Abstract
Study Objectives:
It has been suggested that there might be a pathophysiological link and overlap between primary aldosteronism (PA) and obstructive sleep apnea (OSA). Therefore, in a prospective study, we evaluated the frequency of PA in hypertensive patients suspected of having OSA.
Methods:
We included 207 consecutive hypertensive patients (mean age 53.2 ± 12.1 years, 133 M, 74 F) referred for polysomnography on the basis of one or more of the following clinical features: typical OSA symptoms, resistant or difficult-to-treat hypertension, diabetes, or cardiovascular disease. PA was diagnosed based on thew saline infusion test.
Results:
Moderate-to-severe OSA was diagnosed in 94 patients (45.4% of the whole group). PA was diagnosed in 20 patients with OSA (21.3%) compared with 9 patients in the group without OSA (8.0%; P = .006). PA was also frequent in patients in whom symptoms of OSA were a sole indication for PA screening (15.4%) and in patients with and without resistant hypertension (24.5% and 17.8%, respectively). Most patients with PA and OSA were diagnosed with bilateral adrenal hyperplasia (18 patients, 90%). There were no major differences in clinical characteristics between patients with OSA with PA and those without PA. In multivariate models, moderate-to-severe OSA predicted the presence of PA (odds ratio 2.89, P = .018).
Conclusions:
Patients with clinically important moderate-to-severe OSA are characterized by a relatively high frequency of PA. Our results support the recommendations to screen patients with moderate-to-severe OSA for PA, regardless of the presence of other indications for PA screening.
Citation:
Dobrowolski P, Kołodziejczyk-Kruk S, Warchoł-Celińska E, et al. Primary aldosteronism is highly prevalent in patients with hypertension and moderate to severe obstructive sleep apnea. J Clin Sleep Med. 2021;17(4):629–637.
Keywords: obstructive sleep apnea, primary aldosteronism, hypertension
BRIEF SUMMARY
Current Knowledge/Study Rationale: Prolonged exposure to elevated aldosterone concentrations is associated with target organ damage and high cardiovascular risk in patients with primary aldosteronism (PA). The results of studies evaluating the association between obstructive sleep apnea and the presence of PA are contradictory.
Study Impact: We showed that patients with clinically important moderate-to-severe obstructive sleep apnea are characterized by a relatively high frequency of PA. There are no distinct features that indicate which patients with obstructive sleep apnea should be screened for PA; therefore, moderate-to-severe obstructive sleep apnea should be considered an indication for PA screening.
INTRODUCTION
Obstructive sleep apnea (OSA) is considered a contributing factor to the development of hypertension and is especially associated with resistant hypertension,1 and there is evidence that untreated OSA increases the risk of cardiovascular morbidity and mortality.2–4 Moreover, intermittent nocturnal hypoxia and hypoxemia, a pathognomic feature in OSA, might activate the renin-angiotensin system.5
There is also growing evidence to indicate that prolonged exposure to elevated aldosterone concentrations is associated with target organ damage in the heart, kidney, and arterial wall and with a high cardiovascular risk in patients with primary aldosteronism (PA).6,7 It has been postulated that aldosterone worsens OSA by promoting the accumulation of fluid within the neck area, which then contributes to increased upper airway resistance.8 Therefore, it has been suggested that there might be a pathophysiological link and overlap between PA and OSA.6,9,10
The association between symptoms of OSA and the presence of PA has been shown in two studies.11,12 In those studies, however, OSA was not evaluated by means of polysomnography (PSG) in all patients. Despite the limitations of the available data, the Endocrine Society guidelines recommend screening of hypertensive patients with OSA for PA.13 More recently, Buffolo et al reported that PA was present in 8.9% of a multiethnic cohort of OSA patients (11.8% of European and 5.9% Chinese ethnicity). Moreover, it was demonstrated that in White patients, there is a significant relationship between apnea-hypopnea index (AHI) and serum aldosterone levels.14 It should be noted that in that study the frequency of PA in OSA patients was not compared with controls without OSA.15
The aim of the present study was to evaluate prospectively the frequency of PA in consecutive patients suspected of having OSA who were referred for PSG. We also aimed to evaluate specific clinical features of PA in patients with OSA.
METHODS
Patients
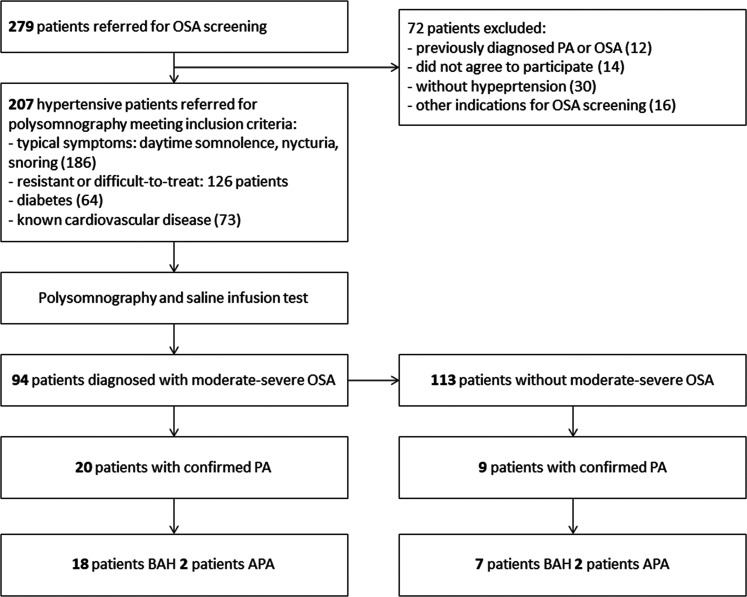
We included in our study 207 consecutive hypertensive patients referred for PSG on the basis of one or more of the following clinical features suggestive for OSA: (1) typical symptoms (daytime somnolence, nocturia, snoring), (2) resistant or difficult-to-treat hypertension, (3) diabetes type 2, and (4) known cardiovascular disease. Some patients qualified for inclusion based on more than one feature. Patients with previously diagnosed OSA or secondary hypertension were excluded from the study. The population recruitment description is presented in Figure 1.
Figure 1. Flowchart of the study population. Some patients had more than one inclusion criteria.
APA = aldosterone-producing adrenal adenoma, BAH = bilateral adrenal hyperplasia, OSA, obstructive sleep apnea, PA = primary aldosteronism.
Study procedure
The detailed clinical protocol included the following: (1) clinical and biochemical evaluation, (2) PSG, and (3) saline infusion test. In patients with a positive saline infusion test, further diagnostic procedures for PA subtyping were performed. The study was approved by the ethics committee of the National Institute of Cardiology, Warsaw, and informed written consent was obtained from each patient.
Patient evaluation
All patients underwent standard clinical evaluation. Body weight and height, as well as neck and waist circumferences, were measured. An enlarged neck circumference was defined as ≥ 41 cm or ≥ 43 cm for women and men, respectively.16 Body mass index was calculated, and obesity was considered BMI ≥ 30 kg/m2. Abdominal obesity was defined as waist circumference > 102 cm and > 88 cm for men and women, respectively.17 Current antihypertensive medications, history of diabetes, history of cardiovascular disease (coronary artery disease, including myocardial infraction, heart failure, transient ischemic attack or stroke, arrhythmias, including atrial fibrillation and ventricular arrhythmias), as well as lowest known serum potassium concentration, were also documented.
Office blood pressure measurements and control of hypertension
Blood pressure (BP) was measured by a trained nurse using an automated device (Omron 705IT, Omron Co., Kyoto, Japan) with the patient in the sitting position after a 5-minute rest. Based on upper-arm circumference, an appropriately sized cuff was placed on the arm with the lower edge of the cuff 2 cm above the antecubital fossa. Three consecutive readings were performed. If the difference between readings was higher than 10 mm Hg, further measurements were taken to obtain three consecutive consistent readings. The average of these three readings was calculated and used for further analysis. Hypertension was defined as office BP ≥ 140 and/or ≥ 90 mm Hg on two separate occasions or being on antihypertensive treatment. Difficult-to-treat hypertension was defined as treatment with at least four antihypertensive medications irrespective of BP control. Resistant hypertension was defined as office BP ≥ 140 and/or ≥ 90 mm Hg while receiving at least three antihypertensive medications.17
Laboratory methods
Biochemical evaluation of blood samples taken after overnight fasting was determined by routine methods and included sodium, potassium, lipids, blood count, fasting plasma glucose, and creatinine. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula.18 Hypokalemia was defined as serum potassium concentration lower than < 3.5 mmol/L. Glucose and lipid metabolism abnormalities were diagnosed on the basis of the 2007 European Society of Hypertension/European Society of Cardiology guidelines: normal fasting plasma glucose, < 5.6 mmol/L; impaired fasting plasma glucose, 5.6–6.9 mmol/L; abnormal glucose tolerance was diagnosed if plasma glucose 2 hours after ingestion of 75 g glucose was 7.8–11.0 mmol/L; diabetes was diagnosed if fasting plasma plasma glucose (two measurements) were ≥ 7.0 mmol/L or 2 hours plasma glucose > 11.0 mmol/L.17 For diagnosis of metabolic syndrome, three of five criteria had to be met: (1) office BP ≥ 130/≥85 mm Hg, (2) abdominal obesity, (3) high-density lipoprotein cholesterol M < 1.0 mmol/L, F < 1.2 mmol/L, (4) triglycerides > 1.7 mmol/L, and (5) fasting plasma glucose ≥ 5.6 mmol/L or use of antidiabetic medication.17
Evaluation for PA and PA subtyping
The following examinations were performed: (1) All included patients underwent a saline infusion test (intravenous infusion of 2 L of 0.9% saline for 4 hours in a sitting position. Postinfusion plasma aldosterone level > 10 ng/dL confirmed a diagnosis of PA providing plasma low renin level in the baseline evaluation.13 (2) To determine the subtype of PA, adrenal vein sampling (AVS) was performed in all patients with a diagnosis of PA. A continuous intravenous infusion of cosyntropin, a synthetic adrenal cortical stimulating hormone (50 μg/h), was started at least 60 minutes before the procedure was begun. AVS was performed sequentially and was regarded as successful if the selectivity index was higher than 5:1. A cutoff of the cortisol-corrected aldosterone ratio from high side to low side of more than 4:1 was used to indicate unilateral aldosterone excess. With these cutoffs, AVS was used for detecting unilateral or bilateral aldosterone hypersecretion.13 All the performed AVS in patients included in this study were successful (the 98% success rate in our center has been reported19). (3) Adrenalectomy was performed in patients with aldosterone-producing adenoma (APA). After surgery, patients were followed up according to the Primary Aldosteronism Surgical Outcome protocol and criteria.20 Plasma aldosterone and renin concentrations were assessed using chemiluminescent immunoassays (Liaison, DiaSorin, Italy). Medication treatment was tailored according to current guidelines, including withdrawing spironolactone, diuretics, and other drugs when appropriate before evaluations. None of the patients received spironolactone/eplerenone during evaluation. We defined typical indications for PA screening other than OSA as (1) BP ≥ 150 and/or ≥ 100 mm Hg, (2) resistant HT, (3) the presence or history of spontaneous or diuretic-induced hypokalemia.13
Polysomnography
The Epworth Sleepiness Scale was evaluated before the sleep study; excessive daytime sleepiness was defined as ≥11 points.21 The diagnosis of OSA was made by standard attended PSG using an Alice 5 (Respironics Inc., Murrysville, Pennsylvania) device. The electroencephalogram, electrooculogram, electromyogram of chin muscles, as well as electrocardiogram, were simultaneously recorded. Oral-nasal airflow (with thermal and pressure sensing device), thoracoabdominal respiratory movements, body position, snoring, and oximetry were also obtained. The PSG recordings were scored manually using 30-second epochs according to Rechtschaffen and Kales’ criteria22 for sleep and wake determination and sleep staging. Abnormal respiratory events were evaluated according to the standard criteria of the American Academy of Sleep Medicine Task Force.23 Apneas were defined as a cessation of airflow for at least 10 seconds, with ongoing effort (obstructive apnea) or loss of effort (central apnea), and hypopneas were defined as more than a 50% decrease in airflow from baseline in amplitude of a valid measure of breathing during sleep for 10 seconds or longer, associated with either an oxygen desaturation of ≥ 3% or an arousal. The AHI, indicating the number of apneic and hypopneic episodes per hour of sleep and oxygen desaturation index (ie, number of oxygen desaturation of ≥ 3% events per hour of sleep) were calculated. Other measures obtained were minimal, nocturnal oxygen saturation was average, and the percent of sleep time spent with oxygen saturation < 90%. Clinically significant moderate-to-severe OSA was diagnosed when the AHI was > 15 events/h.
Statistical analysis
Data analysis was carried out using statistical software PASW Statistics 18 (SPSS Inc., Chicago, Illinois). Data are expressed as mean ± standard deviation, as median and interquartile range, and frequency as a percentage. The values of variables between groups were compared (continuous and discrete variables): Student’s t test, Mann-Whitney test, Kruskal-Wallis test, or univariate analysis of variance analysis with post hoc test of Duncan’s multiple range test; and categorical variables: chi2 test or Fisher’s exact test.
To assess the independent association of several variables, including the presence of OS with PA, a stepwise logistic regression model was performed using removal testing based on the probability of the Wald statistic. Because there were a total of 29 cases of PA (primary outcome) in our sample, we were limited in how many variables could be entered into the logistic regression model to avoid overfitting. As such, if two or more variables were highly correlated with each other, only one would be entered into the model. For example, AHI and oxygen desaturation index were significant in univariate analysis; however, we included only the category of moderate-severe OSA vs no-mild OSA. Similarly, neck circumference, abdominal obesity, and body mass index were significantly different among the groups. We included neck circumference in the model because not only it can represent a measure of central adiposity, but it can also include a measure of rostral fluid shifts. We also chose not to include the models’ variables that were significant in univariate analysis but had less biologic plausibility for PA (ie, low high-density lipoprotein values, glucose metabolism alterations). Age was included in the model, even though it was not significant in univariate analysis. Collinearity in the final model was excluded using the variance inflation factors analysis. P < .05 was considered statistically significant.
RESULTS
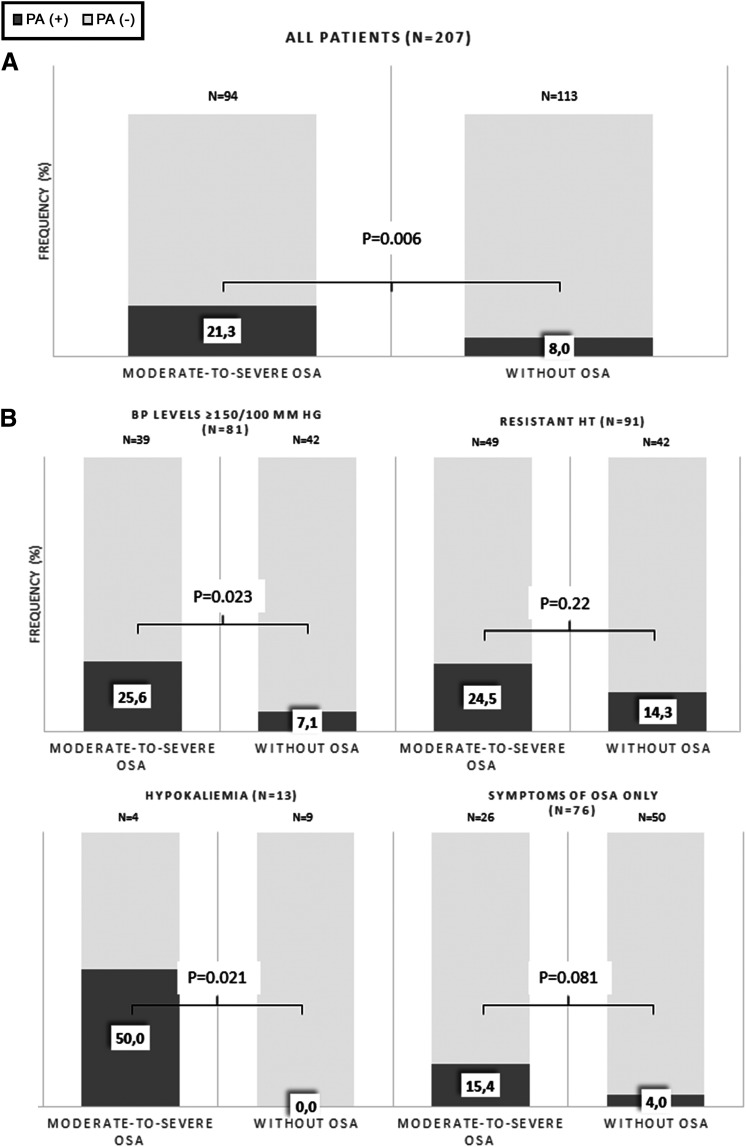
We included in our study 207 consecutive hypertensive patients (mean age 53.2 ± 12.1 years, 133 M, 74 F). Moderate-to-severe OSA was diagnosed in 94 patients (45.4% of the whole group). PA was diagnosed in 20 (21.3%) patients with OSA and in nine (8.0%) patients without OSA (P = .006) (Figure 2A). Among PA patients, only two patients, one patient with OSA and one patient without OSA, were characterized by aldosterone-to-renin ratio values < 30: 26.2 and 27.1, respectively. Bilateral adrenal hyperplasia was most common PA form, and it was diagnosed in 18 patients with OSA (90%) and in 7 patients without OSA (77.8%). Among 29 patients with PA, bilateral adrenal hyperplasia was most common PA form, and it was diagnosed in 18 patients with OSA (90%) and in 7 patients without OSA (77.8%). In the four patients with APA, complete biochemical success and partial clinical success was observed at 1-year follow-up according to the Primary Aldosteronism Surgical Outcome criteria.20
Figure 2. Frequency of PA in patients with or without OSA.
(A) Frequency of PA in patients with or without moderate-to-severe OSA in the whole group. (B) Frequency of PA in patients with or without moderate-to-severe OSA in in relation to the presence of indications for PA screening. BP = blood pressure, CVD = cardiovascular disease, HT = hypertension, OSA = obstructive sleep apnea, PA = primary aldosteronism.
We evaluated the prevalence of PA in OSA patients in relation to the presence of typical indications for PA screening (Figure 2B). The frequency of PA in OSA patients with BP values ≥ 150/100 mm Hg, resistant hypertension, or hypokalemia was 25.6%, 24.5%, and 50%, respectively (Figure 2B). In 26 OSA patients, no indications for OSA screening, other than OSA symptoms, were noted. In this group, PA was diagnosed in four patients (15.4%) (Figure 2B).
Patients with OSA and PA compared with patients with OSA without PA were characterized by higher sodium concentration and a greater number of antihypertensive drugs (Table 1). There were no differences in increased neck circumference, Epworth Sleepiness Scale score, AHI, mean and minimal SpO2, and sleep time spent with oxygen below 90% saturation between patient with OSA and PA compared with patients with OSA without PA (Table 2). The prevalence of PA was nonsignificantly higher in patients with severe than with moderate OSA (25.5% and 16.3%, P = .27). Patients with moderate to severe OSA, compared with patients without OSA, were characterized by a higher frequency of difficult-to-treat hypertension and resistant hypertension (58.5 vs 33.6%; P = .001 and 52.1 vs 37.2%; P = .031). There was no difference in the prevalence of difficult-to-treat and resistant hypertension in OSA patients with and without PA.
Table 1.
Clinical and biochemical parameters of patients in relation to the presence of OSA and/or PA.
| Variables | OSA (-) PA (-) (n = 104) | OSA (+) PA (-) (n = 74) | OSA (-) PA (+) (n = 9) | OSA (+) PA (+) (n = 20) | P OSA (+) PA (-) vs OSA (+) PA (+) | P Value* |
|---|---|---|---|---|---|---|
| Age (y) | 49.9 ± 12.6 | 56.8 ± 10.2 | 50.1 ± 14.2 | 58.9 ± 10.1 | .42 | .064† |
| Males (%) | 54.8 | 68.9 | 77.8 | 90.0 | .058 | .011‡ |
| Blood pressure levels and control | ||||||
| SBP (mm Hg) | 148.4 ± 23.8 | 146.3 ± 21.1 | 145.0 ± 17.3 | 152.0 ± 19.0 | .27 | .35† |
| DBP (mm Hg) | 88.2 ± 13.6 | 86.1 ± 12.5 | 86.4 ± 9.0 | 89.2 ± 11.4 | .32 | .45† |
| HR (bpm) | 76.0 ± 10.8 | 76.2 ± 11.7 | 74.1 ± 13.5 | 75.3 ± 9.9 | .75 | .58† |
| No. of antihypertensive drugs | 3 (2–4) | 4 (2–5) | 3 (2–4.5) | 4 (3–5) | .014 | .002§ |
| Difficult-to-treat HT (%) | 32.7 | 55.4 | 44.4 | 70.0 | .24 | .002‡ |
| Resistant HT (%) | 34.6 | 50.0 | 66.7 | 60.0 | .43 | .033‡ |
| Obesity and metabolic syndrome components | ||||||
| BMI (kg/m2) | 30.0 ± 5.5 | 33.1 ± 4.6 | 32.0 ± 4.2 | 35.0 ± 4.9 | .11 | .064† |
| Obesity (%) | 44.2 | 75.7 | 55.6 | 90.0 | .17 | <.001‡ |
| Abdominal obesity (%) | 68.3 | 94.6 | 88.9 | 95.0 | .94 | <.001‡ |
| Glucose (mmol/L) | 6.0 ± 1.6 | 6.5 ± 1.9 | 7.7 ± 5.6 | 6.6 ± 1.3 | .89 | .069† |
| Diabetes or IFG (%) | 56.7 | 73.0 | 66.7 | 90.0 | .11 | .013‡ |
| Diabetes (%) | 20.2 | 40.5 | 55.6 | 40.0 | .96 | .007‡ |
| Low HDL (%) | 28.8 | 47.3 | 55.6 | 60.0 | .31 | .010‡ |
| High Tg (%) | 29.8 | 41.9 | 33.3 | 30.0 | .33 | .39‡ |
| Metabolic syndrome (%) | 59.6 | 85.1 | 88.9 | 100.0 | .067 | <.001‡ |
| Biochemical evaluations | ||||||
| Na (mmol/L) | 141.7 ± 2.6 | 141.4 ± 2.3 | 141.9 ± 2.0 | 142.9 ± 2.2 | .010 | .058† |
| K (mmol/L) | 4.2 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.3 | 4.2 ± 0.5 | .96 | .59† |
| Creatinine (μmol/L) | 80.2 ± 18.5 | 88.9 ± 25.0 | 91.1 ± 21.1 | 92.6 ± 32.3 | .59 | .10† |
| eGFR (ml/min/1.73m2) | 87.4 ± 19.1 | 80.2 ± 20.9 | 80.6 ± 18.4 | 83.4 ± 28.2 | .58 | .31† |
| Baseline renin concentration (pg/ml) | 11.1 (5.2–24.6) | 10.0 (5.0–204) | 4.0 (3.7–7.1) | 3.8 (2.7–6.7) | <.001 | <.001§ |
| Baseline aldosterone level (pg/ml) | 180 (107–255) | 169 (123–250) | 206 (182–351) | 252 (178–293) | .002 | .006§ |
| Aldosterone postinfusion (pg/ml) | 44 (29–61) | 42 (31–67) | 164 (106–211) | 125 (105–154) | <.001 | <.001§ |
| ARR | 14 (6–33) | 16 (8–29) | 47 (37–68) | 54 (38–77) | <.001 | <.001§ |
| Cardiovascular disease | ||||||
| CAD (%) | 14.4 | 31.1 | 33.3 | 25.0 | .60 | .051‡ |
| Heart failure (%) | 1.9 | 6.8 | 11.1 | 15.0 | .24 | .071‡ |
| Arrhythmias (%) | 9.6 | 14.9 | 11.1 | 20.0 | .58 | .53‡ |
| Stroke/TIA (%) | 6.7 | 4.1 | 0.0 | 10.0 | .29 | .62‡ |
| Any CVD (%) | 26.9 | 41.9 | 44.4 | 50.0 | .52 | .078‡ |
The results are reported as mean ± standard deviation or median (25th–75th percentiles) and frequency as a percentage. *For comparison between four groups. †Univariate analysis of variance with post hoc Duncan’s multiple range test. ‡Chi-square test. §Kruskal-Wallis Test. ARR = aldosterone-to-renin ratio, BMI = body mass index, CAD = coronary artery disease, CVD = coronary vascular disease, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoproteins, HR = heart rate, HT = hypertension, IFG = impaired fasting glycemia, K = potassium, LDL-C = low-density lipoproteins, Na = sodium, OSA = obstructive sleep apnea, PA = primary aldosteronism, SBP = systolic blood pressure, TCh = total cholesterol, Tg = triglycerides, TIA = transient ischemic attack.
Table 2.
Sign and symptoms of OSA and polysomnographic parameters in relation to the presence of OSA or PA.
| Variables | OSA (-) PA (-) n = 104 | OSA (+) PA (-) n = 74 | OSA (-) PA (+) n = 9 | OSA (+) PA (+) n = 20 | P OSA (+) PA (-) vs OSA (+) PA (+) | P * |
|---|---|---|---|---|---|---|
| Increased neck circumference (%) | 40.4 | 67.6 | 44.4 | 85.0 | .13 | <.001† |
| ESS | 7 (5–9.75) | 6 (4–10.5) | 4 (1.5–10) | 7 (4–10) | .93 | .67‡ |
| Total sleep time (h) | 6.9 ± 1.5 | 6.9 ± 1.7 | 6.6 ± 1.4 | 6.7 ± 1.7 | .68 | .62§ |
| AHI (events/h) | 5.6 ± 4.1 | 36.6 ± 19.4 | 5.2 ± 4.9 | 37.9 ± 15.8 | .78 | <.001‡ |
| ODI (events/h) | 6.8 ± 6.8 | 35.1 ± 21.4 | 6.5 ± 7.2 | 32.5 ± 15.9 | .78 | <.001‡ |
| Mean SpO2, (%) | 94.5 ± 1.8 | 92.7 ± 2.0 | 93.8 ± 1.3 | 92.8 ± 2.1 | .76 | <.001‡ |
| Min SpO2, (%) | 87.1 ± 5.9 | 78.3 ± 9.5 | 87.4 ± 3.2 | 78.0 ± 8.2 | .90 | <.001‡ |
| T90 (%) | 10.3 ± 37.2 | 45.9 ± 61.0 | 9.3 ± 22.3 | 44.0 ± 54.5 | .90 | <.001‡ |
| No. of microarousals (/h) | 28.3 ± 15.2 | 41.5 ± 30.6 | 24.3 ± 16.2 | 38.9 ± 20.3 | .91 | <.001‡ |
The results are reported as mean ± standard deviation or median (25th–75th percentiles) and frequency as a percentage. Increased neck circumference was defined as circumference ≥ 43 cm for men and ≥ 41 cm for women. *For comparison between four groups. †Chi-square test. ‡Kruskal-Wallis Test. §Univariate analysis of variance with post hoc Duncan’s multiple range test. AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, PA = primary aldosteronism, SpO2 = peripheral capillary oxygen saturation, T90 = sleep-time spent with oxygen saturation.
In the multivariate logistic regression model, the factors independently associated with the presence of PA were moderate-to-severe OSA, male sex, and serum sodium concentration (Table 3). No independent relationship was found between the presence of moderate-to-severe OSA and BP levels, the number of antihypertensive medications, or the presence of difficult-to-treat or resistant hypertension. When oxygen desaturation index ≥ 15 events/h was included in the model instead of AHI, there was no independent relationship between oxygen desaturation index ≥ 15 events/h and PA presence.
Table 3.
Univariate and multivariate logistic regression model for the variables associated with the presence of primary aldosteronism in patients suspected of OSA.
| Variables Included in Multivariable Model | Univariate | P | Multivariate | P |
|---|---|---|---|---|
| Age (y) | 1.03 (0.99–1.06) | .16 | ||
| Moderate-to-severe OSA | 3.12 (1.35–7.24) | .008 | 2.89 (1.20–6.96) | .018 |
| Sex* | 4.05 (1.35–12.14) | .012 | 3.74 (1.21–11.60) | .022 |
| Resistant HT | 2.35 (1.05–5.28) | .038 | — | — |
| Sodium concentration for 1 mmol/L increment | 1.19 (1.00–1.41) | .047 | 1.23(1.02–1.48) | .027 |
| Increased neck circumference† | 2.45 (1.03–5.83) | .042 | — | — |
Values presented as odds ratios (95% confidence intervals). *Men. †Neck circumference ≥ 41 cm for woman or ≥ 43 cm for men. HT = hypertension, OSA = obstructive sleep apnea.
DISCUSSION
The major finding of our study is that in the group of consecutive patients with newly diagnosed moderate-to-severe OSA primary aldosteronism is present in 21.3% of patients. The frequency of PA in patients with clinically significant moderate-to-severe OSA is higher than in patients in whom OSA has been excluded by PSG. Even in patients who were tested for PA only because of OSA, the frequency of PA was elevated to 15.4%. Our study also indicates that in OSA patients with PA, there is a predominance of the bilateral form of PA.
To date, the frequency of PA in OSA patients has been addressed in only a few studies.6,9 Calhoun et al in 114 patients with resistant hypertension assessed the high probability of OSA by means of Berlin Questionnaire (questionnaire-based assessment without sleep recording).12 Subjects at a high risk of sleep apnea were almost twice as likely to have PA (36% vs 19%). In this study, no confirmatory test for PA was performed.12 The frequency of PA in OSA patients was evaluated also in the study by Di Murro et al.11 These authors included 325 consecutive patients with newly diagnosed hypertension. Based on the Epworth Sleepiness Scale score, 71 patients had an excessive daytime sleepiness. PSG established OSA in 53 patients. In the OSA group, 18 patients (34%) were affected by bilateral PA. The results of this study should be interpreted with caution because PSG was performed only in selected patients with excessive daytime sleepiness based on the Epworth Sleepiness Scale Score. It is therefore uncertain from this study to know how many patients with documented OSA by PSG had PA.11
Recently, the frequency of PA in OSA patients was evaluated in the HYPNOS study. Buffolo et al found in 203 screened OSA patients a prevalence of PA of 8.9%. Inclusion criteria were hypertension and a diagnosis of OSA with an AHI ≥ 15 events/h or AHI ≥ 5 events/h together with symptoms requiring continuous positive airway pressure treatment.14 A difference in the prevalence of PA was found between White and Chinese patients: 11.8% vs 5.9%, respectively.14 It should be noted that there are significant ethnic phenotype differences between European and Asian OSA patients that might have influenced the results.24,25 Buffolo et al used a control group from other previous studies that was not screened for OSA, which may explain why they did not find an increased prevalence of PA in OSA patients (8.9% vs 5.9% in general hypertensive population and 11.2% of the referral centers). It should be taken into consideration that moderate-to-severe OSA is highly prevalent in hypertensive patients at the prevalence ranges from 20% in those newly diagnosed to 45% in patients with resistant hypertension.26,27 Therefore, it is difficult to estimate whether these comparisons reflect the true difference in PA prevalence between hypertensive patients with and without OSA.
In our study, moderate-to-severe OSA independently predicted the presence of PA. There were no major differences in clinical characteristics between patients with OSA and PA and patients with OSA without PA. Therefore, our data do not allow us to specify which OSA patients should be screened for PA in the first instance. Our results in general support the Endocrine Society’s recommendation to screen OSA patients for PA and indicate that patients with moderate-to-severe OSA in particular should be screened.13
It should also be noted that in patients with moderate-to-severe OSA without any typical indication for PA screening apart from symptoms of OSA, PA was present in 15.4% of patients. The study by Ruhle et al showed that despite current recommendations, only 3.0% of hypertensive patients with OSA are screened for PA,28 stressing the necessity to consider screening for PA more often in patients with OSA.
The role of fluid redistribution during supine sleep as an important contributor to OSA has been suggested. It has been hypothesized that high aldosterone promotes renal sodium and water retention, which shifts at night in the supine position and worsens OSA by the accumulation of fluid in the neck. It then contributes to increased upper airway resistance,8,29–31 which might explain why PA accompanying OSA might influence its severity.
This hypothesis, that high aldosterone concentrations worsen the course of OSA, has been tested by Gaddam et al, who administered spironolactone for 8 weeks in 12 patients with resistant hypertension and moderate-to-severe OSA in an observational study. The reductions in total AHI, supine AHI, and AHI during rapid eye movement sleep were noted in all 12 patients.32 These effects on the course of OSA of aldosterone antagonists were also confirmed in two other studies.33,34 It might be also expected that causal treatment of PA may ameliorate the course of OSA. The effect of PA treatment on the course of OSA was evaluated in one observational study, in which 21 patients with PA coexisting with OSA underwent PSG at baseline and 3 months after treatment of PA (ie, with aldosterone antagonists or adrenalectomy). At follow-up, significant reductions in AHI were observed.35
In our study, most PA patients with OSA were diagnosed with bilateral adrenal hyperplasia, which confirms previous observations. 11,14 It also should be noted that there was no difference in the APA prevalence between patients with OSA and those without OSA. Moreover, the prevalence of APA in the OSA group was relatively low (2.1%). This finding is difficult to explain and requires further studies; however, as mentioned, improvement in the OSA course in patients with APA has been reported,35 which may suggest a causative relationship.
The strengths of our study include the large number of recruited patients based on the clinical indications for OSA screening. We also used in all patients a confirmatory test for the diagnosis of PA. We also subtyped by AVS and followed up on the patients with PA according to the ES guidelines and Primary Aldosteronism Surgical Outcome consensus.13,20 It should be emphasized that, in contrast to previous studies on the prevalence of PA in OSA, this is the first study to use PSG in all the included patients.
One of the limitations of the study is that there is no universal consensus on cutoffs of PA confirmation and AVS subtyping; therefore, we used the most rigorous criteria to ensure correctness of the diagnosis. A relatively high number of false-negative results for saline infusion test has been reported; however, we used a seated saline infusion test, which is characterized by 87% sensitivity and 94% specificity.36 When interpreting our results, internight variability of AHI should be taken into consideration. Recently, Sforza et al estimated the internight change of AHI to be no more than 3 events/h. The greatest changes were observed in patients with mild OSA. Based on this study, up to seven patients with mild OSA would have been reclassified as moderate-to-severe OSA if a second PSG had been performed.37
In conclusion, our study shows that patients with clinically important moderate-to-severe OSA are characterized by a high prevalence of PA. There are no distinct features that indicate which patients with moderate-to-severe OSA should be screened for PA. Moreover, PA was frequent in OSA patients without typical indications for PA screening (eg, resistant hypertension, hypokalemia). Therefore, moderate-to-severe OSA should be considered an independent indication for PA screening.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by the National Institute of Cardiology, Warsaw, Poland. Grant no. 2.20/VII/14. The sponsor of the study had no role in the study design, data collection, data analysis, and manuscript writing. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- APA
aldosterone-producing adenoma
- AVS
adrenal vein sampling
- BP
blood pressure
- OSA
obstructive sleep apnea
- PA
primary aldosteronism
- PSG
polysomnography
REFERENCES
- 1.Chahal CA, Somers VK. Secondary hypertension: obstructive sleep apnea. J Am Soc Hypertens. 2015;9(3):244–247. 10.1016/j.jash.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) . Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. 10.1161/CIRCULATIONAHA.117.029400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrowolski P, Klisiewicz A, Florczak E, et al. Independent association of obstructive sleep apnea with left ventricular geometry and systolic function in resistant hypertension: the RESIST-POL study. Sleep Med. 2014;15(11):1302–1308. 10.1016/j.sleep.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 4.Dobrowolski P, Florczak E, Klisiewicz A, et al. Pulmonary artery dilation indicates severe obstructive sleep apnea in patients with resistant hypertension: the Resist-POL Study. Pol Arch Med Wewn. 2016;126(4):222–229. [DOI] [PubMed] [Google Scholar]
- 5.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension. 2010;56(3):369–377. 10.1161/HYPERTENSIONAHA.110.152108 [DOI] [PubMed] [Google Scholar]
- 6.Prejbisz A, Kołodziejczyk-Kruk S, Lenders JWM, Januszewicz A. Primary aldosteronism and obstructive sleep apnea: is this a bidirectional relationship? Horm Metab Res. 2017;49(12):969–976. 10.1055/s-0043-122887 [DOI] [PubMed] [Google Scholar]
- 7.Prejbisz A, Warchoł-Celińska E, Lenders JW, Januszewicz A. Cardiovascular risk in primary hyperaldosteronism. Horm Metab Res. 2015;47(13):973–980. 10.1055/s-0035-1565124 [DOI] [PubMed] [Google Scholar]
- 8.Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26(5):281–287. 10.1038/jhh.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecori A, Buffolo F, Pieroni J, et al. Primary aldosteronism and obstructive sleep apnea: casual association or pathophysiological link? Horm Metab Res. 2020;52(6):366–372. 10.1055/a-1133-7255 [DOI] [PubMed] [Google Scholar]
- 10.Prejbisz A, Florczak E, Klisiewicz A, et al. Relationship between primary aldosteronism and obstructive sleep apnoea, metabolic abnormalities and cardiac structure in patients with resistant hypertension. Endokrynol Pol. 2013;64(5):363–367. 10.5603/EP.2013.0019 [DOI] [PubMed] [Google Scholar]
- 11.Di Murro A, Petramala L, Cotesta D, et al. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2010;11(3):165–172. 10.1177/1470320310366581 [DOI] [PubMed] [Google Scholar]
- 12.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125(1):112–117. 10.1378/chest.125.1.112 [DOI] [PubMed] [Google Scholar]
- 13.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 14.Buffolo F, Li Q, Monticone S, et al. Primary aldosteronism and obstructive sleep apnea: a cross-sectional multi-ethnic etudy. Hypertension. 2019;74(6):1532–1540. 10.1161/HYPERTENSIONAHA.119.13833 [DOI] [PubMed] [Google Scholar]
- 15.Wolf J, Narkiewicz K. Primary aldosteronism and obstructive sleep apnea: a cross-sectional multi-ethnic study. Hypertension. 2019;74(6):1305–1306. 10.1161/HYPERTENSIONAHA.119.13935 [DOI] [PubMed] [Google Scholar]
- 16.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3(5):509–514. [PubMed] [Google Scholar]
- 17.Mancia G, De Backer G, Dominiczak A, et al. European Society of Cardiology . 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187. 10.1097/HJH.0b013e3281fc975a [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 19.Kądziela J, Prejbisz A, Michałowska I, et al. A single-centre experience of the implementation of adrenal vein sampling procedure: the impact on the diagnostic work-up in primary aldosteronism. Kardiol Pol. 2017;75(1):28–34. [DOI] [PubMed] [Google Scholar]
- 20.Williams TA, Lenders JWM, Mulatero P, et al. Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. 10.1016/S2213-8587(17)30135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Technique and Scoring System from Sleep Stages of Human Subjects. US Department of Health, Education, and Welfare Public Health Service NIH/NIND: Washington, DC; 1968. [Google Scholar]
- 23.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. 10.1093/sleep/22.5.667 [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Keenan BT, Wiemken AS, et al. Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea. Sleep. 2020;43(5). zsz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Driscoll DM, Landry SA, Pham J, et al. The physiological phenotype of obstructive sleep apnea differs between Caucasian and Chinese patients. Sleep. 2019;42(11):zsz186. 10.1093/sleep/zsz186 [DOI] [PubMed] [Google Scholar]
- 26.Prejbisz A, Florczak E, Pręgowska-Chwała B, et al. Relationship between obstructive sleep apnea and markers of cardiovascular alterations in never-treated hypertensive patients. Hypertens Res. 2014;37(6):573–579. 10.1038/hr.2014.43 [DOI] [PubMed] [Google Scholar]
- 27.Florczak E, Prejbisz A, Szwench-Pietrasz E, et al. Clinical characteristics of patients with resistant hypertension: the RESIST-POL study. J Hum Hypertens. 2013;27(11):678–685. 10.1038/jhh.2013.32 [DOI] [PubMed] [Google Scholar]
- 28.Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery. 2019;165(1):221–227. 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 29.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56(6):1077–1082. 10.1161/HYPERTENSIONAHA.110.154427 [DOI] [PubMed] [Google Scholar]
- 30.Acelajado MC, Calhoun DA. Aldosteronism and resistant hypertension. Int J Hypertens. 2011;2011:837817. 10.4061/2011/837817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan BM, Li J. Role of aldosterone blockade in resistant hypertension. Semin Nephrol. 2014;34(3):273–284. 10.1016/j.semnephrol.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Gaddam K, Pimenta E, Thomas SJ, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24(8):532–537. 10.1038/jhh.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Zhang H, Cai M, et al. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens. 2016;38(5):464–468. 10.3109/10641963.2015.1131290 [DOI] [PubMed] [Google Scholar]
- 34.Krasińska B, Miazga A, Cofta S, et al. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Pol Arch Med Wewn. 2016;126(5):330–339. [DOI] [PubMed] [Google Scholar]
- 35.Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum Hypertens. 2017;31(9):561–567. 10.1038/jhh.2017.28 [DOI] [PubMed] [Google Scholar]
- 36.Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):4113–4124. 10.1210/jc.2018-01394 [DOI] [PubMed] [Google Scholar]
- 37.Sforza E, Roche F, Chapelle C, Pichot V. Internight variability of apnea-hypopnea index in obstructive sleep apnea using ambulatory polysomnography. Front Physiol. 2019;10:849. 10.3389/fphys.2019.00849 [DOI] [PMC free article] [PubMed] [Google Scholar]