Abstract
Study Objectives:
To the best of our knowledge, there has not as yet been any study on the effects of the COVID-19 pandemic on patients with narcolepsy, in particular, in relation to its impact on sleep schedules, symptoms, the need for medication, work, income, and quality of life. This study therefore aimed to explore these factors and their possible influence on sleep, circadian timing, and narcolepsy symptoms during the pandemic.
Methods:
Patients with narcolepsy who had been in quarantine for at least 3 months completed a 36-question online survey. Questions targeted the conditions of the quarantine, sleep-related behaviors, and factors known to affect sleep and circadian rhythms (work status, income, appetite, narcolepsy symptoms, and medication), as well as the quality of life during the quarantine period.
Results:
The routines of the participants had been altered by quarantine, with changes in their place of work, and an increase in narcolepsy symptoms, such as cataplexy, sleep paralysis, hallucinations, nocturnal awakenings, and sleepiness. Sleep and wake times changed, resulting in altered sleep patterns in most of the sample. No association between changes in the place of work and narcolepsy symptoms was found. Regarding medication, the participants used fewer antidepressant pills but took more stimulants. Appetite was increased and self-reported quality of life decreased during the period.
Conclusions:
During the quarantine, the patients with narcolepsy reported changes in their bedtime and waking-up schedules, suggesting a tendency to circadian misalignment. In Brazil, the effects of the COVID-19 outbreak have gone beyond the direct action of the virus because of the collateral damage it has caused in respect to unemployment, financial hardship, and a reduction in quality of life. These impacts have been amplified in Brazil because of the level of social inequality found in the country, and they have particularly affected vulnerable patients with rare diseases, such as the narcolepsy population.
Citation:
Rodrigues Aguilar AC, Frange C, Huebra L, et al. The effects of the COVID-19 pandemic on patients with narcolepsy. J Clin Sleep Med. 2021;17(4):621–627.
Keywords: sleep, narcolepsy, sleep-wake schedules, quality of life, COVID-19, quarantine
BRIEF SUMMARY
Current Knowledge/Study Rationale: The quarantine by the pandemic due to COVID-19 impacted the current society in an intense and permanent way. Narcolepsy is a chronic sleep disorder, associated with morbidity and an impaired health-related quality of life. The present study is the first to describe the impact of the pandemic on sleep schedules, symptoms, need for medication, work, and quality of life in patients with narcolepsy.
Study Impact: The tendency to circadian misalignment during the pandemic, found in this narcoleptic population, reinforces the importance to investigate circadian rhythm disorders in these patients, especially in this new situation. These findings can be used as an evidence base to promote actions to treat circadian misalignment in patients with narcolepsy.
INTRODUCTION
Pneumonia associated with a new type of coronavirus disease (COVID-19) emerged in Wuhan, China, in December 2019.1,2 Since the emergence of the 2019-nCoV outbreak in China, many questions remain unanswered; however, the potential of the virus to spread from human to human was quickly established.3 Mass “quarantine” has been effective in containing epidemic outbreaks in recent decades, most recently in the severe acute respiratory syndrome epidemic in 2003 and the Ebola outbreak in 2014; for this reason, it was defined as the best strategy to contain and mitigate the virus around the word.4 On February 26, the Brazilian Ministry of Health confirmed the country’s first case of COVID-19, and 2 days later, around 182 suspected cases occurred in 16 Brazilian states. On April 5, the state of São Paulo ordered the closure of malls, fitness centers, stores, and offices that operated within the metropolitan region of the city, resulting in a compulsory quarantine, a strategy taken by other states in the following days.5
Quarantine is the restriction and separation of people who have potentially been exposed to an infectious disease to certify if they become unwell, thereby reducing the risk of transmissibility.6 Quarantine results in social isolation, with a reduction in exposure to sunlight, lower social and physical activity levels, as well as changes in individual daily routines. Social cues provided by the external environment outside our homes impact our circadian timing system.7–9 The circadian timing system is a cellular “clock,” working as a timer to signal metabolic processes, synchronized by a “master clock” in the central nervous system.10
Our circadian system is entrained by many activities but is regulated mainly by the light-dark cycle. As diurnal animals, humans conduct their activities, such as exercise and feeding, primarily during daylight hours, in contrast to sleep and rest throughout the night. Social isolation and the interruption of individual and family daily routines may interfere in exposure to the light-dark cycle, particularly sunlight, and result in chronic circadian rhythm disruption. In addition, exposure to abnormal work-rest cycles, changes in workplaces (from the office to the home), and isolation may induce a change in the phase relationship between the sleep-wake cycle and the endogenous circadian timing system, which may be compounded by a sense of uncertainty and anxiety symptoms resulting from factors such as concerns about the risk of infection, the possibility of death, and the unknown course of the disease. Any dysregulation of routine, especially in patients who need to have their routine regulated by exogenous factors (work, physical activity, meals) and medications, may result in circadian misalignment and, in the case of patients with narcolepsy, worsen their symptoms, sleep, and quality of life.
Narcolepsy is a chronic sleep disorder characterized by a pentad of symptoms: excessive daytime sleepiness (EDS), cataplexy, sleep paralysis, hypnopompic and/or hypnagogic hallucinations, and disrupted nighttime sleep.11 It is associated with substantial morbidity and an impaired health-related quality of life.12 According to the third edition of the International Classification of Sleep Disorders, narcolepsy can be classified as type 1 and 2. Narcolepsy type 1 (NT1) is characterized by cataplexy and cerebrospinal fluid hypocretin-1 deficiency owing to loss of orexinergic neurons in the lateral hypothalamus. In narcolepsy type 2, there is an absence of cataplexy, and cerebrospinal fluid hypocretin-1 concentration is either not measured or is greater than 110 pg/mL.13,14 EDS represents the most common and disabling symptom, whereas cataplexy is specific to NT1.11 Among other narcolepsy symptoms, the reported prevalence of hypnagogic or hypnopompic hallucinations is about 60%, the prevalence of sleep paralysis is approximately 50%, and about 80% of patients report disrupted nighttime sleep.11,15
A recent study about the COVID-19 quarantine reported that individuals are going to bed later and getting up earlier, with consequent poor sleep quality, decreased total sleep time, and experiencing a circadian misalignment, characterized by differences in sleep between social and biological time.9 These differences are also called social jetlag, a chronic state of a discrepancy between biological and social clocks. A few previous studies have described the adverse effects of social jetlag on physical health in the general population, such as obesity, diabetes/prediabetes, and other metabolic syndromes, but it is unclear whether complications are caused by the insufficient sleep or by circadian misalignment.7 In respect to social jetlag, patients with narcolepsy are particularly susceptible to these changes because of the higher risk of circadian misalignment associated with the condition.
During quarantine and isolation, the sleep-wake cycle may be disturbed not only by circadian misalignment but also by sleep homeostasis, a drive that builds up an increased need for sleep in response to extended wake periods, independent of the time of day.16 Recently, social jetlag was associated with symptoms of circadian misalignment in patients with narcolepsy.17 This is a significant concern as quarantine can worsen the previous unbalance between sleep and wake transitions.
This study describes the impact of quarantine and isolation resulting from the COVID-19 pandemic on patients with narcolepsy in relation to work, finances, bedtime, and morning wake-up schedules, narcolepsy symptoms, the need for medication, and quality of life.
METHODS
Ethical aspects
The study was approved by the Ethics Committee of our Institution (no. 0639/2020, CAAE: 33453620.0.0000.5505). Recruitment took place in June 2020.
Participants
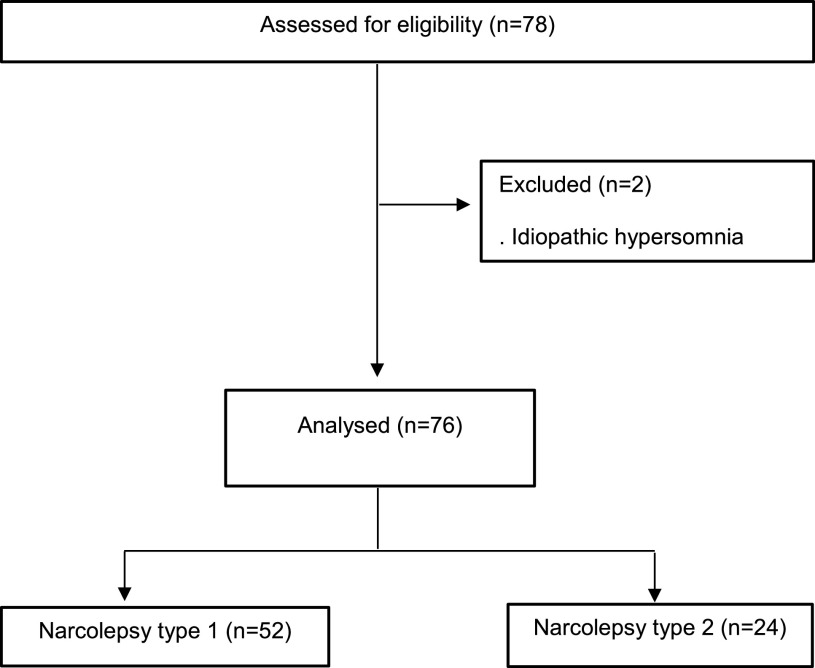
The sample consisted of patients with a diagnosis of narcolepsy, recruited from the Excessive Daytime Sleepiness outpatient clinic of the Universidade Federal de São Paulo, Escola Paulista de Medicina (Unifesp/EPM), São Paulo, Brazil. The participants were invited to participate via messaging applications (WhatsApp Messenger and e-mail) to answer an online survey giving information about changes in their place of work, salary profiles, sleep habits, quality of life, appetite, and severity of daytime sleepiness, assessed using the Epworth Sleepiness Scale (ESS), before and during the quarantine period (Figure 1). The ESS was used to evaluate EDS. The score ranges from 0–24, with 0–9 indicating “no sleepiness symptoms,” whereas scores > 9 are “suggestive of daytime sleepiness.”18 The inclusion criteria were as follows: men and women aged between 18 and 70, a diagnosis of NT1 or 2 according to the American Academy of Sleep Medicine guidelines,19 having attended our outpatient department in the previous 12 months, and gave consent by signing an electronic consent form. The exclusion criteria were diagnostic uncertainty and not fully completing the questionnaire.
Figure 1. The Consolidated Standards of Reporting trials (CONSORT) flow chart (n = 76).
Questionnaire
The questionnaire was created by a team of physicians specializing in sleep medicine and comprised 36 questions. The initial eight questions were about identification, age, sex, the type of narcolepsy, and COVID-19. Of the other 28 questions, four were about workplace and income level, four were about the respondents’ usual bedtime and morning wake-up schedules, eight were from ESS, 10 were about narcolepsy symptoms, one was about perceived quality of life, and one question was about appetite.18
Statistical analysis
Data analysis was performed using SPSS version 20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, New York; IBM Corp. released 2011). The demographic and clinical characteristics of the participants, as well as sleep variables, were summarized using descriptive statistics. Data are presented as mean ± standard deviation or percentage for continuous variables, and frequency for categorical ones (including ordinal variables). Nonnormal and nonhomogeneous variables were determined by the Shapiro-Wilk’s test and Levene’s test, respectively. Based on the nonnormality and heterogeneity of the data, nonparametric tests were used. Data before and after the pandemic were compared using the paired Wilcoxon test (Z) for ordinal data, and chi-square (χ2) for categorical data. Statistical significance was set at P < .05.
RESULTS
Participants and infection by the severe acute respiratory syndrome-CoV-2
A total of 78 patients completed the questionnaire, and two were excluded for having a diagnosis of idiopathic hypersomnia accordingly to the Consolidated Standards of Reporting trials (Figure 1). The final sample comprised 76 narcoleptic patients, both sexes, aged between 18 and 70 years, mean age of 36.9 years old, and 68.7% were diagnosed with NT1. No patient in our sample had received a confirmed diagnosis of COVID-19; 85.5% had experienced no flulike symptoms, and 14.5% had experienced flulike symptoms without laboratory confirmation of COVID-19 in the previous 3 months. The demographic characteristics are depicted in Table 1.
Table 1.
Sociodemographic and corona virus 19 (COVID-19) characteristics of the sample (n = 76).
| Narcolepsy | |
| Narcolepsy type 1 | 68.5 (52) |
| Narcolepsy type 2 | 31.5 (24) |
| Sex, % (n) | |
| Female | 68.4 (52) |
| Male | 31.6 (24) |
| Age, y | 36.9 ± 11.7 |
| Epworth Sleepiness Scale score | 17.9 ± 4.8 |
| Education level | |
| Incomplete elementary school | 3.9 (3) |
| Incomplete high school | 3.9 (3) |
| High school | 17.1 (13) |
| Incomplete higher education | 23.7 (18) |
| Higher education | 26.3 (20) |
| Postgraduate | 25 (19) |
| COVID-19 | |
| Healthy, no symptoms | 85.5 (65) |
| Symptoms, no confirmation | 14.5 (11) |
Values presented as mean ± standard deviation or % (n). Epworth Sleepiness Scale scores 0 to 9 = no sleepiness symptoms; ≥10 = suggestive of daytime sleepiness and requires medical assistance.
Workplace and financial profile
Comparison of individuals who worked from home with those who worked in an outside office showed a statistically significant increase from 17.1% to 39.5% in working from home and a statistically significant reduction from 55.3% to 14.5% in those who worked outside home (P < .01) (Table 2). There was also a statistically significant increase in the number of unemployed (P < .05). No statistically significant differences for monthly income (salary) were found between those before and those during the quarantine (P > .05).
Table 2.
Comparison of workplace, income, and medication before and during the corona virus 19 (COVID-19) pandemic (n = 76).
| Workplace, income, and medication | Before pandemic | During pandemic | P Value |
|---|---|---|---|
| Work status, % (n) | |||
| Home office, flexible hours | 14.5 (11) | 32.9 (25) | .048 |
| Home office, restricted time | 2.6 (2) | 6.6 (5) | |
| Worked on the company’s physical space, flexible hours | 18.4 (14) | 7.9 (6) | |
| Worked on the company’s physical space, restricted time | 36.8 (28) | 6.6 (5) | |
| Not working | 27.6 (21) | 46.1 (35) | |
| Income (monthly, R$), % (n) | |||
| < 1 Minimal salary | 25.0 (19) | 36.8 (28) | .061 |
| 1–5 Minimal salary | 61.8 (47) | 48.7 (37) | |
| 5–10 Minimal salary | 9.2 (7) | 11.8 (9) | |
| > 10 Minimal salary | 3.9 (3) | 2.6 (2) | |
| Medication, % (n) | |||
| Stimulant | 25.0 (19) | 34.2 (26) | .013 |
| Antidepressant | 7.9 (6) | 5.3 (4) | |
| l-carnitine | 1.3 (1) | 1.3 (1) | |
| Stimulant + antidepressant | 32.9 (25) | 5.3 (4) | |
| Stimulant + l-carnitine | 3.9 (3) | 1.3 (1) | |
| Stimulant + antidepressant + l-carnitine | 7.9 (6) | 2.6 (2) | |
| None | 21.1 (16) | 7.9 (6) | |
| Did not stop or change dosage | 0 (0) | 42.1 (32) |
Minimal salary in Brazilian currency (R$, Reais). Wilcoxon (Z) test.
Bedtime and morning wake-up schedules
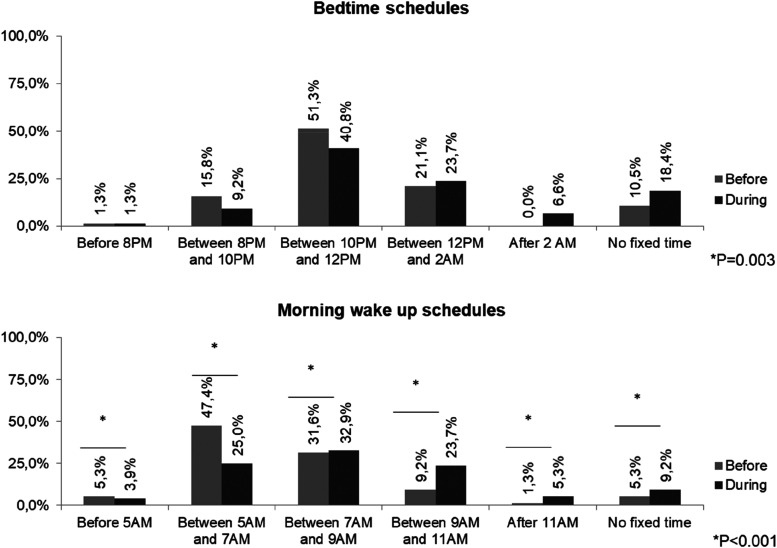
A statistically significant number of the patients were going to bed later or had no fixed bedtime during the pandemic (P < .05). We found similar results in respect to wake times, with a reduction in patients who tended to awaken early, an increase in the number of those who woke up late and in those who had no fixed wake-up time (P < .01). Comparing the periods before and during the pandemic, 42.1% of the patients had changed their bedtime preference, 21.8% had no difference in EDS, and 78.2% had worsened EDS (P < .01) (Figure 2).
Figure 2. Bedtime and morning wake-up schedules of narcoleptic patients before and during the corona virus 19 (COVID-19) pandemic (n = 76).
Narcolepsy symptoms
The mean ESS score of our sample was 17.8. During the pandemic, 96.1% of our patients presented EDS, characterized by an ESS score of 10 points or higher (Table 1). The frequency of self-reported narcolepsy symptoms during the quarantine in the sample are reported in the Table 3. There was a statistically significant association between changes in bedtime schedules and the main narcolepsy symptom: 19% observed changes in EDS, with 69% reporting worsened EDS during quarantine (χ2 = 10.22; df = 2; P < .01). Concerning the other narcolepsy symptoms, we found statistically significant associations between bedtime schedules and hallucinations, as 11% of the sample had a reduction in their hallucinations (X2 = 10.75; df = 3; P < .01; adjusted residual > 2). No statistically significant associations were found between bedtime schedules and sleep paralysis (χ2 = 5.2; df = 3; P = .16) or nocturnal awakenings (χ2 = 6.95; df = 3; P = .07). We analyzed cataplexy symptoms only in NT1 patients (52), and no statistically significant associations with bedtime schedules (X2 = 6.99; df = 3; P = .07) or with wake-up schedules (χ2 = .30; df = 3; P = .96) were found. Regarding morning wake-up schedules, we did not find statistically significant associations with EDS (although a trend was observed [χ2 = 1.21, df = 2, P = .54, adjusted residual > 3]), hallucinations (χ2 = 4.1, df = 3, P = .25); sleep paralysis (χ2 = 4.78, df = 3, P = .19), and nocturnal awakenings (χ2 = 1.61, df = 3, P = .66).
Table 3.
Frequency of self-reported narcolepsy symptoms in the sample (n = 76).
| Narcolepsy symptoms | |
|---|---|
| Scheduled naps before pandemic | |
| Yes | 42.1% |
| No | 57.9% |
| Scheduled naps during pandemic | |
| Increased | 60.5% |
| Decreased | 3.9% |
| No changes | 35.5% |
| Sleepiness during pandemic | |
| No changes | 38.2% |
| Improved | 9.2% |
| Worsened | 52.6% |
| Cataplexy during pandemic* | |
| No changes | 61.5% |
| Improved | 13.5% |
| Worsened | 17.3% |
| Never had | 7.7% |
| Sleep paralysis during pandemic | |
| No changes | 40.8% |
| Improved | 5.3% |
| Worsened | 17.1% |
| Never had | 36.8% |
| Hallucinations during pandemic | |
| No changes | 40.8% |
| Improved | 5.3% |
| Worsened | 30.3% |
| Never had | 23.7% |
| Nocturnal awakenings during pandemic | |
| Do not wake up during the night | 14.5% |
| Increased | 43.4% |
| Decreased | 6.6% |
| No changes | 35.5% |
| Quality of life during pandemic | |
| Unchanged | 18.4% |
| Worsened | 51.3% |
| Improved | 17.1% |
| Does not know | 13.2% |
| Appetite during pandemic | |
| No changes | 28.9% |
| Decreased | 9.2% |
| Increased | 61.8% |
n = 52, only narcolepsy type 1 was analyzed.
Medication
There was a statistically significant increase in the use of stimulants during the quarantine period in patients who used it as monotherapy (Z = −2.48, P = .0135) and a statistically significant reduction in the use of antidepressants, stimulants associated with antidepressants, or l-carnitine (Z = −2.48, P = .0135). No statistically significant changes in the l-carnitine dosage were found as only one pregnant patient was taking it. In 42.1% of our sample, no change in medication dosage or cessation of medication occurred during the pandemic (Table 2). No statistically significant association was found between changes in the use of any medication with bedtime (χ2 = .76, df = 1, P = .38) or wake-up time schedules (χ2 = 3.59; df = 1; P = .58).
Regarding the use of medicaments and narcolepsy symptoms, our data demonstrated that those using stimulants did not present any changes in narcolepsy symptoms, such as sleepiness (χ2 =; df =; P = ), hallucinations (χ2 = 8.65; df = 6; P = .19), sleep paralysis (χ2 = 6.85; df = 6; P = .34), sleep fragmentation (χ2 = 6.68; df = 6; P = .35), or cataplexy (χ2 = 8.13; df = 6; P = .23).
Quality of life
Regarding the question about quality of life before and during quarantine, almost one-fifth (n = 14) of the patients did not report any changes in their quality of life, more than half (n = 39) reported worsening, 13 patients reported quality of life had improved, and 10 patients did not know the answer to this question.
DISCUSSION
To the best of our knowledge, this is the first study of patients with narcolepsy to examine the effects of the COVID-19 outbreak on bedtime and morning wake-up schedules, symptoms, medication, place of work, income, and quality of life during a 3-month-quarantine period. No patient in our sample had a confirmed diagnosis of COVID-19. This can be explained by Brazilian government policy, which sanctions testing only for those with severe symptoms.
Many of the patients with narcolepsy changed their regular workplace, with an increasing number working from home during the pandemic. These changes in workplace environment probably impacted bedtime and waketime, but no consistent associations were found between bedtime and waketime schedules and narcolepsy symptoms. This finding can be explained by the nature of our convenience sample, as the patients had been followed up regularly in our outpatient clinic for at least 12 months, and some of them have been attending for several years. This may also be one of the reasons for the relatively good adaptation to the adversities of the quarantine, as they had already been given advice by neurologists specializing in sleep medicine about sleep hygiene techniques, self-management of their sleep and symptoms, and also the importance of scheduled naps. In addition, it is plausible that the intensity of work of the participants may have been reduced because of the pandemic, at least during this first 3 months of quarantine. It is also possible that the workload may have stayed the same or even increased, but being at home could have mitigated some symptoms associated with greater tiredness, as in this new context there is less time spent on commuting, which can be a significant burden in the city of São Paulo. Working from home may have influenced the increase in the use of stimulants in those who use them as monotherapy; however, no increase was noted in the use of other medications (antidepressants and l-carnitine), with a reduction in the use of antidepressants and medications in combination. The most feasible explanation for this reduction is that the patients rationed their medication, perhaps for fear of not having access to prescriptions during quarantine and isolation (as the outpatient clinic is still closed).
In Brazil, a significant rise in unemployment has occurred as a result of the pandemic, with more than eight million people losing their jobs in the first 3 months of quarantine. Some of our patients lost their jobs, but curiously this did not influence monthly income when comparing before and during quarantine/isolation, perhaps because of the strategies adopted by the Brazilian government to provide emergency aid to those who became unemployed because of the pandemic. Therefore, it is less likely that unemployment could explain the reduction in the use of medications in our sample.
Recently, many studies have been published about the psychosocial impacts of quarantine, demonstrating a higher prevalence of anxiety, acute panic, obsessive behaviors, paranoia, posttraumatic stress disorder, and depression.20 We believe it has directly impacted sleep, quality of life, and appetite in the narcolepsy population. Inadequate sleep is often a risk factor for weight gain, especially during self-quarantine.21
Patients reported an increase in sleep fragmentation. About a third reported an increase in hypnagogic and hypnopompic hallucinations, and in more than half of the sample, the number of naps increased. Although it was less prevalent, there was also an increase in sleep paralysis, and cataplexy among patients with NT1 increased. These findings may be explained by the significant reduction in the use of antidepressants. Further, the higher EDS perception with a disproportionate and unusual schedule of stimulants can also lead to higher frequency of hallucinations, sleep paralysis, sleep fragmentation, and cataplexy, consequently resulting in more EDS.
The patients in our sample changed their bedtime and morning wake-up schedules, and the perceived increase in EDS could be explained by a circadian rhythm unbalance caused by these changes, which in turn led to the disproportionate use stimulants with an unusual schedule. The circadian timing system depends on exogenous factors (zeitgebers) to provide the stimuli to set or reset the biological clock of an organism. The changes in the patients’ routine caused by working from home, prolonged periods indoors, fewer outdoor activities, indeterminate mealtimes, and the loss of regular social interactions could have resulted in a loss of these zeitgebers and could easily explain our findings.
The presence of EDS in narcolepsy patients has not yet been fully elucidated; however, one possible explanation is a lack of wake-promoting neurons owing to insufficient excitatory drive caused by hypocretin deficiency.22 Hypocretin neurons are innervated by neurons of the central nervous system, and their absence may account for the disrupted circadian rhythm often observed in narcolepsy. Another possible reason for EDS is a higher sleep drive in narcolepsy patients, and this may be exacerbated by the changes to sleep schedule associated with the pandemic.23,24 Unfortunately, almost all studies about this issue have been designed in relation to normal sleep-wake characteristics. In the case of narcolepsy, there is already an abnormal condition with a strong tendency to circadian misalignment.
This study has several limitations. There was no sleep diary or actigraphy to define circadian rhythm patterns. Questionnaires about quality of life, depression, or anxiety were not used. Unfortunately, we could not compare some narcolepsy symptoms, such as sleepiness, cataplexy, sleep paralysis, hallucinations, sleep fragmentation, quality of life, and appetite, before vs during quarantine owing to lack of data. The idea was to identify the main changes affecting patients with narcolepsy during the quarantine, with a simple real-world questionnaire designed to try to understand the needs of the patients and improve follow-up and treatment during this challenging time.
There is a lack of studies addressing circadian misalignment and other changes in this specific population during this pandemic period. Our results can be used as an evidence base to promote actions to treat narcolepsy patients in this new situation. These actions should include reinforcing the importance of creating a daily exercise routine, increasing exposure to sunlight, and eating and napping at regular times. Moreover, this study allows us to better understand the behavior of patients with narcolepsy during the quarantine imposed by the coronavirus pandemic and we hope will encourage further studies in this specific population.
DISCLOSURE STATEMENT
The final version of the manuscript has been read and approved by all authors, who have each provided the attention necessary to ensure the integrity of the work. This work was supported by the Associação Fundo de Incentivo à Pesquisa and the São Paulo Research Foundation (FAPESP no. 2018/18952-1 to C.F.). The sponsors had no role in the design or conduct of this research. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
ABBREVIATIONS
- COVID-19
coronavirus disease 2019
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- NT1
narcolepsy type 1
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EYP, Ng MY, Khong PL. COVID-19 pneumonia: what has CT taught us? Lancet Infect Dis. 2020;20(4):384–385. 10.1016/S1473-3099(20)30134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. 10.1016/S0140-6736(20)30460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo CML, Silva GAS, Melo ARS, Freitas AC. COVID-19 pandemic outbreak: the Brazilian reality from the first case to the collapse of health services. Ann Acad Bras Cienc. 2020;92(4):e20200709. 10.1590/0001-3765202020200709 [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27(2):taaa020. 10.1093/jtm/taaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno JP, Crowley SJ, Alfano CA, Hannay KM, Thompson D, Baranowski T. Potential circadian and circannual rhythm contributions to the obesity epidemic in elementary school age children. Int J Behav Nutr Phys Act. 2019;16(1):25. 10.1186/s12966-019-0784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gualano MR, Lo Moro G, Voglino G, Bert F, Siliquini R. Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. Int J Environ Res Public Health. 2020;17(13):4779. 10.3390/ijerph17134779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley S, Colas des Francs C, Aussert F, et al. [The effects of quarantine for SARS-CoV-2 on sleep: an online survey]. Encephale. 2020;46(3S):S53–S59. 10.1016/j.encep.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erren TC, Lewis P. SARS-CoV-2/COVID-19 and physical distancing: risk for circadian rhythm dysregulation, advice to alleviate it, and natural experiment research opportunities. Chronobiol Int. 2020;37(7):1106–1109. 10.1080/07420528.2020.1772811 [DOI] [PubMed] [Google Scholar]
- 11.Kallweit U, Schmidt M, Bassetti CL. Patient-reported measures of narcolepsy: the need for better assessment. J Clin Sleep Med. 2017;13(5):737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955-965. 10.5664/jcsm.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito E, Inoue Y. [The International Classification of Sleep Disorders, third edition. American Academy of Sleep Medicine. Includes bibliographies and index]. Nihon Rinsho. 2015;73(6):916–923. [PubMed] [Google Scholar]
- 14.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. 10.1038/79690 [DOI] [PubMed] [Google Scholar]
- 15.Mamelak M. Narcolepsy and depression and the neurobiology of gammahydroxybutyrate. Prog Neurobiol. 2009;89(2):193–219. 10.1016/j.pneurobio.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 16.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 17.Kayaba M, Sasai-Sakuma T, Inoue Y. Clinical significance of social jetlag in patients with excessive daytime sleepiness. Chronobiol Int. 2018;35(12):1637–1646. 10.1080/07420528.2018.1499666 [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 20.Dubey S, Biswas P, Ghosh R, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14(5):779–788. 10.1016/j.dsx.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zachary Z, Brianna F, Brianna L, et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14(3):210–216. 10.1016/j.orcp.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuñez A, Rodrigo-Angulo ML, Andrés ID, Garzón M. Hypocretin/Orexin neuropeptides: participation in the control of sleep-wakefulness cycle and energy homeostasis. Curr Neuropharmacol. 2009;7(1):50–59. 10.2174/157015909787602797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–6300. 10.1523/JNEUROSCI.0586-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Sagili H. Etiopathogenesis and neurobiology of narcolepsy: a review. J Clin Diagn Res. 2014;8(2):190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]