA expression profiling analysis, molecular docking and retrospective study.
Materials and methods
Encyclopedia of Traditional Chinese Medicine (ETCM) database was used to determine the meridian tropism, active components and target genes of XG-1. Gene ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis were conducted by R Cluster Profiler package (3.14.3). Single cell RNA sequencing (scRNA-seq) data of human lung (GSE122960) was downloaded from Gene Expression Omnibus (GEO) database and analyzed by R Seurat package (3.1.2). Cytoscape (3.7.2) was used to construct the interaction network. The main ingredients in XG-1 were identified by HPLC- Q-TOF- MS and used for molecular docking with COVID-19 3CL hydrolytic enzyme and angiotensin converting enzyme II (ACE2). A retrospective study of 47 close contact participants from Dongtang Community of Hunan Province was conducted to evaluated the preventive effect of XG-1.
Results
According to the network pharmacology analysis, XG-1 formula was closely related to lung-, spleen- and stomach-meridians and include a total of 206 active components and 853 target genes. GO and KEGG pathway enrichment revealed that XG-1 mainly regulated cellular amino acid metabolism process and neuroactive ligand-receptors interaction. The scRNA-seq profiling showed that angiotensin converting enzyme 2 (ACE2) was principally expressed in alveolar type 2 epithelial cells (AT2). 153 genes were up-regulated in AT2 cells expressing ACE2 and 12 genes were obtained by intersecting with XG-1 target genes, of which 3 were related to immunity. Five main chemical ingredients were detected in XG-1 sample by HPLC-Q-TOF-MS. The molecular docking showed that Rutin, Liquiritin and Astragaloside Ⅳ had a good affinity with COVID-19 3CL hydrolytic enzyme and ACE2. Compared with participants who didn't take XG-1, preventive treatment with XG-1gradules resulted in a significant lower rate of testing positive for SARS-CoV-2 nucleic acid (P < 0.0001).
Conclusion
The present study showed that XG-1 exerts a preventive effect in close contacts against COVID-19. The underlying mechanism may be related to modulate immunity response through multiple components, pathways, and several target genes co-expressed with ACE2. These findings provide preliminary evidences and methodological reference for the potential preventive mechanism of XG-1 against COVID-19.
Keywords: Coronavirus disease 2019, Network pharmacology, Single-cell RNA Sequencing, Chinese medicine preventive formula, Close contacts, Molecular docking
Abbreviations: XG-1, Xin guan No.1 preventive prescription; COVID-19, Coronavirus Disease 2019; GO, Gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; scRNA-seq, Single cell RNA sequencing; ACE2, angiotensin-converting enzyme Ⅱ; TCM, traditional Chinese medicine; AT2, alveolar type 2 epithelial cells; HPLC-Q-TOF-MS, High-Performance Liquid Chromatography-Quadrupole-Time of Flight-Mass Spectrometry; PDB, protein data bank; CGA, Chlorogenic acid; KD-1, Phillyrin; AS-IV, Astragaloside IV
Graphical abstract
1. Introduction
In December 2019, an acute pneumonia caused by a new coronavirus (SARS-CoV-2) broke out, which named as coronavirus disease 2019 (COVID-19). The World Health Organization (WHO) declared a public health emergency of international concern on January 30, 2020 (World Health Organization, 2020a). The COVID-19 epidemic has rapidly spread to the world and the current situation is still severe (World Health Organization, 2020b). Clinical symptoms of COVID-19 often manifest as fever, dry cough, muscle pain or fatigue. A part of patients is accompanied by nasal congestion, runny nose, diarrhea and other upper respiratory or digestive tract symptoms. Patients usually died of acute respiratory distress syndrome, septic shock, coagulation dysfunction or multiple organ failure (Huang et al., 2020).
SARS-CoV-2 has been sequenced and it's reported that SARS-CoV-2 has a similar receptor binding domain (RBD) structure with SARS-CoV spike (S) protein, and with 76.5% homology in amino acid sequence (Lu et al., 2020; Xu, X. et al., 2020b). Human angiotensin-converting enzyme II (ACE2) has a strong binding affinity to SARS-CoV S protein and is a functional receptor for SARS-CoV where the virus enters host cells in vitro or in vivo and replicates the virus (Kuba et al., 2005; Li et al., 2003, 2005). Research showed that SARS-CoV-2 can identify human ACE2 more effectively than SARS-CoV, which could regulate the capacity and efficiency of human-to-human transmission (Wan et al., 2020). Overexpression of ACE2 in HeLa cells of different species (human, pig and civet) can cause infection and replication of SARS-CoV-2, which directly proves that SARS-CoV-2 and ACE2 binds to promote virus entry and affected replication on the surface of host cells (Zhou et al., 2020).
Traditional Chinese medicine (TCM) has a long history of fighting against epidemic disease and established a complete theoretical system of infectious diseases intervention. Previous studies have shown that TCM therapy exerted an essential role in combating coronavirus pneumonia such as severe acute respiratory syndrome (SARS) and H1N1 influenza (Leung, 2007; Zhang and Chen, 2008). Evidences also have demonstrated that TCM synergy therapy could alleviate clinical symptoms such as fever, control the progression of disease, reduce the dosage of hormone and the occurrence of complications in patients with COVID-19 (Ni et al., 2020; Wu et al., 2020). Xin guan No.1 (XG-1) is a preventive prescription proposed by Hunan Provincial Administration of TCM for the prevention of COVID-19 (Hunan Provincial Administration of Traditional Chinese Medicine, 2020). It's based on YuPingFeng San, a classic and famous prescription with immune enhancement and inflammation inhibitory effects (Li, Y. et al., 2017b; Liu et al., 2017; Wang et al., 2016; Zhou et al., 2019). It had been used to protect the at-risk hospital workers and showed a significant preventive effect during the SARS epidemic (Lau et al., 2005). A recent study has demonstrated that XG-1 has a certain regulatory effect on the immune function of young and elderly healthy people (Liu, L. et al., 2020a). XG-1 consists of 8 traditional Chinese herbs including Astragalus mongholicus Bunge (Huang Qi), Atractylodes macrocephala Koidz. (Bai Zhu), Forsythia suspensa (Thunb.) Vahl (Lian Qiao), Glycyrrhiza uralensis Fisch. ex DC. (Gan Cao), Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. (Fang Feng), Lonicera japonica Thunb. (Jin Yin Hua), Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) and Acorus calamus var. angustatus Besser (Shi Chang Pu). At the peak of the COVID-19 epidemic in Hunan Province, close contacts with the confirmed cases were under high risks of infection. Although the XG-1 formula has been widely used in Hunan Province to prevent COVID-19 invasion, its bioactive ingredients and their underlying preventive mechanism remains to be elucidated.
Network pharmacology is a method based on systems biology and system pharmacology that analyzes the network of biological systems and selects specific signal nodes for drug molecule design. Chinese herbal compound is the main form of TCM for disease prevention and treatment, with the characteristics of multiple components, multiple targets and multiple pathways. It was demonstrated that network pharmacology has been widely applied to recognize the active ingredients and potential targets of herbals, to predict pharmacological mechanisms of herbal formulas (Wu et al., 2019; Yu et al., 2020). Single-cell sequencing has been a hot topic in recent years and single-cell RNA sequencing (scRNA-Seq) technology is the most mature and widely used (Shalek et al., 2013). Its purpose is to comprehensively measure the expression level of genes in cells, and to improve the accuracy of our understanding of cellular gene function (Stubbington et al., 2017). Molecular docking is a computational tool used to predict the binding capacity and binding mode of receptor-drug molecular complexes. It's confirmed that the combination of the S protein of SARS-CoV-2 and ACE2 in the human body could help the virus invade the body and cause disease. The crystal structure of SARS-CoV-2 3CL hydrolase is reported and considered to be an effective target of SARS-CoV-2, which provides a basis for screening active ingredients against COVID-19 (Lu et al., 2020; Elmezayen et al., 2020).
Therefore, in our study, network pharmacology was applied to screen out the active ingredients and action targets of XG-1, and a single-cell RNA expression profiling of human lung was performed to cluster analysis. The co-action targets between ACE2 expressing cells and XG-1 bioactive components were identified via computationally analysis. The main active components in XG-1 were identified by HPLC-Q-TOF-MS, and the binding energy of the key components with COVID-19 3CL hydrolase and ACE2 was predicted through molecular docking. A retrospective study was conducted to verify the preventive efficacy of XG-1 treatment in the close contacts. We hope our study can provide a preliminary evidence of the potential precautionary mechanism of XG-1 against COVID-19.
2. Materials and Methods
2.1. Information collection of XG-1
XG-1 consists of 8 herbs (Table 1 ). “HUANG QI”, “BAI ZHU”, “LIAN QIAO”, “GAN CAO”, “FANG FENG”, “JIN YIN HUA”, “GUANG HUO XIANG” and “SHI CHANG PU” were searched in the Chinese Medicine Encyclopedia (ETCM, http://www.nrc.ac.cn:9090/ETCM/) database to obtain the contained compounds and the target genes (Xu et al., 2019).
Table 1.
Number of compounds contained in herbs of XG-1 and corresponding putative targets.
| Chinese name | Scientific name | Number of active ingredients | Number of targets |
|---|---|---|---|
| HUANG QI | Astragalus mongholicus Bunge | 27 | 461 |
| BAI ZHU | Atractylodes macrocephala Koidz. | 20 | 368 |
| LIAN QIAO | Forsythia suspensa (Thunb.) Vahl | 49 | 344 |
| GAN CAO | Glycyrrhiza uralensis Fisch. ex DC. | 133 | 340 |
| FANG FENG | Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. | 32 | 338 |
| JIN YIN HUA | Lonicera japonica Thunb. | 47 | 209 |
| GUANG HUO XIANG | Pogostemon cablin (Blanco) Benth. | 59 | 196 |
| SHI CHANG PU | Acorus calamus var. angustatus Besser | 18 | 41 |
2.2. Annotation of target pathway
In order to further observe the biological function of the target, Gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differentially expressed genes were performed using the Cluster Profiler package (3.14.3) in R platform (Yu et al., 2012). The results with the threshold P < 0.05 and in the top 20 entries were visualized into a bubble chart.
2.3. Single-cell RNA expression profiling cluster analysis
The human lung tissue single cell transcriptome sequencing data (GSE122960) was downloaded from the GEO database, and the Seurat software package in R (3.6.2) ((http://satijalab.org/seurat/, version: 3.1.2) was used to perform cell clustering. Low-quality cells that express more than 10% of mitochondrial genes in cells with less than 200 RNAs were filtered out, then the 2000 most highly mutated genes were calculated and analyzed by PCA dimensionality reduction. For the top 10 PCs with explanatory power, Find Clusters was performed to identify each cell cluster, and t-distribution random neighbor embedding (tSNE) was used for clustering and visualization, then the initial clusters were checked and merged. Find All Markers function and Wilcoxon rank sum tests were used to analyze and screen differentially expressed marker genes. Differentially expressed genes were expressed in at least 25% of the cells in the cluster, and the fold change is greater than 0.25 (Log Scale), which was considered to be a marker gene. The tSNE curve and violin curve were generated using Seurat.
2.4. Target network construction and analysis
Cytoscape (http://www.cytoscape.org/, version: 3.7.2) was used to construct the network and analyze the network (Shannon et al., 2003). In a network, nodes represent active ingredients, targets, or pathways.
2.5. High -performance liquid chromatography-quadrupole-time of flight-mass spectrometry (HPLC-Q-TOF-MS)
HPLC- Q-TOF -MS was used to control quality and identify the main chemical constituents of XG-1 granules. Rutin (Lot: 1000801–201811, purity: 91.7%), Liquiritin (Lot:111610-201607, purity: 93.1%), Chlorogenic acid (Lot:110753-201817, purity: 96.8%) and Astragaloside IV (Lot:110781-201717, purity: 96.9%) standards were purchased from National Institutes for Food and Drug Control, China. Phillyrin (Lot:110821-200609, purity: 93.5%) standard was purchased from National Institute for the Pharmaceutical and Biological Products Control, China. All of these standards were used as quality control of XG-1.
Sample preparation: 0.3 g of XG-1 prescription was accurately weighed, then placed in a stoppered Erlenmeyer flask. 4 mL of water was precisely added and weighed then ultrasonicated for 40 min. Weighed again when it was cooled and the lost weight was supplied with water. Shaked well and then filter through a 0.22 μm microporous membrane to obtain the sample solution.
Standard preparation: 1 mg of each standard substance was accurately weighed and placed in an EP tube then 4 mL of methanol was added to dissolve.
Chromatographic conditions: An Agilent 1290 HPLC system (Agilent, USA) with an Inertsil ODS-2 Rapid Resolution HT column (4.6 × 250 mm, 5 μm, Shimadzu, Japan) was used for HPLC analysis at the flow of 0.5 mL/min and the temperature of the column was 30 °C. The injection volume was 10 μL, with wavelength 210 nm, and full wavelength scan range was 190–400 nm. The gradient elution procedure was shown in Table 3 .
Table 3.
The gradient elution procedure in high-performance liquid chromatography.
| Time (min) | Acetonitrile (%) | Ultrapure water (%) |
|---|---|---|
| 0 | 10 | 90 |
| 40 | 50 | 50 |
| 50 | 70 | 30 |
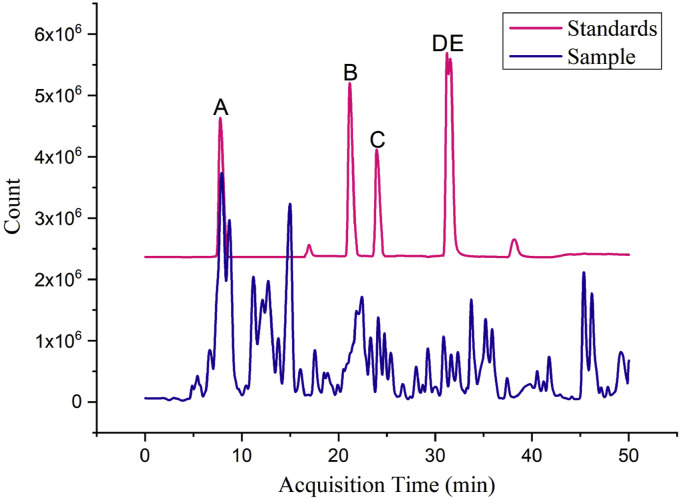
Mass spectrometry conditions: All the samples were analyzed with ESI source in negative mode. The operating parameters of MS were: drying gas, 8 L/min; gas temperature, 320 °C; sheath gas temperature, 350 °C; nebulizer, 35 psig; sheath gas flow, 10 L/min; nozzle voltage, 1.0 kV; V Cap, 3.5 kV; fragmentor, 110 V; skimmer voltage, 65 V; OCT 1 RF Vpp, 750 V; MS scan range, m/z 100–1000 Da. The detection results were shown in Fig. 1 and the quantitative analysis information of active ingredients in XG-1 was shown in Table 4 .
Fig. 1.
The major chemical components contained in XG-1 preparation identified by HPLC- Q-TOF -MS. A: Chlorogenic acid, the retention time occurs at 7.9 min; B: Rutin, the retention time occurs at 21.1 min; C: Liquiritin, the retention time occurs at 23.9 min; D: Phillyrin, the retention time occurs at 31.6 min; E: Astragaloside Ⅳ, the retention time occurs at 32.2 min.
Table 4.
Quantitative analysis information of five active ingredients in XG-1.
| Active ingredients | RT (min) | Origin | Area | Concentration (mg/mL) |
|---|---|---|---|---|
| Chlorogenic acid | 7.9 | Lonicera japonica Thunb. | 293567589 | 0.4951 |
| Rutin | 21.1 | Lonicera japonica Thunb. | 16313870 | 0.0852 |
| Liquiritin | 23.9 | Glycyrrhiza uralensis Fisch. ex DC. | 37725807 | 0.1894 |
| Phillyrin | 31.6 | Forsythia suspensa (Thunb.) Vahl | 2772346 | 1.5404 |
| Astragaloside Ⅳ | 32.2 | Acorus calamus var. angustatus Besser | 855149 | 0.0013 |
2.6. Molecular docking
AutoDock Vina 4.2.6 was used for molecular docking (Trott and Olson, 2010). The key active ingredients of XG-1 as ligands and the SARS-CoV-2 3CL hydrolase and ACE2 as receptors. The PDB format file of 3D structure of SARS-CoV-2 3CL hydrolase (PDB ID: 6LU7) and ACE2 (PDB ID: 1R42) were downloaded from the RSCB PDB database (http://www.rcsb.org/). AutoDock Tools 1.5.6 was used to remove water molecules in the SARS-CoV-2 3CL hydrolase protein and ACE2, separate the protein, add non-polar hydrogen, calculate the Gasteiger charge, and save it as a pdbqt file. Three key active ingredients of XG-1, Rutin (Compound CID: 5280805), Liquiritin (Compound CID: 503737) and Astragaloside Ⅳ (Compound CID: 13943297), were downloaded from PubChem database (https://pubchem.ncbi.nlm.nih.gov) to obtain the SDF format file of 2D structure. After checking the spatial structure in Chem3D, the energy is minimized and converted to pdb format. Chem3D was used to convert them into a mol2 file and AutoDock Tools 1.5.6 program was used to load the structure, add atomic charges and assign atomic types. All flexible bonds are rotatable by default and saved in pdbqt format as a docking ligand.
2.7. Retrospective study
2.7.1. Ethical statement
The retrospective study in this research was reviewed and approved by the Ethics Committee of the Dongtang Community Health Service Center in Changsha (No. 202001), and informed consent was obtained. All information was kept anonymously and complied with national laws and institutional requirements.
2.7.2. Study design and participants
Participants were selected that close contacts who resident in Dongtang community of Changsha City in Hunan Province between February 13 and March 12, 2020. The SARS- CoV-2 nucleic acid test of pharyngeal swab sample was conducted by real-time polymerase chain reaction (RT-PCR) on the day when they're traced to be the close contact of the confirmed COVID-19 cases. Individuals only who possess negative nucleic acid results enrolled in our study. Close contacts should meet one of the following criteria: (1) contacts who live, study or work together with the COVID-19 confirmed patients; (2) contacts who were unprotected exposed to the COVID-19 confirmed patients within 1 m within 2 days before the onset of patient's symptom. Exclusion criteria included: (1) contacts with suspected COVID-19 cases; (2) younger than 18 years old; (3) pregnant woman. The diagnosis of confirmed or suspected COVID-19 cases was in accordance with the “Clinical Guidance of Diagnosis and Treatment for COVID-19 (Trial version 8)” (General Office of the National Health Commission, 2020).
2.7.3. Treatments and data collection
All participants were subject to isolation and observation for 14 days according to relevant regulations. During the quarantine period, SARS- CoV-2 nucleic acid tests were performed by RT-PCR every 7 days. Each participant received the preventive treatment of XG-1 granules (dissolved in 150 mL boiled water with twice a day) for 14 days. The granules were purchased from the First Affiliated Hospital of Hunan University of Traditional Chinese Medicine and the quality was in accordance with the “Pharmacopoeia of the People's Republic of China (Edition, 2015)”. Following indicators were observed and recorded: 1. The number of participants taking traditional Chinese medicine; 2. The outcomes of SARS-CoV-2 nucleic acid testing; 3. Adverse reactions during the treatment. Additionally, the baseline characteristics including age, gender, contact time and relationship of participants were collected.
2.8. Statistical analysis
Statistical analysis of the data was performed using the IBM Social Science Statistical Software Package (SPSS, version 24). Continuous variables were calculated with Mean ± standard deviation, and the percentage of categorical variables. Comparisons between groups were performed using Student's t-test or Mann Whitney U test (the normal distribution of the data has been verified by the Kolmogorov-Smirnov test) and the chi-square test for continuous variables (Fisher's exact test was used when appropriate).
3. Results
3.1. Meridian tropism network of XG-1
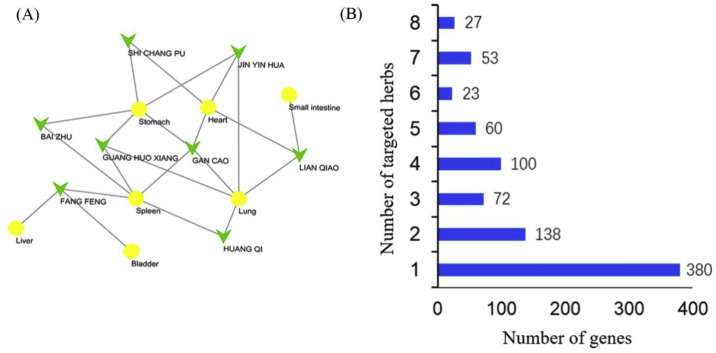
An herb-meridian network was constructed based on the herb information of XG-1 compound, as shown in Fig. 2 A. Among all 8 herbs in XG-1, the most affinity meridians were Lung (5), Spleen (5) and Stomach (5), followed by heart (4), Small intestine (1), Bladder (1) and Liver (1). Therefore, XG-1 compound mainly acted on the lungs, spleen and stomach system.
Fig. 2.
Meridian network of XG-1 (A) and numbers of targeted Chinese medicines-genes (B). The green arrow represents the herbs, and the yellow nodes represents the meridians. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Active ingredients and target genes information of XG-1
Totally 385 active ingredients and 2297 targets were found in XG-1 by searching ETCM database, including 27 active ingredients and 461 targets in Astragalus mongholicus Bunge (Huang Qi), 20 active ingredients and 368 targets in Atractylodes macrocephala Koidz. (Bai Zhu), 49 active ingredients and 344 targets in Forsythia suspensa (Thunb.) Vahl (Lian Qiao), 133 active ingredients and 340 targets in Glycyrrhiza uralensis Fisch. ex DC. (Gan Cao), 32 active ingredients and 338 targets in Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. (Fang Feng), 47 active ingredients and 209 targets in Lonicera japonica Thunb. (Jin Yin Hua), 59 active ingredients and 196 targets in Pogostemon cablin (Blanco) Benth. (Guang Huo Xiang) and 18 active ingredients and 41 targets in Acorus calamus var. angustatus Besser (Shi Chang Pu). After removing the duplicate results, a total of 206 active ingredients and 853 targets were obtained (Table 1). By calculating the number of target genes in each Chinese medicine herb, there were 380 genes targeting 1 herb, 128 genes targeting 2 herbs, 72 genes targeting 3 herbs, 100 genes targeting 4 herbs, 60 genes targeting 5 herbs, 23 genes targeting 6 herbs, 53 genes targeting 7 herbs, and 27 genes targeting 8 herbs (Fig. 2B). These 27 genes were as follows: CYP1A1, CYP1A2, CYP2C8, CYP2C9, CYP3A4, GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6, CYP1B1 CYP2C19, CYP2D6, GABRB1, GABRB2, GABRB3, CYP2A6, GABRD, GABRE, GABRP, GABRQ, GABRG1, GABRG2, GABRG3, ALB, and CYP2B6.
3.3. Target pathway analysis
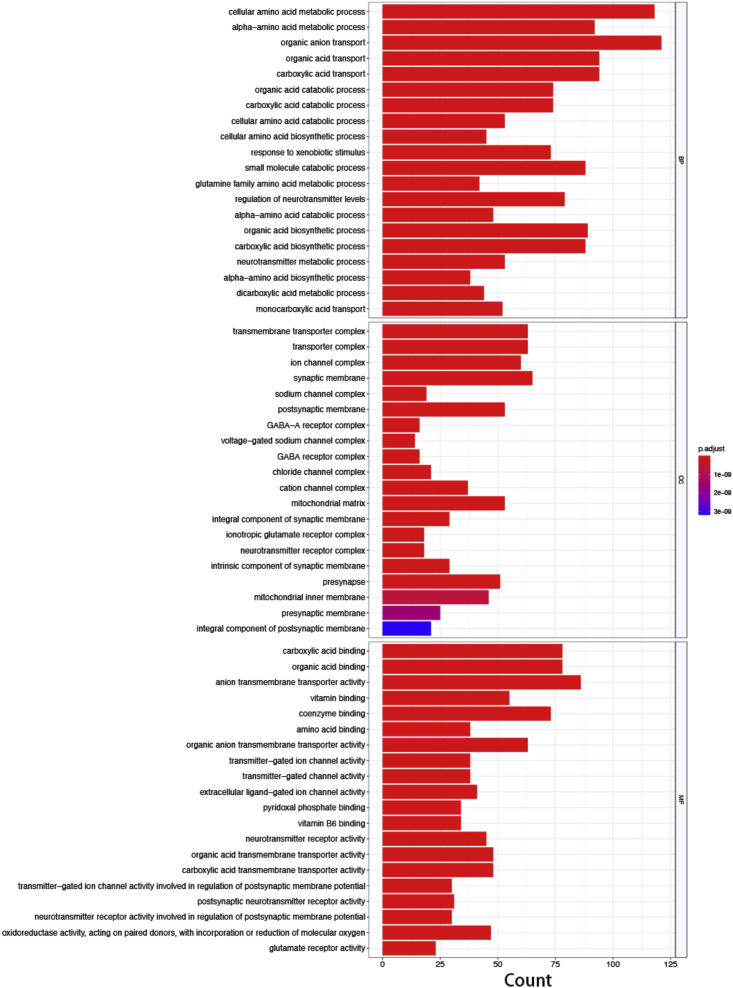
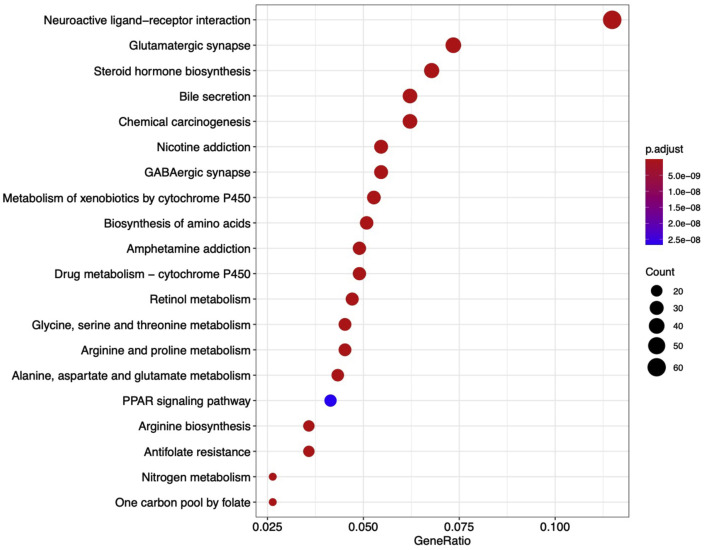
GO analysis and KEGG pathway enrichment analysis were performed based on the 853 target genes screened from the database. In the Top 20 results of GO functional enrichment analysis, the biological process (BP) mainly involving organic anion transport, cellular amino acid metabolism, acid biosynthesis and small molecule catabolic processes; In cell component (CC), targets are mainly enriched in membranes, synapses, channel complexes and other related pathways; In molecular function (MF), the important targets are mainly related to anion transmembrane transporter activity, carboxylic acid binding, organic acid binding, etc. (Fig. 3 ). KEGG pathway enrichment screened 100 signal pathways (P < 0.05), and the Top 20 results showed that the targets of XG-1 were mainly related to neuroactive ligand-receptors, glutamate synapses, steroid hormone synthesis, drug metabolism, calcium signaling pathway, and PPAR signaling pathways (Fig. 4 ).
Fig. 3.
The top 20 pathways for GO enrichment analysis of XG-1. x-axis represents count, indicating the number of target genes belonging to a pathway and the number of the annotated genes located in the pathway. y-axis represents pathway. The color of the column reflects the different P adjust. values. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
The top 20 KEGG pathway enrichment results of XG-1. x-axis represents gene riao, indicating the ratio of the number of target genes belonging to a pathway and the number of the annotated genes located in the pathway. The size of the dot in-dicates the number of target genes in the pathway and the color of the dot reflects the different P adjust. value. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Analysis of single-cell RNA expression profiling in human lungs
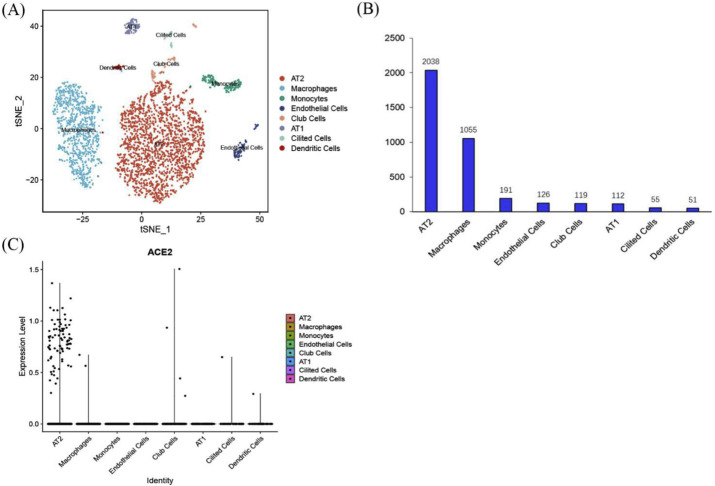
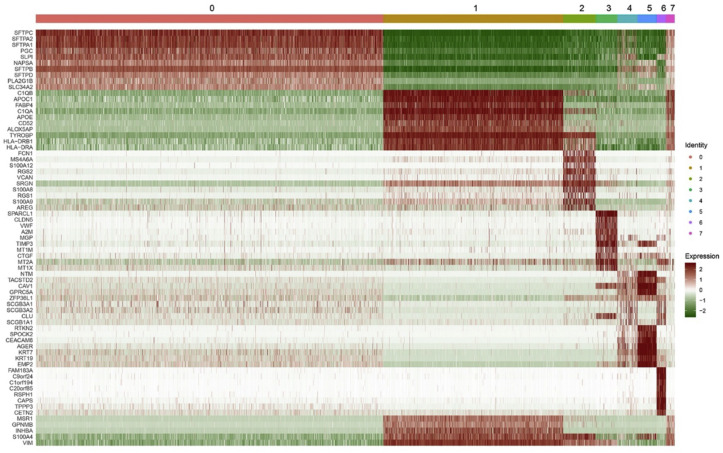
The tSNE classification results showed 8 types of cells, of which 2038 were alveolar type II epithelial cells (AT2), accounting for 54%; 1055 alveolar macrophages, accounting for 28%; 191 monocytes, accounting for 5%; 126 endothelial cells, accounting for 3%; 119 club cells, accounting for 3%; 112 alveolar type I cells (AT1), accounting for 2%; 55 ciliated cells, accounting for 1%; 51 dendritic cells, accounting for 1% (Fig. 5 A and B). As a functional receptor for SARS-CoV S protein, ACE2 is highly expressed in human lung AT2 cells (Fig. 5C). The heat map shows the expression of marker genes in 8 cell clusters, which can observe whether the marker gene expression is specific and determine the cell type based on the marker gene. 585, 858, 564, 826, 168, 1064, 1498 and 154 marker genes were highly expressed in cluster 0 to cluster 7 respectively (Supplementary materials). Representative high expressed marker genes in each cell cluster are displayed (Fig. 6 ).
Fig. 5.
tSNE classification (A) and cell clusters statistics (B) of human lung scRNA-Seq. Single cell clustering diagram of ACE2 expression (C).
Fig. 6.
Heat map of single cell gene expression and eight cell clusters were divided. (0: AT2; 1: Macrophages; 2: Monocytes; 3: Endothelial Cells; 4: Club Cells; 5: AT1; 6: Cilited Cells; 7: Dendritic Cells). Representative high expressed marker genes in each cell cluster are displayed.
3.5. ACE2 expressed AT2 cells intersect with XG-1 target genes
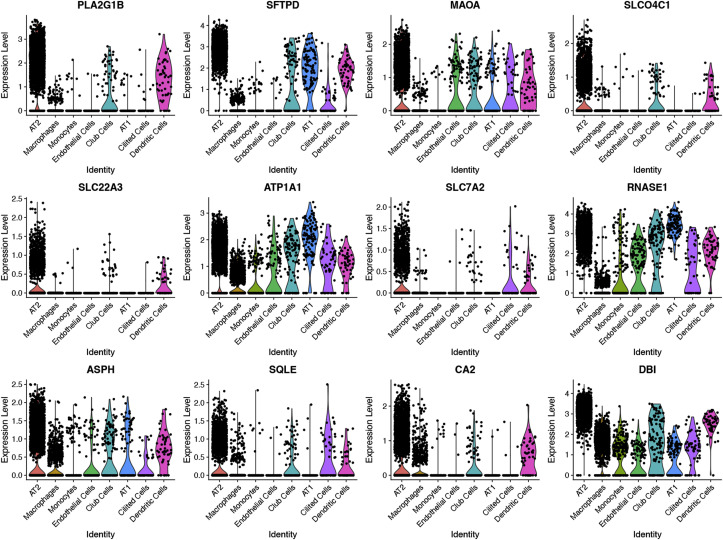
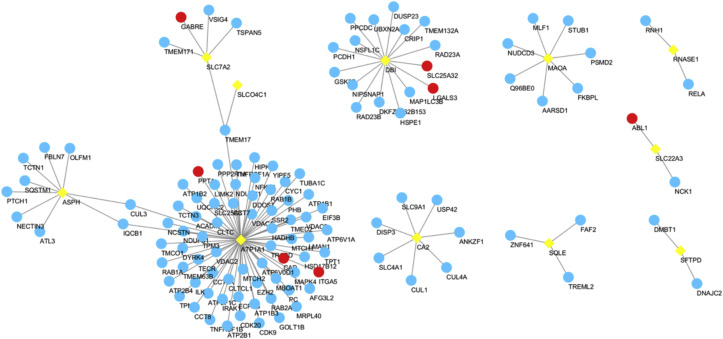
By co-expression analysis of the ACE2 overexpressed AT2 cells and 853 Chinese medicine target genes, 12 intersecting target genes were obtained: PLA2G1B, SFTPD, MAOA, SLCO4C1, SLC22A3, ATP1A1, SLC7A2, RNASE1, ASPH, SQLE, CA2, DBI, with their expression levels in different cells visualized by Violin diagrams (Fig. 7 ). There're some gene-gene networks were established connecting with these interaction genes except PLA2G1B. In the networks, ABL1, ITGA5, CAD, PPT1, GABRE, LGALS3 and SLC25A32 were found to be the target gene in XG-1 (Fig. 8 ). The expression distribution information of the 12 intersection target genes in AT2 cells was shown in Table 2. Among them, PLA2G1B, SFTPD and SLCO4C were related to immunity function.
Fig. 7.
VlnPlot of 12 intersecting target genes expressed in a single cell.
Fig. 8.
The interaction network of the intersection target genes. The yellow diamond represents the intersection target genes; the blue and red circles represent the interaction genes of the intersection target genes. The red ones also indicate that they are the target gene of XG-1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Function and distribution information of 12 intersection genes between ACE2 expressed AT2 cells target genes and XG-1 target genes.
| Gene | Expressed AT2 cells | Percentage of AT2 | Immune |
|---|---|---|---|
| PLA2G1B | 1932 | 94.80% | NK cells DAP12 receptors |
| SFTPD | 2034 | 99.80% | Innate immune response |
| MAOA | 1649 | 80.91% | |
| SLCO4C1 | 1253 | 61.48% | Innate immune system |
| SLC22A3 | 764 | 37.49% | |
| ATP1A1 | 1935 | 94.95% | |
| SLC7A2 | 704 | 34.54% | |
| RNASE1 | 2033 | 99.75% | |
| ASPH | 1539 | 75.52% | |
| SQLE | 845 | 41.46% | |
| CA2 | 1388 | 68.11% | |
| DBI | 2034 | 99.80% |
3.6. Molecular docking
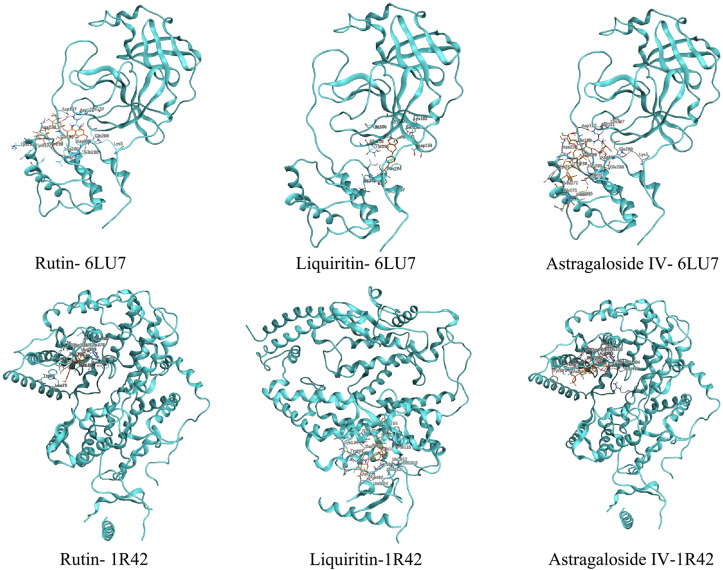
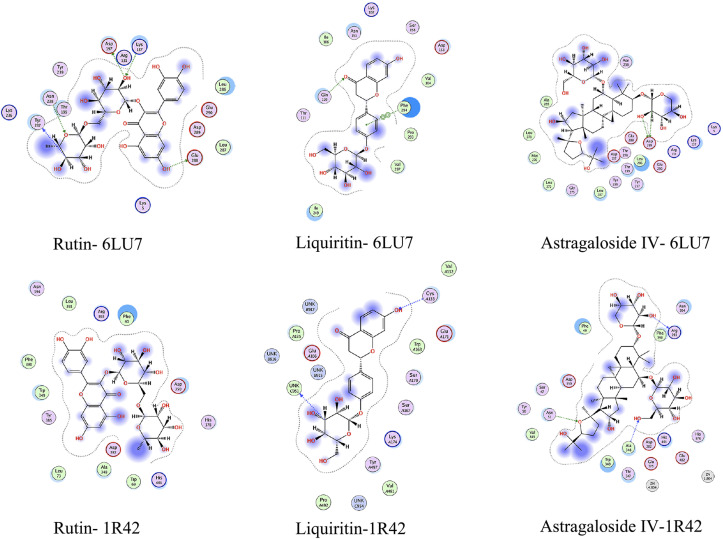
In this study, three key active compounds of Rutin, Liquiritin and Astragaloside Ⅳ were considered as ligands and the SARS-CoV-2 3CL hydrolase and ACE2 were receptors. Their interaction with surrounding key residues and their binding at the active site are shown in Fig. 9, Fig. 10 , and the energy values of the compounds shown in the docking results are shown in Table 5 . The smaller the docking energy means the more stable binding. From the results, it showed that the binding energy of Rutin, Liquiritin and Astragaloside Ⅳ to the SARS-CoV-2 3CL hydrolase (6LU7) were −7.9, −7.7 and −8.6 kJ/mol respectively, and their binding energy to the ACE2 (1R42) were −8.5, −8.5 and −8.8 kJ/mol respectively, indicating these compounds and the receptors may bind and form a stable conformation.
Fig. 9.
3D-interaction molecular docking diagram of the three active ingredients with 6LU7 and 1R42.
Fig. 10.
2D-interaction diagram of the three active ingredients with 6LU7 and 1R42.
Table 5.
Binding affinity of active ingredients in XG-1 with SARS-CoV-2 3CL hydrolase and ACE2 via molecular docking.
| Chemical compound | Molecular formula | Molecular Weight (g/mol) | CAS Number | Binding energy (kJ/mol) (6LU7) |
Binding energy (kJ/mol) (1R42) |
|---|---|---|---|---|---|
| Rutin | C27H30O16 | 610.5 | 153-18-4 | −7.9 | −8.5 |
| Liquiritin | C21H22O9 | 418.4 | 551-15-5 | −7.7 | −8.5 |
| Astragaloside Ⅳ | C41H68O14 | 785 | 84687-43-4 | −8.6 | −8.8 |
3.7. Retrospective study
A total of 47 close contacts were included in this study and the baseline characteristics were summarized in Table 6 . Of total contacts, 18 (38.3%) contacts were males. 36 (76.6%) contacts were adults aged 20–59 years; 12 (25.5%) contacts were spouse and 21 (44.7%) are non-spouse family members of the index cases. Regarding to the infection, 7 (14.9%) contacts were identified to be infected with SARS-COV-2, which included 6 (20.7%) females. The group aged 10–19 years, 50–59 years and over 70 years shared 33.3% attack rate. In addition, the attack rate differs from relationship with index cases: 16.7% for spouse; 19.1% for non-spouse family members; 12.5% for relatives. Participants with more contact frequency has a higher attack rate:18.8% for often; 7.7% for moderate, etc. XG-1 granules were distributed to everyone, the medication taking information was recorded and the data was analyzed. There're 36 participants (76.6%) took XG-1, while 11 (23.4%) refused to take Chinese medicine treatment during the quarantine time. There's no significance in the gender between the participants with taking XG-1 group and without (P = 0.12), while we observed that younger people are more likely to take XG-1 than older people (P = 0.01). Interestingly, regarding to the SARS-CoV-2 nucleic acid testing, of 11 participants who refused to take XG-1, 7 (63.64%) participants were found to be positive, while participants who took XG-1 were all confirmed negative results (P < 0.001). During the quarantine time, few participants expressed some slight symptoms and signs such as headache and insomnia. The characteristics were shown in Table 7 . We followed up all the close contacts who took XG-1 and found nobody detected nucleic acid positive and no respiratory symptoms or adverse effect appeared.
Table 6.
General characteristics of close contacts and attack rates of COVID-19 in contacts with different characteristics.
| Characteristics | Total contacts | Percentage (%) | Total infects | Attack rate (%) |
|---|---|---|---|---|
| Gender | ||||
| Male | 18 | 38.3 | 1 | 5.6 |
| Female | 29 | 61.7 | 6 | 20.7 |
| Age (years) | ||||
| 0–9 | 0 | 0 | 0 | 0 |
| 10–19 | 3 | 6.4 | 1 | 33.3 |
| 20–29 | 13 | 27.7 | 0 | 0 |
| 30–39 | 5 | 10.6 | 0 | 0 |
| 40–49 | 12 | 25.5 | 2 | 16.7 |
| 50–59 | 6 | 12.8 | 2 | 33.3 |
| 60–69 | 5 | 10.6 | 1 | 20.0 |
| ≥70 | 3 | 6.4 | 1 | 33.3 |
| Relationship with index cases | ||||
| Spouse | 12 | 25.5 | 2 | 16.7 |
| Family members (non-spouse) | 21 | 44.7 | 4 | 19.1 |
| Relatives | 8 | 17.0 | 1 | 12.5 |
| Others | 6 | 12.8 | 0 | 0 |
| Contact frequency | ||||
| Often | 32 | 68.1 | 6 | 18.8 |
| Moderate | 13 | 27.7 | 1 | 7.7 |
| Rarely | 2 | 4.2 | 0 | 0 |
Table 7.
Comparison of the characteristics between the groups with or without XG-1.
| Characteristics | With XG-1 (n = 36) | Without XG-1 (n = 11) | P -value | χ2 |
|---|---|---|---|---|
| Gender | ||||
| Male | 16 (44.44%) | 2 (18.18%) | 0.12 | 2.46 |
| Female | 20 (55.56%) | 9 (81.82%) | ||
| Age (years) | 38.53 ± 13.79 | 52.64 ± 20.35 | 0.01 | |
| SARS-CoV-2 nucleic acid detection | ||||
| Positive | 0 (0%) | 7 (63.64%) | <0.0001 | 26.92 |
| Negative | 36 (100%) | 4 (36.36%) | ||
| Signs and symptoms | ||||
| Fever | 0 (0%) | 0 (0%) | ||
| Cough | 0 (0%) | 0 (0%) | ||
| Dyspnea | 0 (0%) | 0 (0%) | ||
| Headache | 0 (0%) | 1 (9.09%) | ||
| Nausea | 0 (0%) | 0 (0%) | ||
| Muscle soreness | 0 (0%) | 0 (0%) | ||
| Mild insomnia | 1 (2.78%) | 2 (18.18%) | ||
4. Discussion
With the spread of COVID-19 epidemic, China suffered a major public health emergency. In the early stage of the outbreak, there're no specific antiviral drugs for COVID-19. TCM treatment combined with symptomatic treatment was the main strategy for patients in China. Studies referring to TCM treatment in combating COVID-19 showed significant therapeutic effects especially in early use (Ai et al., 2020; Shi et al., 2020; Wu et al., 2020). XG-1is a recommended preventive TCM formula and has been widely used to prevent the epidemic. To explore the material basis for the preventive effect of XG-1, network pharmacology approach was integrated to investigate the bioactive ingredients and potential mechanism. According to the network pharmacology study, the meridian information of each herb in XG-1 compound prescription was obtained and a meridian-herbs network of XG-1 was constructed. The meridian theory refers to the position of the herbs (or its chemical ingredients) action in the body, which is summarized through long-term observation of the curative effect in TCM (Zhao et al., 2008). Patients with COVID-19 manifest clinical symptoms such as fever, dry cough, fatigue, loss of appetite and nausea, as well as the abnormal radiological changes in lung tissue (Guan et al., 2020; Yang et al., 2020), while in the meridian network, 5 of 8 herbs in XG-1 have a specific relationship with the meridian of lung, spleen and stomach, indicating that XG-1 could acts on the lungs, spleen and stomach system to alleviate symptoms. Moreover, a total of 206 active ingredients and 853 target genes were obtained by screening the ETCM database. The target genes were collected to perform enrichment analysis. GO enrichment analysis revealed multiple pathways related to biological processes, molecular functions, and cell components; the enrichment results of KEGG pathway showed that XG-1 may exert effects via multiple signaling pathways such as neuroactive ligand-receptor, glutamate synapse, drug metabolism, calcium signaling pathway, and PPAR signaling pathway. Lin et al. stated upregulated gene expressions related to the neuroactive ligand-receptor interaction pathway and elevated internal Ca2+ were detected after H5N1 infection (Lin et al., 2015). Studies found that calcium signaling pathway could control the production of TNF-α and IL-10, which played a key role in immunity function regulation (Planes et al., 2018). Furthermore, calcium signaling pathway, as a mechanosensitive ion channel, could impact the cell stretch to stimulate surfactant secretion in AT II cells (Willner et al., 2003). Another study proved that PPAR expressed in lung macrophages and could affect the development of acute host disease and the restoration of tissue homeostasis after respiratory virus infection (such as respiratory syncytial virus) by regulating lung inflammation (Huang et al., 2019).
ACE2 has been identified as a functional receptor that could effectively bind to the S1 domain of the SARS-CoV spike protein, helping SARS virus to enter host cells and perform replication (Li et al., 2003). Recent studies have confirmed that SARS-CoV-2/2019-nCoV used the same cell receptor- ACE2-as SARS-CoV (Lu et al., 2020; Zhou et al., 2020). ACE2, as a homologue of ACE, could negatively regulate the renin-angiotensin system by balancing ACE activity, causing vasoconstriction and exerting multiple biological functions, which helps to prevent damages from acute lung injury (Imai et al., 2005; Kuba et al., 2006). A previous study has showed that ACE2 was abundantly distribute in the epithelia of the lung and small intestine in humans (Hamming et al., 2004). A recent scRNA-seq analysis from normal lung tissues of 8 adult donors proved that 83% of ACE2 expressing cells were alveolar type II epithelial cells (AT2), which believed that these cells could serve as a possible route for virus entry. In addition, GO enrichment analysis found AT2 expressing ACE2 had high expression of multiple genes related to viral processes, including regulatory genes for viral processes, viral life cycle, viral assembly and viral genome replication (Zhao et al., 2020). Therefore, AT2, as ACE2 expressing target cells, may be the most common and easily damaged cell in SARS-CoV-2 infection. In addition, host immune responses would be triggered during infection. The immune responses include innate immune response and adaptive immune response, both of them playing an indispensable role in defending against pathogens. Owing to only a small number of monocytes-macrophages in the lung express ACE2, how the SARS-CoV-2 infects innate immune cells still unclear. A latest retrospective study reported that T lymphocyte subsets (CD3+, CD4+, and CD8+ T-cell) were remarkably decreased and inflammatory cytokines (SAA, CRP, IL-6, and IL-10) were significantly increased in SARS-CoV-2 infected patients, which was positively related to the deaths of in-hospital and severity of disease (Xu, B. et al., 2020a). Moreover, a survey from lung immune microenvironment by scRNA-seq showed the presence of more CD8+ T-cells effectors and highly expanded clones CD8+ T-cells formed in the mildly COVID-19 patients, suggesting that a rapid and powerful adaptive immune response is potentially crucial for controlling COVID-19 pandemic (Liao et al., 2020).
To determine whether XG-1 acts on AT2 cells expressing ACE2, the scRNA-seq data of human lung was studied. It's found that AT2 cells were the highest expression type and were the ACE2-expressing cell type in the profiling. By labeling the AT2 cell genes expressed by ACE2, it found that 153 genes were up-regulated, and then co-analyzed with the target genes of XG-1 to obtain 12 intersection target genes, indicating that it may be the potential target for XG-1 to prevent COVID-19; Among them, 3 intersection target genes (PLA2G1B, SFTPD, SLCO4C1) were related to immunity, indicating that XG-1 may exert preventive effect by immune regulation.
In this study, HPLC-Q-TOF-MS was used to control quality and identify the main chemical constituents of XG-1 granules. Five chemical components in XG-1 were detected. Rutin and Chlorogenic acid (CGA) from Lonicera japonica Thunb. (Jin Yin Hua), Phillyrin (KD-1) from Forsythia suspensa (Thunb.) Vahl (Lian Qiao), Liquiritin from Glycyrrhiza uralensis Fisch. ex DC. (Gan Cao) and Astragaloside IV (AS-IV) from Astragalus mongholicus Bunge (Huang Qi). Jin Yin Hua is commonly used in Chinese medicine herbs. Numerous compounds such as flavonoids, organic acids, iridoids, triterpenoids and volatile components have been reported from this plant (Song et al., 2014). Rutin is a flavonoids compound and has been proved antioxidant and anti-inflammatory effects through promoting production of antioxidant enzyme and reducing the expression of proinflammatory cytokine such as NF-κB (Chen et al., 2020). CGA is an organic acids compound and as a quality control index component of Jin Yin Hua. Studies have shown that CGA has strong biological activities including antibacterial, antiviral, antitumor, antioxidative, anti-inflammatory and metabolic regulatory activities (Wang et al., 2020). KD-1 is a high content of lignan compounds and is used as an important indicator for the quality control of Lian Qiao. It's investigated that KD-1 could attenuate the inflammatory reaction and pathological changes caused by influenza A virus and LPS-infected mice (Qu et al., 2016; Zhong et al., 2013). Furthermore, KD-1 could protect against SARS-CoV-2 and human coronavirus 229E attack via suppressing the activity of NF-кB signaling pathway (Ma et al., 2020). A recent study revealed that CGA and KD-1 has an effective ability to prevent the combination of SARS-CoV-2-spike protein and ACE2 through the molecular docking method (Yu et al., 2020). AS- IV, a major active ingredient of Huang Qi, possesses anti-inflammatory, immune regulation, antioxidant, anti-apoptotic, anti-tumor and many other bioactive effects (Li et al., 2017a, Li et al., 2017b). Experiment showed that AS-IV has a therapeutic action on inflammation through activating the immune function of Tregs inhibited by high-mobility group box 1 (HMGB1) protein in mice (Li et al., 2016). Liquiritin is a representative ingredient of Gan Cao and it has a neuroprotective, antioxidant and anti-apoptosis effects via regulating multiple pathways (Li et al., 2020).
It's reported that SARS-CoV-2 3CL hydrolase was considered to be an effective target of SARS-CoV-2, which provides a basis for screening active ingredients against COVID-19 (Elmezayen et al., 2020). The binding of ACE2 and the S protein of SARS-CoV-2 in the human body was also proved to lead the virus invading the body and cause disease (Lu et al., 2020). In this study, key active compounds of Chlorogenic acid, Phillyrin, Rutin, Liquiritin and Astragaloside Ⅳ were screened by network pharmacology and HPLC-Q-TOF-MS were verified. Chlorogenic acid and Phillyrin has been explored to block the combination of SARS- CoV-2- spike protein and ACE2 through the molecular docking method (Yu et al., 2020). Therefore, Rutin, Liquiritin and Astragaloside Ⅳ were elected to perform molecular docking with SARS-CoV-2 3CL hydrolase and ACE2. From the results, it showed all the binding energy of the three active compounds to the SARS-CoV-2 3CL hydrolase (PDB: 6LU7) and ACE2 (PDB: 1R42) was all less than −7 kJ/mol, indicating these compounds may bind and form a stable conformation with the receptors to resist SARS-CoV-2.
The epidemiological investigations found that individuals who has a close contact exposure history was more likely confirmed as COVID-19 patient, with 1 out of 4.4–16.5 close contacts being identified to be infected with SARS-COV-2, so that tracing and quarantine the close contacts timely is essential to the epidemic spread and control (Bi et al., 2020; Liu, T. et al., 2020b; Jian et al., 2020). XG-1is a recommended preventive TCM formula and has been widely used in Hunan Province. It's reported that no infection case was found in the population taking the prescription and immune regulatory effect has been proven on adults (Liu, L. et al., 2020a). In order to clarify the preventive effect of XG-1 against COVID-19, a retrospective study was conducted, in which 47 close contacts with COVID-19 confirmed cases were screened. Among them, a total of 36 participants took XG-1 decoction and 11 participants refused to take. 7 participants were confirmed positive SARS-CoV-2 nucleic acid by RT-PCR and none of them took the XG-1 preventive prescription, which indicates the potential efficacy of this preparation as a COVID-19 preventive agent.
This study has some limitations. First, due to the incomplete understanding of the virus and disease pathogenic mechanism as well as the limitations of molecular docking itself, the results obtained may be biased. Second, we performed a community-based single-center retrospective study to assess the preventive effects of XG-1, however, relevant in vitro or in vivo experiments could not be carried out due to the biosecurity problem of the disease. The specific mechanism of action still needs further exploration. Third, the sample size in this study was relatively small that might influence the accuracy in statistical analysis. Thus, larger, multiple-centers and prospective studies are demanded to ascertain the benefit of XG-1 in COVID-19 prevention and control.
5. Conclusion
The present study showed that XG-1 exerts a potential preventive effect in close contacts against COVID-19. The underlying mechanism may relate to modulate immunity response and eliminate inflammatory process through several target genes co-expressed with ACE2. These findings provide preliminary evidences and methodological reference for the potential preventive mechanism of XG-1 against COVID-19.
Author contributions
LL, and MZX proposed and designed the study. ZYY, SSX and SYZ screened the data and conducted the data analysis. KG, PY and JJY collected the data of retrospective study. YQ and MZX performed the quality control. HYW wrote this manuscript, and LL revised it. All of the authors reviewed and approved the submitted version of the paper.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Scientific Research Fund of Hunan Administration of TCM (No. KYGG06 and KYGG07), Hunan Provincial Clinical Medical Technology Innovation Guidance Program (2017SK50315), Hunan Provincial Mental Behavior Disorder Clinical Medicine Research Center (2018SK7002), Hunan Province Innovation Guidance Project (2018SK50705) and Hunan Provincial Science and Technology Major Special Project (2018SK1030).
References
- Ai Z., Zhou S., Li W., Wang M., Wang L., Hu G., Tao R., Wang X., Shen Y., Xie L., Ba Y., Wu H., Yang Y. Fei yan No. 1" as a combined treatment for COVID-19: an efficacy and potential mechanistic study. Front. Pharmacol. 2020;11:581277. doi: 10.3389/fphar.2020.581277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z., Liu X., Wei L., Truelove S.A., Zhang T., Gao W., Cheng C., Tang X., Wu X., Wu Y., Sun B., Huang S., Sun Y., Zhang J., Ma T., Lessler J., Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect. Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Y., Huang C.N., Liao C.K., Chang H.M., Kuan Y.H., Tseng T.J., Yen K.J., Yang K.L., Lin H.C. Effects of Rutin on wound healing in hyperglycemic rats. Antioxidants. 2020;9(11) doi: 10.3390/antiox9111122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmezayen A.D., Al-Obaidi A., Sahin A.T., Yelekci K. Drug repurposing for coronavirus (COVID-19): in silico screening of known drugs against coronavirus 3CL hydrolase and protease enzymes. J Biomol Struct Dyn. 2020:1–13.doi. doi: 10.1080/07391102.2020.1758791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- General Office of the National Health Commission . 2020. Diagnosis and Treatment Plan for COVID-19 (Trial Version 8)http://www.gov.cn/zhengce/zhengceku/2020-08/19/5535757/files/da89edf7cc9244fbb34ecf6c61df40bf.pdf [Google Scholar]
- Guan, W.J., Ni, Z.Y., Hu, Y., Liang, W.H., Ou, C.Q., He, J.X., Liu, L., Shan, H., Lei, C.L., Hui, D.S.C., Du, B., Li, L.J., Zeng, G., Yuen, K.Y., Chen, R.C., Tang, C.L., Wang, T., Chen, P.Y., Xiang, J., Li, S.Y., Wang, J.L., Liang, Z.J., Peng, Y.X., Wei, L., Liu, Y., Hu, Y.H., Peng, P., Wang, J.M., Liu, J.Y., Chen, Z., Li, G., Zheng, Z.J., Qiu, S.Q., Luo, J., Ye, C.J., Zhu, S.Y., Zhong, N.S., China medical treatment expert group for, C., 2020. Clinical characteristics of coronavirus isease 2019 in China. N. Engl. J. Med. 382(18), 1708-1720.doi:10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhu B., Cheon I.S., Goplen N.P., Jiang L., Zhang R., Peebles R.S., Mack M., Kaplan M.H., Limper A.H., Sun J. PPAR-gamma in macrophages limits pulmonary inflammation and promotes host recovery following respiratory viral infection. J. Virol. 2019;93(9) doi: 10.1128/JVI.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunan Provincial Administration of Traditional Chinese Medicine Hunan Traditional Chinese Medicine Letter (2020) No. 23 Notice of the Hunan Provincial Administration of Traditional Chinese Medicine on the promotion of the use of traditional Chinese medicine in government agencies, enterprises, institutions, and schools to prevent new coronavirus infections. 2020. http://tcm.hunan.gov.cn/tcm/xxgk/tzgg/202002/t20200210_11175538.html
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian S.W., Cheng H.Y., Huang X.T., Liu D.P. Contact tracing with digital assistance in Taiwan's COVID-19 outbreak response. Int. J. Infect. Dis. 2020;101:348–352. doi: 10.1016/j.ijid.2020.09.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T.F., Leung P.C., Wong E.L., Fong C., Cheng K.F., Zhang S.C., Lam C.W., Wong V., Choy K.M., Ko W.M. Using herbal medicine as a means of prevention experience during the SARS crisis. Am. J. Chin. Med. 2005;33(3):345–356. doi: 10.1142/S0192415X05002965. [DOI] [PubMed] [Google Scholar]
- Leung P.C. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am. J. Chin. Med. 2007;35(4):575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li J., Huang L., Wang S., Yao Y., Zhang Z. Astragaloside IV attenuates inflammatory reaction via activating immune function of regulatory T-cells inhibited by HMGB1 in mice. Pharm. Biol. 2016;54(12):3217–3225. doi: 10.1080/13880209.2016.1216133. [DOI] [PubMed] [Google Scholar]
- Li L., Hou X., Xu R., Liu C., Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017;31(1):17–36. doi: 10.1111/fcp.12232. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Qin X., Tian J., Gao X., Wu X., Du G., Zhou Y. Liquiritin protects PC12 cells from corticosterone-induced neurotoxicity via regulation of metabolic disorders, attenuation ERK1/2-NF-kappaB pathway, activation Nrf2-Keap1 pathway, and inhibition mitochondrial apoptosis pathway. Food Chem. Toxicol. 2020;146:111801. doi: 10.1016/j.fct.2020.111801. [DOI] [PubMed] [Google Scholar]
- Li Y., Zheng B., Tian H., Xu X., Sun Y., Mei Q., Lin X., Liu L. Yupingfeng Powder relieves the immune suppression induced by dexamethasone in mice. J. Ethnopharmacol. 2017;200:117–123. doi: 10.1016/j.jep.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Lin X., Wang R., Zhang J., Sun X., Zou Z., Wang S., Jin M. Insights into human astrocyte response to H5N1 infection by microarray analysis. Viruses. 2015;7(5):2618–2640. doi: 10.3390/v7052618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zhang X., Li Y.J., He S.G., Wu S.L., Chen C.Y., Tang B., He H.H., Li X.J. The effect of hunan preventing corona virus disease 2019 (COVID-19) No.1 prescription on human cellular immunity and humoral immunity. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2020;26(10):8–11. doi: 10.13862/j.cnki.cn43-1446/r.2020.10.001. [DOI] [Google Scholar]
- Liu X., Shen J., Fan D., Qiu X., Guo Q., Zheng K., Luo H., Shu J., Lu C., Zhang G., Lu A., Ma C., He X. Yupingfeng san inhibits NLRP3 inflammasome to attenuate the inflammatory response in asthma mice. Front. Pharmacol. 2017;8:944. doi: 10.3389/fphar.2017.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Liang W., Zhong H., He J., Chen Z., He G., Song T., Chen S., Wang P., Li J., Lan Y., Cheng M., Huang J., Niu J., Xia L., Xiao J., Hu J., Lin L., Huang Q., Rong Z., Deng A., Zeng W., Li J., Li X., Tan X., Kang M., Guo L., Zhu Z., Gong D., Chen G., Dong M., Ma W. Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerg. Microb. Infect. 2020;9(1):1546–1553. doi: 10.1080/22221751.2020.1787799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. 10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Li R., Pan W., Huang W., Liu B., Xie Y., Wang Z., Li C., Jiang H., Huang J., Shi Y., Dai J., Zheng K., Li X., Hui M., Fu L., Yang Z. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-kappaB) signaling pathway. Phytomedicine. 2020;78:153296. doi: 10.1016/j.phymed.2020.153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., Luan X., Zhao Y., Xu J., Yuan W., Chen H. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm. Sin. B. 2020;10(7):1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes R., Serrero M., Leghmari K., BenMohamed L., Bahraoui E. HIV-1 envelope glycoproteins induce the production of TNF-alpha and IL-10 in human monocytes by activating calcium pathway. Sci. Rep. 2018;8(1):17215. doi: 10.1038/s41598-018-35478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X.Y., Li Q.J., Zhang H.M., Zhang X.J., Shi P.H., Zhang X.J., Yang J., Zhou Z., Wang S.Q. Protective effects of phillyrin against influenza A virus in vivo. Arch Pharm. Res. (Seoul) 2016;39(7):998–1005. doi: 10.1007/s12272-016-0775-z. [DOI] [PubMed] [Google Scholar]
- Shalek A.K., Satija R., Adiconis X., Gertner R.S., Gaublomme J.T., Raychowdhury R., Schwartz S., Yosef N., Malboeuf C., Lu D., Trombetta J.J., Gennert D., Gnirke A., Goren A., Hacohen N., Levin J.Z., Park H., Regev A. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498(7453):236–240. doi: 10.1038/nature12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Lu Y., Zhang Y., Xia L., Ye C., Lu Y., Chen S., Xu Q., Tang B., Yin K., Zhang J., Chen X., Yang Z. Traditional Chinese medicine formulation therapy in the treatment of coronavirus disease 2019 (COVID-19) Am. J. Chin. Med. 2020;48(7):1523–1538. doi: 10.1142/S0192415X20500755. [DOI] [PubMed] [Google Scholar]
- Song Y.L., Ni F.Y., Zhao Y.W., Xie X., Huang W.Z., Wang Z.Z., Xiao W. Research progress on chemical constituents from Lonicerae Flos. Chin. Tradit. Herb. Drugs. 2014;45(24):3656–3664. doi: 10.7501/j.issn.0253-2670.2014.24.027. [DOI] [Google Scholar]
- Stubbington M.J.T., Rozenblatt-Rosen O., Regev A., Teichmann S.A. Single-cell transcriptomics to explore the immune system in health and disease. Science. 2017;358(6359):58–63. doi: 10.1126/science.aan6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.H., Du T.T., Zhang Z.H., Ji M., Hu H.Y., Chen X.G. Advances in research on the pharmacological effects and mechanism of action of chlorogenic acid. Acta Pharm. Sin. 2020;55(10):2273–2280. doi: 10.16438/j.0513-4870.2020-0423. [DOI] [Google Scholar]
- Wang Z., Cai X., Pang Z., Wang D., Ye J., Su K., Sun X., Li J., Cao P., Hu C. Yupingfeng pulvis regulates the balance of T cell subsets in asthma mice. Evid Based Complement Alternat Med. 2016;2016:6916353. doi: 10.1155/2016/6916353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner J., Vordermark D., Schmidt M., Gassel A., Flentje M., Wirtz H. Secretory activity and cell cycle alteration of alveolar type II cells in the early and late phase after irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003;55(3):617–625. doi: 10.1016/s0360-3016(02)03991-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2020. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV)https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov [Google Scholar]
- World Health Organization . 2020. Coronavirus Disease (COVID-19) Outbreak Situation.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- Wu H.Y., Zhang C., Wang Z.H., Zhang S.Y., Li J., Li F., Huang H.Y., Li L. Network pharmacology based analysis on the molecular biological mechanisms of Xin Hui Tong Formula in coronary heart disease treatment. Digital Chinese Medicine. 2019;2(2):86–96. doi: 10.1016/j.dcmed.2019.09.003. [DOI] [Google Scholar]
- Wu X.V., Dong Y., Chi Y., Yu M., Wang W. Traditional Chinese Medicine as a complementary therapy in combat with COVID-19-A review of evidence-based research and clinical practice. J. Adv. Nurs. 2020 doi: 10.1111/jan.14673. [DOI] [PubMed] [Google Scholar]
- Xu B., Fan C.Y., Wang A.L., Zou Y.L., Yu Y.H., He C., Xia W.G., Zhang J.X., Miao Q. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.Y., Zhang Y.Q., Liu Z.M., Chen T., Lv C.Y., Tang S.H., Zhang X.B., Zhang W., Li Z.Y., Zhou R.R., Yang H.J., Wang X.J., Huang L.Q. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(D1):D976–D982. doi: 10.1093/nar/gky987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in wuhan, China. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.W., Wang L., Bao L.D. Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID-19) based on molecular docking method. J Funct Foods. 2020;71:104016. doi: 10.1016/j.jff.2020.104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Chen D. Anticomplementary principles of a Chinese multiherb remedy for the treatment and prevention of SARS. J. Ethnopharmacol. 2008;117(2):351–361. doi: 10.1016/j.jep.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li Y., Wang X., Sun W. The experimental study of Cortex Eucommiae on meridian tropsim: the distribution study of aucubin in rat tissues. J. Pharmaceut. Biomed. Anal. 2008;46(2):368–373. doi: 10.1016/j.jpba.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Zhong W.T., Wu Y.C., Xie X.X., Zhou X., Wei M.M., Soromou L.W., Ci X.X., Wang D.C. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-kappaB activation in acute lung injury mice. Fitoterapia. 2013;90:132–139. doi: 10.1016/j.fitote.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Zhou C.J., Ma F., Liao W.J., Song L.J., Yu D., Song Y.N., Hu T.Y., Liu Z.Q., Liu Z.G., Zhang X.W., Yang P.C. Restoration of immune suppressor function of regulatory B cells collected from patients with allergic rhinitis with Chinese medical formula Yupingfeng San. Am J Transl Res. 2019;11(3):1635–2164. [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]