Abstract
Background
This study compared the effects of a bean-based and a white rice-based breakfast diet on postprandial glucose and insulin levels in Chinese patients with type 2 diabetes mellitus (T2DM).
Material/Methods
We recruited 63 patients with T2DM. The patients participated in the randomized 2×2 crossover trial. The bean-based diet group and white rice control group were matched for 50 g of available carbohydrate at breakfast. The patients followed the diets for 3 days. Vein blood samples were collected at 0, 30, 60, 120, and 180 min after eating. Data were analyzed using a repeated-measures analysis of variance. The results are expressed as the mean±standard error of mean (SEM) or as the median with interquartile range values.
Results
Compared with the white rice control, postprandial glucose was significantly lower with the bean-based diet treatments at 60 min (P=0.004), 120 min (P=0.000), and 180 min (P=0.000). The insulin levels of the bean-based diet group were significantly higher at 60 min (P=0.013). The C-peptide levels of the bean-based diet group were significantly higher at 30 min (P=0.042) and 60 min (P=0.005) postprandial. The glucose area under the curve (AUC) showed a similar trend (P=0.000). There were no statistically significant differences in the AUC of insulin and C-peptide, except C-peptide AUC at 0 to 60 min (P=0.027).
Conclusions
Compared with a white rice-based breakfast, a bean-based diet significantly reduced postprandial glucose levels and promoted insulin secretion. These results support a dietary approach to reduce postprandial hyperglycemia.
Keywords: Blood Glucose, Diabetic Diet, Soy Foods
Background
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease, and its prevalence is rapidly increasing worldwide. The International Diabetes Federation estimated that 451 million people were living with diabetes in 2017, and this number could rise to 693 million by 2045, especially in low- and middle-income countries, where the greatest increase is expected to occur [1]. The postprandial hyperglycemia associated with T2DM causes oxidative stress, which impairs endothelial cell function and eventually causes microvascular and macrovascular complications [2].
Therefore, the control of postprandial blood glucose is critical in diabetes management. The world-leading health agencies recommend diet and lifestyle changes as the first interventions for T2DM. Diet therapy is an effective and low-cost method for preventing and controlling hyperglycemia [3], and the recent rise of precision nutrition may provide personalized nutritional guidance for more effective prevention and management of T2DM [4]. Medical nutrition therapy is important for reducing hyperglycemia and slowing the development of diabetes complications [5], and the nutrition practice guidelines recommend foods that have a low glycemic index (GI) [6].
In Asia, especially China, white rice is a staple food. Asians derive nearly 60% of their energy intake from carbohydrates [7], and epidemiological research has shown that white rice consumption is positively correlated with the risk of T2DM [8]. White rice has a high GI, can produce a relatively large increase in postprandial glucose, and increases oxidative stress [9]. However, the carbohydrate-rich grain is part of Asian culture and not simply food. Eliminating carbohydrates completely or consuming a low-carbohydrate diet is also not recommended because other health functions may be impaired [10].
Beans are the opposite of rice [11] and have a low GI [12]. They are rich in vegetable protein, dietary fiber, phytoestrogens, and minerals, while being low in cholesterol, so they are often used as a component in diets for weight loss [13]. Consumption of beans has been associated with lower blood glucose and with antioxidant effects [14]. Few studies have focused on mixed meals, but the combination of beans and high-GI foods was shown to produce a glycemic reaction somewhere between the 2 types of foods alone [15]. A meta-analysis showed that a low-GI diet reduced glycoproteins by 7.4% more than a high-GI diet. This benefit was similar to that provided by a new drug and was clinically significant [12]. When used in combination with other diets to reduce GI or to increase dietary fiber, dietary beans can improve medium- to long-term glucose control [11]. GI is an important determinant of mixed breakfast meal blood glucose levels. The GI and carbohydrate content of mixed foods explained 90% of the variation in the average blood glucose response, while protein and fat had little effect [16].
Therefore, adding low-GI foods may reduce the damage caused by high-GI components of a mixed diet. But the acute effects of beans as part of a traditional meal on glucose and insulin response are unclear. This information is critical for guiding recommendations to improve the control of hyperglycemia and reduce the risk of complications [17]. In this study, we aimed to compare the effects of bean-based and white rice-based breakfast diets on postprandial glucose and insulin levels in Chinese patients with T2DM.
Material and Methods
Study Design
This study was a randomized, crossover, controlled clinical trial. It was carried out from September to November 2020 and was performed in accordance with the guidelines established in the 1975 Declaration of Helsinki [18]. The Affiliated Haikou Hospital of Xiangya Medical College, Central South University approved the research protocol (Ethics Application Ref: 2020-267). This trial was registered on the Chinese clinical trial registry website (no. ChiCTR2000038684). All clinicians agreed to this study. All patients signed informed consent before being enrolled in the study.
Study Participants
Inpatients with T2DM were recruited from the Department of Endocrinology, Affiliated Haikou Hospital of Xiangya Medical College, Central South University. Our team of doctors selected the appropriate patients for the study based on the following inclusion criteria: age 20–60 years; blood glucose that met diagnostic criteria for diabetes; glycosylated hemoglobin A1c (HbA1c) <9%; BMI <35 kg/m2; no acute illness; no digestive diseases; no autonomic neuropathy (eg, diabetic stomach paralysis); no cardiac vascular disease; no psychiatric disorder and comprehension impairment; willing to eat study foods; and no allergy to beans or latex. Additional criteria for premenopausal women were regular menstrual cycles and not pregnant, lactating, or planning a pregnancy. Before recruitment, Excel software was used to generate a random order [19], and participants were given 2 test meals in a random order.
Six participants did not complete the study. Two individuals withdrew because they were unable to follow the research procedures. Three individuals had scheduling conflicts with work. One participant had other reasons. Data from these participants were not included in the analysis. We obtained data on blood glucose and insulin analysis from 63 participants who completed the study. In addition, their baseline levels were analyzed.
Breakfast Characteristics
Both types of breakfasts were designed to contain similar energy and carbohydrates based on the China Food Composition [20], the Dietary Guidelines for Chinese Residents [21], and the China Medical Nutrition Therapy Guideline for Diabetes [22]. The breakfasts were prepared by dieticians using the same commercially available food brands in the kitchen of Department of Endocrinology, Affiliated Haikou Hospital of Xiangya Medical College. The breakfasts were provided to the participants in the hospital dining room on the day of each test meal. Two types of breakfast (white rice diet group [WG], bean-based diet group [BG]) were provided within a 3-day intervention period. The white rice diet was used as the control diet. White rice is a basic food in the main meals (breakfast, lunch, and dinner) in China. The 2 breakfasts each provided about 250 kcal of energy and contained 50 g of available carbohydrate. Fifty grams of available carbohydrate is a standard amount used to test glucose response in patients with T2DM [23]. The carbohydrate content was obtained from food composition tables. The white rice diet was prepared with an automatic multifunctional rice cooker (MB-WFS4029, Midea, China). In order to achieve consistent preparation, the amounts of white rice (polished long-grain rice purchased from Fulinmen, COFCO, China) and water were normalized to grams. Drinking water (250 g) was added to dry white rice (65 g), and the mixture was cooked for 30 min [19]. The bean-based diet, prepared by Tongfu Group Co., Ltd., consisted of 300 g of Adzuki beans, soybeans, and black beans. It was heated in a microwave for 30 s at medium power. The food was served at room temperature at 7: 00 am. All participants were instructed to consume no self-selected foods or beverages during the intervention period.
Study Protocol
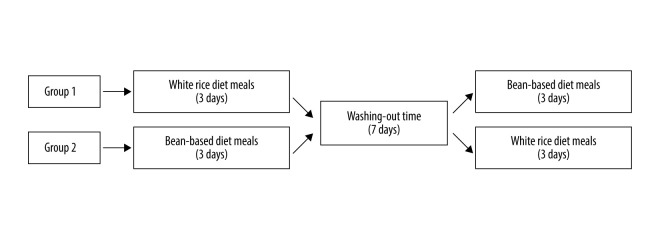
Figure 1 shows a flow chart of the participants included in this study. Eligible participants completed this trial in 2 weeks. Before the study, each individual’s standing height without shoes was measured with a wall-mounted stadiometer. Body mass index (BMI) was calculated by dividing weight by the square of height (kg/m2). The experimental breakfast types included white rice diet and bean-based diet meals. Participants consumed 2 types of breakfast meals for 6 days, with a ≥7-day washout period between each 3-day dietary intervention (Figure 2). Before tests and during washout periods, patients were instructed to maintain their usual medications (metformin, rapid-acting insulin, or non-sulfonylurea), diet, and daily physical activities. The treatment regimen remained unchanged throughout the experiment. During each 3-day intervention period, participants consumed the designated breakfast type and water every morning. Participants received instructions to stop drinking alcohol, consuming caffeine, and pursuing physical activities beyond their daily activities and to fast overnight (12 h) during the period of the research. On the last day of each intervention period, a meal tolerance test was performed. First, blood samples were collected with an indwelling cannula to obtain baseline measurements in the fasting condition. Then patients took their usual medications (other than those that reduce hyperglycemia) with 100 mL of water and had their test breakfast. They were asked to consume the entire breakfast within 15 min and be quiet during the test. Next, vein blood samples were collected at 30, 60, 120, and 180 min after the meal. Blood samples were used to measure glucose, insulin, and C-peptide. The researchers were blinded to the breakfast type during data collection.
Figure 1.
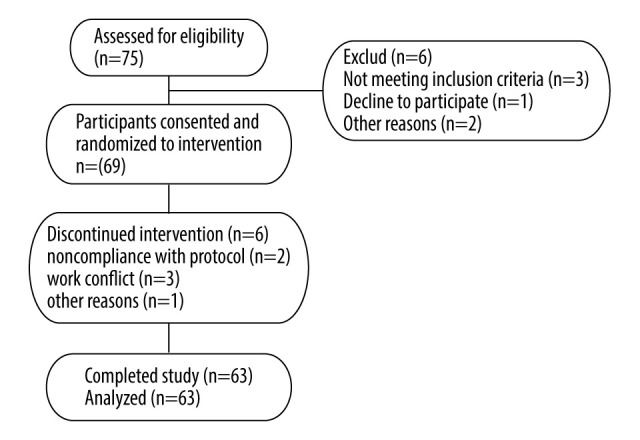
Flow chart for individuals’ enrollment, allocation, intervention, and follow-up.
Figure 2.
Study diagram. Group 1: control → intervention; treatment from white rice diet meals to bean-based diet meals. Group 2: intervention → control; treatment from bean-based diet meals to white rice diet meals.
Biochemical Assays
Each blood sample was collected into Eppendorf tubes and was left at room temperature for 2 h to clot. It was then centrifuged at 4°C for 10 min at 4000 rpm. The available serum was stored at −80°C until testing. The blood glucose, insulin, and C-peptide were determined using an electrochemiluminescence immunoassay method on the Elecsys 2010 analyzer (Roche Diagnostic USA, Indianapolis, IN). The homeostatic model assessment (HOMA) formulas were as follows: the insulin resistance index HOMA (HOMA-IR)=fasting blood glucose (FBG) (mmol/L)×fasting insulin (FINS) (mIU/L)/22.5; pancreatic β-cell function index (HOMA-β)=20×FINS (mIU/L)/[FBG (mmol/L)-3.5] (%) [24]. The area under the curve (AUC) for glucose, insulin, and C-peptide was computed with the trapezoidal rule: AUC was computed with the trapezoidal rule: AUC=(Gn1+Gn2)×(n2-n1)/2, where Gn was the blood glucose, insulin, and C-peptide level at the n timepoint) [25].
Statistical Analysis
A power-based (90%) sample size was calculated with the plasma glucose responses of the test food, which showed that 14 patients were needed for this study (α=0.05) [26]. The sample size used was greater.
All data were statistically analyzed with SPSS 17.0 software. The Shapiro-Wilk test was used to determine whether the data were normally distributed. Between-group comparisons were analyzed by using repeated-measures analysis of variance. Nonnormal distribution data were analyzed by using the Mann-Whitney U test. The values of HOMA-IR and HOMA-β showed nonnormal distributions. Thus, before the statistical analysis, natural logarithms were obtained to meet the normal distribution and were described as absolute values. The data are expressed as mean±SEM or as median with interquartile range values. Chi-square analysis was used to test the rate. The level of statistically significant difference was P<0.05.
Results
Participant Characteristics
Participants included 29 women (46.03%) and 34 men (53.97%), with an average age of 48.83±5.36 years. Their basic data were analyzed, including BMI (21.93±0.38 kg/m2), HbA1C (7.81±0.18%), serum lipid (total cholesterol, 4.96±0.20 mmol/L), total triglycerides, 1.56 [0.855, 2.778] mmol/L), and lipoprotein concentrations (high-density lipoprotein cholesterol, 1.1 [0.93, 1.31] mmol/L; low-density lipoprotein cholesterol, 3.02±0.16 mmol/L). All values were within normal clinical reference ranges, with the exception of HbA1C (Table 1). Body weight did not significantly change during the experiment.
Table 1.
Baseline participant characteristics of study patients with type 2 diabetes (n=63).
| Variable | Value (mean±SEM) | P | ||
|---|---|---|---|---|
| WG→BG (n=31) | BG→WG (n=32) | Total (n=63) | ||
| Age (years) | 47.63±10.28 | 49.23±7.6 | 48.832±5.36 | 0.724 |
| Women, n (%) | 13 (41.9%) | 16 (50%) | 29 (46.0%) | 0.739 |
| Weight (kg) | 57.76±8.12 | 62.83±6.77 | 60.41±1.61 | 0.218 |
| BMI (kg/m2) | 21.39±2.13 | 22.49±1.62 | 21.93±0.38 | 0.761 |
| TC (mmol/L) | 4.78±0.74 | 5.14±1.1 | 4.96±0.20 | 0.373 |
| TG (mmol/L) | 1.54 (0.995, 2.22) | 1.58 (0.815, 3.455) | 1.56 (0.855, 2.778) | 0.887 |
| HDL-C(mmol/L) | 1.09 (0.925, 1.31) | 1.15 (0.94, 1.26) | 1.1 (0.93, 1.31) | 0.915 |
| LDL-C (mmol/L) | 2.99±0.57 | 3.04±0.92 | 3.02±0.16 | 0.913 |
| HbA1C (%) | 7.57±0.91 | 8.03±0.78 | 7.81±0.18 | 0.213 |
BG – bean-based diet group; SEM – standard error of mean; T2DM – type 2 diabetes mellitus; WG – white rice diet group.
Breakfast Characteristics
Participants randomly received the 2 test meals: white rice diet and bean-based diet. We matched the diets in terms of available carbohydrates (49.20 vs 48.75 g) and total energy for breakfast (207 vs 201 kcal) (Table 2).
Table 2.
Energy and macronutrient distribution of provided breakfast treatments.
| Dietary variables | Breakfast treatments | |
|---|---|---|
| White rice diet | Bean-based diet | |
| White rice (g) | 65 | n. p. |
| Beans (g) | 0 | n. p. |
| Total weight (g) | 300 | 300 |
| Energy (kcal) | 207 | 201 |
| Available carbohydrate (g) | 49.20 | 48.75 |
| Total protein (g) | 4.75 | 6.3 |
| Total fat (g) | 0 | 0 |
| Fiber (g) | 0.7 | 10.2 |
n.p. – not provided.
Test Meal Effects on Fasting Glucose, Insulin, and C-Peptide
Fasting glucose (WG vs BG: 6.04±0.29 vs 5.77±0.20, P=0.383), insulin (WG vs BG: 7.05±1.13 vs 8.02±1.09, P=0.107), and C-peptide (WG vs BG: 1.51±0.18 vs 1.51±0.18, P=0.093) were analyzed. The values for log HOMA-IR (WG vs BG: 0.386±0.059 vs 0.282±0.048, P=0.088) and log HOMA-β (WG vs BG: 1.700±0.081 vs 1.828±0.087, P=0.206) were not significantly different between the 2 test meal groups (P>0.05). No significant difference was found between blood glucose and islet function in fasting state between the 2 diet groups (P>0.05) (Table 3).
Table 3.
Fasting blood glucose, insulin, C-peptide, and relevant calculated parameters.
| Fasting variables | Group (mean±SEM) | ||
|---|---|---|---|
| WG | BG | P | |
| Glucose (mmol/L) | 6.04±0.29 | 5.77±0.20 | 0.383 |
| Insulin (mIu/L) | 7.05±1.13 | 8.02±1.09 | 0.107 |
| C peptide (pg/ml) | 1.51±0.18 | 1.75±0.15 | 0.093 |
| Lg HOMA-IR | 0.386±0.059 | 0.282±0.048 | 0.088 |
| Lg HOMA-β | 1.700±0.081 | 1.828±0.087 | 0.206 |
BG – bean-based diet group; SEM – standard error of mean; WG – white rice diet group.
Postprandial Glucose, Insulin, and C-Peptide Responses of Meal Tolerance Testing, and the Respective AUC
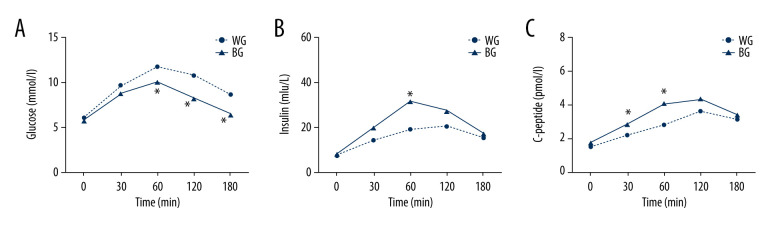
Results from meal tolerance testing showed that postprandial plasma glucose of patients in the bean-based diet group was significantly lower at 60 min (WG vs BG: 11.73±1.74 vs 10.08±1.88, P=0.004), 120 min (WG vs BG: 10.74±2.21 vs 8.28±1.71, P=0.000) and 180 min (WG vs BG: 8.64±2.15 vs 6.48±1.36, P=0.000) compared with the white rice control group (Figure 3A). The postprandial insulin levels of patients in the bean-based diet group were significantly higher than those of control group individuals at 60 min (WG vs BG: 18.72±4.94 vs 31.44±9.86, P=0.013) (Figure 3B). The postprandial C-peptide levels of bean-based diet group patients were significantly higher at 30 min (WG vs BG: 2.19±1.04 vs 2.83±1.802, P=0.042) and 60 min (WG vs BG: 2.81±1.36 vs 4.03±1.44, P=0.005) (Figure 3C).
Figure 3.
(A–C) The postprandial glucose, insulin, C-peptide responses of meal tolerance testing. All values are mean±SEM. * P<0.05. BG – bean-based diet group; SEM – standard error of mean; WG – white rice diet group.
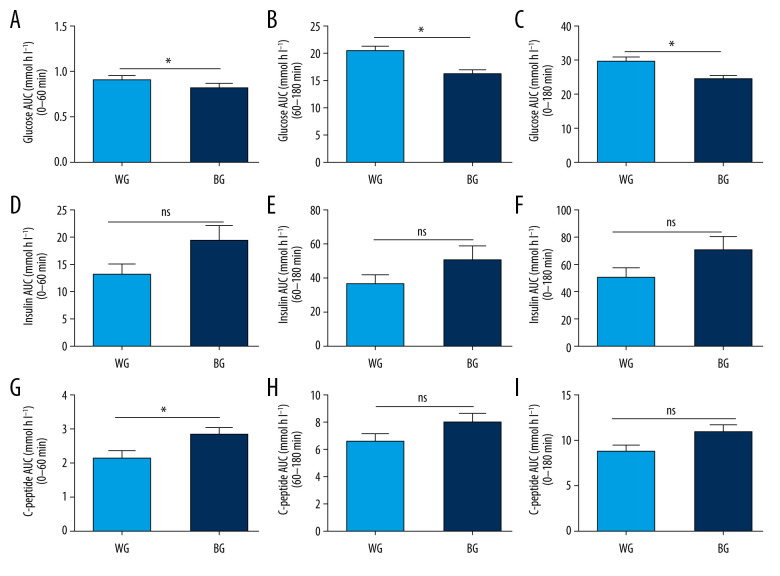
Compared with the white rice diet meal, bean intake remarkably reduced glucose AUC at 0–60 min (WG vs BG: 9.24±0.29 vs 8.35±0.29, P=0.000), 60–180 min (WG vs BG: 20.93±0.79 vs 16.56±0.62, P=0.000), and 0–180 min (WG vs BG: 30.17±1.02 vs 24.91±0.83, P=0.000) (Figure 4A–4C), and significantly increased C-peptide AUC at 0–60 min (WG vs BG: 2.17±0.22 vs 2.86±0.21, P=0.027). However, no statistically significant differences (P≥0.05) were observed for insulin AUC at 0–60 min (WG vs BG: 13.49±8.89 vs 19.82±12.15, P=0.050), 60–180 min (WG vs BG: 37.25±5.29 vs 51.65±8.01, P=0.141), and 0–180 min (WG vs BG: 50.74±7.10 vs 71.48±10.28, P=0.104) (Figure 4D–4F) or for C-peptide AUC at 60–180 min (WG vs BG: 6.63±0.59 vs 8.10±0.65, P=0.100) and at 0–180 min (WG vs BG: 8.81±0.79 vs 10.97±0.83, P=0.067) (Figure 4G–4I). The above results imply that a bean-based meal was beneficial for promoting early-phase endogenous insulin secretion and reducing postprandial blood glucose.
Figure 4.
(A–I) The respective area under the curve (AUC) of glucose, insulin, and C-peptide. All values are mean±SEM. * P<0.05. BG – bean-based diet group; ns – not significant; SEM – standard error of mean; WG – white rice diet group.
Discussion
The present study compared the effects of a bean-based and a white rice-based breakfast diet on postprandial glucose and insulin levels in Chinese patients with T2DM. Compared with the white rice control group, participants consuming the bean-based diet had significantly reduced postprandial glucose at 60, 120, and 180 min. The bean-based diet group had significantly higher insulin levels at 60 min and significantly higher C-peptide levels at 30 and 60 min after the meal.
Rice is a principal staple and energy source food of more than half of the world’s population and has important nutrition and health significance. Rice is a medium-high GI food, with the fiber content reduced by 80% in the refining processes [27]. Hu et al [28] found that consuming 1 serving of white rice a day increased the risk of diabetes by 11% in the general population. This result implied that rice is a significant contributor to diabetes, which motivated us to find another widely accepted diet combination that could reduce the risk of diabetes.
Common beans are a traditional part of the diet in Latin America, the Mediterranean, and the Middle East. Beans could lower blood glucose because they have a low GI and components that have an antidiabetic effect [29]. Consuming a low-GI diet can diminish the risk of developing T2DM [30]. A traditional Mexican diet with beans played the same role in T2DM [32], and the conventional dietary, including beans, benefited health, diminished chronic complications of T2DM, and improved patient compliance [31–33]. Therefore, we decided to explore the clinical role of beans and white rice in glucose metabolism.
In this study, we observed no statistically significant differences in glucose, insulin, C-peptide, and calculated HOMA-IR and HOMA-β between the 2 groups before they ate. These findings indicated that the 3-day intervention had no significant effect on the fasting state. We speculated that beans have less of an effect on fasting blood sugar, which controls postprandial blood sugar by increasing α-glucosidase and α-amylase inhibition activity to delay the absorption of glucose and control the postprandial hyperglycemia [34–36]. Another possible reason was insufficient intervention time.
Next, we found that bean-based meals reduced postprandial blood sugar responses, which was consistent with previous research [19]. Results from the meal tolerance tests suggested that postprandial blood glucose and AUC of BG patients were significantly lower than those of WG patients at 60, 120, and 180 min. The results implied that complex foods were effective in reducing the acute postprandial glycemic response. The postprandial glucose peak occurred at 1 h after eating, consistent with findings in individuals with overweight and obesity [37]. The glucose concentration of BG patients declined to nearly baseline level at 180 min. The common bean protein was related to antioxidant, anti-inflammatory, and antihypertensive effects; tumor cell inhibition; and reduction of hyperglycemia due to inhibiting α-amylase, α-glucosidase, and dipeptidyl peptidase 4 activities [24,38,39]. In mixed foods, the combination of carbohydrates and bean products significantly reduced the GI of white rice [40].
Dietary fiber stimulates secretion of gastrointestinal hormones, such as cholecystokinin and glucagon-like peptide-1, that can promote insulin secretion [41]. In addition, phytoestrogens can reduce fat accumulation [42]. To investigate the effect of beans on insulin secretion, we examined the insulin and C-peptide levels. We found that a bean-based diet has a more decisive role in promoting endogenous insulin secretion, compared with white rice meals in T2DM. The peak value of C-peptide in all patients was at 2 h, but that of insulin in patients consuming the bean-based diet was at 1 h. The delayed peaks of insulin secretion were consistent with the manifestation of T2DM [43], but based on the trend, the bean-based meal may have led to an earlier peak in insulin production. We reached this conclusion by statistically significant C-peptide AUC at 0–60 min and insulin secretion peak at 60 min. Consistent with previous research results, cooked common beans can maintain β-cell mass and protect against β-cell damage in rats with diabetes [44]. A common bean extract ameliorated glucose tolerance and insulin sensitivity by regulating insulin response and protecting β cells from hyperglycemia [45]. In addition, breakfast composition is important. Partial replacement of rapidly available carbohydrate with low-GI types of foods at breakfast can improve metabolic wellness [46].
There were some limitations in this study. First, the sample size was relatively small. Second, the subjects were inpatients, so healthy control and prediabetes groups were not included. A subsequent study of intestinal flora included 3 groups: healthy volunteers and prediabetic and diabetic adults. Third, confounding factors exist in clinical studies, and a crossover design was used to avoid the influence of the order of dietary administration and the different physical conditions of the patients. The crossover test requires a sufficient washout period to eliminate the residual effects of the first-stage interventions. Therefore, we adopted a short-term effect test of 3 days of intervention to avoid the effects of interventions as much as possible. Finally, except for the effect of food ingredients on the results, this study lacked investigation of further mechanisms. Despite the limitations, the results of this study provide a clinical basis for our further study on the improvement of glucose metabolism through intestinal flora.
Conclusions
This clinical study demonstrated that a bean-based diet attenuated the glycemic response and promoted insulin secretion compared with a white rice meal. Beans should be promoted for their beneficial role in diabetes. In addition, studies on the effects of food on postprandial glucose response and the mechanisms related to intestinal flora can provide information for precision nutrition medicine in the future. The diet of diabetic patients deserves further study by clinicians.
Acknowledgments
The authors thank the staff of Department of Endocrinology, Affiliated Haikou Hospital of Xiangya Medical College, Central South University. The authors thank all patients for their cooperation.
Footnotes
Ethics Statement
The Affiliated Haikou Hospital of Xiangya Medical College, Central South University approved the research protocol (Ethics Application Ref: 2020-267).
Conflict of Interest
None.
Source of support: This study was supported by the National Natural Science Foundation of China (no. 81471053), and the Key Science and Technology Project of Hainan province (grant no. ZDXM2015084)
References
- 1.Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Nguelefack TB, Fofie CK, Nguelefack-Mbuyo EP, Wuyt AK. Multimodal α-glucosidase and α-amylase inhibition and antioxidant effect of the aqueous and methanol extracts from the trunk bark of Ceiba pentandra. Biomed Res Int. 2020;2020 doi: 10.1155/2020/3063674. 3063674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6(5):416–26. doi: 10.1016/S2213-8587(18)30037-8. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 6.Briggs Early K, Stanley K. Position of the Academy of Nutrition and Dietetics: The role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet. 2018;118(2):343–53. doi: 10.1016/j.jand.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Cui Z, Dibley MJ. Trends in dietary energy, fat, carbohydrate and protein intake in Chinese children and adolescents from 1991 to 2009. Br J Nutr. 2012;108(7):1292–99. doi: 10.1017/S0007114511006891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sar S, Marks GC. Estimated effects of white rice consumption and rice variety selection on incidence of type 2 diabetes in Cambodia. Public Health Nutr. 2015;18(14):2592–99. doi: 10.1017/S1368980014003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 10.Wee MSM, Henry CJ. Reducing the glycemic impact of carbohydrates on foods and meals: Strategies for the food industry and consumers with special focus on Asia. Compr Rev Food Sci Food Saf. 2020;19(2):670–702. doi: 10.1111/1541-4337.12525. [DOI] [PubMed] [Google Scholar]
- 11.Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52(8):1479–95. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- 12.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–67. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine KR, Yang D, Gadbury GL, et al. Results of soy-based meal replacement formula on weight, anthropometry, serum lipids & blood pressure during a 40-week clinical weight loss trial. Nutr J. 2003;2:14. doi: 10.1186/1475-2891-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oomah BD, Corbé A, Balasubramanian P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J Agric Food Chem. 2010;58(14):8225–30. doi: 10.1021/jf1011193. [DOI] [PubMed] [Google Scholar]
- 15.Hutchins AM, Winham DM, Thompson SV. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br J Nutr. 2012;108(Suppl 1):S52–65. doi: 10.1017/S0007114512000761. [DOI] [PubMed] [Google Scholar]
- 16.Wolever TM, Yang M, Zeng XY, et al. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr. 2006;83(6):1306–12. doi: 10.1093/ajcn/83.6.1306. [DOI] [PubMed] [Google Scholar]
- 17.O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–94. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.Thompson SV, Winham DM, Hutchins AM. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr J. 2012;11:23. doi: 10.1186/1475-2891-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Nutrition and Food Safety. China CDC 2005 China Food Composition. Peking University Medical Press; 2005. [Google Scholar]
- 21.Wang SS, Lay S, Yu HN, Shen SR. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J Zhejiang Univ Sci B. 2016;17(9):649–56. doi: 10.1631/jzus.B1600341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Jiang H, Tao YX, Shu XL. Development and interpretation of China medical nutrition therapy guideline for diabetes (2010) Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011;33:253–56. doi: 10.3881/j.issn.1000-503X.2011.03.009. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 23.Josse AR, Kendall CW, Augustin LS, et al. Almonds and postprandial glycemia – a dose-response study. Metabolism. 2007;56(3):400–4. doi: 10.1016/j.metabol.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Wang Q, Li S, et al. Convenient food made of extruded adzuki bean attenuates inflammation and improves glycemic control in patients with type 2 diabetes: A randomized controlled trial. Ther Clin Risk Manag. 2018;14:871–84. doi: 10.2147/TCRM.S161649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Q, Wu Y, Yang M, et al. Nr2e1 ablation impairs liver glucolipid metabolism and induces inflammation, high-fat diets amplify the damage. Biomed Pharmacother. 2019;120:109503. doi: 10.1016/j.biopha.2019.109503. [DOI] [PubMed] [Google Scholar]
- 26.de Carvalho CM, de Paula TP, Viana LV, et al. Plasma glucose and insulin responses after consumption of breakfasts with different sources of soluble fiber in type 2 diabetes patients: A randomized crossover clinical trial. Am J Clin Nutr. 2017;106(5):1238–45. doi: 10.3945/ajcn.117.157263. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DJ, Wolever TM, Jenkins AL, et al. The glycaemic response to carbohydrate foods. Lancet. 1984;2(8399):388–91. doi: 10.1016/s0140-6736(84)90554-3. [DOI] [PubMed] [Google Scholar]
- 28.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. BMJ. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petlevski R, Hadzija M, Slijepcevic M, Juretic D. Effect of ‘antidiabetis’ herbal preparation on serum glucose and fructosamine in NOD mice. J Ethnopharmacol. 2001;75(2–3):181–84. doi: 10.1016/s0378-8741(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 30.Schulze MB, Liu S, Rimm EB, et al. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80(2):348–56. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Cruz A, Bacardi-Gascon M, Turnbull WH, et al. A flexible, low-glycemic index Mexican-style diet in overweight and obese subjects with type 2 diabetes improves metabolic parameters during a 6-week treatment period. Diabetes Care. 2003;26(7):1967–70. doi: 10.2337/diacare.26.7.1967. [DOI] [PubMed] [Google Scholar]
- 32.Mattei J, Hu FB, Campos H. A higher ratio of beans to white rice is associated with lower cardiometabolic risk factors in Costa Rican adults. Am J Clin Nutr. 2011;94(3):869–76. doi: 10.3945/ajcn.111.013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999;70(3 Suppl):464S–74S. doi: 10.1093/ajcn/70.3.464s. [DOI] [PubMed] [Google Scholar]
- 34.Valencia-Mejía E, Batista KA, Fernández JJA, Fernandes KF. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.) Food Res Int. 2019;121:238–46. doi: 10.1016/j.foodres.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 35.Yao Y, Cheng X, Ren G. α-Glucosidase inhibitory activity of protein-rich extracts from extruded adzuki bean in diabetic KK-Ay mice. Food Funct. 2014;5(5):966–71. doi: 10.1039/c3fo60521c. [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Xu B, Cai W. Active substances and in vitro anti-diabetic effects of a traditional folk remedy Bian-Que Triple-Bean Soup as affected by the boiling time. Food Funct. 2013;4(4):635–43. doi: 10.1039/c3fo30303a. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins AL, Jenkins DJ, Wolever TM, et al. Comparable postprandial glucose reductions with viscous fiber blend enriched biscuits in healthy subjects and patients with diabetes mellitus: Acute randomized controlled clinical trial. Croat Med J. 2008;49(6):772–82. doi: 10.3325/cmj.2008.49.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo J, Cai W, Wu T, Xu B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016;201:350–60. doi: 10.1016/j.foodchem.2016.01.101. [DOI] [PubMed] [Google Scholar]
- 39.Yu Z, Yin Y, Zhao W, et al. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012;135(3):2078–85. doi: 10.1016/j.foodchem.2012.06.088. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama M, Tang AC, Wakaki Y, Koyama W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr. 2003;57(6):743–52. doi: 10.1038/sj.ejcn.1601606. [DOI] [PubMed] [Google Scholar]
- 41.Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138(3):439–42. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 42.Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol. 2009;304(1–2):30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Zhao X, Zhou R, et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J Diabetes Investig. 2018;9(6):1288–95. doi: 10.1111/jdi.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández-Saavedra D, Mendoza-Sánchez M, Hernández-Montiel HL, et al. Cooked common beans (Phaseolus vulgaris) protect against β-cell damage in streptozotocin-induced diabetic rats. Plant Foods Hum Nutr. 2013;68(2):207–12. doi: 10.1007/s11130-013-0353-1. [DOI] [PubMed] [Google Scholar]
- 45.Kim M, Kim DK, Cha YS. Black adzuki bean (Vigna angularis) extract protects pancreatic β cells and improves glucose tolerance in C57BL/6J mice fed a high-fat diet. J Med Food. 2016;19(5):442–49. doi: 10.1089/jmf.2015.3598. [DOI] [PubMed] [Google Scholar]
- 46.Maki KC, Phillips-Eakley AK, Smith KN. The effects of breakfast consumption and composition on metabolic wellness with a focus on carbohydrate metabolism. Adv Nutr. 2016;7(3):613S–21S. doi: 10.3945/an.115.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]