Abstract
Background
Hepatocellular carcinoma (HCC) occurs frequently in China, with high morbidity and mortality. Cell division cycle 20 homolog (CDC20) is reportedly related to many cancers. In this study, we discuss a potential link of CDC20 expression to HCC patients’ prognoses.
Material/Methods
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to assess CDC20 expression in HCC and the paired noncancerous tissues. Chi-square analysis was used to assess potential association of CDC20 expression with clinicopathologic profiles among HCC patients. The overall survival for HCC patients with different CDC20 expressions was assessed using the Kaplan-Meier method. To evaluate the prognostic value for HCC patients, Cox regression analyses were performed.
Results
The expression of CDC20 was elevated among HCC specimens compared with adjacent noncancerous ones (P<0.05). The expression of CDC20 was significantly related to differentiation (P<0.001), tumor node metastasis stage (P<0.001), and lymphatic metastasis (P<0.001). Moreover, HCC patients with high CDC20 expression had dismal overall survival rates compared with low CDC20 expression (P<0.05). CDC20 alone could forecast HCC prognoses according to multivariable Cox regression analysis (hazard ratio=2.354, 95% confidence interval=1.177–4.709, P=0.016).
Conclusions
Overexpressed CDC20 may act as a reliable biomarker for dismal prognoses among HCC patients.
Keywords: Carcinoma, Hepatocellular; Cdc20 Proteins; Prognosis
Background
Hepatocellular carcinoma (HCC) is a primary histologic type of liver malignancy in humans. The incidence of HCC is fifth in the world and it has become the third leading cause of cancer-related death in the world [1,2]. The incidence and mortality for HCC show an annual upward tendency in China. The carcinogenesis of HCC is a complex process regulated by various genes, including suppressor genes and oncogenes. Environmental factors can lead to a high incidence of HCC, too, such as hepatitis B virus/hepatitis C virus infection, cirrhosis, and alcoholic liver disease [3–5]. With surgery and adjuvant treatment technologies, the treatment of HCC has made great progress. However, because of the high recurrence and metastasis rates, the 5-year survival of HCC patients is still very low [6,7]. Thus, there is an urgent need to find novel and effective biomarkers for the prognosis of HCC.
The cell division cycle 20 homolog (CDC20) is a cell-cycle checkpoint control factor that can bind with Cdh1 directly to activate the anaphase-promoting complex (APC) [8,9]. CDC20 assumes crucial functions of cells in anaphase of mitosis [10,11]. Hence, its dysregulation may have considerable impacts on cellular growth and oncogenesis [12]. Reportedly, overexpressed CDC20 may be correlated with an unsuitably acting spindle assembly checkpoint [13,14]. More and more research has revealed that CDC20 is a carcinogen that promotes cancer development. Recent reports have unveiled that CDC20 expression is upregulated in various malignancies such as breast cancer, HCC, cervical cancer, prostate cancer, and gastric cancer [15–19]. Li et al [20] found that CDC20 was elevated in tumor tissues and positively related to sex, tumor differentiation, and tumor node metastasis (TNM) stage of HCC. CDC20 could also promote the proliferation of HCC [9,20]. Therefore, we hypothesized that CDC20 might play an important role in the prognosis of HCC. However, few studies have reported the prognostic role of CDC20 in HCC until now.
We investigated the potential correlation of clinicopathologic characteristics with CDC20 expression and assessed its potential significance in HCC prognosis.
Material and Methods
Cases and Sample Collection
A total of 139 HCC and paired noncancerous tissue specimens was collected from HCC patients that were all confirmed by pathologists from Harrison International Peace Hospital. We acquired permission from the Ethics Committee of Harrison International Peace Hospital, and written consents were also signed by eligible patients and their families. No patient had undergone chemotherapy or radiotherapy ahead of surgery. Patients diagnosed by histopathologic biopsy as HCC were included in this study. Individuals with other tumors or a history of tumor, liver or kidney diseases, other lymphatic system disorders, or diseases influencing our results were excluded from this study. All the tissues were immediately placed in liquid nitrogen and afterward maintained at −80°C until ribonucleic acid (RNA) was extracted. The patients were followed for up for 5 years; every 3 months in the first year, then every 6 months in the subsequent 2 years, and then annually. The clinicopathologic features are listed in Table 1, including age, sex, tumor size, differentiation, TNM stage, and lymphatic metastasis.
Table 1.
Association of CDC20 gene expression with clinicopathologic features of HCC patients.
| Features | No. N=139 | CDC20 expression | P-values | |
|---|---|---|---|---|
| Low (n=65) | High (n=74) | |||
| Age (years) | ||||
| <60 | 69 | 34 | 35 | 0.556 |
| ≥60 | 70 | 31 | 39 | |
| Sex | ||||
| Men | 74 | 33 | 41 | 0.585 |
| Women | 65 | 32 | 33 | |
| Tumor size | ||||
| <5 cm | 62 | 30 | 32 | 0.731 |
| ≥5 cm | 77 | 35 | 42 | |
| Differentiation | ||||
| High | 60 | 43 | 17 | <0.001 |
| Low-moderate | 79 | 22 | 57 | |
| Tumor node metastasis stage | ||||
| I–II | 59 | 40 | 19 | <0.001 |
| III–IV | 80 | 25 | 55 | |
| Lymphatic metastasis | ||||
| Yes | 73 | 23 | 50 | <0.001 |
| No | 66 | 42 | 24 | |
RNA Separation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated with Trizol reagent (Invitrogen) following the manufacturer’s instructions. The first-strand complementary deoxyribonucleic acid (cDNA) was synthesized by TransScript and cDNA synthesis kit from TransGen Biotech (Beijing, China). In our study, RT-PCR was completed with Bio-Rad iQ5 multicolor real-time PCR detection system (Bio-Rad, Hercules, CA). Expression of CDC20 was determined relative to that of β-actin. The primers of CDC20 and β-actin were as follow:
-
CDC20 forward 5′-TCGCATCTGGAATGTGTGCT-3′ and
reverse 5′-CCCGGGATGTGTGACCTTTG-3′,
-
β-actin forward 5′-TGACGTGGACATCCGCAAAG-3′ and
reverse 5′-CTGGAAGGTGGACAGCGAGG-3′ [20].
The relative expression for CDC20 among 139 paired samples was normalized against the β-actin internal control and calculated using the 2−ΔΔCt method.
Statistical Analysis
Data synthesis was accomplished with SPSS software (SPSS 19.0) and GraphPad Prism 5. Expression level of CDC20 was given as mean±SD. CDC20 expression was compared by the t test. The association of CDC20 expression with clinicopathologic traits among HCC patients was determined via the chi-square test. The Kaplan-Meier method with the log-rank test was performed to estimate survival rates and the survival differences of the HCC patients. Cox regression analysis explored the influence of CDC20 expression and the clinicopathologic variables on survival. P<0.05 represented statistical significance.
Results
Upregulation of CDC20 in HCC Tissues
qRT-PCR was performed to examine CDC20 expression in 139 HCC tissues and matched noncancerous tissues. The HCC cases were categorized into high- and low-expression classes. Figure 1 shows that CDC20 expression exhibited an upward tendency among HCC tissues compared with matched noncancerous ones (P<0.05).
Figure 1.
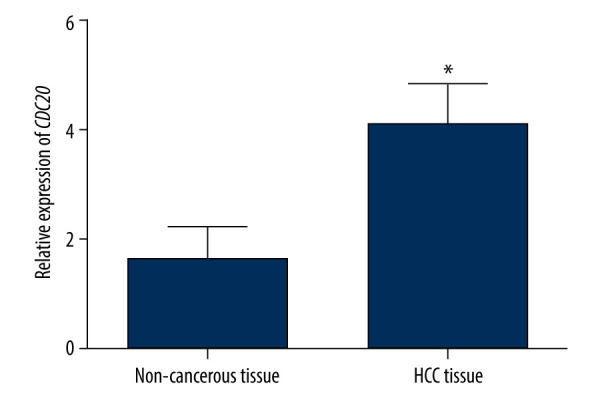
CDC20 expression level between hepatocellular carcinoma tissues and paired adjacent noncancerous tissues. * P<0.05.
Connection of CDC20 Expression with Clinicopathologic Traits of HCC Patients
In addition, we explored a possible link of CDC20 expression with clinicopathologic traits among HCC patients. As shown in Table 1, the results revealed that CDC20 expression was obviously related to differentiation (P=0.003), TNM stage (P=0.000), and lymphatic metastasis (P=0.001). However, no significant correlation was found with age, sex, and tumor size of the HCC patients (all P>0.05).
Correlation of CDC20 Expression with HCC Patients’ Prognoses
The potential connection of CDC20 expression with survival among HCC patients was evaluated by Kaplan-Meier survival analysis. Figure 2 shows that HCC patients with high CDC20 expression faced worse overall survival rates than low-expressing ones (log-rank test P=0.004). Hence, CDC20 is a novel and reliable predictor for HCC prognoses.
Figure 2.
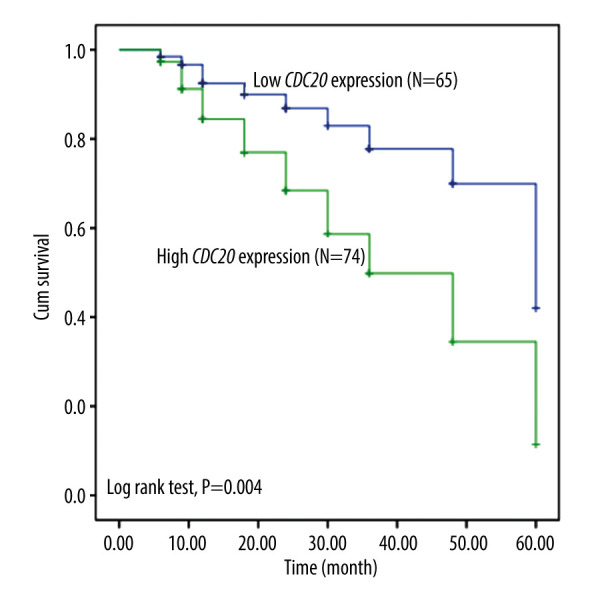
Kaplan-Meier analysis for hepatocellular carcinoma patients based on the expression of CDC20.
Prognosis of CDC20 Expression in HCC Patients
To estimate CDC20 correlation with HCC patients’ prognoses, we performed Cox regression analysis. As listed in Table 2, CDC20 expression alone could signify overall survival among HCC patients (hazard ratio=2.354, 95% confidence intervaI: 1.177–4.709, P=0.016). It indicated that increased expression of CDC20 had an adverse influence on HCC.
Table 2.
Univariable and multivariate Cox regression analyses for CDC20 in HCC patients.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| CDC20 | 2.464 (1.245–4.876) | 0.010* | 2.354 (1.177–4.709) | 0.016* |
| Age | 0.670 (0.369–1.219) | 0.190 | – | – |
| Sex | 0.834 (0.460–1.512) | 0.550 | – | – |
| Tumor size | 0.863 (0.474–1.573) | 0.631 | – | – |
| Differentiation | 1.930 (1.007–3.700) | 0.048* | – | – |
| TNM stage | 1.304 (0.689–2.469) | 0.415 | – | – |
| Lymphatic metastasis | 1.944 (1.049–3.602) | 0.035* | ||
HCC – hepatocellular carcinoma; HR – hazard ratio; CI – confidence interval; TNM – tumor node metastasis.
Statistically significant.
Discussion
HCC is a type of primary liver cancer and is the fourth most common cancer in men and the fourth leading cause of cancer-related death among men and women in China [21]. Its onset has shown an upward tendency in developed countries in recent years [22]. Because of frequent intrahepatic relapse and frequently associated cirrhosis [23–25], HCC patients have very poor prognoses. It has become a pressing sociomedical problem.
CDC20 influences cell division, and is also a major cofactor for the APC via interaction with A-box, D-Box, or KEN-box, the specific constituents in the substrates [26,27]. In recent years, overexpression of CDC20 has been found in many human cancers, and is associated with poor prognosis. For example, Kato et al claimed that CDC20 expression was high among non-small-cell lung cancer tissues and CDC20 alone could signify disease prognoses [28]. Choi et al demonstrated CDC20 elevation among urothelial bladder cancer (UBC) cases, and high CDC20 expression correlated with shorter recurrence-free survival and poorer overall survival in UBC patients [29]. Karra et al reported for the first time a strong connection of high CDC20 and securin immunoexpression with dismal prognoses among breast cancer patients. The results indicated that CDC20 might be a promising candidate for clinical application in breast cancer prognostication [30]. This role of CDC20 has also been found in many other cancers, including pancreatic cancer and colorectal cancer [31,32], as well as in HCC [20]. Li et al investigated the impacts of CDC20 on HCC progression, and they showed CDC20 elevation in HCC samples; CDC20 small interfering RNA transfection in HCC cells could decrease cellular multiplication while raising the cell numbers in G2/M phase [20]. However, few studies have reported the clinical prognostic performance of CDC20 in HCC cases.
Our results conformed to those from earlier research. We found CDC20 expression was heightened among HCC tissues compared with matched noncancerous tissues by qRT-PCR. The CDC20 expression level was significantly related to differentiation, TNM stage, and lymphatic metastasis, but had no significant correlation with age, sex, and tumor size of the patients with HCC. That was in accordance with a previous study. Li et al found that CDC20 was elevated in tumor tissues and positively related to sex, tumor differentiation, and TNM stage of HCC. CDC20 could promote the proliferation of HCC [9,20]. Kaplan-Meier survival analysis showed that HCC patients with high CDC20 expression had poorer survival rates than those with low ones. We also explored the prognostic value of CDC20 expression among HCC patients. Cox regression analysis demonstrated that except for differentiation and lymphatic metastasis, CDC20 expression alone could signify lower survival among HCC patients. In other words, overexpression of CDC20 predicts poor prognoses among HCC patients.
Numerous studies have found CDC20 acts as an oncoprotein during oncogenesis, and inactivation of CDC20 might be effective in treating malignancies. Several CDC20 inhibitors have been discovered, such as toxoid-antitoxoid mixture esterase (TAME), pro-TAME, and 2-[benzyl-(2-nitro-benzenesulfonyl)-amino]-N-hydroxy-3-methyl-N-propyl-butyramide [33,34]. The investigation of CDC20 inhibitors might help to elucidate the functional mechanism of CDC20 in human cancers. However, we did not study the related mechanism for CDC20 affecting HCC, which is a limitation of our study. The effects of CDC20 in trends in the stages of disease progression were not explored in this study. Therefore, in future research we will pay more attention to the mechanism of CDC20 and try to find related inhibitors of CDC20 in HCC.
Conclusions
In summary, CDC20 expression exhibited a rising tendency among HCC tissues and could act as a prognostic indicator for HCC, but further and more correlational research needs to be conducted.
Footnotes
Source of support: Departmental sources
References
- 1.Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: A global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–79. doi: 10.1586/17474124.2015.1028363. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–59. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budny A, Kozlowski P, Kaminska M, et al. [Epidemiology and risk factors of hepatocellular carcinoma]. Pol Merkur Lekarski. 2017;43:133–39. [in Polish] [PubMed] [Google Scholar]
- 6.Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: Analysis of the National Cancer Database. J Clin Oncol. 2018;36:600–8. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–58. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F, Lin Y, Cui P, et al. Cdc20/p55 mediates the resistance to docetaxel in castration-resistant prostate cancer in a Bim-dependent manner. Cancer Chemother Pharmacol. 2018;81:999–1006. doi: 10.1007/s00280-018-3578-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Zhang J, Wan L, et al. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol Ther. 2015;151:141–51. doi: 10.1016/j.pharmthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfarsi LH, Ansari RE, Craze ML, et al. CDC20 expression in oestrogen receptor positive breast cancer predicts poor prognosis and lack of response to endocrine therapy. Breast Cancer Res Treat. 2019;178:535–44. doi: 10.1007/s10549-019-05420-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Huang H, Liu A, et al. Cell division cycle 20 (CDC20) drives prostate cancer progression via stabilization of beta-catenin in cancer stem-like cells. EBioMedicine. 2019;42:397–407. doi: 10.1016/j.ebiom.2019.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S, Castillo V, Sliva D. CDC20 associated with cancer metastasis and novel mushroom-derived CDC20 inhibitors with antimetastatic activity. Int J Oncol. 2019;54:2250–256. doi: 10.3892/ijo.2019.4791. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Choi JW, Lee JH, et al. Spindle assembly checkpoint MAD2 and CDC20 overexpressions and cell-in-cell formation in gastric cancer and its precursor lesions. Hum Pathol. 2019;85:174–83. doi: 10.1016/j.humpath.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Mondal G, Sengupta S, Panda CK, et al. Overexpression of Cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis. 2007;28:81–92. doi: 10.1093/carcin/bgl100. [DOI] [PubMed] [Google Scholar]
- 15.Tang J, Lu M, Cui Q, et al. Overexpression of ASPM, CDC20, and TTK confer a poorer prognosis in breast cancer identified by gene co-expression network analysis. Front Oncol. 2019;9:310. doi: 10.3389/fonc.2019.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuang L, Yang Z, Meng Z. Upregulation of BUB1B, CCNB1, CDC7, CDC20, and MCM3 in tumor tissues predicted worse overall survival and disease-free survival in hepatocellular carcinoma patients. Biomed Res Int. 2018;2018 doi: 10.1155/2018/7897346. 7897346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gayyed MF, El-Maqsoud NM, Tawfiek ER, et al. A comprehensive analysis of CDC20 overexpression in common malignant tumors from multiple organs: Its correlation with tumor grade and stage. Tumour Biol. 2016;37:749–62. doi: 10.1007/s13277-015-3808-1. [DOI] [PubMed] [Google Scholar]
- 18.Mao Y, Li K, Lu L, et al. Overexpression of Cdc20 in clinically localized prostate cancer: Relation to high Gleason score and biochemical recurrence after laparoscopic radical prostatectomy. Cancer Biomark. 2016;16:351–58. doi: 10.3233/CBM-160573. [DOI] [PubMed] [Google Scholar]
- 19.Ding ZY, Wu HR, Zhang JM, et al. Expression characteristics of CDC20 in gastric cancer and its correlation with poor prognosis. Int J Clin Exp Pathol. 2014;7:722–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Gao JZ, Du JL, et al. Increased CDC20 expression is associated with development and progression of hepatocellular carcinoma. Int J Oncol. 2014;45:1547–55. doi: 10.3892/ijo.2014.2559. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 22.Zhu ZX, Huang JW, Liao MH, et al. Treatment strategy for hepatocellular carcinoma in China: Radiofrequency ablation versus liver resection. Jpn J Clin Oncol. 2016;46:1075–80. doi: 10.1093/jjco/hyw134. [DOI] [PubMed] [Google Scholar]
- 23.Iizuka N, Oka M, Yamada-Okabe H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet. 2003;361:923–29. doi: 10.1016/S0140-6736(03)12775-4. [DOI] [PubMed] [Google Scholar]
- 24.Bodzin AS, Busuttil RW. Hepatocellular carcinoma: Advances in diagnosis, management, and long term outcome. World J Hepatol. 2015;7:1157–67. doi: 10.4254/wjh.v7.i9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe A, Ramalho M, AlObaidy M, et al. Magnetic resonance imaging of the cirrhotic liver: An update. World J Hepatol. 2015;7:468–87. doi: 10.4254/wjh.v7.i3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H. Cdc20: A WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Wu MS, Ma QY, Liu DD, et al. CDC20 and its downstream genes: Potential prognosis factors of osteosarcoma. Int J Clin Oncol. 2019;24:1479–89. doi: 10.1007/s10147-019-01500-3. [DOI] [PubMed] [Google Scholar]
- 28.Kato T, Daigo Y, Aragaki M, et al. Overexpression of CDC20 predicts poor prognosis in primary non-small cell lung cancer patients. J Surg Oncol. 2012;106:423–30. doi: 10.1002/jso.23109. [DOI] [PubMed] [Google Scholar]
- 29.Choi JW, Kim Y, Lee JH, et al. High expression of spindle assembly checkpoint proteins CDC20 and MAD2 is associated with poor prognosis in urothelial bladder cancer. Virchows Arch. 2013;463:681–87. doi: 10.1007/s00428-013-1473-6. [DOI] [PubMed] [Google Scholar]
- 30.Karra H, Repo H, Ahonen I, et al. Cdc20 and securin overexpression predict short-term breast cancer survival. Br J Cancer. 2014;110:2905–13. doi: 10.1038/bjc.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo W, Zhong K, Wei H, et al. Long non-coding RNA SPRY4-IT1 promotes cell proliferation and invasion by regulation of Cdc20 in pancreatic cancer cells. PLoS One. 2018;13:e0193483. doi: 10.1371/journal.pone.0193483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Wang Y, Wang X, et al. CDK1 and CDC20 overexpression in patients with colorectal cancer are associated with poor prognosis: Evidence from integrated bioinformatics analysis. World J Surg Oncol. 2020;18:50. doi: 10.1186/s12957-020-01817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes A, Maes K, De Raeve H, et al. The anaphase-promoting complex/cyclosome: A new promising target in diffuse large B-cell lymphoma and mantle cell lymphoma. Br J Cancer. 2019;120:1137–46. doi: 10.1038/s41416-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang J, Thyagarajan-Sahu A, Krchnak V, et al. NAHA, a novel hydroxamic acid-derivative, inhibits growth and angiogenesis of breast cancer in vitro and in vivo. PLoS One. 2012;7:e34283. doi: 10.1371/journal.pone.0034283. [DOI] [PMC free article] [PubMed] [Google Scholar]


