Abstract
Study Objectives:
Minimal focus has been placed on variations in health care delivery for obstructive sleep apnea (OSA). This study compared positive airway pressure usage in developing countries (Brazil and Mexico) vs. a developed country (United States) and investigated the impact of a patient engagement tool (myAir; ResMed, San Diego, CA) on adherence.
Methods:
Deidentified data from the AirView database (ResMed) for patients receiving positive airway pressure therapy with wirelessly connected Air10 (AirSense and AirCurve) devices in Brazil, Mexico, and the United States were analyzed. Adherence was defined using US Center for Medicare and Medicaid Services (CMS) criteria (usage ≥ 4 h/night on ≥ 70% of nights in the first 90 days).
Results:
The analysis included 4,181,490 patients (Brazil: 31,672; Mexico 16,934; United States: 4,132,884). CMS adherence over 90 days was slightly lower in Latin America vs. the United States (Brazil: 71.7%; Mexico: 66.4%; United States: 74.0%). Significantly fewer patients were using the patient engagement tool in Brazil (8.1%) and Mexico (2.8%) vs. the United States (26%; both P < .001). Patients registered to use an engagement tool had a higher rate of CMS adherence and were twice as likely to achieve CMS adherence. Average daily usage and days with usage > 4 hours in the first week were the strongest predictors of CMS adherence. Across all countries, > 80% of patients meeting CMS criteria at 3 months were still using positive airway pressure therapy at 1 year, with 1-year adherences rates of > 75%.
Conclusions:
Short-term and long-term positive airway pressure adherence rates in Brazil and Mexico were similar to those achieved in the United States. Patients who registered to use an engagement tool consistently had better adherence than those who did not.
Citation:
Drager LF, Malhotra A, Yan Y, et al. Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs developed countries: a big data study. J Clin Sleep Med. 2021;17(4):703–709.
Keywords: adherence, sleep apnea, treatment, telemedicine, wearables, lung
BRIEF SUMMARY
Current Knowledge/Study Rationale: Minimal focus has been placed on differences in the use of positive airway pressure (PAP) therapy for obstructive sleep apnea in developing compared with developed countries. This study compared the use of PAP therapy in developing countries (Brazil and Mexico) with that in a developed country (United States) and investigated the impact of a patient engagement tool on adherence.
Study Impact: Short-term and long-term PAP adherence rates in Brazil and Mexico were similar to those achieved in the United States. Patients who registered to use an engagement tool to track PAP therapy consistently had better adherence than those who did not.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition with major neurocognitive and cardiovascular sequelae.1,2 We recently estimated that up to 1 billion patients worldwide have OSA,3 emphasizing the global impact of this disease. Rates of OSA are also high in less developed regions, with a reported prevalence of moderate to severe OSA in Brazil of 32.9–38.4% depending on the definitions used.4,5 There do not appear to be any published data on the prevalence of OSA in Mexico, but a recent cross-sectional study using a national probabilistic sample showed that > 25% of adults aged > 20 years were at high risk for OSA.6
There are currently few data available regarding the use of positive airway pressure (PAP) therapy in less developed areas. A recent retrospective multicenter study conducted at 9 sleep centers across Argentina, Brazil, Chile, Colombia, Mexico, and Peru showed that 56% of 880 patients with moderate to severe OSA initiated PAP and 14% discontinued therapy during the first year (mainly due to intolerance).7 These data are limited by a lack of objective PAP adherence, as only self-reported use was available.7
Assessment of PAP therapy usage is complicated to some extent by differences in health care delivery and economics as they relate to the treatment of OSA. In the United States, diagnostic sleep testing and therapy is covered for some patients if they have adequate insurance but sometimes requires copayment. In Brazil, there is minimal coverage for sleep diagnostics and PAP therapies. Thus, patients often pay out of pocket for OSA management. In contrast, surgical therapy for OSA is often covered in Brazil, which may favor this therapeutic approach based on economic pressures. In Mexico, coverage for OSA diagnostics and therapeutics is variable, leading to differences in patient burden depending on the specific financial situation.
This study compared the use of PAP therapy in developing countries (Brazil and Mexico) with that in a developed country (United States) and identified predictors of PAP usage in these 3 countries. We also investigated whether a patient engagement tool, which has been associated with improved adherence in the United States,8,9 influenced PAP usage in the other countries.
METHODS
Study design and participants
Similar to a previous study,10 this retrospective study utilized deidentified data from a large cloud-based database of PAP users to determine adherence to therapy over the period October 2014 to November 2018. A home medical equipment provider (HME) was responsible for obtaining patient consent to be enrolled to use the AirView system; all included patients had registered to use telemonitoring and provided consent for their data to be used in future analyses. The HME gave permission to deidentify protected health information in accordance with 45 C.F.R. x 164.514(b) and to analyze these deidentified data. Patients were included if they were adults aged ≥ 18 but < 100 years, had started treatment on the AirSense/AirCurve 10 (ResMed Corp.) PAP platform within Brazil, Mexico, or the United States between October 1, 2014 and November 1, 2018, were registered on the cloud database by their HME, and had used a single PAP modality for at least 1 session that was ≥ 1 h during the first 90 days of therapy. The 1-hour threshold was chosen to minimize selection bias while ensuring that machine-calculated parameters such as the apnea-hypopnea index (AHI) and respiratory rate were averaged over a sensible minimum timeframe.10 The study was reviewed by an Institutional Review Board and deemed exempt from Institutional Review Board oversight (Pro 00031753, Advarra Institutional Review Board, Columbia, MD). All data were analyzed in a secure, anonymized database physically separate from the main production server.
PAP delivery characteristics by country
All PAP in Brazil is self-funded; there is no public or insurance funding for this therapy. In Mexico, the public health system covers a CPAP device and mask, while health insurance provides some coverage and requires a copayment. In the United States, patients with insurance coverage receive fully funded PAP therapy for OSA, subject to US Center for Medicare and Medicaid Services (CMS) usage criteria for prescription renewal (device usage for > 4 hours on ≥ 70% of nights in a consecutive 30-day period in the first 90 days of therapy).
Technology
AirView (ResMed Corp, San Diego, CA) is a Health Insurance Portability and Accountability Act (HIPAA)-compliant web-based solution for health care specialists intended to transfer and display device and therapeutic information that has been transmitted remotely from a patient's PAP device. All AirSense/AirCurve 10 platform PAP devices (ResMed Corp, San Diego, CA) have an on-board mobile communications chip that automatically connects to the secure cloud infrastructure. Patient adherence and therapy data are uploaded daily, typically within 1 hour after the end of each PAP session. All AirView communication and storage is encrypted to meet required international privacy and security standards. The AirView database is hosted in a secure facility in the United States. Adherence data are automatically provided to the HMEs to facilitate patient care, and the patient can also nominate their physician to access the data. The information provided includes usage data and therapy issues (eg, high mask leak, high residual AHI or central apnea index).
The platform became available in August 2014 and was rapidly adopted, such that older devices currently represent < 0.2% of those set up in the United States. Mobile communications costs are covered by the HME as part of their population management process. The patient engagement tool, myAir (ResMed Corp, San Diego, CA), became available in October 21, 2014, and the iOS app became available August 2016. Patients do not pay for access to myAir irrespective of HME signup. The system is designed for patients and provides real-time daily feedback to the patient about their PAP use, while also providing them with coaching on the basis of the data collected. Notifications include a myAir score (calculated based on usage hours, mask seal [as a measure of leak], mask on-off time, and the number of respiratory events per hour). Personalized coaching and reinforcement messages are sent via e-mail and are designed to increase self-management skills, recognize success, and identify and resolve basic treatment issues. The messages generally provide tips on making PAP therapy more comfortable or provide encouragement when patients reach specific therapy milestones (eg, usage averaging > 4 h/night).
Endpoints
The primary outcome was short- and long-term overall adherence rate. Short-term adherence was defined using the US CMS criteria (device usage for > 4 hours on ≥ 70% of nights in a consecutive 30-day period in the first 90 days of therapy). Time to achieve CMS adherence was defined as the number of days from the first day of PAP use to the day on which the adherence threshold was met. Long-term adherence refers to continuation of PAP therapy from day 91 to 1 year. This criterion was because United States funding rules mean that patients who do not achieve short-term adherence do not qualify to continue with PAP therapy. Therefore, only those who met CMS criteria at 90 days were eligible for inclusion in the analysis of long-term adherence. In this analysis, patients were considered to have stopped PAP therapy (ie, therapy termination) if there were 30 consecutive days of zero device usage. Secondary outcomes included the rate of registration to use of a patient engagement strategy and its contribution to achievement of CMS adherence and predictors of PAP usage.
Statistical analysis
Statistical analyses were performed using the R language (R development core team). The first day of therapy was taken as the patient AirView set-up date. Missing adherence data were imputed as zero. For other data fields, including clinical metrics and respiratory events, missing data were not imputed and therefore not included in calculations of mean values, standard deviations, and proportions. Comparisons between the 3 countries were performed using chi-square, Kruskal–Wallis, and analysis of variance tests, as appropriate. Long-term adherence was visualized using Kaplan Meier curves. A univariate logistic regression model was developed to determine the following: whether registration for myAir was associated with better achievement of CMS adherence and the therapeutic metrics associated with CMS adherence, including initial pressure settings and the first week of device usage, count of compliant days, daily usage, residual AHI, obstructive apnea index, monitoring pressure and mask leak.
RESULTS
Participants
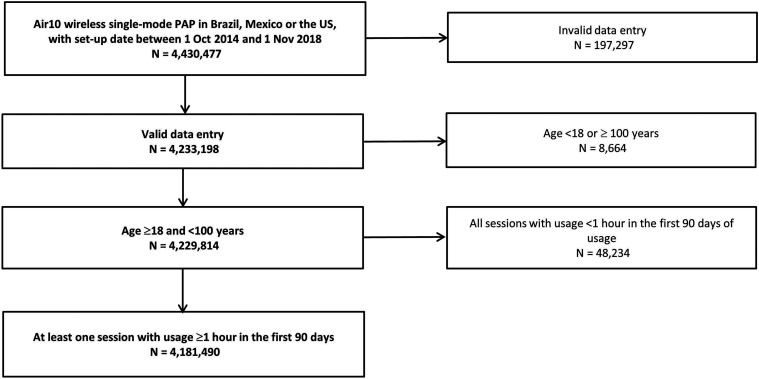
A total of 4,181,490 patients was included in the final analysis, representing 94% of all available AirView records (Figure 1). Although age distribution of patients from different countries was broadly similar (see Figure S1 (307.4KB, pdf) ), the mean age of patients from Mexico was slightly higher than that for patients from Brazil and the United States (Mexico: 59.3 ± 13.1; Brazil: 58.2 ± 14.1; United States: 58.2 ± 14.0 years; P < .001).
Figure 1. Flow diagram.
PAP = positive airway pressure.
Adherence
Among individuals who used their PAP device for at least 1 session that was ≥ 1 h in duration, CMS adherence rates at 90 days were 71.7% in Brazil, 66.4% in Mexico, and 74.0% in the United States (Table 1). Average PAP usage was also slightly lower in Latin America (especially in Mexico) compared with the United States (Table 1). The proportion of patients who registered to use the myAir patient engagement tool was also lower in Brazil (8.1%) and Mexico (2.8%) than in the United States (26%; both P < .001). Patients from Brazil had higher residual AHI, obstructive apnea index, and mask leak during PAP therapy (Table S1 (307.4KB, pdf) ).
Table 1.
Positive airway pressure usage over the first 90 days of therapy, by country.
| Adherence Variables (in first 90 days) | Brazil (n = 31,672) | Mexico (n = 16,934) | United States (n = 4,132,884) | P Value |
|---|---|---|---|---|
| CMS adherence achieved, n (%) | 22,702 (71.7) | 11,251 (66.4) | 3,057,187 (74.0) | < .001b |
| Days to achieve CMS adherence, days | ||||
| Mean ± SD | 28.48 ± 12.41 | 29.20 ± 13.63 | 28.08 ± 12.81 | < .001c |
| Median (IQR) | 24.0 (22.0, 28.0) | 24.00 (22.0, 29.0) | 23.0 (21.0, 28.0) | < .001d |
| Proportion of days with nonzero usage, % | ||||
| Mean ± SD | 79.7 ± 24.1 | 74.3 ± 28.5 | 80.0 ± 26.6 | < .001c |
| Median (IQR) | 90.0 (70.0, 97.8) | 86.7 (57.8, 97.8) | 93.3 (71.1, 98.9) | < .001d |
| Proportion of days adherent (usage ≥ 4 h), % | ||||
| Mean ± SD | 67.0 ± 29.4 | 60.7 ± 33.9 | 67.5 ± 32.5 | < .001c |
| Median (IQR) | 75.6 (47.8, 92.2) | 71.1 (31.1, 91.1) | 80.0 (45.6, 95.6) | < .001d |
| Average usage per session, ha | ||||
| Mean ± SD | 5.91 ± 1.61 | 5.75 ± 2.05 | 5.98 ± 2.03 | < .001c |
| Median (IQR) | 6.07 (5.01, 6.97) | 6.01 (4.58, 7.12) | 6.18 (4.77, 7.36) | < .001d |
| Average daily usage across all days, h | ||||
| Mean ± SD | 4.90 ± 2.15 | 4.59 ± 2.55 | 5.13 ± 2.56 | < .001c |
| Median (IQR) | 5.23 (3.47, 6.50) | 4.93 (2.53, 6.56) | 5.52 (3.37, 7.04) | < .001d |
CMS = Centers for Medicare and Medicaid, IQR = interquartile range, SD = standard deviation. aTotal usage over 90 days/days with usage > 0; bchi-square test; canalysis of variance test; dKruskal–Wallis test.
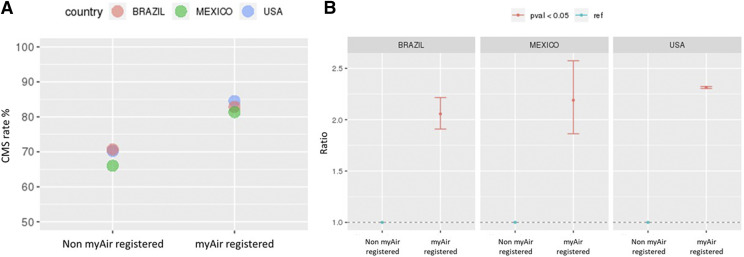
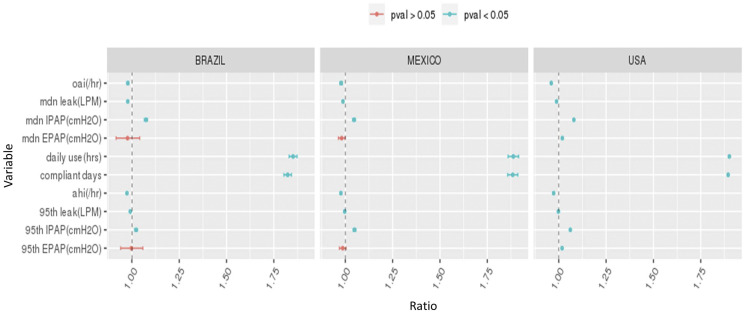
The rate of CMS adherence at 90 days was consistently higher in patients who did vs. did not have access to the patient engagement tool (Figure 2A). Engagement tool usage was associated with > 2-fold increase the probability of achieving CMS adherence (Figure 2B). Across all countries, rates of CMS adherence varied by patient age; the lowest adherence rates were seen in younger adults (Figure S2 (307.4KB, pdf) ). This finding was particularly the case the United States and less so in Mexico based on univariate analysis with age 55–60 years as the reference (Figure S3 (307.4KB, pdf) ). Also on multivariate analysis, the strongest predictors of CMS adherence at 90 days were average daily usage and days with PAP usage of ≥ 4 hours, particularly during the first week of therapy; other significant predictors of PAP usage were residual AHI, mask leak, and inspiratory positive airway pressure (Figure 3).
Figure 2. Adherence rates.
Centers for Medicare and Medicaid (CMS) adherence in patients who did vs. did not register to use the myAir tool (A) and likelihood of achieving CMS adherence (based on a univariate logistic regression model with non-myAir registered as the reference (B).
Figure 3. Predictors of adherence.
Predictors of Centers for Medicaid and Medicare (CMS) adherence on univariate logistic regression analysis. AHI = apnea-hypopnea index, EPAP = expiratory positive airway pressure, IPAP = inspiratory positive airway pressure, LPM = liters/min, mdn = median, OAI = obstructive apnea index.
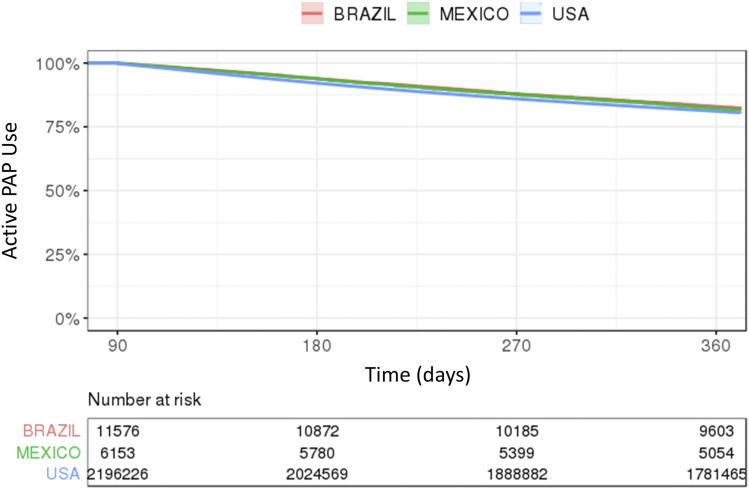
In patients who achieved CMS adherence criteria at 90 days, 1-year therapy continuation rates were 83.0% in Brazil, 82.1% in Mexico, and 81.1% in the United States, and 1-year adherence rates were > 75% (Figure 4).
Figure 4. Proportion remaining on therapy.
Kaplan Meier curves showing the proportion of patients remaining on positive airway pressure therapy from day 91 to day 360 in patients who achieved Centers for Medicaid and Medicare (CMS) adherence at 90 days. PAP = positive airway pressure.
DISCUSSION
To our knowledge, this is the first large-scale study to assess how PAP usage patterns differ between developing and developed countries. We found that PAP usage was similar in Mexico and Brazil, and users in both these countries had adherence rates that were only slightly lower than those in the United States. These findings suggest that PAP therapy for OSA might be appropriate/effective even in less developed countries, particularly given that the majority of patients who were adherent at 90 days were still using therapy at the 1-year follow-up. The requirement to achieve CMS criteria for device resupply and reimbursement might have been a factor motivating higher real-world adherence in the United States, but these criteria do not apply in Mexico or Brazil where adherence rates were only slightly below those achieved in the United States.
Another finding of our study was that registration to use a patient engagement tool was associated with improved adherence compared with usual care, consistent with existing data from the United States.9,10 In our analyses, by far the strongest predictors of PAP adherence in the first 90 days were average daily usage and days with usage of > 4 h/night, especially during the first week of therapy.
The main study findings highlighted much better use of PAP than expected based on current literature. Several observational and randomized studies have reported fair or poor adherence with PAP therapy, especially in OSA patients with mild to no daytime symptoms.11–13 Such data, and the lack of multidisciplinary teams to manage OSA,14 have contributed to the belief that PAP is efficient at abolishing nocturnal respiratory events but has suboptimal effectiveness in clinical practice.15 However, until recently, the majority of current data in the field and experience in clinical practice are based on data manually downloaded from PAP machines. In contrast, our data represent the results of a variety of real-life influences on adherence to PAP therapy.
Use of telemonitoring is becoming an increasing part of PAP therapy for OSA as new “connected” devices become available. Data from a randomized trial showed that telemonitoring reduced the delay to first technical intervention in PAP-treated patients and that this early activation of troubleshooting was associated with improved adherence at 3 months.16 Nevertheless, the inconsistent nature of published data in this new field means that the impact of telemonitoring on PAP adherence is the subject of ongoing debate.17–19 Our data support a role for telemonitoring, especially use of a patient engagement tool, in optimizing adherence to PAP therapy. Although the number of patients registering to use the patient engagement tool in each country was relatively small, our data suggest that this approach might be beneficial across a range of countries.
In terms of adherence in different countries, it could be argued that patients who are self-funding their PAP therapy (such as those in Brazil) and use an engagement tool might be more motivated to use the device than those whose costs are covered by insurance or public health systems (as in the United States and Mexico). If this were the case, we might have expected better PAP usage in Brazil than compared with Mexico or the United States, but this finding was not the case. Perhaps the variable nature of access to therapy made the impact of the “healthy user effect” less clear. Conversely, lack of funded access to PAP could have a negative impact on the number of patients able to access therapy, and there may be a major financial barrier to starting PAP therapy at all. Overall, the motivation provided by financial demands may not be sufficient to optimize PAP usage, making our finding that registration to use a patient engagement tool was associated with robust adherence to PAP therapy particularly important. Nevertheless, overall adherence to PAP therapy is governed by a range of interacting factors, including biomedical, health system, psychological, and social aspects.20 Therefore, financial factors might impact individuals differently and may be confounded by other factors (eg, socioeconomic status). For example, the ability to obtain treatment despite financial hurdles could be associated with other variables that might influence adherence, including coping skills, greater resourcefulness, and better health literacy. The contribution of influences such as these to our study findings cannot be determined due to the limited data available from the database queried. We also have no data on the PAP care provider (eg, sleep center or elsewhere and publicly vs. privately funded). Therefore, our findings should be interpreted as a high-level overview of general differences between the 3 countries analyzed.
Another factor that might have influenced the results of our study is potential differential availability of the technology required to register and use the patient engagement tool. Patients in developing countries with the resources to access reliable internet connectivity and who have a mobile device for app access mean that the subgroup of patients using the patient engagement tool in our study could be more likely to come from higher socioeconomic groups, meaning that they might not be representative of the general population in these countries. Differential access to health care between individuals of different socioeconomic statuses in developing countries is another factor that is likely to introduce selection bias to our analysis. If the sample includes a greater proportion of higher socioeconomic status patients from developing countries, adherence rates in these countries might be overestimated.
Univariate analysis of our data from a very large number of patients showed that early use of PAP therapy was the strongest predictor of longer-term adherence. This finding is consistent with a good body of evidence showing that the pattern of adherence to PAP therapy is established early after treatment initiation.21–29 Mask leak was another predictor of adherence in our study, as has been documented previously.30 Furthermore, data showing that lower maximal PAP level was associated with low PAP adherence31 are supported by our finding of a significant association between inspiratory positive airway pressure and use of PAP.
Our study has a number of strengths including its novelty and sample size. However, we acknowledge a number of important limitations. Firstly, we report a retrospective observational study, not a randomized trial; thus, our findings provide information on correlation rather than causation. However, the real-world nature of the data means that the results should have good external validity. Secondly, the nature of the database used in terms of privacy issues and recorded data means that we were not able to adjust for important covariates such as baseline AHI, socioeconomic status, race/ethnicity, and comorbidities. In addition, differences between regions in each country and differences between countries with respect to health care systems and financial factors (eg, insurance and public funding) could result in differences between the populations accessing a PAP device in each country, meaning that those included in our study might represent a small subset of the total OSA population. Also lacking are data on the setting for PAP delivery (ie, public vs. private). However, home medical equipment suppliers are not expected to be fundamentally different between countries. We also have no information on use of other treatments for OSA, such as weight loss, upper airway surgery, or oral appliance use. Therefore, more patients may be receiving acceptable treatment for OSA than our estimates of PAP usage might suggest. As such, the adherence rates reported in this study likely reflect a “worst-case scenario” in terms of the number of effectively treated patients with OSA.
In conclusion, although some between-country differences were evident, 90-day PAP adherence in Brazil and Mexico in our patient sample was close to that achieved in the United States. Based on our findings, the best way to ensure long-term adherence to PAP would be to optimize therapy in the first week. Support programs that enable patients to track their PAP therapy appear to be beneficial in all the countries studied and should be further investigated and utilized. Additional research on adherence rates and the effect of telemonitoring interventions across different countries with different economic status and health care systems is needed to clarify further the widespread applicability of PAP therapy and telemedicine solutions in patients with OSA and to inform approaches for optimizing treatment.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by ResMed. Editorial assistance by Nicola Ryan was funded by ResMed. Dr Drager received consulting fees from ResMed. Prof. Malhotra is funded by the National Institutes of Health. Prof. Malhotra received funding from Merck and Livanova for medical education. ResMed, Inc. provided a philanthropic donation to the UC San Diego in support of a sleep center. Ms. Yan and Drs. Armitstead, Nunez, and Benjafield are employees of ResMed. Prof. Pépin is partially supported by MIAI @ Grenoble Alpes (ANR-19-P3IA-0003), and his department has received research support from ResMed, Philips Respironics, and Fisher and Paykel. Dr Woehrle has received consulting and speaker’s fees from ResMed and Inspire Medical. Prof. Cistulli has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding; has received research support from ResMed, SomnoMed, and Zephyr Sleep Technologies; is a consultant to Zephyr Sleep Technologies and Narval; and has a pecuniary interest in SomnoMed related to a 2004 role in research and development (2004).
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors acknowledge editorial assistance with manuscript preparation by Nicola Ryan, independent medical writer. Author contributions: AVB is the guarantor of the paper. All authors developed the study objectives and study design. YY performed the statistical analysis, which was reviewed by LFD, AM, YY, and AVB. The first draft of the manuscript was prepared by LFD and AM. All authors reviewed and revised the manuscript before submission, and approved the final submitted version.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CMS
US Center for Medicare and Medicaid Services
- CPAP
continuous positive airway pressure
- HME
home medical equipment provider
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
REFERENCES
- 1.Farrell PC, Richards G. Recognition and treatment of sleep-disordered breathing: an important component of chronic disease management. J Transl Med. 2017;15(1):114. 10.1186/s12967-017-1211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610. 10.1378/chest.11-2214 [DOI] [PubMed] [Google Scholar]
- 3.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. 10.1016/j.sleep.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 5.Drager LF, Santos RB, Silva WA, et al. OSA, short sleep duration, and their interactions with sleepiness and cardiometabolic risk factors in adults: the ELSA-Brasil Study. Chest. 2019;155(6):1190–1198. 10.1016/j.chest.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Guerrero-Zúñiga S, Gaona-Pineda EB, Cuevas-Nasu L, et al. [Prevalence of sleep symptoms and risk of obstructive sleep apnea in Mexico]. Salud Publica Mex. 2018;60(3):347–355. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira JF, Poyares D, Simonelli G, et al. Accessibility and adherence to positive airway pressure treatment in patients with obstructive sleep apnea: a multicenter study in Latin America. Sleep Breath. 2020;24(2):455–464. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest. 2018;153(4):843–850. 10.1016/j.chest.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharjee R, Benjafield AV, Armitstead J, et al. Adherence in children using positive airway pressure therapy: a big data analysis. Lancet Digit Health. 2020;2(2):e94–e101. 10.1016/S2589-7500(19)30214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cistulli PA, Armitstead J, Pepin JL, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. 10.1016/j.sleep.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy RD, Antic NA, Heeley E, et al.SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 12.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al.Spanish Sleep And Breathing Network . Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2019;1:61–72. 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- 14.Pépin JL, Baillieul S, Tamisier R. Reshaping sleep apnea care: time for value-based strategies. Ann Am Thorac Soc. 2019;16(12):1501–1503. 10.1513/AnnalsATS.201909-670ED [DOI] [PubMed] [Google Scholar]
- 15.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. 10.1164/rccm.201212-2223OC [DOI] [PubMed] [Google Scholar]
- 16.Pépin JL, Tamisier R, Hwang D, Mereddy S, Parthasarathy S. Does remote monitoring change OSA management and CPAP adherence? Respirology. 2017;22(8):1508–1517. 10.1111/resp.13183 [DOI] [PubMed] [Google Scholar]
- 17.Hoet F, Libert W, Sanida C, Van den Broecke S, Bruyneel AV, Bruyneel M. Telemonitoring in continuous positive airway pressure-treated patients improves delay to first intervention and early compliance: a randomized trial. Sleep Med. 2017;39:77–83. 10.1016/j.sleep.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 18.Turino C, de Batlle J, Woehrle H, et al. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. Eur Respir J. 2017;49(2):1601128. 10.1183/13993003.01128-2016 [DOI] [PubMed] [Google Scholar]
- 19.Hwang D, Chang JW, Benjafield AV, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The Tele-OSA randomized trial. Am J Respir Crit Care Med. 2018;197(1):117–126. 10.1164/rccm.201703-0582OC [DOI] [PubMed] [Google Scholar]
- 20.Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence--new concepts? Sleep Med Rev. 2014;18(2):123–139. 10.1016/j.smrv.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. 10.1093/sleep/20.4.278 [DOI] [PubMed] [Google Scholar]
- 22.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–1114. 10.1164/ajrccm.159.4.9807111 [DOI] [PubMed] [Google Scholar]
- 23.Krieger J. Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep. 199215, 6, Suppl:S42–S46. 10.1093/sleep/15.suppl_6.S42 [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal L, Gerhardstein R, Lumley A, Guido P, Day R, Syron ML, Roth T. CPAP therapy in patients with mild OSA: implementation and treatment outcome. Sleep Med. 2000;1(3):215–220. 10.1016/S1389-9457(00)00012-5 [DOI] [PubMed] [Google Scholar]
- 25.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149(1):149–154. 10.1164/ajrccm.149.1.8111574 [DOI] [PubMed] [Google Scholar]
- 26.Sanders MH, Gruendl CA, Rogers RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest. 1986;90(3):330–333. 10.1378/chest.90.3.330 [DOI] [PubMed] [Google Scholar]
- 27.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 28.Popescu G, Latham M, Allgar V, et al. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2 week trial to identify factors associated with long term use. Thorax. 2001;56:727e33. 10.1136/thorax.56.9.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbull CD, Bratton DJ, Craig SE, Kohler M, Stradling JR. In patients with minimally symptomatic OSA can baseline characteristics and early patterns of CPAP usage predict those who are likely to be longer-term users of CPAP. J Thorac Dis. 2016;8(2):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valentin A, Subramanian S, Quan SF, Berry RB, Parthasarathy S. Air leak is associated with poor adherence to autoPAP therapy. Sleep. 2011;34(6):801–806. 10.5665/SLEEP.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nsair A, Hupin D, Chomette S, Barthélémy JC, Roche F. Factors influencing adherence to auto-CPAP: an observational monocentric study comparing patients with and without cardiovascular diseases. Front Neurol. 2019;10:801. 10.3389/fneur.2019.00801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.