To the Editor:Cystic fibrosis (CF) is caused by mutations in the CFTR (CF transmembrane conductance regulator) gene (1). The most common CFTR mutation in populations of European descent is F508del, with up to 90% of people with CF (pwCF) having one or more F508del alleles (2–4).
Two pivotal phase 3 studies of a triple-combination regimen consisting of small-molecule CFTR modulators elexacaftor (ELX)/tezacaftor (TEZ)/ivacaftor (IVA) showed unprecedented clinical efficacy in pwCF 12 years old or older heterozygous for the F508del-CFTR mutation and a minimal function mutation (F/MF; an MF mutation results in either no CFTR protein or a CFTR protein that does not respond to IVA and TEZ/IVA in vitro) or homozygous for F508del (F/F) (5–7). ELX/TEZ/IVA was generally safe and well tolerated in both studies (5, 6).
Eligible participants from both pivotal phase 3 studies could elect to participate in an ongoing, phase 3, open-label extension (OLE) study to evaluate the long-term safety and efficacy of ELX/TEZ/IVA. We report the results of an interim analysis of the OLE performed after the last ongoing participant had completed the Week 24 visit. Final results from this study will be published after study completion. These interim results have been accepted for oral presentation (8).
Methods
Participants who completed the last visit of the 24-week treatment period in the F/MF pivotal study (NCT03525444) or of the 4-week treatment period in the F/F pivotal study (NCT03525548) and who met other eligibility criteria could enroll in the OLE (NCT03525574). In the OLE, all participants receive ELX 200 mg/TEZ 100 mg/IVA 150 mg each morning and IVA 150 mg each evening.
The primary objective of the OLE is to evaluate long-term safety and tolerability of ELX/TEZ/IVA in pwCF with one or more F508del mutations. Secondary objectives include evaluating long-term efficacy and pharmacodynamics (PD) of ELX/TEZ/IVA. The primary endpoint is safety as assessed by adverse events (AEs), clinical laboratory values, vital signs, ECGs, and pulse oximetry. Secondary endpoints include absolute change from parent study baseline in FEV1% predicted (ppFEV1), sweat chloride (SwCl) concentration, body mass index (BMI), and CF Questionnaire-Revised (CFQ-R) respiratory domain (RD) score in addition to the cumulative number of pulmonary exacerbations (PEx).
An interim analysis with prespecified analyses was conducted when the last ongoing participant reached their Week 24 visit (i.e., the data cutoff). Safety and efficacy data sets included all participants who received one or more doses of ELX/TEZ/IVA during the OLE. Safety data include events up to the data cutoff for the OLE interim analysis. Parent study baseline (before ELX/TEZ/IVA treatment) was used as the baseline for the efficacy analyses; for F/F participants, the baseline in the parent study was assessed after a 4-week run-in period with TEZ/IVA. ppFEV1, SwCl, BMI, and CFQ-R RD data were analyzed using a mixed-effects model for repeated measures with absolute change from baseline as the dependent variable. PEx event rate was calculated starting from each participant’s first dose of ELX/TEZ/IVA (whether during the parent study or OLE) using a negative binomial regression model.
Participants enrolling from the F/F pivotal study entered the OLE earlier and therefore in this interim analysis, had longer follow-up than those entering from the F/MF pivotal study. For F/F participants, the most recent ppFEV1 and BMI were obtained at Week 36, whereas the most recent SwCl concentration and CFQ-R RD score were obtained at Week 24.
Results
A total of 506 participants received ELX/TEZ/IVA in this study (n = 399 F/MF; n = 107 F/F). Participant demographics were similar to those observed in the pivotal studies (5, 6). A total of 471 (93.1%) participants experienced AEs (Table 1); AE rates in the ELX/TEZ/IVA arm of the larger, longer F/MF pivotal study, which was the primary source of phase 3 safety data, are provided for comparison (5, 9). The majority of participants had mild or moderate AEs that were similar to those observed in the pivotal studies (5, 6). The most common AEs included infective PEx of CF (exposure-adjusted rate, 49.6 per 100 participant-years), cough (44.3), and oropharyngeal pain (25.7). A total of 80 (15.8%) participants experienced serious AEs (exposure-adjusted rate, 27.5 per 100 participant-years), with infective PEx of CF, hemoptysis, and distal intestinal obstruction syndrome as the more common events. Seven participants (1.4%) had AEs resulting in treatment discontinuation; these AEs included liver events (n = 4), depression (n = 1), rash (n = 1), and tinnitus and contusion (n = 1 [in the same participant]). AEs of elevated transaminases occurred in 36 (7.1%) participants, and laboratory elevations in alanine aminotransferase or aspartate aminotransferase >3×, >5×, and >8× the upper limit of normal occurred in 32 (6.3%), 11 (2.2%), and 3 (0.6%) participants, respectively.
Table 1.
Adverse Events
| OLE Study (N = 506; Mean Duration of Exposure: 37.2 wk) |
F/MF Pivotal Study* ELX/TEZ/IVA Arm (n = 202; Mean Duration of Exposure: 23.6 wk) |
|||
|---|---|---|---|---|
| Participants with AEs [n (%)] | Event Rate per 100 Participant-Years | Participants with AEs [n (%)] | Event Rate per 100 Participant-Years | |
| Any AE | 471 (93.1) | 739.9 | 188 (93.1) | 1,096.0 |
| AEs by maximum severity | ||||
| Mild | 180 (35.6) | ND | 67 (33.2) | ND |
| Moderate | 238 (47.0) | ND | 102 (50.5) | ND |
| Severe | 51 (10.1) | ND | 19 (9.4) | ND |
| Life-threatening† | 2 (0.4) | ND | 0 | ND |
| AEs leading to treatment discontinuation | 7 (1.4) | 3.3 | 2 (1.0) | 3.0 |
| AEs leading to treatment interruption | 29 (5.7) | 13.7 | 19 (9.4) | 26.0 |
| AEs leading to death | 0 | 0 | 0 | 0 |
| Most common AEs in the OLE study (occurring in ≥10% of participants) | ||||
| Infective pulmonary exacerbation of CF | 127 (25.1) | 49.6 | 44 (21.8) | 64.9 |
| Cough | 118 (23.3) | 44.3 | 34 (16.8) | 38.9 |
| Oropharyngeal pain | 74 (14.6) | 25.7 | 20 (9.9) | 27.0 |
| Nasopharyngitis | 69 (13.6) | 21.6 | 22 (10.9) | 30.0 |
| Headache | 66 (13.0) | 24.9 | 35 (17.3) | 48.9 |
| Sputum increased | 63 (12.5) | 20.6 | 40 (19.8) | 46.9 |
| Upper respiratory tract infection | 60 (11.9) | 18.3 | 24 (11.9) | 30.0 |
| Fatigue | 51 (10.1) | 16.3 | 9 (4.5) | 9.0 |
| SAEs | 80 (15.8) | 27.5 | 28 (13.9) | 36.9 |
| Most common SAEs (occurring in ≥1% of participants) | ||||
| Infective pulmonary exacerbation of CF | 42 (8.3) | 12.2 | 11 (5.4) | 12.0 |
| Hemoptysis | 5 (1.0) | 1.5 | 2 (1.0) | 2.0 |
| Distal intestinal obstruction syndrome | 5 (1.0) | 1.5 | 1 (0.5) | 1.0 |
| Any rash event | 50 (9.9) | 15.8 | 22 (10.9) | 30.0 |
| SAEs | 1 (0.2) | 0.3 | 3 (1.5) | 3.0 |
| Leading to treatment interruption | 5 (1.0) | 1.3 | 4 (2.0) | 4.0 |
| Leading to treatment discontinuation | 1 (0.2) | 0.3 | 1 (0.5) | 1.0 |
| ALT or AST increase‡ | ||||
| >3× to ≤5× ULN | 21 (4.2) | ND | 11 (5.4) | ND |
| >5× to ≤8× ULN | 8 (1.6) | ND | 2 (1.0) | ND |
| >8× ULN | 3 (0.6) | ND | 3 (1.5) | ND |
| Elevated transaminase AEs‡ | ||||
| Any AEs | 36 (7.1) | 16.5 | 22 (10.9) | 42.9 |
| SAEs | 2 (0.4) | 1.0 | 0 | 0 |
| Leading to treatment interruption | 11 (2.2) | 5.1 | 2 (1.0) | 3.0 |
| Leading to treatment discontinuation | 3 (0.6) | 1.5 | 0 | 0 |
Definition of abbreviations: AE = adverse event; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CF = cystic fibrosis; ELX = elexacaftor; F/MF = heterozygous for the F508del-CFTR mutation and a minimal function CFTR mutation; IVA = ivacaftor; ND = not determined; OLE = open-label extension; SAE = serious adverse event; TEZ = tezacaftor; ULN = upper limit of normal.
Some of the data from the F/MF pivotal study were previously published (5) but are provided here for comparison.
The life-threatening AEs were a suicide attempt and a pulmonary hemorrhage.
In the placebo arm of the F/MF pivotal study, 8 (4.0%) participants had an elevated transaminase AE. Laboratory elevations in ALT or AST of >3× to ≤5× ULN, >5× to ≤8× ULN, and >8× ULN were seen in 8 (4.0%), 1 (0.5%), and 2 (1.0%) participants, respectively.
Key efficacy and PD data from the OLE and parent studies are provided in Figure 1. In F/MF participants, the mean absolute changes from baseline (95% confidence interval [CI]) in ppFEV1 at Week 24 were 14.9 (13.5–16.3) and 14.3 (12.9–15.7) percentage points in those who had been in the respective placebo (n = 189) or ELX/TEZ/IVA (n = 180) groups in the F/MF pivotal study. Among F/MF participants, the estimated PEx event rate per 48 weeks (95% CI) was 0.30 (0.24–0.39) (n = 403). In F/F participants, the mean absolute changes from baseline (95% CI) in ppFEV1 at Week 36 were 12.8 (10.1–15.4) and 11.9 (9.3–14.5) percentage points in those who had been in the TEZ/IVA (n = 49) or ELX/TEZ/IVA (n = 51) groups, respectively, in the F/F pivotal study. Among F/F participants, the estimated PEx event rate per 48 weeks (95% CI) was 0.30 (0.20–0.45) (n = 107). Efficacy in these and all other secondary endpoints tested was comparable with and maintained from parent studies.
Figure 1.
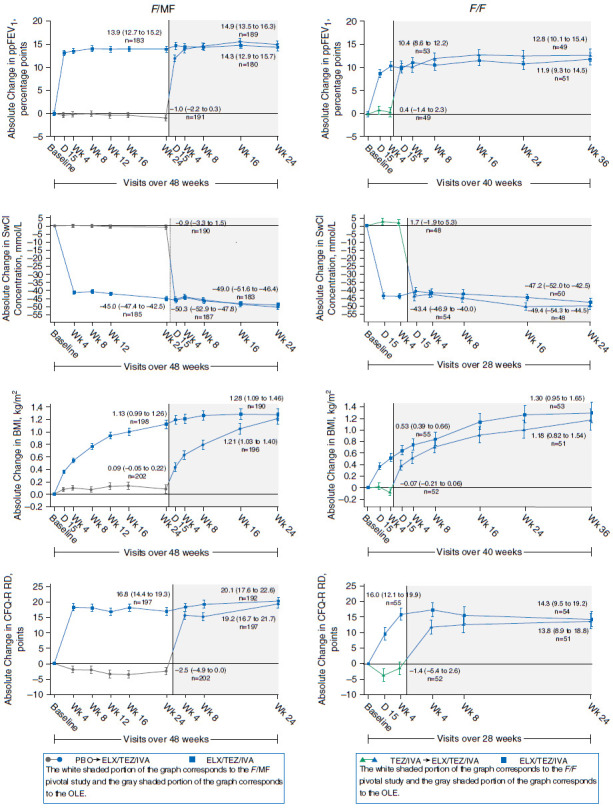
Mixed-effects model for repeated measures analysis of absolute change from baseline in FEV1% predicted (ppFEV1), sweat chloride (SwCl), body mass index (BMI), and Cystic Fibrosis Questionnaire-Revised respiratory domain (CFQ-R RD) by visit. The graphed data represent the least squares mean (SE) absolute change from parent study baseline by mixed-effects model for repeated measures at each visit. The data labels on each plot represent the least squares mean (95% confidence interval) absolute change from parent study baseline and the number of evaluable participants for that visit. For participants heterozygous for the F508del-CFTR mutation and a minimal function CFTR mutation (F/MF) (5), the data labels correspond with the Week 24 visit of the F/MF pivotal study and the Week 24 visit of the open-label extension. For participants homozygous for the F508del-CFTR mutation (F/F) (6), the data labels correspond with the Week 4 visit of the F/F pivotal study and the Week 24 (SwCl and CFQ-R RD) or Week 36 visit (ppFEV1 and BMI) of the open-label extension. Baseline for F/F participants occurred after a 4-week run-in with tezacaftor (TEZ)/ivacaftor (IVA). SwCl and CFQ-R RD were assessed through Week 24 in all participants. The ppFEV1 and BMI were assessed through Week 24 in participants with F/MF genotypes and through Week 36 in participants with the F/F genotype. For participants with F/MF genotypes, n = 203 for those who received placebo in the 24-week F/MF pivotal study and n = 196 for those treated with elexacaftor (ELX)/TEZ/IVA. For participants with the F/F genotype, n = 52 for those treated with TEZ/IVA and n = 55 for those treated with ELX/TEZ/IVA in the 4-week F/F pivotal study. OLE = open-label extension; PBO = placebo.
Discussion
Safety results from this interim analysis were consistent with the initial 24-week placebo-controlled F/MF pivotal study, with similar or lower exposure-adjusted event rates observed in the OLE (Table 1) (5). ELX/TEZ/IVA was generally safe and well tolerated. Most AEs were consistent with common manifestations of CF and were not treatment limiting (3, 10). In participants who received ELX/TEZ/IVA in parent studies, improvements in efficacy and PD measures, including ppFEV1, SwCl concentration, BMI, CFQ-R RD score, and PEx event rate, were maintained or continued to improve further over 24 weeks (F/MF genotypes) or 36 weeks (F/F genotype) of additional treatment. These results validate the durability of ELX/TEZ/IVA efficacy responses, with no emerging safety concerns. Among participants who had received placebo or TEZ/IVA in the respective parent studies, initiation of ELX/TEZ/IVA rapidly led to marked improvements in these efficacy measures that were consistent with the results seen in the ELX/TEZ/IVA arms of those parent studies. Thus, the results of this combined-group interim analysis demonstrate the safety and sustained efficacy of long-term ELX/TEZ/IVA treatment in pwCF 12 years old or older with one or more F508del alleles.
Supplementary Material
Acknowledgments
Acknowledgment
Editorial coordination and support were provided by Morgan Deng, Pharm.D., who is an employee of Vertex Pharmaceuticals, Inc., and may own stock or stock options in that company. Medical writing and editorial support were provided by Samantha Keller, Ph.D., and Karen Kaluza Smith, Ph.D., C.M.P.P., of ArticulateScience LLC, and were funded by Vertex Pharmaceuticals, Inc.
Footnotes
Supported by Vertex Pharmaceuticals Inc., which participated in the design, statistical analysis, and interpretation of the data and provided editorial and writing assistance.
Author Contributions: The study sponsor (Vertex Pharmaceuticals, Inc.) designed the protocol in collaboration with the academic authors. Site investigators collected the data, which were analyzed by the sponsor. All authors contributed to data interpretation, conception, drafting, and/or revisions to the manuscript, and all approved the final version submitted for publication. All authors had full access to the study data, and M.G. had the final responsibility for the decision to submit for publication.
Data sharing statement: Vertex is committed to advancing medical science and improving the health of people with cystic fibrosis. This includes the responsible sharing of clinical trial data with qualified researchers. Proposals for the use of these data will be reviewed by a scientific board. Approvals are at the discretion of Vertex and will be dependent on the nature of the request, the merit of the research proposed, and the intended use of the data. Please contact CTDS@vrtx.com if you would like to submit a proposal or need more information.
Originally Published in Press as DOI: 10.1164/rccm.202008-3176LE on September 24, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med. 2020;201:1193–1208. doi: 10.1164/rccm.201910-1943SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8:65–124. doi: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Trust UK Cystic Fibrosis Registry 2018 Annual Data Report London, United Kingdom: Cystic Fibrosis Trust; 2019 [accessed 2020 Dec 10]. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reporting-and-resources [Google Scholar]
- 5.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griese M, Costa S, Linnemann RW, Mall MA, McKone EF, Polineni D, et al. A phase 3, open-label extension study of elexacaftor/tezacaftor/ivacaftor: interim analysis of safety and efficacy in people with cystic fibrosis and F508del/minimal function (F/MF) or F508del/F508del (F/F) genotypes. Presentation at the 43rd Annual European Cystic Fibrosis Conference Virtual; September 24–25, 2020. [Google Scholar]
- 9.Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res. 2019;5:00082-2019. doi: 10.1183/23120541.00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elborn JS. Cystic fibrosis. Lancet. 2016;388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


