Abstract
Study Objectives:
To determine if polysomnographic cardiorespiratory outcomes are associated with and could have the potential to predict the presence of postoperative adverse respiratory events in children with neuromuscular disease undergoing any surgical procedure.
Methods:
A retrospective cohort study was conducted at a tertiary pediatric institution. The study population included individuals with neuromuscular disease admitted for a surgical intervention under general anesthetic who had undergone a polysomnogram within 1 year before surgical intervention. Polysomnographic indices and postoperative adverse respiratory events were collected through chart review. Multivariable logistic regression was used to model postoperative adverse respiratory events, where PSG results were considered primary predictors.
Results:
Postoperative adverse respiratory events occurred in 25/61 (41%) of individuals and consisted mainly of desaturations requiring intervention 33 (73%), airway obstruction 15 (33%), and atelectasis (22%). Results from the unadjusted and adjusted logistic regression models indicated that saturation nadir and bulbar dysfunction individually were independent risk factors for postoperative adverse respiratory events with the highest areas under the receiver operating characteristic curve. A multivariable prediction model using these 2 risk factors provided an area under the receiver operating characteristic curve of 0.74 (95% confidence interval, 0.65–0.83).
Conclusions:
Knowing that nocturnal oxygen saturation nadir and the presence of bulbar dysfunction are potential predictors of postoperative adverse respiratory events is useful for future counseling of families and surgical planning, in an effort to improve perioperative management and reduce adverse events.
Citation:
Fishman H, Hamid JS, Barrowman N, Momoli F, Maclusky I, Katz SL. Associations between polysomnography measurements and postoperative adverse respiratory events in children with neuromuscular disease. J Clin Sleep Med. 2021;17(4):757–765.
Keywords: neuromuscular disease, pediatrics, polysomnography, complications, pulmonary, postoperative
BRIEF SUMMARY
Current Knowledge/Study Rationale: Aside from pulmonary function tests, which are limited by age and cognitive ability, few studies have evaluated other indices capable of determining risk for postoperative adverse respiratory events in individuals with neuromuscular disease. If clinically predictive, polysomnographic indices could be used in counseling and surgical planning in an effort to improve perioperative management.
Study Impact: Nocturnal oxygen saturation nadir and the presence of bulbar dysfunction were identified as potential predictors of postoperative adverse respiratory events. To reduce the risk of postoperative adverse events, preoperative screening may include an overnight oximetry and an evaluation of aspiration risk.
INTRODUCTION
Neuromuscular (NM) disorders encompass a wide variety of diseases that share a common symptom: respiratory muscle weakness. Pulmonary function gradually declines over time, further exacerbated by stress or increased metabolic demands. Individuals with NM disease more frequently require general anesthesia and surgical interventions and have a higher risk of postoperative adverse respiratory events, the most common being aspiration pneumonia and respiratory failure. The higher complication rate is attributed to reduced lung compliance, sleep-disordered breathing (SDB), and decreased cough strength with poor airway clearance, exacerbated by pain and immobilization.1–6
The ability to predict which individuals are at highest risk of postoperative adverse respiratory events would assist in preoperative planning and counseling of families, postoperative management, and disposition planning. Previous studies in individuals with NM disorders have identified an increased risk of postoperative complications and a need for prolonged postoperative ventilation when vital capacity is lower on preoperative spirometry.4,5,7–11 However, in children with NM disease, the presence of cognitive impairments and young age significantly limits the spectrum of these patients who can perform pulmonary function testing. Alternative measures are therefore needed to identify children with NM disorders at the highest risk of postoperative adverse respiratory events.
Polysomnography (PSG) is frequently performed in children with NM disease and provides comprehensive information on respiratory function and gas exchange during sleep, which in some respects mimics the effects of anesthesia. In children and adults with OSA, PSG parameters can predict the risk of postoperative respiratory adverse events.12,13 For instance, in a retrospective study of 151 pediatric patients undergoing adenotonsillectomy with a preoperative PSG, desaturations requiring supplemental oxygen therapy along with other postoperative adverse events were of higher frequency in patients with a significantly higher AHI (31.8 vs 14.1 events/h; P = .001), a higher hypopnea index (22.6 vs 8.9 events/h; P = .004), and a lower nadir oxygen saturation.12 A similar study with 83 pediatric patients performed by Hill et al14 showed that among other risk factors, an AHI greater than 24 events/h and an oxygen saturation < 90% were predictive of postoperative complications, which included the need for CPAP or bilevel PAP, reintubation, pulmonary edema, oxygen, and airway support. As seen in these studies, data from PSG may be predictive of postoperative respiratory outcomes in a population with NM disease. A review of the literature identified only 1 previous study using PSG to predict pulmonary complications after scoliosis repair.15 Such a surgery, focusing on the thorax and upper abdomen, reduces lung volume and may have high rates of pulmonary complications compared with other surgical procedures. An evaluation of outcomes of a more exhaustive list of surgical procedures would enable the elucidation of postoperative respiratory complication frequency by surgical type, which may differ.
Our aim was to determine whether any polysomnographic cardiorespiratory parameters are associated with and could have the potential to predict the presence of postoperative adverse respiratory events in pediatric patients with NM disease undergoing any surgical procedure.
METHODS
This retrospective cohort study took place at the Children’s Hospital of Eastern Ontario (CHEO), a tertiary pediatric institution in Ottawa, Ontario, Canada. Inclusion criteria were as follows: (1) children with confirmed neuromuscular disease, (2) children younger than age 18 years, (3) admission to CHEO for a surgical intervention under general anesthetic, (4) PSG at CHEO within 1 year before surgical intervention; (4) patient care during April 1, 2010–December 31, 2017. PSG data were included up to 1 year before surgical intervention because a majority of patients only have annual PSGs given the capacity of the sleep laboratory, and in the absence of any clinical changes. The term “neuromuscular disease” was expanded to include the following diagnostic codes: Duchenne muscular dystrophy (DMD), Becker muscular dystrophy, congenital muscular dystrophy, congenital myopathy, myotonic dystrophy, spinal muscular atrophy, Bethlem myopathy, fascioscapulohumeral muscular dystrophy, limb girdle muscular dystrophy, nemaline rod myopathy, and cerebral palsy. Institutional approval was obtained from the Research Ethics Board (CHEOREB# 18/26) at CHEO.
Individuals were identified from CHEO electronic health records using diagnostic codes from the International Classification of Diseases, 10th revision, with Canadian enhancements. Demographic and clinical data were collected from electronic charts and paper charts obtained through health records.
The demographic information and medical history collected from the study sample included the following: age, sex, neuromuscular condition, wheelchair use, presence of bulbar dysfunction (based on clinical assessment), presence of scoliosis, use of noninvasive positive pressure ventilation (NIV), and use of an assistive cough device (manual lung volume recruitment or mechanical insufflation-exsufflation).
PSGs were conducted and scored in accordance with the American Academy of Sleep Medicine9 for pediatric PSGs (XL-TEK, Oakville, Ontario, Canada). Sleep studies were not routinely ordered as part of the preoperative assessment but were independently performed based on the suspicion of SDB (symptoms or a rapid decline in pulmonary function). Information collected from the PSG included the central apnea-hypopnea index, the obstructive apnea-hypopnea index, the percentage of total sleep time with carbon dioxide (CO2) > 50 mmHg, the lowest recorded oxygen saturation, the mean baseline saturation during sleep, and the therapeutic intervention, if any. Indices from the diagnostic and titration PSGs were used. The CO2 value was collected from the end-tidal monitor. If the end-tidal value was not available (eg, poor patient tolerance, poor tracing, use of NIV), then the transcutaneous CO2 was used instead. The end-tidal CO2 monitor used was the Capnostream 20 (Covidien, Minneapolis, MN) and the transcutaneous CO2 monitor was either a TCM4 series (Radiometer, Brea, CA) or a SenTec V-Sign System (SenTec Inc, Lincoln, RI).
The type of surgical intervention and the duration of surgery were recorded. Surgical intervention was further evaluated by surgical specialty, such as otolaryngology, musculoskeletal, gastrointestinal, central nervous system, urology, and cardiovascular. Information on postoperative adverse respiratory events that developed postoperatively at any time before discharge was also collected, including pneumonia, atelectasis, pneumothorax, hypoxemia, desaturations requiring intervention, aspiration, and airway obstruction. Events occurring in the postanesthetic recovery unit, inpatient ward, and intensive care unit (ICU) were considered. Interventions to address respiratory complications, including airway support, reintubation, mechanical ventilation, NIV, use of supplemental oxygen, and antibiotics were recorded. Finally, length of hospital stay and duration of ICU admission were also collected.
The data were compiled in a REDCap database. Patient information was deidentified and stored on a locked computer in a code-accessed room in the hospital.
Statistical analyses
Descriptive statistics were used to summarize the study population, particularly the demographic and clinical variables. The unit of analysis was unique general anesthetic events. Where multiple procedures were performed under the same general anesthetic, the major surgical procedure was considered. Descriptive statistics with respect to surgeries performed were also provided. Continuous variables were summarized using median and interquartile range (IQR), and frequency and percentages were used to summarize categorical variables. For each of the cardiorespiratory parameters, along with age and sex, univariable logistic regression for postoperative adverse respiratory events was carried out. For each parameter, the odds ratio with 95% confidence interval was estimated, as was the area under the receiver operating characteristic curve as a measure of predictive performance. Given the constraints of sample size, a multivariable prediction model was obtained by selecting the parameters with the 2 highest areas under the receiver operating characteristic curve. Odds ratios and 95% confidence intervals were estimated, as was an overall area under the receiver operating characteristic curve. To examine whether the predictive ability of sleep parameters differed between patients receiving respiratory support preoperatively vs those who were not, the model was expanded by including an interaction term and a main effect for respiratory support. In the event that evidence for the interaction was not strong (P > .2), the interaction term was removed. All analyses were performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was determined at α = .05.
As mentioned in the paragraph above, the unit of analysis was general anesthetic events and hence data included multiple surgical procedures from the same individual if that person had multiple independent surgical events in the time period studied. As such, we first considered a linear mixed model, where correlations across multiple surgeries (within patients) were adjusted. Nevertheless, the correlation was very weak and the traditional multivariable logistic regression was chosen for analyzing our data.
RESULTS
A total of 61 patients met the inclusion criteria. During the study period, a total of 125 surgical procedures were performed under 111 general anesthetic instances. Each individual underwent at least 1 surgery, with some having undergone multiple surgeries under the same anesthetic and in subsequent visits. Only the major surgery under the same anesthetic was considered in the analysis because such multiple surgeries within the same anesthetic were removed. The results presented in the rest of this article are based on the 111 surgeries associated with the unique anesthetic instances.
The number of unique anesthetic instances that a patient underwent during the study period ranged from 1–7, where almost half (49.2%; n = 30) of the individuals had 2 surgeries, 18.03% (n = 11) of patients had 3 surgeries, 3 had 4 surgeries (4.9%), 4 had 5 surgeries (6.6%), 1 had 6 surgeries (1.6%), and 1 had 7 surgeries (1.6%).
The study sample consisted of mainly boys (37/61; 60.7%), with a median age of 9 years (IQR, ages 4–14 years; Table 1). The most common neuromuscular conditions were cerebral palsy (24/61; 39.3%), followed by DMD (10/61; 16.4%) and congenital myotonic dystrophy (7/61; 11.5%). The majority of patients had bulbar dysfunction (32/61; 52.5%). An overall description of the 61 patients is presented in Table 1, along with whether or not they experienced a postoperative adverse respiratory event in any general anesthetic event.
Table 1.
Descriptive statistics for all individuals (n = 61) undergoing surgery, categorized by whether the surgeries resulted in postoperative adverse respiratory events.
| Characteristic | Overall (n = 61) | Complications (n = 25) | No Complications (n = 36) |
|---|---|---|---|
| Female | 24 (39.3) | 11 (44.0) | 13 (36.1) |
| Age, y, median (IQR) | 9.0 (4.0–14.0) | 9.0 (3.0–14.0) | 9.0 (5.0–14.0) |
| Scoliosis | 17 (27.9) | 8 (47.1) | 9 (52.9) |
| Neuromuscular condition | |||
| Cerebral palsy | 24 (39.3) | 15 (60.0) | 9 (25.0) |
| DMD | 10 (16.4) | 2 (8.0) | 8 (22.2) |
| Congenital muscular dystrophy | 5 (8.2) | 2 (8.0) | 3 (8.3) |
| Congenital myotonic dystrophy | 7 (11.5) | 2 (8.0) | 5 (13.9) |
| Nemaline rod myopathy | 2 (3.3) | 1 (4.0) | 1 (2.8) |
| Spinal muscular atrophy type 1 | 1 (1.6) | 0 (0.0) | 1 (2.8) |
| Mitochondrial storage disease | 1 (1.6) | 1 (4.0) | 0 (0.0) |
| Other | 11 (18.0) | 2 (8.0) | 9 (25.0) |
| Use of assisted ventilation | |||
| CPAP | 2 (3.3) | 1 (4.0) | 1 (2.8) |
| Bilevel PAP | 21 (34.4) | 11 (44.0) | 10 (27.8) |
| Tracheostomy and ventilation | 1 (1.6) | 0 (0.0) | 1 (2.8) |
| Other | 5 (8.2) | 3 (12.0) | 2 (5.6) |
| Assistive cough device | |||
| MIE | 4 (6.6) | 2 (8.0) | 2 (5.6) |
| LVRAB | 8 (13.1) | 3 (12.0) | 5 (13.9) |
| Both MIE and LVRAB | 1 (1.6) | 0 (0.0) | 1 (2.8) |
| Chest physiotherapy (percussion) | 6 (9.8) | 3 (12.0) | 3 (8.3) |
| Bulbar dysfunction | 32 (52.5) | 18 (72.0) | 14 (38.9) |
| Wheelchair use | 45 (77.6)mv=3 | 19 (82.6)mv=2 | 26 (74.3)mv=1 |
Values are n (%) except where noted. IQR = interquartile ratio, DMD = Duchenne muscular dystrophy, LVRAB = lung volume recruitment Ambu-bag, MIE = mechanical insufflation-exsufflation, MV = missing values.
Of the 61 individuals who underwent surgery, 25 (40.9%) had postoperative adverse respiratory events (Table 1). In our study sample, girls (11/25; 45.8%) and boys (14/25; 37.8%) had similar rates of postoperative adverse respiratory events. The age distribution did not seem to differ between those with complications and those with no complications. Individuals with cerebral palsy had more postoperative adverse respiratory events than those with DMD, those with bulbar dysfunction had more postoperative adverse respiratory events than those without bulbar dysfunction, and patients with wheelchair use had more postoperative adverse respiratory events than those who remained ambulatory. The use of assisted ventilation (ie, NIV) was equivalent between those with and those without postoperative adverse respiratory events. Only 1 individual received invasive ventilation, and this patient had no postoperative adverse respiratory events. Patients who used a lung volume recruitment Ambu-bag and the combination of both mechanical insufflation-exsufflation and a lung volume recruitment Ambu-bag had fewer postoperative adverse respiratory events.
The 111 general anesthetic events were further evaluated according to surgical type (Table 2). The median length of surgery was 1.7 hours (IQR, 1.0–2.5 hours), and the majority of the surgeries were either otolaryngology, musculoskeletal, or gastrointestinal. The highest percentage of postoperative adverse respiratory events (18/34; 52.9%) was observed among patients undergoing ENT surgeries, which was also the most common surgical subtype performed (34/111; 30.6%). There were only 3 surgeries related to central line insertion/removal, and all of these resulted in postoperative adverse respiratory events.
Table 2.
Surgical information based on 111 unique anesthetic events.
| Surgery Type and Subtype | Overall (n = 111) | Complications (n = 45) | No Complications (n = 66) |
|---|---|---|---|
| Duration, h, median (IQR) | 1.7 (1.0–2.5)mv=2 | 2.0 (1.5–3.0) | 1.5 (1.0–2.0)mv=2 |
| ENT, n (%) | 34 (30.6) | 18 (52.9) | 16 (47.1) |
| Adenotonsillectomy | 6 (17.6) | 3 (50.0) | 3 (50.0) |
| Myringotomy tube insertion | 2 (5.9) | 2 (100.0) | 0 (0.0) |
| Salivary ligation/Botox | 4 (11.8) | 2 (50.0) | 2 (50.0) |
| Supraglottoplasty | 1 (2.9) | 1 (100.0) | 0 (0.0) |
| Other | 22 (64.7) | 11 (50.0) | 11 (50.0) |
| MSK, n (%) | 31 (27.9) | 10 (32.3) | 21 (67.7) |
| Scoliosis repair | 8 (25.8) | 4 (50.0) | 4 (50.0) |
| Tendon release | 8 (25.8) | 2 (25.0) | 6 (75.0) |
| Other | 19 (61.3) | 5 (26.3) | 14 (73.7) |
| GI, n (%) | 29 (26.1) | 13 (44.8) | 16 (55.2) |
| G-tube insertion | 14 (48.3) | 5 (35.7) | 9 (64.3) |
| Fundoplication | 7 (24.1) | 4 (57.1) | 3 (42.9) |
| Other | 10 (34.5) | 5 (50.0) | 5 (50.0) |
| CNS, n (%) | 13 (11.7) | 6 (46.2) | 7 (53.8) |
| Shunt insertion/revision | 5 (38.5) | 2 (40.0) | 3 (60.0) |
| Other | 8 (61.5) | 4 (50.0) | 4 (50.0) |
| Urology, n (%) | 9 (8.1) | 1 (11.1) | 8 (88.9) |
| Orchidopexy | 6 (66.7) | 0 (0.0) | 6 (100.0) |
| Other | 3 (33.3) | 1 (33.3) | 2 (66.7) |
| CVS, n (%) | 3 (2.7) | 3 (100.0) | 0 (0.0) |
| Central line insertion/removal | 3 (100.0) | 3 (100.0) | 0 (0.0) |
Proportions for surgery subtype are calculated using the total number of surgeries within each surgery type as the denominator. All of the CVS surgeries involved central line insertion/removal. CNS = central nervous system, CVS = cardiovascular, ENT = otolaryngology, IQR = interquartile ratio, MSK = musculoskeletal, MV = missing values.
The 3 most common postoperative adverse respiratory events were desaturations requiring intervention (73.3%), airway obstruction (33.3%), and atelectasis (22.2%; Table 3). The most commonly used therapeutic interventions were supplemental oxygen (75.6%) and NIV (37.8%). The “other” category consisted of deep suctioning and chest physiotherapy. A subgroup analysis was performed to illustrate differences in pulmonary complications and respiratory interventions between patients with cerebral palsy and patients with other neuromuscular conditions (Table 3).
Table 3.
Frequency and type of postoperative adverse respiratory events (n = 45) and the interventions used to manage them based on 111 unique anesthetic events.
| Complications and Interventions | n (%) | Cerebral Palsy (n = 21) | No Cerebral Palsy (n = 24) |
|---|---|---|---|
| Pulmonary complication | |||
| Pneumonia | 8 (17.8) | 2 (9.5) | 6 (25.0) |
| Atelectasis | 10 (22.2) | 3 (14.3) | 7 (29.2) |
| Pneumothorax | 1 (2.2) | 0 (0.0) | 1 (4.2) |
| Hypoxemia | 6 (13.3) | 1 (4.8) | 5 (20.8) |
| Desaturations requiring intervention | 33 (73.3) | 17 (81.0) | 16 (66.7) |
| Aspiration | 2 (4.4) | 1 (4.8) | 1 (4.2) |
| Airway obstruction | 15 (33.3) | 10 (47.6) | 5 (20.8) |
| Other | 8 (17.8) | 2 (9.5) | 6 (25.0) |
| Respiratory intervention | |||
| Airway support | 8 (17.8) | 4 (19.0) | 4 (16.7) |
| Reintubationa | 3 (6.7) | 1 (4.8) | 2 (8.3) |
| Mechanical ventilation | 13 (28.9) | 3 (14.3) | 10 (41.7) |
| Supplemental oxygen | 34 (75.6) | 19 (90.5) | 15 (62.5) |
| Antibiotics | 7 (15.6) | 2 (9.5) | 5 (20.8) |
| CPAP | 3 (6.7) | 3 (14.3) | 0 (0.0) |
| NIV | 17 (37.8) | 8 (38.1) | 9 (37.5) |
| Other | 19 (42.2) | 6 (28.6) | 13 (54.2) |
Based on n = 45 unique anesthetic events with postoperative respiratory complications. aReintubation at any point in the postoperative period. NIV = noninvasive ventilation.
Anesthetic events resulting in no postoperative adverse respiratory events had a median length of hospital stay of 1 day (IQR, 1.0–5.0 days) compared with a median of 7 days (IQR, 4.0–19.0 days) for those with postoperative adverse respiratory events. A difference was also seen when evaluating median ICU length of stay. Anesthetic events with no postoperative adverse respiratory events had a median length of ICU stay of 0.0 hours (IQR, 0.0–0.0 hours) compared with a median of 18.0 hours (IQR, 0.0–96.0 hours) for anesthetic events with postoperative adverse respiratory events.
The preoperative PSG results showed that there were no differences in the median obstructive apnea-hypopnea index among patients with postoperative adverse respiratory events (0.1 events/h; IQR, 0.0–2.2 events/h) and among those without postoperative adverse respiratory events (0.1 events/h; IQR, 0.0–0.7 events/h; Table 4). The central apnea-hypopnea index was found to be slightly higher (2.3 events/h; IQR, 0.7–5.8 events/h) among individuals with no postoperative adverse respiratory events compared with patients with respiratory events (1.8 events/h; IQR, 0.3–6.6 events/h). The lowest saturation (nadir) among patients with postoperative adverse respiratory events was 84.2% (IQR, 73.6%–89.6%), slightly lower than those with no respiratory events (89%; IQR, 84.2%–92.0%). The mean saturation was similar in both groups, with a median mean saturation of 97.1% (IQR, 96.3%–98.0%) among those with postoperative adverse respiratory events and 97.4% (IQR, 96.7%–98.1%) among those with no events.
Table 4.
PSG results, categorized by the absence or presence of postoperative adverse respiratory events.
| Characteristic | Complications (n = 45) | No Complications (n = 66) |
|---|---|---|
| OAHI (events/h) | 0.1 (0.0–2.2)mv=1; range, 0–78.3 | 0.1 (0.0–0.7); range, 0–7.7 |
| CAHI (events/h) | 1.8 (0.3–6.6); range, 0–15.8 | 2.3 (0.7–5.8); range, 0–20.2 |
| Hypoventilation (% TST with CO2 > 50 mm Hg) | 0.1 (0.0–0.7)mv=14; range, 0–40.3 | 0.4 (0.0–4.2)mv=22; range, 0–58.3 |
| Lowest saturation (%) | 84.2 (73.6–89.6); range, 40.8–98.1 | 89.0 (84.2–92.0); range, 39.5–96.4 |
| Mean saturation (%) | 97.1 (96.3–98.0); range, 90.6–99.8 | 97.4 (96.7–98.1); range, 95–99.4 |
CAHI = central apnea-hypopnea index, CO2 = carbon dioxide, MV = missing values, OAHI = obstructive apnea-hypopnea index, PSG = polysomnography, TST = total sleep time.
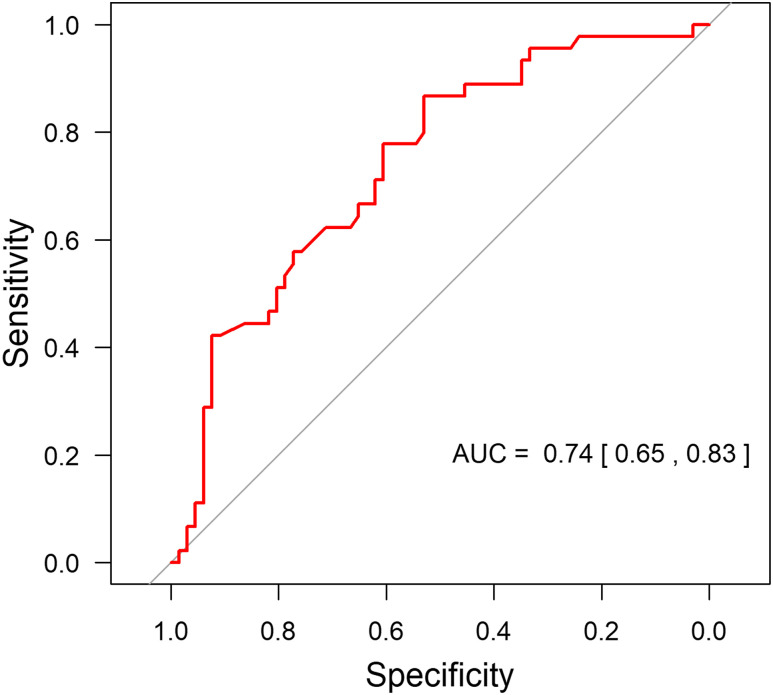
Results from the unadjusted and adjusted logistic regression models are presented in Table 5. The results indicate that the saturation nadir and bulbar dysfunction individually were independent risk factors for postoperative adverse respiratory events, with the highest areas under the receiver operating characteristic curve. A multivariable prediction model using these 2 risk factors was constructed. There was little evidence of an interaction between the saturation nadir and preoperative respiratory support (P = .51). After removing the interaction term, we found little evidence of a main effect (P = .43). Figure 1 shows that the multivariable prediction model provided an area under the receiver operating characteristic curve of 0.74 (95% confidence interval, 0.65–0.83).
Table 5.
Factors predictive of postoperative respiratory complications.
| Variables | Unadjusted OR (95% CI) | AUC | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Age per y | 0.94 (0.87–1.02) | 0.58 | — | — |
| Female | 1.64 (0.75–3.57) | 0.56 | — | — |
| OAHI (events/h)a | 1.15 (1.03–1.39) | 0.54 | — | — |
| CAHI (events/h) | 0.98 (0.89–1.07) | 0.52 | — | — |
| Hypoventilationb (% TST with CO2 > 50 mm Hg) | 0.97 (0.89–1.02) | 0.60 | — | — |
| Mean saturation (%) | 0.80 (0.59–1.06) | 0.53 | — | — |
| Lowest saturation (%) | 0.96 (0.92–0.99) | 0.64 | 0.96 (0.92–0.99) | .01 |
| Bulbar dysfunction | 3.49 (1.55–8.30) | 0.64 | 3.61 (1.55–8.93) | .004 |
| Cerebral palsy | 2.16 (0.98–4.83) | 0.59 | — | — |
Results from unadjusted and adjusted logistic regression models based on 111 unique anesthetic events. aOne missing value. bThirty-six missing values. AUC = area under the receiver operating characteristic curve, CAHI = central apnea-hypopnea index, CI = confidence interval, CO2 = carbon dioxide, OAHI = obstructive apnea-hypopnea index, OR = odds ratio, TST, total sleep time.
Figure 1. ROC curve.
Multivariate prediction model using saturation nadir and bulbar dysfunction provided an AUC of 0.74 (95% CI, 0.65–0.83). AUC = area under the receiver operating characteristic curve, CI = confidence interval, ROC = receiver operating characteristic.
DISCUSSION
Our study identified a low oxygen saturation nadir during sleep and bulbar dysfunction as potential predictors of postoperative adverse respiratory events in children with NM disease. Our study is the first to evaluate postoperative adverse respiratory events in a population of children exclusively with NM disorders and considering a range of respiratory events. A literature search revealed only 1 previous study addressing PSG findings as a predictor of postoperative complications in children. In a retrospective study of 110 children undergoing scoliosis surgery (61 of whom had neuromuscular scoliosis), none of the PSG parameters predicted the risk of prolonged ventilation.15 However, the study differed from ours in that it limited surgical interventions to any type of scoliosis repair and only evaluated prolonged postoperative mechanical ventilation as a postoperative adverse respiratory event, whereas our study looked at a more extensive list of pulmonary complications.
We hypothesized that PSG parameters would predict postoperative adverse respiratory events because sleep is closely representative of the physiologic stress that is placed on individuals with NM disease when they are administered sedating drugs and paralytic agents perioperatively. Sleeping in a recumbent position, particularly during REM sleep when an individual is more reliant on a weakened diaphragm, will more readily identify patients with poor respiratory reserve. Respiratory control is also significantly affected in many individuals with NM disease, further contributing to SDB and a blunted hypercarbic response.3
Contrary to our hypothesis, neither the median obstructive apnea-hypopnea index, the central apnea-hypopnea index, nor the presence of hypoventilation were predictive of postoperative complication risk. The median obstructive apnea-hypopnea index of 0.1 events/h (Table 4) for such a high-risk population was surprisingly low. This finding may be explained by the proportion of patients who required some modality of assistive ventilation at baseline. As per the baseline characteristics of the patient population (Table 1), a total of 29 patients (47%) were managed with either CPAP, bilevel PAP, invasive ventilation, or some other modality such as supplemental oxygen or high-flow nasal cannula. Therefore, in approximately half of the study population, PSG performed within 1 year of surgical intervention represented titration studies as opposed to diagnostic studies.
Interestingly, the rates of postoperative respiratory complications seemed to be similar between those with and without NIV at baseline. These results contrast with those found by Khirani et al,16 who reported no respiratory complications after scoliosis surgery in children with NM disease after preoperative use of NIV and cough assist.
A robust pool of literature exists detailing operative complications in individuals with DMD undergoing spinal surgery.5,17–20 This is because nearly all (70%–90%) individuals with DMD develop scoliosis after losing independent ambulation. Failure to repair scoliosis in DMD can lead to poor quality of life, reduced pulmonary reserve, and increased hospitalization rates.21 Scoliosis surgery in children with NM disorders is associated with an increased hospital length of stay, prolonged intubation, and higher in-hospital mortality.10,17,22 Thoracic surgery decreases lung volumes, expiratory flow rates, and oxygenation postoperatively as a result of the surgery itself, anesthesia, pain, and immobilization.10 Respiratory complications, including aspiration pneumonia and respiratory failure, are often cited as the most common complications.1,3,5 Postoperative adverse respiratory events rates in the literature are predominantly based on postoperative scoliosis surgery among individuals with neuromuscular scoliosis (mainly DMD) and are highly variable, ranging from 0%–31%.5,6,10,11,23–25 Our higher postoperative adverse respiratory events rate (40.5%) may be related to a greater diversity of neuromuscular diseases, a larger variety of surgical interventions, and a selection bias for children with more advanced disease. The most commonly documented postoperative adverse respiratory events in other studies were pneumonia, atelectasis, and acute respiratory failure necessitating the need for prolonged mechanical intubation (> 72 hours).6,10,12,22–27 Other complications reported in the literature that were not found in our chart review included hemothorax, pulmonary edema, and pleural effusion. The median hospital stay of 7 days and ICU stay of 18 hours reported in our study is similar to that reported in the literature, with a mean hospital length of stay ranging from 2–26 days, and mean ICU stays ranging from 2.5–8 days1,11,18–20,22,26
Previous studies evaluating postoperative respiratory complications after scoliosis repair included the diagnosis of cerebral palsy because it falls within the category of neuromuscular scoliosis. This subgroup of patients was also included in our analysis because this cohort of patients shares many common themes of respiratory management with patients who have other neuromuscular disorders. These include upper airway obstruction, chest wall restriction, hypoventilation, poor airway clearance, and chronic lower airway disease.3 Patients with cerebral palsy have a higher incidence of SDB, specifically increased obstructive and central apnea indices, and more frequent and severe desaturation events. This cohort tends to have reduced vital capacity because of spasticity and/or dyssynchrony of the respiratory musculature. In addition to hypertonia, patients can also present with hypotonia of the pharyngeal musculature. This group is known to have a higher incidence of oropharyngeal motor problems and poor handling of oral secretions.28–30 However, given the static nature of cerebral palsy compared to the progressive nature of other neuromuscular conditions, a subgroup analysis was performed to compare the 2 groups (Table 3).
In our study, supplemental oxygen was the most common intervention for postoperative adverse respiratory events, namely desaturations. Note that oxygen should be used with caution in this population because it can mask hypoventilation. Upper airway obstruction was typically treated with airway support (ie, laryngeal mask airway) and CPAP. Atelectasis was treated with supplemental oxygen, mechanical insufflation-exsufflation, and chest physiotherapy. The fourth most common complication, pneumonia, was managed with a combination of antibiotics, supplemental oxygen, chest physiotherapy, and cough assist.
A higher risk of postoperative complications was seen in our study in those with bulbar dysfunction, which is a common feature of cerebral palsy. Complication rates in our population were the highest in those with cerebral palsy. This may be related to the involvement of the central nervous system and the associated impairment of the airway protective mechanisms.11 Aspiration is often caused by oropharyngeal weakness, incoordination, and failure of the protective laryngeal closure. The risk of postoperative adverse respiratory events may therefore be mitigated by prompt clearing of upper airway secretions with suctioning and reduction of salivary load preoperatively with the use of, eg, salivary Botox, salivary duct ligation, and atropine. Swallowing evaluation may also be useful to identify the risk of aspiration.
The limitations of this study are that it is a retrospective review and there was no designated control group. Hospital discharge records and operative reports may be subject to coding errors and omissions. The study had a limited sample size because of the rarity of NM disease; this led to the examination of all surgical interventions under general anesthesia as opposed to focusing on 1 specific intervention, such as postoperative scoliosis repair. The PSG indices from titration studies were included in the analysis with other diagnostic studies because they were felt be representative of a patient’s baseline respiratory status, albeit on assistive support. Thus our population did include 2 groups of patients: those with untreated SDB and those with SDB managed with assistive therapy. Another limitation of this study is the inability to distinguish between the absence of SDB at baseline from the absence of SDB as it is appropriately managed with ventilatory support. Although we did not detect differences in the predictive ability of PSG findings to identify postoperative adverse respiratory events between children with and without preoperative respiratory support, this predictive ability may be further investigated in future studies with a larger sample size.
There may have been overrepresentation of the select individuals with multiple events under general anesthesia, although we aimed to control for this possibility with the use of a linear mixed model accounting for within-individual correlations. There may have been a selection bias for children with more advanced disease to undergo preoperative PSG, because such testing is not a routine preoperative investigation. The time interval of up to 1 year between the PSG and the surgical intervention may have overlooked deterioration in the polysomnographic indices because of natural disease progression and/or improvement as a result of other treatment modalities. However, a majority of patients with NM disease in our practice are followed with annual PSGs unless there are significant interval clinical changes. In the context of pulmonary complications, we were unable to differentiate the need for mechanical ventilation as a true postoperative adverse respiratory event from whether there was a predetermined management plan to have a patient remain intubated postoperatively. Finally, our study included a heterogeneous population of children with diverse diagnoses, stage of disease, ambulatory status, preoperative use of NIV, and nutritional status. Each of these factors may have contributed to postoperative adverse respiratory events risk, thereby limiting the generalization of the study findings to children with all categories and stages of NM disease. Because this was a retrospective study, future work includes validation of our modeling in a larger, prospective study.
CONCLUSIONS
In pediatric patients with NM conditions undergoing surgical procedures, nocturnal oxygen saturation nadir and the presence of bulbar dysfunction were identified as potential predictors of postoperative adverse respiratory events. This information is useful for future counseling of families and surgical planning, in an effort to improve perioperative management and reduce postoperative adverse respiratory events.
A preoperative oximetry in lieu of a PSG may be sufficient to screen for individuals with NM disorders at higher risk of postoperative complications, although further research is required.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada. The authors report no conflicts of interest.
ABBREVIATIONS
- CHEO
Children’s Hospital of Eastern Ontario
- CO2
carbon dioxide
- DMD
Duchenne muscular dystrophy
- ICU
intensive care unit
- IQR
interquartile range
- NIV
noninvasive ventilation
- NM
neuromuscular
- PSG
polysomnography
- SDB
sleep-disordered breathing
REFERENCES
- 1.Karmaniolou I, Krishnan R, Galtrey E, Cleland S, Vijayaraghavan R. Perioperative management and outcome of patients with Rett syndrome undergoing scoliosis surgery: a retrospective review. J Anesth. 2015;29(4):492–498. 10.1007/s00540-015-1974-3 [DOI] [PubMed] [Google Scholar]
- 2.Mathieu J, Allard P, Gobeil G, Girard M, De Braekeleer M, Bégin P. Anesthetic and surgical complications in 219 cases of myotonic dystrophy. Neurology. 1997;49(6):1646–1650. 10.1212/WNL.49.6.1646 [DOI] [PubMed] [Google Scholar]
- 3.Blatter JA, Finder JD. Perioperative respiratory management of pediatric patients with neuromuscular disease. Paediatr Anaesth. 2013;23(9):770–776. 10.1111/pan.12214 [DOI] [PubMed] [Google Scholar]
- 4.Jenkins JG, Bohn D, Edmonds JF, Levison H, Barker GA. Evaluation of pulmonary function in muscular dystrophy patients requiring spinal surgery. Crit Care Med. 1982;10(10):645–649. 10.1097/00003246-198210000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Chong HS, Moon ES, Park JO, et al. Value of preoperative pulmonary function test in flaccid neuromuscular scoliosis surgery. Spine Phila Pa 1976. 2011;36(21):E1391–E1394. 10.1097/BRS.0b013e31820cd489 [DOI] [PubMed] [Google Scholar]
- 6.Chong HS, Padua MR, Kim JS, et al. Usefulness of noninvasive positive-pressure ventilation during surgery of flaccid neuromuscular scoliosis. J Spinal Disord Tech. 2015;28(8):298–300. 10.1097/BSD.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 7.Berry R, Brooks R, Gamaldo CE, et al.et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0.1. Darien, IL: American Academy of Sleep Medicine; 2013. [Google Scholar]
- 8.Modi HN, Suh SW, Hong JY, Park YH, Yang JH. Surgical correction of paralytic neuromuscular scoliosis with poor pulmonary functions. J Spinal Disord Tech. 2011;24(5):325–333. 10.1097/BSD.0b013e3181f9f6fc [DOI] [PubMed] [Google Scholar]
- 9.Chng SY, Wong YQ, Hui JH, Wong HK, Ong HT, Goh DY. Pulmonary function and scoliosis in children with spinal muscular atrophy types II and III. J Paediatr Child Health. 2003;39(9):673–676. 10.1046/j.1440-1754.2003.00266.x [DOI] [PubMed] [Google Scholar]
- 10.Lin LC, Jong YJ. Pulmonary function assessment in patients with spinal muscular atrophy type II and type III. Acta Paediatr Taiwan. 2004;45(1):15–18. [PubMed] [Google Scholar]
- 11.Yuan N, Skaggs DL, Dorey F, Keens TG. Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2005;40(5):414–419. 10.1002/ppul.20291 [DOI] [PubMed] [Google Scholar]
- 12.Jaryszak EM, Shah RK, Vanison CC, Lander L, Choi SS. Polysomnographic variables predictive of adverse respiratory events after pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 2011;137(1):15–18. 10.1001/archoto.2010.226 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Bustamante A, Bartels K, Clavijo C, et al. Preoperatively screened obstructive sleep apnea is associated with worse postoperative outcomes than previously diagnosed obstructive sleep apnea. Anesth Analg. 2017;125(2):593–602. 10.1213/ANE.0000000000002241 [DOI] [PubMed] [Google Scholar]
- 14.Hill CA, Litvak A, Canapari C, et al. A pilot study to identify pre- and peri-operative risk factors for airway complications following adenotonsillectomy for treatment of severe pediatric OSA. Int J Pediatr Otorhinolaryngol. 2011;75(11):1385–1390. 10.1016/j.ijporl.2011.07.034 [DOI] [PubMed] [Google Scholar]
- 15.Yuan N, Skaggs DL, Davidson Ward SL, Platzker AC, Keens TG. Preoperative polysomnograms and infant pulmonary function tests do not predict prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2004;38(3):256–260. 10.1002/ppul.20021 [DOI] [PubMed] [Google Scholar]
- 16.Khirani S, Bersanini C, Aubertin G, Bachy M, Vialle R, Fauroux B. Non-invasive positive pressure ventilation to facilitate the post-operative respiratory outcome of spine surgery in neuromuscular children. Eur Spine J. 2014;23(Suppl 4):S406–S411. 10.1007/s00586-014-3335-6 [DOI] [PubMed] [Google Scholar]
- 17.Cognetti D, Keeny HM, Samdani AF, et al. Neuromuscular scoliosis complication rates from 2004 to 2015: a report from the Scoliosis Research Society Morbidity and Mortality database. Neurosurg Focus. 2017;43(4):E10. 10.3171/2017.7.FOCUS17384 [DOI] [PubMed] [Google Scholar]
- 18.Barsdorf AI, Sproule DM, Kaufmann P. Scoliosis surgery in children with neuromuscular disease: findings from the US National Inpatient Sample, 1997 to 2003. Arch Neurol. 2010;67(2):231–235. 10.1001/archneurol.2009.296 [DOI] [PubMed] [Google Scholar]
- 19.Pesenti S, Blondel B, Peltier E, et al. Experience in perioperative management of patients undergoing posterior spine fusion for neuromuscular scoliosis. BioMed Res Int. 2016;2016:3053056. 10.1155/2016/3053056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thacker M, Hui JHP, Wong HK, Chatterjee A, Lee EH. Spinal fusion and instrumentation for paediatric neuromuscular scoliosis: retrospective review. J Orthop Surg (Hong Kong). 2002;10(2):144–151. 10.1177/230949900201000207 [DOI] [PubMed] [Google Scholar]
- 21.Finder JD, Birnkrant D, Carl J, et al.Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004;170(4):456–465. 10.1164/rccm.200307-885ST [DOI] [PubMed] [Google Scholar]
- 22.Bendon AA, George KA, Patel D. Perioperative complications and outcomes in children with cerebral palsy undergoing scoliosis surgery. Paediatr Anaesth. 2016;26(10):970–975. 10.1111/pan.12981 [DOI] [PubMed] [Google Scholar]
- 23.Sinclair JL, Reed PW. Risk factors for perioperative adverse events in children with myotonic dystrophy. Paediatr Anaesth. 2009;19(8):740–747. 10.1111/j.1460-9592.2009.03079.x [DOI] [PubMed] [Google Scholar]
- 24.Modi HN, Suh SW, Hong JY, Cho JW, Park JH, Yang JH. Treatment and complications in flaccid neuromuscular scoliosis (Duchenne muscular dystrophy and spinal muscular atrophy) with posterior-only pedicle screw instrumentation. Eur Spine J. 2010;19(3):384–393. 10.1007/s00586-009-1198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Kishk I, Kozer E, Hod-Feins R, et al. Pediatric scoliosis surgery—is postoperative intensive care unit admission really necessary? Paediatr Anaesth. 2013;23(3):271–277. 10.1111/pan.12108 [DOI] [PubMed] [Google Scholar]
- 26.Duckworth AD, Mitchell MJ, Tsirikos AI. Incidence and risk factors for post-operative complications after scoliosis surgery in patients with Duchenne muscular dystrophy: a comparison with other neuromuscular conditions. Bone Joint J. 2014;96-B(7):943–949. 10.1302/0301-620X.96B7.33423 [DOI] [PubMed] [Google Scholar]
- 27.Almenrader N, Patel D. Spinal fusion surgery in children with non-idiopathic scoliosis: is there a need for routine postoperative ventilation? Br J Anaesth. 2006;97(6):851–857. 10.1093/bja/ael273 [DOI] [PubMed] [Google Scholar]
- 28.Zucconi M, Bruni O. Sleep disorders in children with neurologic diseases. Semin Pediatr Neurol. 2001;8(4):258–275. 10.1053/spen.2001.29477 [DOI] [PubMed] [Google Scholar]
- 29.Kotagal S. Sleep in neurodevelopmental and neurodegenerative disorders. Semin Pediatr Neurol. 2015;22(2):126–129. 10.1016/j.spen.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Kontorinis G, Thevasagayam MS, Bateman ND. Airway obstruction in children with cerebral palsy: need for tracheostomy? Int J Pediatr Otorhinolaryngol. 2013;77(10):1647–1650. 10.1016/j.ijporl.2013.07.017 [DOI] [PubMed] [Google Scholar]