Abstract
BACKGROUND:
Lumbar disc herniation (LDH) occurs owing to the inability of the posterior longitudinal ligament (PLL) to preserve the disc material within the intervertebral space. There is apparently no study that has investigated the histopathological changes occurring in both PLL and disc material in patients with LDH.
OBJECTIVE:
Investigate and compare the histopathological changes occurring in PLL and disc material of the patients who underwent a surgical operation for LDH.
DESIGN:
Descriptive, cross-sectional.
SETTING:
Pathology and neurosurgery departments of a tertiary health care institution
PATIENTS AND METHODS:
The study included patients who underwent surgical operation for LDH from January 2018 to May 2019 and whose PLL and disc material were removed together, and had disc degeneration findings that were radiologically and histologically concordant.
MAIN OUTCOME MEASURES:
PLL degeneration scores according to the histopathological findings, changes in disc materials according to the MRI findings, disc degeneration scores according to the histo-pathological findings.
SAMPLE SIZE:
50.
RESULTS:
MRI and histological examinations showed fully degenerated black discs (Grade 2) in 12 patients, partially degenerated discs (Grade 1) in 29 patients and fresh/acute discs (Grade 0) in 9 patients. The PLL showed grade 0 degeneration in 2 patients, grade 1 degeneration in 23 patients, and grade 2 degeneration in 25 patients. PLL degeneration grades were higher than the disc degeneration grades (P=.002).
CONCLUSION:
Longitudinal ligament degeneration can play a significant role in the pathogenesis of LDH. To the best of our knowledge, this study represents the first to focus on the histopathological changes occurring in both the PLL and disc material in patients with LDH.
LIMITATIONS:
Small sample, retrospective
CONFLICT OF INTEREST:
None.
INTRODUCTION
Lumbar disc herniation (LDH) is one of the most frequently diagnosed disorders in neurosurgery.1 One of the causes of the clinical symptoms in LDH is that the disc protrudes from the posterior longitudinal ligament (PLL).2 Previous studies focusing on the etio-pathogenesis of LDH mainly evaluated the histopatho-logical changes in the disc material.3–5 However, the clinical symptoms are usually related to the compressive effect of the disc on the radix or dural sac. Furthermore, the clinical symptoms and compression of the neural structures are unrelated to the histopathological changes in the disc material.
The PLL supports the mobility, stability, and flexibility of the spine. It also protects the spinal cord by restricting flexion and lateral tilt movements.6,7 The PLL prevents the disc from protruding and plays a key role in the pathogenesis of LDH.8 This study aimed to investigate and compare the histopathological changes that occur in the PLL and disc material in patients with LDH to understand the etiopathogenesis of this disorder.
PATIENTS AND METHODS
This study included patients who underwent surgery for LDH between January 2018 and May 2019. The inclusion criteria were as follows: 1) patients with sufficient clinical data, 2) patients with sufficient pathological material for analysis (fragmented samples were excluded), 3) patients whose PLL and disc material were excised together, and 4) patients had disc degeneration findings radiologically and histologically concordant. All the hematoxylin and eosin-stained slides were re-evaluated. The histopatho-logical parameters that were evaluated and graded for PLL included capillary proliferation, amount of chronically inflamed cells, changes in the collagen fibers (fibroblastic proliferation, fibrosis), and the presence of calcification and/or ossification (Grade 0, 1, and 2) (Table 1, Figure 1). Cyst formation and mucoid degeneration were also investigated during the histopathological assessment of the PLL, as these findings were not observed, they were not included in the tables. Any histological scoring system to assess the morphological changes of PLL degeneration in patients diagnosed with LDH has not existed so far. When determining the parameters, we used the microscopic findings of PLL degeneration mentioned in various diseases affecting the PLL.9 As no other method for comparison of the PLL and disc materials was available in the literature; a scoring system was required for performing this comparison. We used a scoring system that used the sum of the scores of the pathological changes. All procedures performed in this study followed the Declaration of Helsinki and the study was approved by the local clinical research ethics committee (approval date and number: 2019-11/133). Informed consent was obtained from all the participants.
Table 1.
Histopathological scoring of posterior longitudinal ligament degeneration (Grade 0: 0, Grade 1: 1-3, Grade 2: 4-6).
| Histopathological parameters | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Chronic inflammation | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Capillary proliferation | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Changes in collagen fibers (fibroblastic proliferation, fibrosis) | 0 (none) | 1 (available) | - |
| Calcification/ossification | 0 (none) | 1 (available) | - |
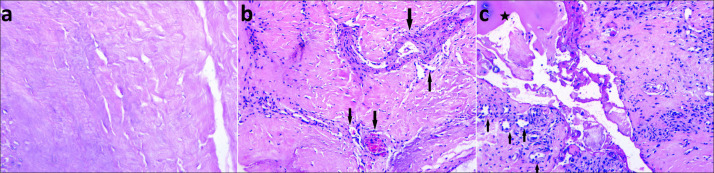
Figure 1: The grading of PLL degeneration according to the histopathological findings. (a: Histologic image shows a normal posterior longitudinal ligament; Grade 0, b: Mild capillary proliferation and mild lymphocyte infiltration (arrows) in the ligament; Grade 1, c: Intense capillary proliferation, intense lymphocyte infiltration (arrows), collagen is dense and has a fibrous appearance, ossification is seen in the ligament (star); Grade 2) (H&E, ×200; H&E, ×100; H&E, ×100).
Grading of the disc degeneration was based on both radiological and histopathological findings that were concordant for each patient (Grade 0, 1, and 2) (Figures 2 and 3). The scoring was based on the most intense microscopic area representing the sample in sufficient quantity. We excluded very fragmented biopsy specimens, since they were considered undiagnostic. The de-generations observed in the disc material and PLL were compared to evaluate their etiopathogenetic roles.
Figure 2: Changes in disc materials according to the MRI findings (a: Mild degenerative changes that did not demonstrate any degenerative findings on MRI were evaluated as Grade 0, b: Mild-to-moderate smaller discs showing “black and white areas” on MRI; partial degenerative changes observed on MRI examination were evaluated as Grade 1. c: Marked degenerative progression, significantly smaller disc volume, and changes in excised disc materials deemed as “black discs” were evaluated as Grade 2.
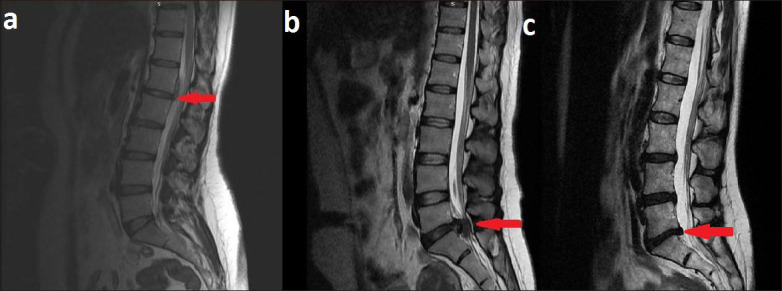
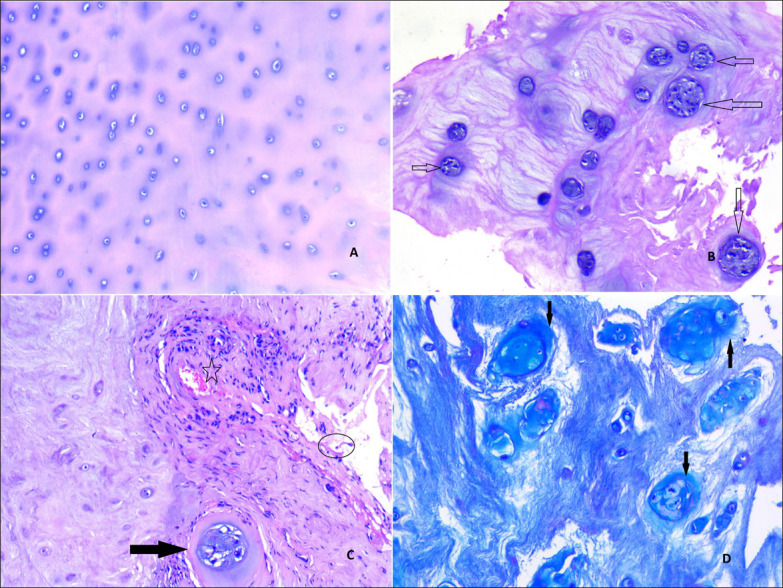
Figure 3: The grading of disc degeneration according to the histopathological findings (a: Microscopic image shows an area from a non-degenerated disc; Grade 0, b: Chondrocyte proliferation, clones of chondrocytes are seen (arrows) from a Grade 1 disc, c: A clone of chondrocytes (arrow), clefts (circle), neovascularization and lymphocyte precipitate (star) in continuity with the disc tissue; Grade 2) (H&E, ×100), d: Alcian Blue-PAS Staining (×100).
A semi-quantitative scoring was performed for the histological evaluation of the disc materials, and three categories (Grade 0, 1, and 2) were defined as shown in Table 2. The parameters of the scoring system included chondrocyte proliferation, tears/clefts, granular changes, mucoid changes, and neovascularization. The available histomorphological studies on disc material are predominantly based on autopsy tissue samples in experimental animal models and human post-mortem cohorts. Histopathological scoring systems for disc degeneration are not yet integrated into routine pathology of surgical materials. While creating the histopathological degeneration scoring of the surgically obtained disc samples, we modified the criteria developed by Rowas et al.10 We selected the first four parameters from the Rowas et al grading scheme to score disc degeneration. We added the neovascularization parameter because abnormal vascularization was seen in the degenerated discs.8 Quantitative histological assessment was not performed because of the small number of patients; however, the available data were included for completion. The histopathological changes observed in the disc materials were classified based on the findings detected on magnetic resonance imaging (MRI) examinations, which were performed without using a contrast agent. Height loss, osteophyte formation, and diffuse sclerosis were used to assess the disc degeneration (Table 3).11 Mild histopathological degenerative changes in the disc materials that did not demonstrate any degenerative findings on MRI were evaluated as “grade 0.” Mild-to-moderate smaller discs showing “black and white areas” on MRI, partial degenerative changes observed both in histopathological and MRI examinations were evaluated as “grade 1.” Marked degenerative progression, a significantly smaller disc volume, and changes in the excised disc materials considered as “black discs” were evaluated as “grade 2.”
Table 2.
Histopathological scoring of disc degeneration (Grade 0: 0, Grade 1: 1-5, Grade 2: 6-10).
| Histopathological parameters | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Chondrocyte proliferation | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Tears/clefts | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Granular changes | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Mucoid changes | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Neovascularization | 0 (none) | 1 (mild/moderate) | 2 (intense) |
Table 3.
Magnetic resonance imaging, scoring of disc degeneration (Grade 0 (fresh/acute disc): 0, Grade 1 (partially degenerated): 1-3, Grade 2 (black disc): 4-6).
| Radiological (MRI) parameters | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Height loss | 0 (none) | 1 (mild/moderate) | 2 (severe) |
| Osteophyte formation | 0 (none) | 1 (mild/moderate) | 2 (intense) |
| Endplate sclerosis | 0 (none) | 1 (mild/moderate) | 2 (severe) |
Statistical analysis was performed using IBM SPSS for Windows, version 21.0 (IBM SPSS Statistics for Windows, version 21.0, Armonk, NY: IBM Corp., USA). Comparisons were made using the chi-square test. A P value of <.05 was considered to be statistically significant. The explanatory statistics of the variables are presented as numbers (n) and percentages (%).
Two researchers assessed the grading 0, 1. 2. Calculation of the inter- and intra-rater reliability for the assessment of the PLL degeneration and disc degeneration in 50 cases was done. Categorical data were analyzed using a chi-square test, and 2x2 post hoc chi-square tests were performed for pairwise differences.
RESULTS
The 50 patients included in the study had a mean age of these patients was 48.1 years (22 men and 28 women). The mean age of the patients with acute disc herniation was 33.5 years. MRI and histological examinations showed fully degenerated black discs (grade 2) in 12 patients (24%), partially degenerated discs (grade 1) in 29 patients (58%), and fresh/acute discs (grade 0) in nine patients (18%). The PLL materials showed grade 0 degeneration in two patients (4%), grade 1 degeneration in 23 patients (46%), and grade 2 degeneration in 25 patients (50%). Grades of the disc and PLL degenerations are shown in Table 1 and Table 2. The number of cases, according to the grades, is shown in Table 4. Calculation of the interrater reliability for the assessment of the PLL and disc degenerations in 50 cases showed substantial rater agreement (kappa statistics: PLL degeneration=.674; disc degeneration=.708). The intraobserver reliability test showed excellent intra-rater agreement (kappa statistics: 1. evaluator=.888; 2. evaluator=.897).
Table 4.
The comparison of the states of disc degeneration and posterior longitudinal ligament degeneration.
| PLL degeneration | Disc degeneration | Disc degeneration/LDH diagnosis | PLL degeneration/LDH diagnosis | |
|---|---|---|---|---|
| Grade 0 | 2 | 9 | 9/50 (18) | 2/50 (4) |
| Grade 1 | 23 | 29 | 29/50 (58) | 23/50 (46) |
| Grade 2 | 25 | 12 | 12/50 (24) | 25/50 (50) |
Data are number (%).
Of the PLLs that were attached to the discs with grade 2 degeneration, which were considered fully degenerated, none showed grade 0 degeneration, while 67.0% and 33.0% showed grade 1 and grade 2 degeneration, respectively. Further, 8% of the grade 2 PLLs were attached to grade 0 disc materials, 76% to grade 1 disc materials, and 16% to grade 2 disc materials.
The difference between the degeneration grade of PLL and disc were statistically significant (Table 5). The PLL degeneration grades were statistically significantly higher than the disc degeneration grades (P=.002). When the number of patients with the same disc degeneration grade were compared by the PLL degeneration, the chi-square test results showed that the number of patients with grade 1 PLL degeneration was significantly higher than those with grade 2 PLL degeneration, in cases of non-degenerated (grade 0) discs (P=.003). The number of patients with grade 2 PLL degeneration was significantly higher than those with grade 1 and 0 PLL degeneration, in cases of partial degenerated (grade 1) discs (P=.001). The number of patients with grade 2 PLL degeneration was significantly lower than those with grade 1 PLL degeneration, in cases of severe degenerated (grade 2) discs (P=.001).
Table 5.
Comparison of disc degeneration and posterior longitudinal ligament degeneration.
| Disc degeneration grades (ddg) | |||||
|---|---|---|---|---|---|
| PLL degeneration grades (pdg) | 0 | 1 | 2 | Total | P value (pdg, ddg) |
| 0 | 0 | 2 | 0 | 2 | - |
| 1 | 7 | 8 | 8 | 23 | Pa (1, 0)= .003 |
| 2 | 2 | 19 | 4 | 25 | Pb (2, 1)=.001, Pc (2, 2)=.001 |
| Total | 9 | 29 | 12 | 50 | Pd=.002 |
Data are numbers of patients. Overall chi-square P value: .008, degrees of freedom: 2. Crosstabs with Pearson chi-square test (Pa, Pb, Pc) and dependent-sample t-test (Pd):
Grade 1 PLL degeneration was significantly higher than those with grade 2, with non-degenerated (grade 0) discs.
Grade 2 PLL degeneration was significantly higher than those with grade 1 and 0, with partial degenerated (grade 1) discs.
Grade 2 PLL degeneration was significantly lower than those with grade 1, with severe degenerated (grade 2) discs.
PLL degeneration grades were statistically significantly higher compared to disc degeneration grades.
DISCUSSION
LDH typically affects adults, and it is possible to encounter disc degeneration in adults, with or without evidence of disc herniation. Various factors, including mechanical influences, environmental factors, occupational exposures, traumatic injuries, smoking, and changes related to aging, have been implicated in the etiopathogenesis of disc degeneration.12,13 Inflammation of the disc tissue plays a significant role in the process of accelerated disc degeneration.14–16 Genetic factors are increasingly proven; whereas, environmental factors are relatively less important.17 Solovieva et al have indicated that disc degeneration is a multifactorial process involving synergistic effects in a gene-environment interaction.18
The ligaments are functional under stretch or stress. If the mechanical compression exceeds a critical threshold, injuries can occur. This leads to morphofunctional changes and movement disorders.19,20 The key factor in the development of disc herniation is the protrusion of the PLL, or herniation of the disc material outside the PLL.21–24 Hence, the main pathogenetic factor of disc herniation could be the failure of the PLL to keep the disc material within the intervertebral space. Therefore, the histopathological changes that occur in the PLLs may shed a light on the pathogenesis of this disorder. Various histopathological findings have been investigated in cases involving PLL degeneration.9 Fibroblast proliferation, capillary proliferation, chondrocyte infiltration owning to the metaplasia of collagen fibers, and small ossifications are the main histopathological changes observed in the degenerated ligament.
The PLL, a connective tissue covering the dorsal surface of the vertebral body, is located within the vertebral canal and extends from the cervical spine to the sacrum. The PLL contains smooth and longitudinal fibers.6,7 When mechanical influences are considered, the mechanical effects of the vertical pressure experienced by the disc material and PLL are clearly different. The compressive force that the PLL sustains while subjected to vertical forces is quite lower as its surface area is much smaller than the area of the disc.
To the best of our knowledge, no study has investigated the pathological features of the PLL or compared the disc and PLL degenerations in patients with LDH. This study evaluated the PLLs and disc materials of patients who underwent surgery for LDH and whose PLLs were excised together with the disc material. The PLL excision is not a routine component of discectomy and most of the PLL materials evaluated in this study were attached to the disc material in the course of surgery.
The comparison of the histopathological changes observed in the disc materials and PLLs revealed that disc herniation becomes more prevalent as the grade of PLL degeneration increases. Only two patients (4.0%) had grade 0 PLL degeneration, while the remaining 48 patients (96.0%) had grade 1 or grade 2 PLL degeneration. No statistically significant relationship between disc degeneration and the development of disc herniation was observed. Since the protruding or extruding disc material was dislocated from the intervertebral space, the disc material may be exposed to further degeneration, as it will not be able to maintain its normal physiological status. Accordingly, in patients with herniation, the disc material was evaluated as grade 1 or grade 2, except for acute disc herniation. In this study, 41 (82%) patients had grade 1 and grade 2 disc degeneration.
Fully degenerated discs, which correspond to grade 2 degeneration, contain less fluid, collagen, and aggre-cans, and they are usually smaller in volume.25 Therefore, in patients with fully degenerated discs, the compressive force that the disc can exert on the PLL will be less in terms of volume, and disc herniation will be less frequent. Furthermore, these patients usually present with degeneration-related non-radicular symptoms, rather than radicular symptoms, which are more common in disc herniation.
In this study, disc herniation was infrequent among patients with grade 0 PLLs, who comprised only 4.0% of the total patients. We suggest that the disc herniation that occurred in this group may be related to the inability of the PLL to resist the disc-induced pressure. Such cases may appear when the compressive force exerted on the PLL by the disc material, which itself is subjected to sudden vertical force, overcomes the resistance of the PLL. Therefore, these patients usually present with slightly degenerated or grade 0 disc materials. Hence, these patients have a significantly lower mean age and typically present with acute disc herniation. In this study, the mean age of the patients with acute disc herniation was 33.5 years.
LDH occurs owing to the inability of the PLL to preserve the disc material within the intervertebral space. Most of the studies investigating the histopathological aspect of LDH focused on the degenerated disc materials.3–5,26 In fact, we emphasize that the main factor in the pathogenesis may not be the degeneration of the disc material, but the PLL as it can no longer fulfill its protective function. The degeneration of the disc material is categorized as degenerative disc disease. Protruding or extruding disc materials, if not linked to acute disc herniation, cannot maintain their normal physiological process as their localization changes. Therefore, degenerated discs are more prevalent among patients who undergo surgery.
In patients with LDH, detailed histological data on the degeneration of the PLL tissue is mandatory to select the right therapeutic options. Better recognition of the biological nature of the degenerated PLL may allow use of biomaterials or cell-based transplantation therapies that have developed rapidly in recent years.20 This may be more valuable in patients presenting with relapse.
Özcan-Ekşi et al reported that severe disc degeneration in the lumbar spine was closely related to end-plate changes by examining the MRI images.27 In this study, surgical tissue samples representing endplate changes could not be obtained with sufficient accuracy in all patients owing to the retrospective nature of the study. This was a limitation of the study. The evaluation of chondrocyte proliferation, mucoid changes, and neovascularization was reliable and simple. In a few cases, tears and clefts owing to the mostly fragmented feature of the samples, and granular changes due to “wash out” effects were difficult to determine.
There were no other studies to compare with our study. Our study showed that PLL degeneration can play a significant role in the pathogenesis of LDH. To the best of our knowledge, this study is the first step toward histopathological changes occurring in both the PLL and disc material in patients with LDH. Considering the lack of the studies on this topic, we believe that our study provides a morphological basis for future quantitative, comprehensive, and prospective studies.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to Omer Faruk Elmas and Emine Muge Acar, who offered support in the preparation and design of the manuscript.
Funding Statement
None.
REFERENCES
- 1.Wu JC, Mummaneni PV. Lumbar disc herniation and surgical management. World neurosurg. 2010;74(6):572-3. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg MS. Handbook of Neurosurgery. 6th edition. Thieme medical publisher; 2006;pp:289-312. [Google Scholar]
- 3.Pytel P, Wollmann RL, Fessler RG, Krausz TN, Montag AG. Degenerative spine disease: pathologic findings in 985 surgical specimens. Am J Clin Pathol. 2006;125(2):193-202. [DOI] [PubMed] [Google Scholar]
- 4.Amin RM, Andrade NS, Neuman BJ. Lumbar Disc Herniation. Curr Rev Musculoskelet Med. 2017;10(4):507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens F, Vajkoczy P, Jadik S, Hegewald A, Stieber J, Hes R. Patients at the Highest Risk for Reherniation Following Lumbar Discectomy in a Multicenter Randomized Controlled Trial. JB JS Open Access. 2018 Jun 28;3(2):e0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SB, Chang JC, Lee GS, Hwang JC, Bae HG, Doh JW. Morphometric Study of the Lumbar Posterior Longitudinal Ligament. J Korean Neurosurg Soc. 2018;61(1): 89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salaud C, Ploteau S, Hamel O, Armstrong O, Hamel A. Morphometric study of the posterior longitudinal ligament at the lumbar spine. Surg. Radiol. Anat. 2018;40(5): 563-9. [DOI] [PubMed] [Google Scholar]
- 8.Martin MD, Boxell CM, Malone DG. Patho-physiology of lumbar disc degeneration: a review of the literature. Neurosurg focus. 2002;13(2):1-6. [DOI] [PubMed] [Google Scholar]
- 9.Sato R, Uchida K, Kobayashi S, Yayama T, Kokubo Y, Nakajima H, et al. Ossification of the posterior longitudinal ligament of the cervical spine: histopathological findings around the calcification and ossification front. J Neurosurg Spine. 2007;7(2): 174-83. [DOI] [PubMed] [Google Scholar]
- 10.Rowas SA, Haddad R, Gawri R, Al Ma’awi AA, Chalifour LE, Antoniou J, Mwale F. Effect of in utero exposure to diethylstilbestrol on lumbar and femoral bone, articular cartilage, and the intervertebral disc in male and female adult mice progeny with and without swimming exercise. Arthritis Res Ther. 2012 Jan 23;14(1):R17. doi: 10.1186/ar3696. PMID: 22269139; PMCID: PMC3392807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke HJ, Rohlmann F, Neidlinger-Wilke C, Werner K, Claes L, Kettler A. Validity and interobserver agreement of a new radio-graphic grading system for intervertebral disc degeneration: Part I. Lumbar spine. European Spine Journal. 2006; 15(6): 720-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debnath UK. Etiology and Risk Factors of Lumbar Intervertebral Disc (IVD) Degeneration. Res Med Eng Sci. 2018;4(5):1-10. [Google Scholar]
- 13.Oichi T, Taniguchi Y, Oshima Y, Tanaka S, Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3(1):e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine. 2007;32:2812-9. [DOI] [PubMed] [Google Scholar]
- 15.Murakami H, Yoon ST, Attallah-Wasif ES, Tsai KJ, Fei Q, Hutton WC. The expression of anabolic cytokines in intervertebral discs in age-related degeneration. Spine 2006; 31:1770-4. [DOI] [PubMed] [Google Scholar]
- 16.Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J 2007; 16:447-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teles Filho RV, Abe GDM, Daher MT. Genetic Influence in Disc Degeneration-Systematic Review of Literature. Revista brasileira de ortopedia. 2020; 55(2):131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, Ala-Kokko L, et al. COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene-environment interaction. Spine. 2002; 27:2691-6. [DOI] [PubMed] [Google Scholar]
- 19.Borges MCD, Errero TK, Rosa CT, Bernardino GR, Brancalhão RMC, Ribeiro LFC, et al. Evaluation of longitudinal ligament of the spine of Wistar rats in an experimental model of Suit therapy. Fisioter Pesqui. 2016; 23(2): 148-54. [Google Scholar]
- 20.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker MH, Anderson DG. Molecular basis of intervertebral disc degeneration. Spine J. 2004;4(6):158-66. [DOI] [PubMed] [Google Scholar]
- 22.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006. Apr;88 Suppl 2:10-4. doi: 10.2106/JBJS.F.00019. PMID: 16595436. [DOI] [PubMed] [Google Scholar]
- 24.Asan Z. Lumbar disc herniations causing contralateral radicular symptoms: Can they be explained by hypotenusal theory? World Neurosurg. 2018;114:e1297-e1301. [DOI] [PubMed] [Google Scholar]
- 25.Choi YS. Pathophysiology of Degenerative Disc Disease. Asian Spine J. 2009;3(1): 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitfield PC, Gherle MS. Treatment options and pathophysiology of degenerative spine disease. Surgery. 2018;36(7):362-9. [Google Scholar]
- 27.Özcan-Ekşi EE, Ekşi MŞ, Akçal MA. Severe lumbar intervertebral disc degeneration is associated with modic changes and fatty infiltration in the paraspinal muscles at all lumbar levels, except for L1-L2: A cross-sectional analysis of 50 symptomatic women and 50 age-matched symptomatic men. World Neurosurgery. 2019;122:e1069-e1077. [DOI] [PubMed] [Google Scholar]