Abstract
Study Objectives:
Obstructive sleep apnea (OSA) and central sleep apnea (CSA) are common in infants with laryngomalacia. The purpose of this study was to evaluate developmental changes in sleep-related breathing disorders over time in infants with laryngomalacia and understand the effect of supraglottoplasty (SGP) and nonsurgical treatment.
Methods:
This is a retrospective review of infants with laryngomalacia who had at least 2 diagnostic polysomnography studies performed from January 2000 and May 2015. We included infants who had either OSA or CSA. Comparison of sleep and respiratory parameters by age group (0–6, 6–12, and >12 months old) was performed in both SGP and non-SGP groups using a mixed-effect regression model. A log-normal mixed model was used to explore the changes in sleep and respiratory parameters with age. The time to resolution of CSA and OSA was analyzed using nonparametric survival analysis.
Results:
A total of 102 infants were included; 57 had only OSA and 45 had both CSA and OSA. There were significant decreases in apnea-hypopnea index, obstructive index, central apnea index, and arousal index with increasing age in both SGP and non-SGP groups. The mean age at resolution of CSA (central apnea index < 5) was 7.60 months old for SGP and 12.57 months old for non-SGP (P < .05). There were no significant differences in the mean age at resolution of OSA (obstructive index < 1; 35.18 [SGP] vs 41.55 months [non-SGP]; P = .60) between SGP and non-SGP groups. Infants with neurologic disease, congenital anomalies, or genetic syndromes required significantly more time to resolve OSA (28.12 [normal] vs 53.13 [neurological] vs 59.53 months [congenital anomalies and genetic]; P < .01).
Conclusions:
Both OSA and CSA improve in infants with laryngomalacia with increasing age regardless of SGP. The mechanism underlying these changes may involve airway growth and maturation of respiratory control. Time to resolution of OSA is affected by the presence of neurologic diseases, congenital anomalies, and genetic syndromes. Further studies are needed to confirm these findings and to evaluate long-term outcomes in this population.
Citation:
Ratanakorn W, Brockbank J, Ishman S, Tadesse DG, Hossain MM, Simakajornboon N. The maturation changes of sleep-related respiratory abnormalities in infants with laryngomalacia. J Clin Sleep Med. 2021;17(4):767–777.
Keywords: obstructive sleep apnea, central sleep apnea, developmental changes of sleep apnea, laryngomalacia
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep-related breathing disorders including obstructive and central sleep apnea are common in infants with laryngomalacia. Several studies have shown the effectiveness of surgical and medical interventions and their effects on sleep-related breathing disorders; however, there are limited data on developmental changes and natural courses of sleep-related breathing disorders in infants with laryngomalacia.
Study Impact: The study reports a large cohort of infants with laryngomalacia with initial and follow-up sleep studies over time, and the findings indicate that both obstructive and central sleep apnea improve over time regardless of the type of treatment (surgical vs nonsurgical management). The resolution of obstructive sleep apnea is affected by the presence of neurologic diseases and congenital anomalies and genetic syndromes.
INTRODUCTION
Laryngomalacia is the most common cause of stridor, accounting for more than 60% of congenital laryngeal anomalies.1 The condition results in dynamic collapse of supraglottic structures during inspiration, causing stridor, and can lead to significant upper airway obstruction and feeding disturbances in infants afflicted by this condition. A recent theory of the etiology of congenital laryngomalacia is focused on a neuromuscular etiology, consisting of immaturity or abnormal integration of peripheral nerves, brain stem nuclei, and pathways responsible for swallowing and maintenance of airway patency.2 This theory is supported by findings of increased laryngopharyngeal sensory thresholds in patients with moderate to severe laryngomalacia compared with those with mild disease. This correlates with the weak laryngeal tone seen in infants with laryngomalacia because the alteration of laryngeal afferent is associated with changes in laryngeal motor function.2
Obstructive sleep apnea (OSA) is a known common sleep-related breathing disorder in infants with laryngomalacia.3,4 A previous study from our group demonstrated that central sleep apnea (CSA) is relatively common in infants with laryngomalacia, most notably in infants with neurologic disorders, infants with history of apparent life-threatening events, premature infants, and infants aged less than 3 months.5 CSA in otherwise healthy infants tends to improve with age as the maturation of the central nervous system occurs.6 The pathophysiology of CSA in infants emphasizes on the role of carbon dioxide apneic threshold, which is much closer to the eucapnia level than that reported in adults,7 and on the concept of loop gain, a term first used in engineering. The loop gain is described as a negative feedback system in which a disturbance leads to an increase in alveolar ventilation from a steady state. This increase in ventilation in turn reduces carbon dioxide, which evokes a negative corrective action to suppress the disturbance. The ratio of corrective ventilatory response and the ventilatory disturbance define the loop gain of the system. In the high loop gain system, the response is greater or equal to the disturbance, which results in an unstable ventilatory system and predisposition to CSA.8
The development of OSA in infants with laryngomalacia3,4 may be secondary to the high compliance seen in the infant’s upper airway in which a 2-cm H2O change in luminal pressure results in a 50% reduction in cross-sectional area.9 These rapid changes in the caliber of the airway contribute to ventilatory instability and obstructive cycling. In addition, laryngeal anatomical abnormalities can result in increased upper airway resistance during sleep and may contribute to the development of OSA. Sleep-dependent laryngomalacia is a related condition that has increasingly been acknowledged as a potential cause for OSA. It is characterized by dynamic supraglottic airway collapse that becomes clinically significant only during sleep.10
Approximately 10% of infants with laryngomalacia have severe OSA, hypoxemia, or failure to thrive and may require surgical intervention.11 Supraglottoplasty (SGP) is an endoscopic procedure designed to modify the anatomy of the supraglottic larynx to reduce collapse and prolapse into the airway. It has become a preferred primary surgical intervention for this condition. Children with severe symptoms often benefit significantly from SGP, with reported surgical success rates as indicated by significant improvement or complete resolution of symptoms, approaching 95% in some series.12–14 SGP for sleep-dependent laryngomalacia has been reported as an effective treatment for OSA as measured by improvements in polysomnographic (PSG) parameters after surgery,10,15–17 with complete resolution reported in about 60% of cases. In infants with laryngomalacia who are not surgical candidates, low-flow supplemental oxygen has been used as an effective treatment for OSA in our center.18
There are several publications regarding PSG findings in patients with laryngomalacia and outcomes after SGP.10,13,15 However, there is limited information on the maturation changes in respiratory parameters and overall sleep architecture in this population. Therefore, the purpose of this study was to evaluate the longitudinal changes in OSA, CSA, and sleep architecture in infants with laryngomalacia who were treated with SGP compared with a nonsurgical group (non-SGP).
METHODS
Study design
We performed a retrospective review of the electronic medical record and database of PSG studies of infants with laryngomalacia at Cincinnati Children’s Hospital Medical Center. The study was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center. Data were obtained from all infants who were diagnosed with laryngomalacia and had at least two full-night PSGs performed from January 2000 through May 2015. The diagnosis of laryngomalacia was confirmed by transnasal fiberoptic laryngoscopy, including findings of prolapsing supra-arytenoid tissue during inspiration, shortening of the aryepiglottic folds, and an omega-shaped or retroflexed epiglottis. Only infants who had a central apnea index (CI) greater than 5 events/h or an obstructive index (obstructive apnea-hypopnea index or OI) greater than 1 event/h on an initial study were included. Infants who underwent a split-night or oxygen titration study were excluded. Split-night studies were excluded because they contained limited diagnostic study time. The short duration may affect the estimation of respiratory parameters. In addition, one of our aims was to assess longitudinal changes in sleep architecture in infants with laryngomalacia. Assessment of sleep architecture from split-night studies will not be accurate. Infants who underwent SGP before an initial PSG and those who had other upper airway surgeries were also excluded. It is a standard practice at our institution for infants with laryngomalacia to have their first PSG within the first 6 months of life and then every 3–6 months until all of sleep and respiratory parameters are normalized (OI < 1 and CI < 5) or markedly improved (OI < 5). However, the follow-up PSGs in each individual were done based on multiple factors including physician and family preferences and availability of sleep laboratory.
PSG
The PSG was performed in accordance with the American Academy of Sleep Medicine guidelines. The standard infant montage was used, and these variables were recorded simultaneously: body position, left and right electrooculogram (ROC/A1,LOC/A2), 4-channel electroencephalogram (O1A2,O2A1, C4A1,C3A2), chin electromyogram, electrocardiogram, pulse oximetry and pulse waveform, thoracic and abdominal inductance plethysmography, nasal thermistor, end-tidal pCO2 monitoring (BCI®, Capnocheck®, Smiths Medical, St Paul, MN), and transcutaneous pO2 and pCO2 (Tina TCM4/40, Radiometer, Copenhagen, Denmark). Scoring of sleep stages and respiratory events were done by registered PSG technologists and validated by a board-certified sleep physician. Sleep efficiency was defined by the percentage of total sleep time divided by the time in bed. An arousal was defined as a shift in the electroencephalogram pattern to frequencies of 8 to 13 Hz or above 16 Hz for a minimum of 3 seconds. Arousals in rapid eye movement sleep were only scored if there was a concurrent increase in submental electromyogram amplitude. The arousal index was expressed as the number of arousals per hour of sleep.19
An apnea was defined as at least a 90% reduction in airflow from baseline for at least 2 respiratory cycles. An obstructive apnea was defined as cessation of airflow in the presence of continued or increased respiratory effort. A central apnea was defined as an absence of both airflow and respiratory effort as indicated by movement of the chest wall or abdomen. A hypopnea was defined as a 30% or greater reduction in airflow associated with reduced respiratory effort and accompanied by an oxygen desaturation of at least 3% or an arousal. The OI was defined as the number of obstructive apneas, mixed apneas, and obstructive hypopneas per hour. The CI was defined as the number of central apneas per hour. OSA was diagnosed if the OI was more than 1 event/h. CSA was diagnosed if CI was more than 5 events/h. Because there are limited normative data in infants and there are no standard cutoffs for making diagnosis of OSA and CSA in infants, we apply the same cutoff criteria for diagnosis of OSA and CSA as those used for children. The pediatric definition of OSA may overestimate OSA in infants and identify many healthy infants as having OSA.
Data analysis
Demographic, comorbidities, and PSG data were expressed as percentage for discrete variables and as median (lower quartile, upper quartile) for continuous variables. A comparison between OSA alone and OSA/CSA groups was performed by independent t test for continuous data or by χ2 (or Fisher exact) test for discrete data. Patients were divided into 3 age groups: 0–<6, 6–12, and >12 months old. Comparisons of sleep and respiratory parameters between each age group were done in both SGP and non-SGP groups using a mixed-effects regression model. The random effects were assigned to address the repeated measurements of sleep and respiratory parameters, and a compound symmetry correlation structure was assumed for the dependency in repeated data. The least square mean estimates with standard error were acquired from the mixed-effects regression models. The reported P values from pairwise comparison were adjusted for multiplicity using the Tukey-Kramer method. To determine the effect of age on sleep and respiratory parameters, we fitted a mixed-effect log-normal regression model with age as a covariate.
The times to resolution of OSA (OI < 1) and CSA (CI < 5) was analyzed using survival analysis specifically using Kaplan-Meier curves. Patients who never had the resolution were considered as censored events at the last observed age. The ages at resolution of OSA and CSA were correlated with treatment, presence of other congenital anomalies or syndromes, and presence of neurologic diseases using the log-rank test. Kaplan-Meier curves were generated to compare the patients’ age at resolution of OSA and CSA for SGP and non-SGP groups. P < .05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) and R Studio version 4.0.0 (RStudio Team, Vienna, Austria) were used for statistical analysis.
RESULTS
Of 316 laryngomalacia infants who had full-night PSG performed, we excluded 51 infants who underwent SGP before an initial PSG. Hence, 265 infants had at least 1 PSG before intervention. Of these 265 infants, 203 infants had sleep-related breathing disorders, 138 infants had only OSA (OI > 1), 9 infants had only CSA (CI > 5), and 56 infants had both OSA and CSA. Thus, the prevalence of sleep-related breathing disorders in our cohort of infants with laryngomalacia who underwent a diagnostic study was 76.6%.
Of those 265 infants who had at least 1 PSG before intervention, we excluded 155 infants who had only 1 PSG performed and 8 infants who did not have either OSA or CSA on the initial study. Therefore, 102 infants met the criteria for entry into analysis. Of these, 57 (56%) infants had OSA alone and 45 (44%) infants had both OSA and CSA. All infants had their first PSG within the first 6 months of life and then every 3–6 months until all of sleep and respiratory parameters were normalized (OI < 1 and CI < 5) or markedly improved (OI < 5).
Patient demographics and baseline characteristics
The clinical characteristics and baseline PSG variables of infants with OSA alone and infants with both OSA and CSA (OSA/CSA) are shown in Table 1. There were no significant differences in sex or race between the 2 groups. Infants with both OSA/CSA underwent their first PSG earlier than infants with only OSA, with a median age at the first PSG of 3.60 months in the OSA group and 2.33 months in the OSA/CSA group. In term of respiratory parameters, the median baseline OI was similar between the 2 groups (10.10 [5.20, 14.90] [OSA] vs 10.40 [5.40, 20.50] [OSA/CSA] events/h, P = .74). There was no significant difference in the proportion of mild, moderate, and severe OSA between the 2 groups. The apnea-hypopnea index (AHI) was significantly higher in the OSA/CSA group (12.60 [8.00, 17.80] [OSA] vs 26.00 [14.80, 39.10] [OSA/CSA] per hour, P < .01) because of higher CI. For sleep parameters, sleep efficiency was similar between the 2 groups, although the arousal index was significantly higher in the OSA/CSA group (17.20 [11.90, 21.70] [OSA] vs 22.00 [15.90, 27.60] [OSA/CSA] per hour, P < .01). The percentage of supine position was not different between the 2 groups.
Table 1.
Demographic and baseline patient characteristics for infants with congenital laryngomalacia before treatment.
| OSA (OI > 1) (n = 57) | OSA and CSA (OI > 1 and CI > 5) (n = 45) | P | |
|---|---|---|---|
| Sex, n (% within group) | |||
| Total | 57 | 45 | .32 |
| Male | 27 (47.37) | 26 (57.78) | |
| Female | 30 (52.63) | 19 (42.22) | |
| Age at the first PSG (mo), median [Q1, Q3] | 3.60 [2.10, 8.23] | 2.33 [1.17, 3.80] | <.01 |
| Race, n (% within group) | |||
| Total | 57 | 45 | .32 |
| White | 33 (57.90) | 34 (75.56) | |
| Black | 11 (19.30) | 6 (13.33) | |
| Biracial | 5 (8.77) | 2 (4.44) | |
| Others | 8 (14.03) | 3 (6.67) | |
| Initial PSG parameter, median [Q1, Q3] | |||
| CI (events/h) | 1.70 [0.90, 3.10] | 8.90 [6.20, 16.80] | <.01 |
| OI (events/h) | 10.10 [5.20, 14.90] | 10.40 [5.40, 20.50] | .74 |
| Mild [1–5], n (% within group) | 14 (24.56) | 11 (24.44) | .47 |
| Moderate [>5–15], n (% within group) | 29 (50.88) | 18 (40.00) | |
| Severe [>15], n (% within group) | 14 (24.56) | 16 (35.56) | |
| AHI (events/h) | 12.60 [8.00, 17.80] | 26.00 [14.80, 39.1] | <.01 |
| Sleep efficiency (%) | 0.77 [0.70, 0.82] | 0.76 [0.69, 0.81] | .56 |
| Arousal index (events/h) | 17.20 [11.90, 21.7] | 22.00 [15.90, 27.6] | <.01 |
| Supine position (%) | 0.64 [0.51,0.89] | 0.61 [0.36,0.77] | .14 |
| Comorbidities, n (% within group)* | |||
| Secondary airway lesion | 1 (1.75) | 3 (6.67) | .32 |
| Neurological disease | 12 (21.05) | 10 (22.22) | .9999 |
| GERD | 6 (10.53) | 15 (33.33) | <.01 |
| Pulmonary disease | 0 (0.00) | 1 (2.22) | .44 |
| Congenital heart disease | 3 (5.26) | 5 (11.11) | .30 |
| Congenital anomalies and syndrome | 11 (19.30) | 7 (15.56) | .79 |
| Prematurity | 1 (1.75) | 4 (8.89) | .17 |
| Treatment | |||
| SGP, n (% within group) | 37 (64.91) | 21 (46.67) | .07 |
| Age at SGP (months), median [Q1, Q3] | 4.43 [2.27, 14.17] | 3.23 [2.23, 4.77] | .27 |
| Oxygen supplementation, n (% within group) | 10 (17.54) | 21 (46.67) | <.01 |
| Medication, n (% within group) | |||
| Proton pump inhibitor or H2 blocker | 44 (77.19) | 33 (77.33) | .82 |
| Caffeine | 0 (0.00) | 5 (11.11) | .02 |
| Acetazolamide | 0 (0.00) | 2 (4.44) | .19 |
| CPAP/BPAP | 1 | 1 | .9999 |
Median with lower and upper quartiles for continuous variables are reported. P < .05 was considered statistically significant. AHI = apnea-hypopnea index, BPAP = bilevel positive airway pressure, CI = central apnea index, CPAP = continuous positive airway pressure, CSA = central sleep apnea, GERD = gastroesophageal reflux disease, OI = obstructive apnea index, OSA = obstructive sleep apnea, PSG = polysomnography, SGP = supraglottoplasty. *Each patient may have more than one condition.
Neurologic disease was the most common comorbidity, with a similar prevalence in both groups (P = .9999). Gastroesophageal reflux disease was the second common comorbidity and was more common in the OSA/CSA group (P < .01). Congenital anomalies and syndromes were the third common comorbidity and had a similar prevalence in both groups (P = .79). Down syndrome was the most common congenital anomaly and syndrome found in both groups, followed by Pierre Robin sequence and Prader-Willi syndrome.
There was no significant difference in the rate of SGP performed in infants with OSA alone and those with OSA/CSA (64.91% [OSA] vs 46.67% [OSA/CSA], P = .07). The median age at SGP was 4.43 and 3.23 months in the OSA and OSA/CSA groups, respectively (P = .27). A higher proportion of infants in the OSA/CSA group was treated with supplemental oxygen compared with infants in the OSA group (17.54% [OSA] vs 46.67% [OSA/CSA], P < .01). Proton pump inhibitors and H2 blockers were commonly prescribed for patients in both groups (77.19% [OSA] vs 77.33% [OSA/CSA], P = .82). Caffeine and acetazolamide were prescribed in a few patients who had both CSA and OSA. Noninvasive ventilation was used in only 2 patients. Continuous positive airway pressure was used in 1 patient in the OSA/CSA group, and bilevel positive airway pressure was used in the other patient in the OSA group. Both had neurologic diseases with alveolar hypoventilation and had failed oxygen therapy.
Changes in sleep and respiratory parameters between age groups
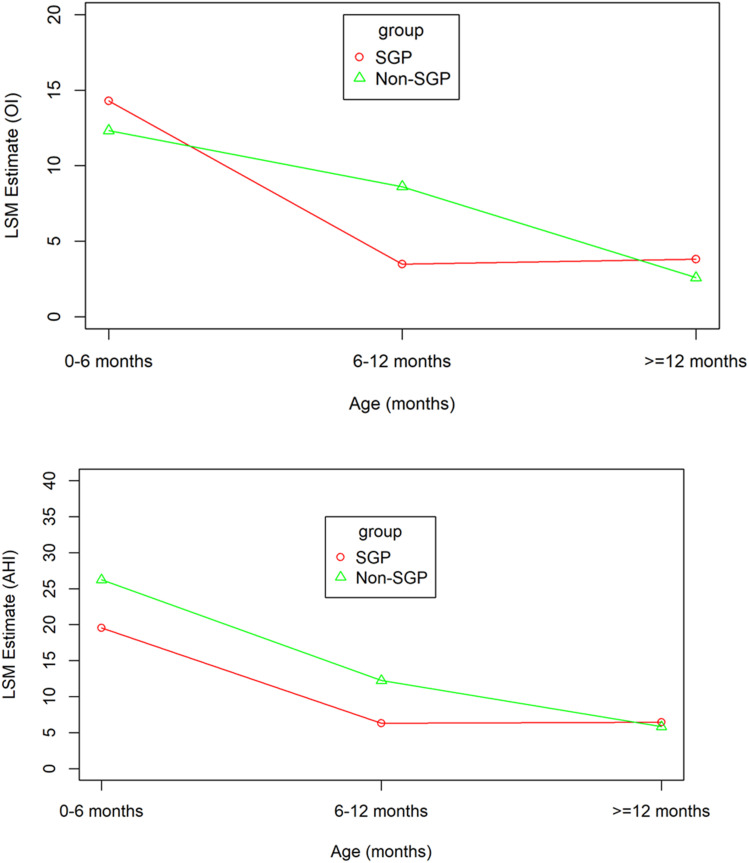
Comparisons of sleep and respiratory parameters between age group in both SGP and non-SGP are shown in Table 2 and Figure 1. AHI was significantly decreased from 0–6 to 6–12 months and from 0–6 to > 12 months in both SGP and non-SGP groups. Similarly, CI was significantly decreased from 0–6 to 6–12 months and from 0–6 to >12 months in both SGP and non-SGP groups. OI was significantly decreased from 0–6 to 6–12 months and from 0–6 to >12 months in only the SGP group. In the non-SPG group, OI was significantly decreased from 0–6 to >12 months but not from 0–6 to 6–12 months. There were no significant changes in maximum end tidal CO2 and mean SpO2 with increasing age. For sleep parameters, there were significant increases in sleep efficiency from 0–6 to >12 months in both SGP and non-SGP groups, but there were no significant increases in sleep efficiency from 0–6 to 6–12 months in either group. Arousal index was significantly decreased from 0–6 to 6–12 months and from 0–6 to >12 months in both SGP and non-SGP groups. There was no significant change in the percentage of supine position with increasing age in either group.
Table 2.
Comparison of sleep and respiratory parameters between age groups.
| Type of Treatment | AGE 0–6 MO [N (SGP) = 45 N (NON-SGP) = 29, LSM ± SE] | AGE 6–12 MO [N (SGP) = 34 N (NON-SGP) = 24, LSM ± SE] | AGE ≥ 12 MO [N (SGP) = 24 N (NON-SGP) = 26, LSM ± SE] | P VALUE | |||
|---|---|---|---|---|---|---|---|
| 0–6 VS 6–12 MO | 0–6 VS ≥12 MO | 6–12 VS ≥12 MO | |||||
| AHI | SGP | 19.55 ± 1.50 | 6.32 ± 2.17 | 6.45 ± 1.97 | <.01 | <.01 | .99 |
| NON-SGP | 26.22 ± 2.64 | 12.24 ± 3.00 | 5.84 ± 2.53 | <.01 | <.01 | .23 | |
| ALL | 21.95 ± 1.40 | 9.12 ± 1.82 | 6.14 ± 1.60 | <.01 | <.01 | .42 | |
| CI | SGP | 5.38 ± 0.75 | 2.19 ± 0.95 | 2.50 ± 0.96 | <.01 | .02 | .97 |
| NON-SGP | 14.10 ± 2.11 | 3.55 ± 2.40 | 3.38 ± 2.00 | <.01 | <.01 | .99 | |
| ALL | 8.44 ± 0.99 | 2.95 ± 1.22 | 2.94 ± 1.12 | <.01 | <.01 | .9999 | |
| OI | SGP | 14.29 ± 1.32 | 3.49 ± 1.80 | 3.81 ± 1.66 | <.01 | <.01 | .99 |
| NON-SGP | 12.33 ± 1.56 | 8.62 ± 1.76 | 2.59 ± 1.50 | .24 | <.01 | .02 | |
| ALL | 13.59 ± 1.01 | 5.78 ± 1.28 | 3.21 ± 1.28 | <.01 | <.01 | .28 | |
| SLEEP EFFICIENCY | SGP | 0.76 ± 0.01 | 0.78 ± 0.02 | 0.82 ± 0.02 | .73 | .04 | .32 |
| NON-SGP | 0.74 ± 0.02 | 0.78 ± 0.02 | 0.81 ± 0.01 | .18 | .01 | .62 | |
| ALL | 0.74 ± 0.01 | 0.80 ± 0.02 | 0.83 ± 0.02 | .06 | <.01 | .48 | |
| AROUSAL INDEX | SGP | 20.82 ± 0.61 | 12.59 ± 0.83 | 11.61 ± 0.77 | <.01 | <.01 | .66 |
| NON-SGP | 20.11 ± 0.90 | 12.81 ± 1.00 | 10.45 ± 0.88 | <.01 | <.01 | .14 | |
| ALL | 20.55 ± 0.51 | 12.62 ± 0.64 | 11.09 ± 0.58 | <.01 | <.01 | .15 | |
| MAX ETCO2 | SGP | 48.52 ± 0.66 | 47.22 ± 0.87 | 48.93 ± 0.82 | .40 | .90 | .29 |
| NON-SGP | 48.49 ± 0.66 | 49.45 ± 0.74 | 49.44 ± 0.66 | .54 | .51 | .9999 | |
| ALL | 48.44 ± 0.46 | 48.48 ± 0.79 | 49.10 ± 0.69 | .99 | .68 | .81 | |
| MEAN SPO2 | SGP | 0.98 ± 0.003 | 0.98 ± 0.004 | 0.97 ± 0.004 | .74 | .07 | .34 |
| NON-SGP | 0.97 ± 0.004 | 0.97 ± 0.003 | 0.96 ± 0.003 | .80 | .06 | .15 | |
| ALL | 0.98 ± 0.002 | 0.97 ± 0.003 | 0.97 ± 0.003 | .22 | .02 | .62 | |
| SUPINE POSITION | SGP | 0.66 ± 0.04 | 0.78 ± 0.10 | 0.58 ± 0.11 | .50 | .79 | .38 |
| NON-SGP | 0.59 ± 0.06 | 0.34 ± 0.09 | 0.37 ± 0.13 | .07 | .28 | .99 | |
| All | 0.63 ± 0.04 | 0.52 ± 0.07 | 0.48 ± 0.09 | .35 | .27 | .94 | |
P value was calculated using Tukey-Kramer adjusted from mixed model. Ages are in months. AHI = apnea-hypopnea index, CI = central apnea index, LSM = least square mean, OI = obstructive apnea-hypopnea index, SE = standard error, SGP = supraglottoplasty. P < .05 was considered statistically significant.
Figure 1. Mixed model.
Significant reduction in OI (top) and AHI (bottom) with increasing age without significant difference between the SGP and non-SGP groups. AHI = apnea-hypopnea index, LSM, least square mean, OI = obstructive index, SGP = supraglottoplasty.
Log-normal mixed model showed negative correlations between age and AHI (P < .01), age and OI (P < .01), and age and CI (P < .01) in both SGP and non-SGP groups (Table 3, Table 4, and Figure 2). Similarly, a negative correlation was observed between age and arousal index (P < .01) in both groups (Table 3, Table 4, and Figure 2). However, there were no significant correlations between age and sleep efficiency or age and mean SpO2 (Table 3 and Table 4).
Table 3.
Log-normal mixed model in patients with congenital laryngomalacia who have undergone supraglottoplasty.
| Characteristic | Age vs AHI | Age vs OI | Age vs CI | Age vs arousal index | Age vs sleep efficiency | Age vs mean SpO2 |
|---|---|---|---|---|---|---|
| Coefficient | −0.03 | −0.05 | −0.06 | −0.01 | 0.003 | −0.0002 |
| R2 | 0.06 | 0.18 | 0.16 | 0.18 | −0.004 | 0.04 |
| P value | <.01 | <.01 | <.01 | <.01 | .55 | .07 |
P < .05 was considered statistically significant. AHI = apnea-hypopnea index, CI = central apnea index, OI = obstructive apnea-hypopnea index.
Table 4.
Log-normal mixed model in patients with congenital laryngomalacia who have not undergone supraglottoplasty.
| Characteristic | Age vs AHI | Age vs OI | Age vs CI | Age vs arousal index | Age vs sleep efficiency | Age vs mean SpO2 |
|---|---|---|---|---|---|---|
| Coefficient | −0.06 | −0.05 | −0.05 | -0.01 | 0.001 | −0.0002 |
| R2 | 0.17 | 0.12 | 0.12 | 0.20 | 0.03 | −0.01 |
| P value | <.01 | <.01 | <.01 | <.01 | .06 | .05 |
P < .05 was considered statistically significant. AHI = apnea-hypopnea index, CI = central apnea index, OI = obstructive apnea-hypopnea index.
Figure 2. Scatter diagram with log-normal mixed model.
Changes with age for AHI (top left), obstructive AHI (top right), central apnea index (bottom left), and arousal index (bottom right) in SGP and non-SGP. AHI = apnea-hypopnea index, SGP = supraglottoplasty
Resolution of sleep-related breathing disorders: survival analysis
The ages at resolution of CSA (CI < 5) and OSA (OI < 1) were calculated using Kaplan-Meier curves. The mean age at resolution of CSA was 9.77 ± 1.13 months old for all patients. The mean age at resolution of CSA was significant lower in the SGP group compared with the non-SGP group (7.58 ± 1.16 [SGP] vs 12.57 ± 1.97 [non-SGP] months, P = .02; Figure 3). The mean age at resolution of OSA was 38.03 ± 4.97 months old for all patients. There was no significant difference in the mean age at resolution of OSA between the SGP and non-SGP group (35.18 ± 6.54 [SGP] vs 41.55 ± 6.86 [non-SGP] months, P = .60; Figure 4). The mean age at resolution of OSA was significantly higher in infants with congenital anomalies and syndromes and infants with neurologic diseases compared with normal infants (28.12 ± 5.01 [normal] vs 59.53 ± 11.55 [congenital anomalies and syndromes] vs 53.13 ± 9.10 [neurologic diseases], P = .003; Figure 4). There was no statistically significant difference in the mean age at resolution of CSA between infants with congenital anomalies and syndromes and infants with neurologic disease compared with normal infants.
Figure 3. Kaplan-Meier curve (central sleep apnea).
Age at resolution of central sleep apnea (CI < 5) in infants with laryngomalacia comparing between SGP and non-SGP (left) and comparing infants with neurologic disease, congenital anomalies and syndrome, and infants with no underlying conditions (right). CI = central apnea index, SGP = supraglottoplasty, surv = survival probability.
Figure 4. Kaplan-Meier curve (obstructive sleep apnea).
Age at improvement of obstructive sleep apnea (OI < 1) in infants with laryngomalacia comparing between SGP and non-SGP (left) and comparing infants with neurological disease, congenital anomalies and syndrome, and infants with no underlying conditions (right). OI = obstructive index, SGP = supraglottoplasty, surv = survival probability.
DISCUSSION
In our cohort, we found that approximately 44% of infants with laryngomalacia and OSA had coexistent CSA. When assessing the natural progression of these conditions with age in those undergoing SGP and those being treated conservatively (non-SGP), AHI and CI improved from 0–6 to 6–12 months and from 0–6 to >12 months in both groups. Although OI improved from 0–6 to >12 months in both groups, OI improved from 0–6 to 6–12 months in only the SGP group. The correlation analysis revealed reduction of AHI, OI, and CI with ages in both SGP and non-SGP groups.
In the current study, the main objectives were to examine longitudinal changes of sleep-related breathing disorders and the time to resolution of CSA and OSA and the effect of SGP. The resolution of CSA was defined as CI < 5 based on the American Academy of Sleep Medicine definition of CSA (>5 central apneas/h), and resolution of OSA was defined as an OI < 1 based on a commonly used cutoff for OSA, although there are limited normative data in infants and young children.20–22 Based on this definition, the mean age at resolution of CSA was significant lower in the SGP group compared with the non-SGP group, whereas there was no significant difference in the mean age at resolution of OSA between the SGP and non-SGP group. In addition, the mean age at resolution of OSA was significantly higher in the infants with congenital anomalies and syndromes and infants with neurologic diseases compared with normal infants.
Infants with laryngomalacia in our cohort had a relatively high proportion of CSA (44%). This is consistent with our previous data showing a high prevalence of CSA in infants with laryngomalacia who had PSG before upper airway surgeries (46.3%).5 The mechanism underlying CSA in infants with laryngomalacia is unknown. One proposed mechanism is based on abnormal sensorimotor integrative function of the larynx theory.2 According to this theory, the brainstem is responsible for both laryngeal tone and respiratory control leading to association between laryngomalacia and CSA. In fact, abnormality of specific areas of brainstem nuclei can lead to weak laryngeal tone, central apnea, and swallowing dysfunction.2 The nucleus tractus solitarius, one of respiratory control neurons, modulates the signals to the phrenic motor neuron pools in the spinal cord that control diaphragm activity and also sends axonal projections to the cell bodies of the recurrent laryngeal nerve in the nucleus ambiguous.23 The delayed development of this site in the brain stem may play a role in the pathophysiology of CSA and reduced laryngeal tone seen in infants with laryngomalacia.
Our study found that congenital anomalies and syndrome was relatively common in infants with laryngomalacia in both OSA alone and OSA/CSA groups. Down syndrome was the most common condition in this category. Compared with the general population, children with Down syndrome had a higher prevalence of OSA.24 Pathophysiologic factors that contribute to its increased prevalence of OSA in this population include the cranio-facial features, generalized hypotonia, and predisposition toward increased body mass index and obesity.25 In addition to OSA, a previous study demonstrated that patients with Down syndrome had increased central respiratory events, which may related to a dysfunction of the central respiratory control at the brain stem level.26 Other common congenital anomalies and syndromes in our cohort were Pierre-Robin sequence and Prader-Willi syndrome. Studies have demonstrated a strong association between Pierre-Robin sequence and OSA.27 The explanation was their anatomical defects, which included micro/retrognathia, glossoptosis, and possible cleft palate. One study described CSA in infants with Pierre-Robin sequence.28 OSA, CSA, and hypoventilation have been reported in patients with Prader-Willi syndrome. CSA is commonly seen in patients with Prader-Willi syndrome during infancy period.29,30 This is thought to be caused by abnormalities in central chemoreceptor sensitivities. OSA is found in older children with Prader-Willi syndrome with adenotonsillar hypertrophy and morbid obesity as the etiologic factors. Previous studies showed that the duration and frequency of central apnea improved with age in Prader-Willi syndrome29,30 but not in Pierre-Robin sequence.28 Interestingly, our previous study identified congenital syndrome including Down syndrome and Pierre Robin sequence as one of risk factors for CSA in infants with laryngomalacia.5
Clinical symptoms of laryngomalacia usually appear within 2 weeks of life, worsen at 4–8 months, improve between 8 and 12 months, and usually resolve by 12–18 months. In most cases, symptoms generally resolve with minimal or no treatment by 18 months of age, although the rate and average time of resolution vary among studies.31 Our study demonstrates that both OSA and CSA in infants with laryngomalacia improved with increasing age. The mean age at resolution of CSA was 9.77 months old, whereas the mean age at resolution of OSA was 38.03 months. The differences between our study and previous studies may be because of the definition of resolution. Both the rate and average time of resolution of laryngomalacia from previous studies were based on clinical improvement especially, self-reported stridor resolution, but not on resolution of OSA. Our study examined the resolution of OSA based on prediction of time when OI < 1 was expected to occur. Based on our study, the resolution of OSA takes longer than clinical improvement. The mechanisms underlying resolution of OSA and CSA may involve airway growth and maturation of respiratory control. Furthermore, our study reveals that resolution of OSA in infants with laryngomalacia was affected by the presence of underlying neurologic diseases and congenital anomalies and syndrome, which were common comorbidities in our infants with laryngomalacia. In both conditions, the time to resolution of OSA was longer. Neurologic diseases may influence vagal nerve function of laryngeal tone, thereby contributing to severity of airway obstruction,12 and may lead to delayed resolution of OSA.
The other main aim of our study was to assess the effect of SGP and nonsurgical treatment on the time to resolution of OSA and CSA. We found that both OSA and CSA in infants with laryngomalacia were improved with increasing ages regardless of SGP. In addition, the mean age at resolution of OSA was not difference between SGP and non-SGP group. However, the initial improvement of OSA was better in the SGP group as evidenced by a significant decrease in OI from 0–6 to 6–12 months in only the SGP group. Although, there was no significant difference in severity of OSA between SGP and non-SGP in our study, the decision to perform surgery is not based on PSG findings alone. Other clinical symptoms such as feeding difficulty, failure to thrive, or witnessed apnea would be an important consideration in the decision-making process for surgery. Therefore, our findings may indicate that some infants with laryngomalacia and sleep-related breathing disorders may be able to avoid surgery and be managed conservatively with nonsurgical interventions such as supplemental oxygen. However, SGP should be considered in those infants with severe clinical symptoms and significant OSA/CSA based on PSG findings, especially in the first 6 months of age. Further studies are needed to identify those infants with laryngomalacia who would benefit the most from surgical intervention.
Infants in the SGP group had an early decline in OI from 0–6 to 6–12 months, but there was no significant further decline in OI after 12 months. The magnitude of improvement in the early stage may be large enough that it is unlikely to see further improvement afterward. In contrast, the nonsurgical group had a delayed improvement in OSA with no significant decrease in OI in the first 12 months but a significant decline in OI after 12 months. These findings may suggest that a surgical correction of airway obstruction in infants with laryngomalacia may accelerate airway growth and/or maturation changes in respiratory control. However, further studies are needed to address this issue.
Interestingly, the mean age at resolution of CSA was significant lower in the SGP group compared with the non-SGP group, whereas there was no significant difference in the mean age at resolution of OSA between the 2 groups. Previous studies reported the benefit of upper airway surgery including adenotonsillectomy and mandibular distraction osteogenesis on improving of CSA in infants and children including those with Down syndrome and Pierre-Rubin sequence.28,32,33 The mechanism underlying resolution of CSA after surgery is unknown. Lee et al28 proposed that there may be a connection between airway obstruction and impaired ventilatory control by the central nervous system. The increased upper airway resistance secondary to obstruction may cause elevated CO2 levels, which trigger central chemoreceptors and create unstable inspiratory and expiratory muscle dynamics. Correction of upper airway obstruction may lead to more stable ventilatory control resulting in quicker resolution of CSA in the SGP group.
The management of CSA in infants includes medications, supplemental oxygen, and positive pressure ventilation. Methylxanthines (caffeine and theophyllines), which have been used mainly for treatment of CSA in premature infants,34 were used in 11.1% of our infants with laryngomalacia and OSA/CSA. Acetazolamide was used in only 2 patients in our study. There is some evidence for the use of acetazolamide as a treatment for CSA.35 Supplemental oxygen is probably the most common and widely prescribed therapy for CSA in prematurity, full-term, and syndromic infants.29,36 The biological mechanism of the therapeutic effects of oxygen on central apnea is unclear. Low-flow oxygen has been effective in premature infants with CSA by reducing ventilatory instability.36 In addition to the effect on CSA, oxygen has been shown to stabilize ventilation and reduce frequency of OSA in adult patients with high loop gain.37 Our recent study showed that the use of supplemental oxygen in infants with OSA resulted in reduction of obstructive respiratory events and improved oxygenation without adversely affecting alveolar ventilation.18 In our institution, infants with OSA who are not surgical candidates are often treated with supplement oxygen, although oxygen is more likely used in infants with coexisting CSA as shown in this study.
Although SGP is now considered the mainstay of treatment for severe laryngomalacia, alternative treatments with acid suppression, high-calorie diets, and swallowing therapy have been shown to be effective, with equivalent weight gain in a recent case-control study.38 Gastroesophageal reflux disease has a strong association with laryngomalacia.12 It remains unclear whether the relationship exists between these 2 conditions or the processes simply share common risk factors. In our study, gastroesophageal reflux disease was the second most common comorbidity identified in our infants with laryngomalacia and was more likely to be diagnosed in the OSA/CSA group. Most infants were clinically diagnosed, and acid suppression therapy (proton pump inhibitor or H2-blocker) was prescribed to either confirmed or clinically diagnosed patients with gastroesophageal reflux disease.
Continuous positive airway pressure has been used in the treatment of OSA, CSA, and periodic breathing in infants.39 Continuous positive airway pressure improves OSA by splinting the upper airways and alleviating airway obstruction. Continuous positive airway pressure can improve CSA by reducing the loop gain via an increase in the lung volume.40 In those patients with associated hypoventilation, bilevel positive airway pressure support with backup rate may be required to restore normal gas exchange during sleep. In our study, only 2 infants were prescribed noninvasive positive pressure ventilation. Both had neurologic disease with alveolar hypoventilation and had failed oxygen therapy. Because of the difficulty in using noninvasive positive pressure ventilation in infants, at our institution, this therapy is usually reserved for severe cases who are not surgical candidates and fail oxygen therapy.
Our study has several limitations. First, it is a retrospective study, and some clinical information might be incomplete. Second, PSG is not routinely performed in all infants with laryngomalacia. It is likely to be performed in symptomatic infants. In addition, there is a possibility of selection bias because of the different rates of PSG performed in the SGP and non-SGP groups. Third, only patients with follow-up PSG were included. It is a standard practice at our institution for infants with laryngomalacia who have OSA and/or CSA to have a repeated PSG every 3–6 months until all of sleep and respiratory parameters are normalized or markedly improved. However, the follow-up PSGs in each individual were done based on multiple factors, including physician and family preferences and availability of the sleep laboratory. Infants who did not have a follow-up PSG are likely to have a normal sleep study or very mild OSA and/or CSA on the initial study. The timing and availability of follow-up PSGs could affect the survival analysis in calculating the resolution of OSA and CSA.
CONCLUSIONS
To our knowledge, this is the first study that examines the natural courses of OSA and CSA in infants with laryngomalacia and the effect of SGP. Our findings indicate that developmental changes in respiratory pattern occur with ages in infants with laryngomalacia, leading to improvement of obstructive and central sleep apnea over time regardless of SGP. The mechanism underlying these changes may involve airway growth and maturation of respiratory control. The resolution of OSA is affected by the presence of neurologic diseases and congenital anomalies. Further studies are needed to confirm these findings and to evaluate long-term outcomes in this population.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The study was funded by the Cincinnati Children’s Research Foundation. Part of this research was presented as an abstract at the International conference of the American Thoracic Society 2017. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CI
central apnea index
- CSA
central sleep apnea
- OI
obstructive index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SGP
supraglottoplasty
REFERENCES
- 1.Daniel SJ. The upper airway: congenital malformations. Paediatr Respir Rev. 2006;7(Suppl 1):S260–S263. 10.1016/j.prrv.2006.04.227 [DOI] [PubMed] [Google Scholar]
- 2.Thompson DM. Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: a new theory of etiology. Laryngoscope. 2007117(6 Pt 2, Suppl 114:1–33. 10.1097/MLG.0b013e31804a5750 [DOI] [PubMed] [Google Scholar]
- 3.Ramgopal S, Kothare SV, Rana M, Singh K, Khatwa U. Obstructive sleep apnea in infancy: a 7-year experience at a pediatric sleep center. Pediatr Pulmonol. 2014;49(6):554–560. 10.1002/ppul.22867 [DOI] [PubMed] [Google Scholar]
- 4.Gonçalves MT, Sato J, Avelino MA, et al. Polisomnographic findings on children with laryngopathies. Braz J Otorhinolaryngol. 2006;72(2):187–192. 10.1016/S1808-8694(15)30054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanphaichitr A, Tanphaichitr P, Apiwattanasawee P, Brockbank J, Rutter MJ, Simakajornboon N. Prevalence and risk factors for central sleep apnea in infants with laryngomalacia. Otolaryngol Head Neck Surg. 2014;150(4):677–683. 10.1177/0194599814521379 [DOI] [PubMed] [Google Scholar]
- 6.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125(3):872–878. 10.1378/chest.125.3.872 [DOI] [PubMed] [Google Scholar]
- 7.Khan A, Qurashi M, Kwiatkowski K, Cates D, Rigatto H. Measurement of the CO2 apneic threshold in newborn infants: possible relevance for periodic breathing and apnea. J Appl Physiol 1985. 2005;98(4):1171–1176. 10.1152/japplphysiol.00574.2003 [DOI] [PubMed] [Google Scholar]
- 8.Edwards BA, Sands SA, Berger PJ. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Respir Physiol Neurobiol. 2013;185(1):144–155. 10.1016/j.resp.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 9.Isono S, Tanaka A, Ishikawa T, Nishino T. Developmental changes in collapsibility of the passive pharynx during infancy. Am J Respir Crit Care Med. 2000;162(3 Pt 1):832–836. 10.1164/ajrccm.162.3.9911089 [DOI] [PubMed] [Google Scholar]
- 10.Mase CA, Chen ML, Horn DL, Parikh SR. Supraglottoplasty for sleep endoscopy diagnosed sleep dependent laryngomalacia. Int J Pediatr Otorhinolaryngol. 2015;79(4):511–515. 10.1016/j.ijporl.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 11.Ayari S, Aubertin G, Girschig H, et al. Management of laryngomalacia. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130(1):15–21. 10.1016/j.anorl.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 12.Thompson DM. Laryngomalacia: factors that influence disease severity and outcomes of management. Curr Opin Otolaryngol Head Neck Surg. 2010;18(6):564–570. 10.1097/MOO.0b013e3283405e48 [DOI] [PubMed] [Google Scholar]
- 13.Powitzky R, Stoner J, Fisher T, Digoy GP. Changes in sleep apnea after supraglottoplasty in infants with laryngomalacia. Int J Pediatr Otorhinolaryngol. 2011;75(10):1234–1239. 10.1016/j.ijporl.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 14.Escher A, Probst R, Gysin C. Management of laryngomalacia in children with congenital syndrome: the role of supraglottoplasty. J Pediatr Surg. 2015;50(4):519–523. 10.1016/j.jpedsurg.2014.05.035 [DOI] [PubMed] [Google Scholar]
- 15.Digoy GP, Shukry M, Stoner JA. Sleep apnea in children with laryngomalacia: diagnosis via sedated endoscopy and objective outcomes after supraglottoplasty. Otolaryngol Head Neck Surg. 2012;147(3):544–550. 10.1177/0194599812446903 [DOI] [PubMed] [Google Scholar]
- 16.Chan DK, Truong MT, Koltai PJ. Supraglottoplasty for occult laryngomalacia to improve obstructive sleep apnea syndrome. Arch Otolaryngol Head Neck Surg. 2012;138(1):50–54. 10.1001/archoto.2011.233 [DOI] [PubMed] [Google Scholar]
- 17.Katz ES, Mitchell RB, D’Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185(8):805–816. 10.1164/rccm.201108-1455CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockbank J, Astudillo CL, Che D, et al. Supplemental oxygen for treatment of infants with obstructive sleep apnea. J Clin Sleep Med. 2019;15(8):1115–1123. 10.5664/jcsm.7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLean JE, Fitzgerald DA, Waters KA. Developmental changes in sleep and breathing across infancy and childhood. Paediatr Respir Rev. 2015;16(4):276–284. 10.1016/j.prrv.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 21.Daftary AS, Jalou HE, Shively L, Slaven JE, Davis SD. Polysomnography reference values in healthy newborns. J Clin Sleep Med. 2019;15(3):437–443. 10.5664/jcsm.7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chervin RD, Ellenberg SS, Hou X, et al. ; Childhood Adenotonsillectomy Trial . Prognosis for spontaneous resolution of OSA in children. Chest. 2015;148(5):1204–1213. 10.1378/chest.14-2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauda EB. Upper-airway muscle control during development. In: Mathew OP, ed. Respiratory Control and Disorders in the Newborn. Boca Raton, FL: Taylor & Francis; 2003:115–148. [Google Scholar]
- 24.Austeng ME, Øverland B, Kværner KJ, et al. Obstructive sleep apnea in younger school children with Down syndrome. Int J Pediatr Otorhinolaryngol. 2014;78(7):1026–1029. 10.1016/j.ijporl.2014.03.030 [DOI] [PubMed] [Google Scholar]
- 25.Shires CB, Anold SL, Schoumacher RA, Dehoff GW, Donepudi SK, Stocks RM. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int J Pediatr Otorhinolaryngol. 2010;74(7):768–772. 10.1016/j.ijporl.2010.03.050 [DOI] [PubMed] [Google Scholar]
- 26.Ferri R, Curzi-Dascalova L, Del Gracco S, Elia M, Musumeci SA, Stefanini MC. Respiratory patterns during sleep in Down’s syndrome:importance of central apnoeas. J Sleep Res. 1997;6(2):134–141. 10.1046/j.1365-2869.1997.00030.x [DOI] [PubMed] [Google Scholar]
- 27.Anderson IC, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48(5):614–618. 10.1597/10-100 [DOI] [PubMed] [Google Scholar]
- 28.Lee JJ, Thottam PJ, Ford MD, Jabbour N. Characteristics of sleep apnea in infants with Pierre-Robin sequence: Is there improvement with advancing age? Int J Pediatr Otorhinolaryngol. 2015;79(12):2059–2067. 10.1016/j.ijporl.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 29.Urquhart DS, Gulliver T, Williams G, Harris MA, Nyunt O, Suresh S. Central sleep-disordered breathing and the effects of oxygen therapy in infants with Prader-Willi syndrome. Arch Dis Child. 2013;98(8):592–595. 10.1136/archdischild-2012-303441 [DOI] [PubMed] [Google Scholar]
- 30.Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi syndrome. PLoS One. 2014;9(6):e101012. 10.1371/journal.pone.0101012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landry AM, Thompson DM. Laryngomalacia: disease presentation, spectrum, and management. Int J Pediatr. 2012;2012:753526. 10.1155/2012/753526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldassari CM, Kepchar J, Bryant L, Beydoun H, Choi S. Changes in central apnea index following pediatric adenotonsillectomy. Otolaryngol Head Neck Surg. 2012;146(3):487–490. 10.1177/0194599811428118 [DOI] [PubMed] [Google Scholar]
- 33.Thottam PJ, Choi S, Simons JP, Kitsko DJ. Effect of adenotonsillectomy on central and obstructive sleep apnea in children with Down syndrome. Otolaryngol Head Neck Surg. 2015;153(4):644–648. 10.1177/0194599815587877 [DOI] [PubMed] [Google Scholar]
- 34.Henderson-Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev. 2010;12:CD000140. 10.1002/14651858.CD000140.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philippi H, Bieber I, Reitter B. Acetazolamide treatment for infantile central sleep apnea. J Child Neurol. 2001;16(8):600–603. 10.1177/088307380101600813 [DOI] [PubMed] [Google Scholar]
- 36.Simakajornboon N, Beckerman RC, Mack C, Sharon D, Gozal D. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics. 2002;110(5):884–888. 10.1542/peds.110.5.884 [DOI] [PubMed] [Google Scholar]
- 37.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162(2):144–151. 10.1016/j.resp.2008.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faria J, Behar P. Medical and surgical management of congenital laryngomalacia: a case-control study. Otolaryngol Head Neck Surg. 2014;151(5):845–851. 10.1177/0194599814541921 [DOI] [PubMed] [Google Scholar]
- 39.Popatia R, Rosen D. Infant with obstructive sleep apnea successfully treated with continuous positive airway pressure. Clin Pediatr (Phila). 2014;53(4):395–396. 10.1177/0009922813479166 [DOI] [PubMed] [Google Scholar]
- 40.Edwards BA, Sands SA, Feeney C, et al. Continuous positive airway pressure reduces loop gain and resolves periodic central apneas in the lamb. Respir Physiol Neurobiol. 2009;168(3):239–249. 10.1016/j.resp.2009.07.006 [DOI] [PubMed] [Google Scholar]