Abstract
The roles of nitrate and nitrite ions as nitric oxide (NO) sources in mammals, complementing NOS enzymes, have recently been the focus of much research. We previously reported that rat skeletal muscle serves as a nitrate reservoir, with the amount of stored nitrate being highly dependent on dietary nitrate availability, as well as its synthesis by NOS1 enzymes and its subsequent utilization. We showed that at conditions of increased NO need, this nitrate reservoir is used in situ to generate nitrite and NO, at least in part via the nitrate reductase activity of xanthine oxidoreductase (XOR). We now further investigate the dynamics of nitrate/nitrite fluxes in rat skeletal muscle after first increasing nitrate levels in drinking water and then returning to the original intake level. Nitrate/nitrite levels were analyzed in liver, blood and several skeletal muscle samples, and expression of proteins involved in nitrate metabolism and transport were also measured. Increased nitrate supply elevated nitrate and nitrite levels in all measured tissues. Surprisingly, after high nitrate diet termination, levels of both ions in liver and all muscle samples first declined to lower levels than the original baseline. During the course of the overall experiment there was a gradual increase of XOR expression in muscle tissue, which likely led to enhanced nitrate to nitrite reduction. We also noted differences in basal levels of nitrate in the different types of muscles. These findings suggest complex control of muscle nitrate levels, perhaps with multiple processes to preserve its intracellular levels.
Keywords: nitrate, nitrite, nitric oxide, skeletal muscle, nitrate reductase activity, xanthine oxidoreductase, soleus, EDL
Introduction.
Nitric oxide (NO) is considered to be among the most important signaling molecules in the mammalian body. NO is a small diatomic molecule but with a large field of effects, including its roles in the vasculature as a potent vasodilator and inhibitor of platelet aggregation, in immune system regulation, in neuronal tissues as a volume neurotransmitter and in regulation of mitochondrial respiration, to name just few [1; 2; 3; 4; 5]. After NO was identified in 1987 to be the mysterious and elusive endothelium-derived relaxing factor (EDRF), NO synthetizing enzymes were discovered and named as nitric oxide synthases (NOS). Originally, the NOS pathway, where NO and citrulline are synthesized from L-arginine in the presence of oxygen, was considered the only physiological pathway of mammalian NO synthesis [6].
The contradiction between the greatest need of NO vasodilatory effects at low oxygen levels and the requirement of oxygen for its synthesis by NOS, combined with the short NO lifetime in vasculature remained unexplained for several decades [7; 8]. One of the obvious explanations was that there are other sources of NO than arginine and other enzymes than NOS involved in NO physiology. Finally, it was realized that heme proteins, such as hemoglobin or myoglobin, serve as important regulators of availability of NO by either oxidizing the excess of NO to nitrate when oxygen is bound to the heme (and therefore, there is less need to increase oxygen availability by increasing vasodilation), or by reducing nitrite, an inauspicious ion present in the bloodstream, directly to NO when the heme is free of oxygen [9; 10; 11; 12; 13]. Later on, other members of nitrite-to-NO reduction pathways (proteins containing heme or molybdopterin compounds) were identified in various mammalian tissues [14; 15; 16].
Previously, using rodents, we showed that skeletal muscle is the largest reservoir of nitrate, which also can be reduced to nitrite and then NO and that the amount of stored nitrate in muscle depends strongly on diet [17; 18]. Further, we found that at least in rodents, there is an unexpected tendency of skeletal muscle to “hoard” nitrate if its supply was previously unusually low [18]. We also showed that skeletal muscle expresses at least two different nitrate transporters [19], and that muscle tissue is able to use the stored nitrate in situ, with increased demand for local NO production such as increasing local blood flow, as in the case of exercise [20; 21]. Rat muscle tissue also expresses xanthine oxidoreductase (XOR), a protein known to be able to reduce nitrate to nitrite and then to NO. This local NO expression is likely to be important for functional hyperemia in working muscle. Based on these results that established a prominent nitrate concentration gradient from muscle to other organs, we hypothesize that rat skeletal muscle also supplies nitrate “on demand” to other tissues.
In the present work, our goal was to follow the dynamics of outflux of nitrate ions from the skeletal muscle into blood and other organs in rats. Using a dietary approach, we provided high amounts of nitrate to rats for 5 days and then we abruptly lowered nitrate levels to the pre-experimental baseline values. We measured nitrate and nitrite levels in skeletal muscle, blood and liver at several time points after high nitrate withdrawal. The liver was chosen as a comparison organ because liver tissue shows the highest known nitrate and nitrite reductase activity [22; 23]. We also followed expression of some of the proteins known to be involved in nitrate metabolism and transport in muscle tissue – XOR, nitric oxide synthase 1 (NOS1), myoglobin and sialin [24]. As expected, when high amounts of nitrate were available in diet, nitrate levels in muscle, blood and liver increased well above the baseline and when amount of dietary nitrate decreased, its levels in skeletal muscle rapidly declined, perhaps to compensate its loss in other organs. To our surprise, nitrate levels in muscle continued to decline over time, to values below observed for the pre-experimental baseline, before slowly returning to the original baseline. At the same time, XOR expression gradually increased in rat muscle tissue, which likely caused the increased nitrate reductase activity of this tissue; we also found a delayed increase of expression of sialin (known nitrate transporter) and NOS1. Since there are indications that NO might be supplied differently in type I and II fibers, based on their different oxygenation (type II being more hypoxic than type I) [25; 26], we collected muscles with different proportions of type I and type II fibers. Our results showed that different muscle groups do, indeed, store different amounts of nitrate, but there was no simple clear correlation between muscle type and level of nitrate, probably due to the fact that most of muscles are mixed type I/II and even in pure type II there are 3 different subtypes that, in theory could have different properties vis a vis nitrate/NO metabolism. Further experiments are needed to elucidate the possible differences of muscle fiber type and their nitrate metabolism on a molecular level.
These findings together suggest complex dynamics of the muscle nitrate reservoir and significant changes and adaptations in nitrate ion trafficking in and out of the muscle cells, based on local and/or global demand for NO. Elucidating the dynamics of this nitrate transport might allow us to better understand the basic physiological processes in these muscles, first, in animals and, ultimately, in humans. At the end, it could also help with design of supporting dietary recommendations for metabolic diseases associated with NO deficiencies and/or for diets designed to improve sarcopenia or athletic performances if some of the findings reported here for rats are confirmed in humans.
Materials and Methods.
Dietary intervention.
All experiments were done under NIDDK animal protocol K049-MMB-17 and in agreement with NIH animal care policies. Animals were housed in a 12h light/dark cycle environment with access to food and drinking tap water ad libitum. Young Wistar rats (n=32, 250±50g, 16 males, 16 females) were fed NIH standard rat diet (NIH07, nitrate 340.3±13.5 nmol/g, nitrite 7.4±0.1nmol/g) for one week. Then high nitrate diet consisting of water containing 1g/l (11.8mM) of sodium nitrate (Sigma, St. Louis, MO) was introduced for 5 days to 28 rats. There was no change of diet for remaining 4 control rats. After 5 days of high nitrate water (day 0, D0), all 32 rats continued to be fed standard diet and tap water (containing 100μM nitrate) up to 21 days. Groups of 4 rats (2 males, 2 females) were killed at time points D0 (immediately after completing 5 days of high nitrate water), 12 hours (D0.5), 1, 4, 7, 14 and 21 days after D0, together with the 4 control rats. Experimental design is shown in Scheme 1.
Scheme 1.
Flow chart of experiment. At baseline (control conditions) rats were consuming tap water and standard chow for one week. High nitrate water (1g/l) was then introduced and rats continued to receive the standard chow for 5 days. On the fifth day, water was switched back to tap water. Arrows show time points at which groups of 4 rats were killed.
Sample collection.
Rats were enclosed in an anesthesia box and anesthetized using 5% isoflurane mixture with air. Anesthetized animals were placed on the pad in supine position and anesthesia continued through a nose cone. The thoracic cavity was opened and ~9–10 ml of blood was collected by cardiac puncture; representing about two-thirds of total blood volume for a rat of this size. Heparinized syringes were used for blood collection. Immediately after withdrawal, blood was mixed with a solution containing potassium ferricyanide, NEM and detergent in final ratio 2:1 as previously described [27] to conserve nitrite from oxidation by hemoglobin. Animals were then perfused using heparin-containing saline to flush the remaining blood out of the tissues. Perfusion continued until no blood was detected in outgoing saline and liver and kidneys were significantly discolored. Samples from liver and five skeletal muscles from both hind legs - gluteus, soleus, extensor digitorum longus (EDL), gastrocnemius, and tibialis anterior (TA) - were then collected, placed into Eppendorf tubes and immediately frozen on dry ice. All samples were stored at −80°C until analysis. According to published analyses of rat muscle fiber type [28], fiber type and relative composition in these collected muscles is the following: soleus: 80% type I, 20% type IIa, extensor digitorum longus (EDL): 11% type IIa, 25% type IIx, 64% type IIb, gluteus: 5% type IIa, 19% type IIx, 76% type IIb., tibialis anterior (TA): 0.4% type I, 10% type IIa, 20% type IIx, 70% type IIb and gastrocnemius: 6% type I, 10% type IIa, 19% type IIx, 64% type IIb.
Determination of nitrite and nitrate.
Nitrite and nitrate levels in whole blood were measured using a standard chemiluminescence method [29]. Samples were deproteinized by dilution with cold methanol 1:1 (sample:methanol, vol/vol), then centrifuged for 5 min at 13,000 rpm and 4°C (AccuSpinMicroR, Fisher Scientific, Pittsburgh, PA), the supernatant was collected and injected into the nitric oxide analyzer (NOA, Sievers, Model 280 NO analyzer, Boulder, CO) using helium as the carrier gas for determination of NO. For nitrate analysis, samples were processed the same way as for nitrite determination and analyzed with NOA using the vanadium(III) chloride (VCl3) chemiluminescence assay.
Tissue samples were weighed, mixed with 1% solution of NP-40 in water and homogenized using GentleMacs (Miltenyi Biotec Inc, Auburn, CA). Proteins were precipitated using methanol (dilution 1:3 sample:methanol, vol/vol) and samples were centrifuged at 3,000 g for 45 min to separate most of the protein. Supernatants were collected and used to determine nitrite and nitrate content by chemiluminescence as described above for blood samples.
Nitrate reductase assay.
Nitrate reduction activity assay was performed as previously described [23]. Briefly, EDL muscle tissue was homogenized using GentleMacs and protein content was measured by the bicinchoninic acid (BCA) assay (Thermo Scientific, #23227) and adjusted to 3mg/ml. The cofactor mixture with or without oxypurinol (200μM) was added to homogenate solution and then 500μM nitrate was added to the reaction tube. The aliquots were taken at 0 and 2h and then analyzed by chemiluminescence for nitrite content. Experiments were performed at 37°C under 2% oxygen in 100mM phosphate buffer, pH6.5.
Western blots.
Western blotting was performed to analyze the levels of XOR, NOS1, sialin, myoglobin and GAPDH protein in soleus and EDL skeletal muscle samples. Muscle tissues were homogenized in RIPA buffer (Sigma, Cat. # R0278) containing protease inhibitors (CalBiochem, #539134) and protein concentration was measured by the (BCA) assay. Denatured samples (20 μg) were run on SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies (Anti-myoglobin: Santa Cruz Biotechnology, sc-25607; Anti-sialin: Alpha Diagnostics, SIAL11-A; Anti-XOR: Abcam, ab133268; Anti-NOS1: BD Transduction Laboratories, 610309; Anti-GAPDH: Cell Signaling, 97166) overnight at 4°C. Goat-anti-mouse or goat-anti-rabbit antibodies conjugated with horseradish peroxidase (Jackson Immunoresearch) were used as secondary antibodies and followed by ECL detection (Thermo Scientific, #34095). Band density was quantified using NIH Image J software.
Results.
Dynamics of nitrate levels in skeletal muscles, blood and liver.
Figure 1A shows the time course of nitrate levels in liver, blood and gluteus muscle of rats after 5 days of high nitrate ingestion (D0) and six time points at 12h, 1, 4, 7, 14 and 21 days after returning to the normal diet (D0.5, D1, D4, D7, D14 and D21, respectively) and control animals on their normal nitrate ingestion levels.
Figure 1.
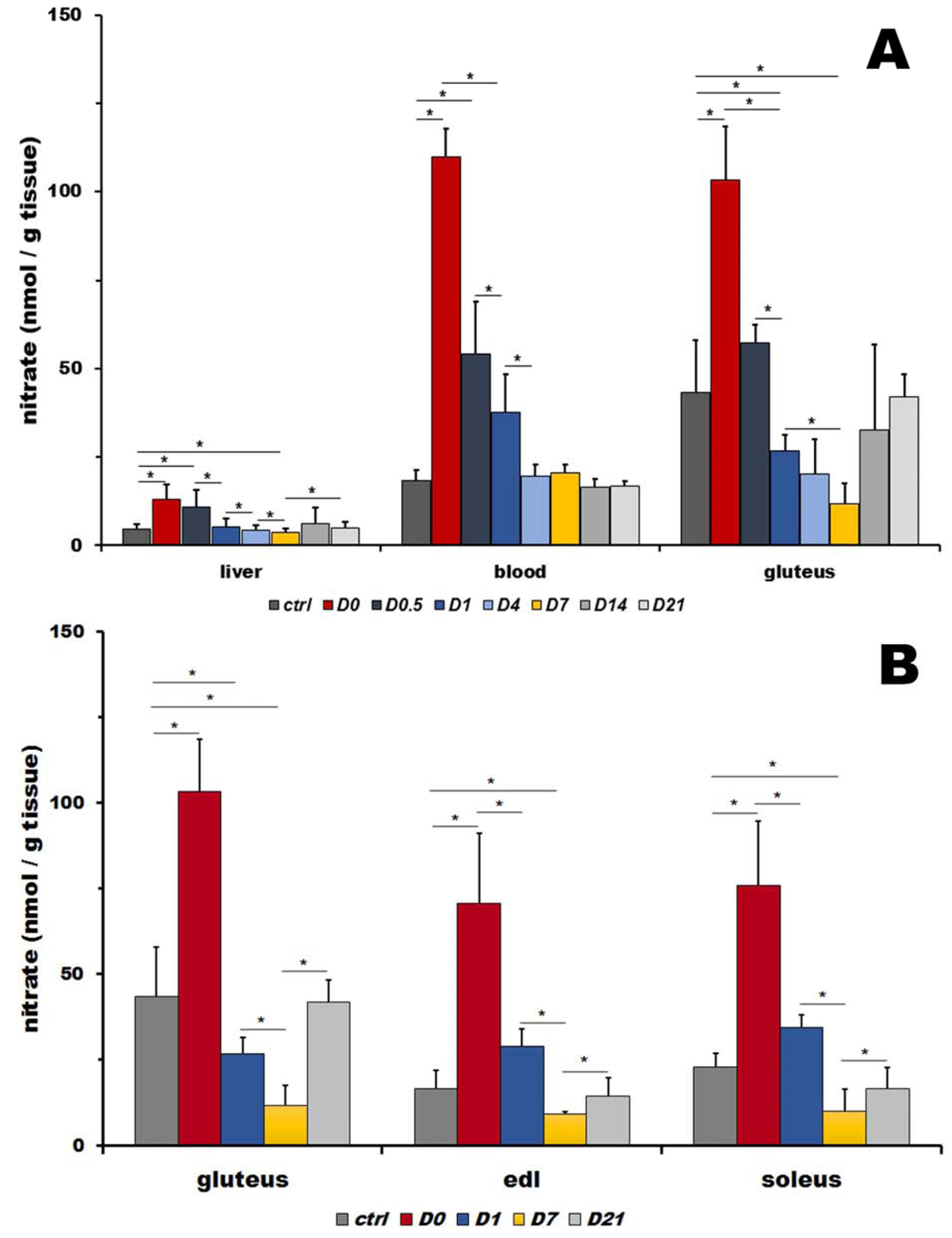
A: Nitrate levels in rat liver, blood and skeletal muscle (gluteus) at basal levels (ctrl), five days after high nitrate supplementation (D0) and after cessation of nitrate supplementation at 12 hours and 1, 4, 7, 14 and 21 days (D0.5, D1, D4, D7, D14 and D21). Bars represents average of 4 rats, data are plot as average ± SD, * denotes p<0.05.
B: Comparison of nitrate levels in rat gluteus (type II fibers type), extensor digitorum longus (EDL, type II fibers) and soleus (predominantly type I fibers) muscles at basal levels (ctrl), five days after high nitrate supplementation (D0) and after cessation of nitrate supplementation at 1, 7 and 21 days (D1, D7, and D21). Bars represent average of 4 rats, data are plot as average ± SD, * denotes p<0.05.
In control rats, nitrate was distributed in nonhomogeneous manner among liver, blood and gluteus, with liver containing the least amount (4.7±1.3 nmol/g tissue) and gluteus the highest amount (43.4±14.6 nmol/g tissue or 9.2-fold increase over its amount in liver tissue), with blood levels of nitrate at mid-levels (18.5±2.9 μM, equivalent to 18.5±2.9 nmol/g tissue or 3.9-fold increased over its amount in liver).
Five days of increased nitrate supply led to significant, 2.8-, 5.9- and 2.4-fold increases of nitrate in liver, blood and gluteus, respectively, when compared to their baseline values.
When the dietary nitrate levels returned to control amounts, levels of both nitrate and nitrite started to decrease, each organ with its own dynamics. Liver nitrate levels decreased back to the control baseline values after one day (D1, 1.1-fold of controls) and blood nitrate after 4 days (D4, 1.1-fold of controls) after cessation of high nitrate supplementation. Interestingly and surprisingly, after a rapid decrease to baseline levels at 12 hours after high nitrate withdrawal (D0.5, 1.3-fold of controls), nitrate in gluteus continued to decrease further with a minimum reached at 7 days, when its level was significantly lower than that observed at baseline (D7, 0.3-fold of controls). It was only at day 14 that nitrate levels returned to the control baseline values again (D14, 0.8-fold of controls) and were stable by day 21.
Figure 1B compares nitrate levels in three different muscles – gluteus (type II, fast twitch fibers), extensor digitorum longus (EDL, type II, fast twitch fibers) and soleus (predominantly type I slow twitch fibers) for controls, day 0 (D0, after 5 days of high nitrate water) and after returning to tap water at day 1, 4, 7, and 21 (D1, D4, D7 and D21, respectively).
Interestingly, baseline levels of nitrate differ significantly between these three types of muscles, with gluteus containing significantly higher amounts of nitrate compared to EDL and soleus (1.9- and 2.6-fold, respectively), thus not strongly correlating with fiber types.
As expected, 5 days of nitrate supplementation significantly increased levels of nitrate in all measured muscles - gluteus (2.4-fold from 43.4±14.6 nmol/g to 103.2±15.3nmol/g tissue), EDL (4.3-fold from 16.6±5.4 to 70.7±20.4 nmol/g tissue) and soleus (3.3-fold from 22.8±4.0 to 75.9±48.8 nmol/g tissue).
After nitrate supplementation ceased, nitrate decreased in all three muscles following the pattern observed in gluteus muscle (described above), with the minimum observed at day 7 (D7) (reaching 0.6- and 0.4-fold of control value for EDL and soleus, respectively). Interestingly, the absolute values of nitrate levels at their minimum at D7 are very similar for all three muscles – 11.7±5.9, 9.2±0.5 and 10.0±6.4 nmol/g tissue for gluteus, EDL and soleus, respectively).
Return to baseline values is observed at day 21 with nitrate levels of 41.9±6.5, 14.4±5.2 and 16.5±6.1 nmol/g tissue for gluteus, EDL and soleus, respectively.
To determine if fiber type could be one of the deciding factors for skeletal muscle nitrate storage capacity, we also measured nitrate levels in two additional skeletal muscles, gastrocnemius and tibialis anterior (TA), at the baseline. Nitrate level in TA was 12.1±3.5 nmol /g tissue and gastrocnemius contained 18.8±9.2 nmol /g tissue of nitrate.
Dynamic of nitrite levels in skeletal muscles, blood and liver.
Figure 2A shows the time course of nitrite levels in liver, blood and gluteus muscle of animals at baseline, after 5 days of high nitrate diet (D0) and six time points, 12h, 1, 4, 7, 14 and 21 days, after returning to the normal diet (D0.5, D1, D4, D7, D14 and D21, respectively). Distribution of nitrite at control conditions was mostly uniform, with 0.20±0.07, 0.20±0.05 and 0.28±0.05 nmol /g tissue of nitrite measured in liver, blood and gluteus, respectively.
Figure 2.
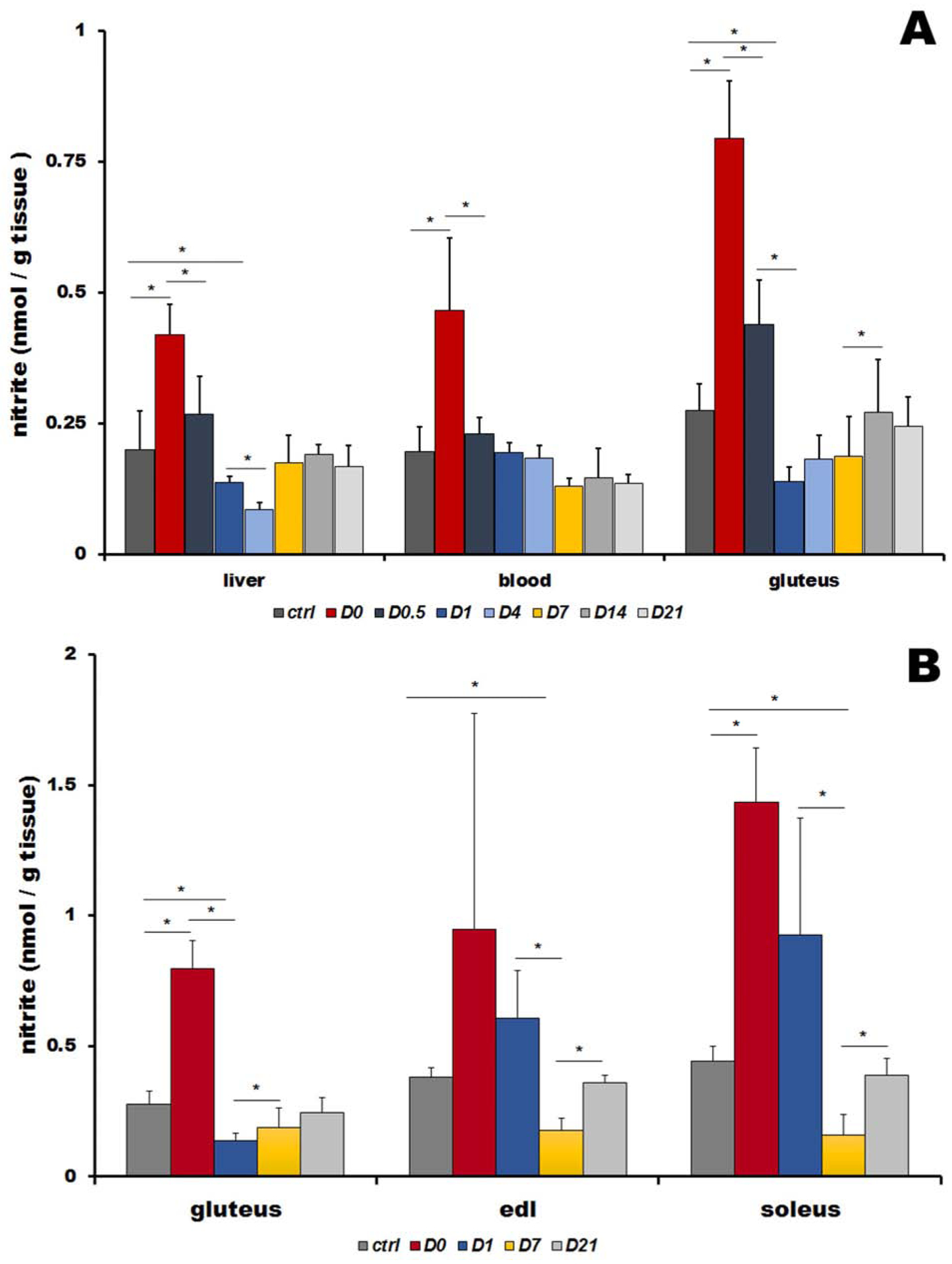
A: Nitrite levels in rat liver, blood and skeletal muscle (gluteus) at basal levels (ctrl), five days after high nitrate supplementation (D0) and after cessation of nitrate supplementation at 12 hours and 1, 4, 7, 14 and 21 days (D0.5, D1, D4, D7, D14 and D21). Bars represents average of 4 rats, data are plot as average ± SD, * denotes p<0.05.
B: Comparison of nitrite levels in rat gluteus (type II fibers), extensor digitorum longus (EDL, type II fibers) and soleus (predominantly type I fibers) muscles at basal levels (ctrl), five days after high nitrate supplementation (D0) and after cessation of nitrate supplementation at 1, 7 and 21 days (D1, D7, and D21). Bars represent average of 4 rats, data are plot as average ± SD, * denotes p<0.05.
Similar to nitrate, significantly lower nitrite levels than in controls were observed after cessation of nitrate supplementation, in gluteus at day 1 (D1, 0.5-fold of controls), days 4 and 7 (D4, D7, 0.7-fold of controls) and in liver at day 4 (D4, 0.4-fold of controls). After reaching its minimum at day 7, gluteus nitrite level returned to baseline at day 14, timing similar to nitrate. Liver nitrite increased back to its baseline values faster, within only 7 days. Nitrite in blood stabilized back to baseline values even faster, after 12 hours, but after day 4 its levels stabilized at a new, slightly lower baseline level (D7-D21, 0.7-fold of ctrl).
Figure 2B compares nitrite levels in three different muscles – gluteus (type II fiber muscle), extensor digitorum longus (EDL, type II fiber muscle) and soleus (type I fiber muscle) for controls, day 0 (D0, after 5 days of high nitrate water) and after returning to tap water at day 1, 4, 7, and 21 (D1, D4, D7 and D21, respectively).
Baseline levels of nitrite significantly differ in these three types of muscles, with gluteus containing the lowest amounts of nitrite (0.28±0.05 nmol/g tissue) compared to EDL (0.38±0.04 nmol/g tissue) and soleus (0.44±0.06 nmol/g tissue), the opposite of the finding with nitrate levels.
Five days of nitrate supplementation (at time-point D0) significantly increased levels of nitrite in all three muscle – gluteus (2.9-fold from 0.28±0.05 to 0.80±0.11 nmol/g tissue), EDL (2.5-fold from 0.38±0.04 to 0.95±0.83 nmol/g tissue) and soleus (3.3-fold from 0.44±0.06 to 1.43±0.21 nmol/g tissue).
After termination of high nitrate supplementation, nitrite decreased with a similar pattern in all three muscles, with minimum values (again, significantly lower than previous baseline value) reached at day 7 (D7) - 0.7– 0.6- and 0.4-fold of ctrl values for gluteus, EDL and soleus, respectively. It is intriguing that all three nitrite level values at D7 are practically identical - 0.19±0.08, 0.18±0.05 and 0.16±0.08 nmol/g tissue for gluteus, EDL and soleus, respectively. Return to baseline values is evident at day 21 with nitrite values of 0.25±0.06, 0.36±0.03 and 0.39±0.06 nmol/g tissue for gluteus, EDL and soleus, respectively.
To determine if fiber type influenced skeletal muscle nitrite content, we measured nitrite levels in two additional skeletal muscles, gastrocnemius and tibialis anterior (TA), at the baseline. TA contained 0.31±0.04 nmol /g tissue and gastrocnemius 0.26±0.04 nmol /g tissue of nitrite.
Expression of proteins involved in nitrate metabolism in EDL and soleus muscles.
Figure 3 shows expression levels of proteins involved in the nitrate-nitrite-NO cycle – XOR, NOS1, sialin and myoglobin in EDL and soleus muscles. Panels A and C presents original Western blots in EDL and soleus, respectively, and panels B and D show densitometric evaluation of protein expression levels for EDL and soleus, respectively.
Figure 3.
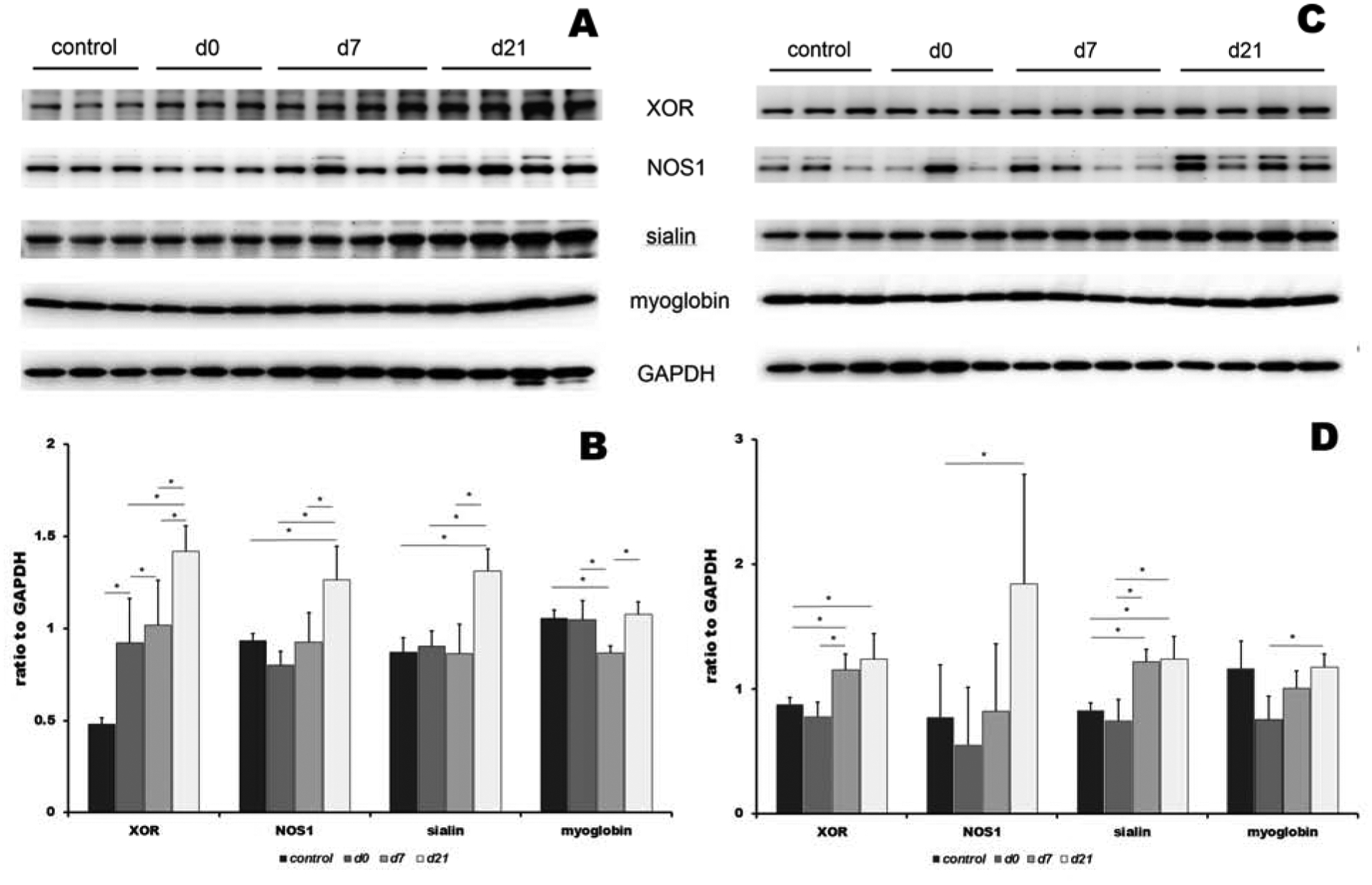
Expression of proteins involved in nitrate cycle at basal level (control), 5 days after high nitrate supplementation (D0) and 7 and 21 days after cessation of nitrate supplementation (D7 and D21) in EDL (panel A and B) and soleus (panel C and D). Panels A and C show original Western blots and panels B and D show densitometric evaluation of protein expression changes. XOR: xanthine oxidoreductase, NOS1: nitric oxide synthase 1 (nNOS), GAPDH: glyceraldehyde 3-phosphate dehydrogenase. Data are plot as average ± SD,* denotes p<0.05
In EDL (panel A and B), XOR levels gradually increased through the whole experimental treatment, from baseline up to day 21 (1.9-, 2.1- and 3-fold increase at D0, D7 and D21, respectively, relative to controls). Levels of NOS1 and sialin increased significantly only at day 21 (1.4- and 1.5-fold for NOS1 and sialin, respectively), and expression of myoglobin remained stable, with the exception of a small dip at day 7 (D7, 0.8-fold relative to ctrl).
In soleus (panel C and D), XOR levels increased at day 7 and 21, albeit not as pronouncedly as in EDL (1.3-, and 1.4-fold increase at D7 and D21 when compared with controls). Sialin levels were also slightly elevated at day 7 and 21 when compared to controls (1.5- and 1.5-fold for D7 and D21, respectively). NOS1 expression slightly decreased at day 0 (0.7-fold), and then rose 2.4-fold by day 21, compared with controls. Expression of myoglobin remained stable, with a small dip at day 0 (D0, 0.6-fold relative to controls).
Nitrate and nitrite levels and nitrate reductase activity in EDL muscle.
Figure 4 shows nitrate reductase activity of EDL at baseline, after 5 days of nitrate supplementation (D0) and at D1, D7 and D21 after withdrawal of high nitrate (grey bars) and at the same time points with the addition of xanthine oxidoreductase (XOR) inhibitor, oxypurinol (green bars).
Figure 4.
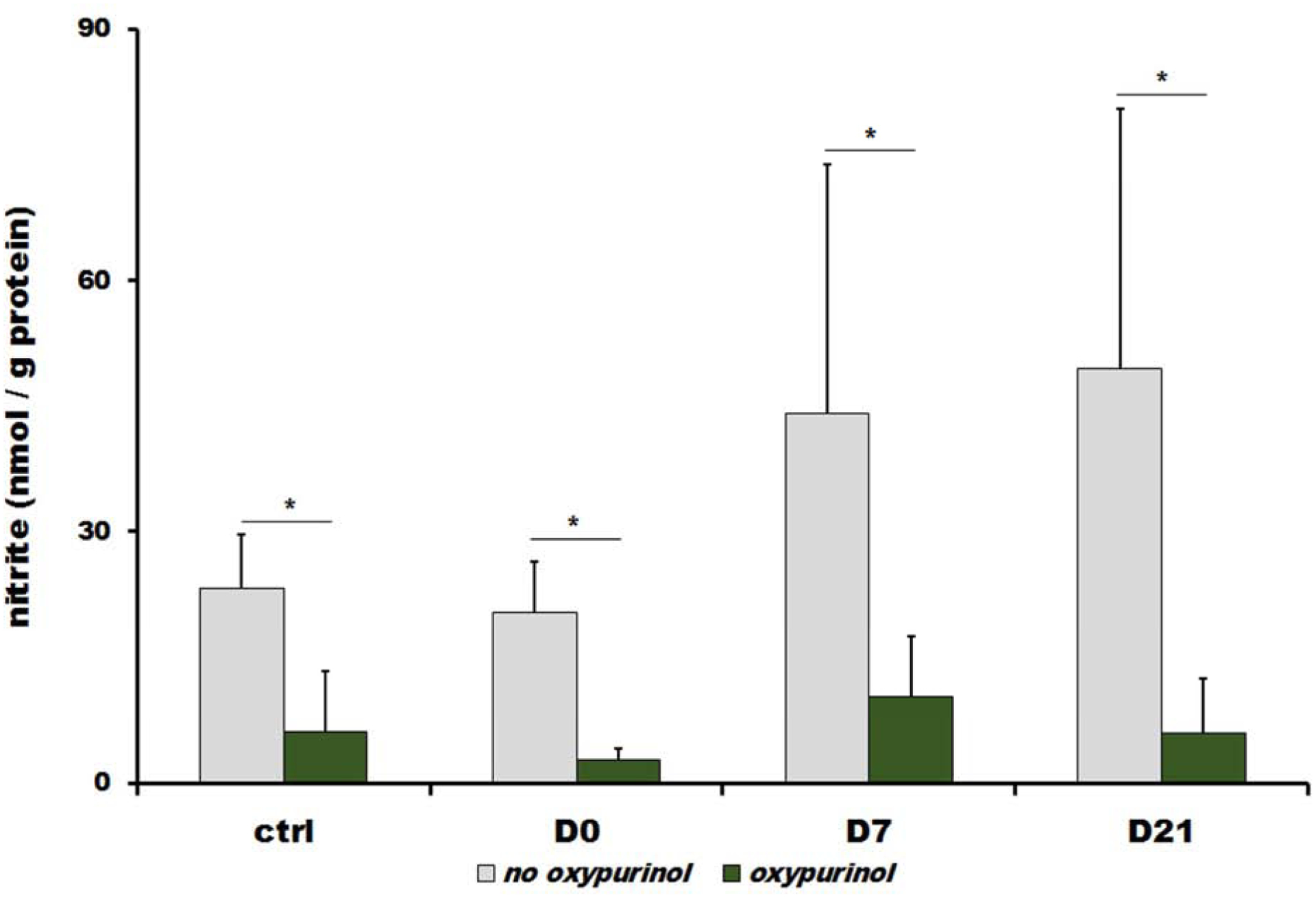
Nitrate reductase activity of extensor digitorum longus (EDL) tissue homogenate in control conditions (ctrl), 5 days after nitrate supplementation (D0) and 7 and 21 days after high nitrate withdrawal (D7 and D21, respectively). Bars represent the amount of nitrite in nmol/g protein reduced by EDL tissue from 500 μM of exogenous nitrate during 2 hours after its addition. Oxypurinol, xanthine oxidoreductase inhibitor, was added at the same time as exogenous nitrate to the final concentration of 200μM. Gray bars represent values measured in sample without oxypurionol and green bars are values measured in the samples containing oxypurinol. EDL homogenates were kept under atmosphere of 2% oxygen, 37°C and at pH 6.5. Bars represent average of 4 rats, data are plot as average ± SD, * denotes p<0.05.
After addition of 500μM of nitrate to EDL muscle homogenate, XOR-related nitrate reductase activity was followed by measuring the amount of newly appeared nitrite that originated from reduction of exogenous nitrate after 2 hours of incubation period at pH 6.5 and 2% oxygen. Results (gray bars) show that significant generation of nitrite from reduction of exogenous nitrate occurred in EDL homogenates at every time point studied with nitrite levels increases of 23.3±6.4, 20.3±6.2, 44.1±29.8 and 49.5±31.0 nmol/g protein after 2 hours for controls, D0, D7 and D21, respectively. Introduction of high dietary nitrate didn’t lead to changes of XOR-related nitrate reduction, but subsequent decrease of dietary nitrate levels triggers increased nitrate reduction at days 7 and 21 (1.9- and 2.1-folds for D7 and D21, respectively, when compared with controls).
Nitrate reduction is greatly inhibited by addition of XOR inhibitor, oxypurinol with levels of nitrite at 6.1±7.2, 2.7±1.4, 10.3±7.2 and 5.9±6.6 nmol/g protein, generated after 2h for controls, D0, D7 and D21, respectively (green bars).
Discussion.
Nitrate and nitrite ions are now understood to be significant storage and precursor forms of NO in mammals. It had been shown recently in animals [30] and, more importantly, in humans [31; 32], that lack of the steady dietary supply of these ions has many detrimental effects for health, especially for cardiovascular health. In the past, several studies showed that, when ingested, about a quarter of dietary nitrate from the bloodstream is transported by salivary glands into the saliva where it is reduced to nitrite by oral bacteria [33]. Most of the remaining dietary nitrate is excreted from the bloodstream, mostly by kidney [34], but small portions of dietary intake appear to be retained by various organs, such as muscle [17]. In addition to dietary nitrate supply, nitrate is also continuously generated in the body by oxidation of NO, and probably nitrite ions, by oxyhemoglobin and by other oxyheme proteins [35].
Until recently, commensal oral bacteria were considered to be the main nitrite suppliers to mammals and their presence and action is, with no doubt of high importance. Just to point few, studies on normal and germ-free mice [36] showed that absence of bacteria combined with NOS inhibition had detrimental effects on the cardiometabolic health. In several human trials, eliminating oral bacteria by using mouthwash suppressed dietary nitrate effect on lowering blood pressure [37; 38; 39; 40]. Mammals themselves were considered only being able to reduce nitrite to NO, using either XOR or deoxy-heme proteins as nitrite reductases; nitrite reduction was assumed to be mainly taking place either in liver (by XOR [22]) or blood (by deoxyHb, for review see [12; 41]). Small amounts of NO oxidation to nitrite in the blood were assumed to be due to a reaction with molecular oxygen, perhaps catalyzed by ceruloplasmin [42]. However, when it was demonstrated that mammalian xanthine oxidoreductase (XOR) is also able to reduce nitrate into nitrite, and that this reaction is enhanced with decreased oxygen and increased acidification (similar to nitrite reduction), it became clear that mammals themselves possess the complete set of enzymes for nitrate-to-nitrite-to-NO reduction [23]. Therefore, high reliance of humans on their oral microbiome to regulate the amount of NO for vascular events, does not exclude the possibility of native mammalian enzymes being equally important in some compartments (organs) or at some physiological conditions. We believe that these three parts of NO formation cycle (oral microbiome, XOR, NOS) have their specific sphere of influence or compartment where their proper functioning is crucial and that they support each other, but are not completely interchangeable. Follow-up research confirmed the idea of possible importance of nitrate and mammalian nitrate reductases for normal physiology [20]. Since then, evidence about the role of both nitrate and nitrite ions as a NO source in mammals is growing [41]. It is likely that both sources of nitrate, diet and NO/nitrite oxidation, are roughly equivalent in their importance and the quantities of nitrate they supply, but this is still uncertain and further research is needed. For these purposes, one can clearly and easily manipulate the amount of bio-available nitrate by dietary changes, exposing the subjects either to low- or high- nitrate containing diets. Most hypotheses about NO metabolic pathways usually consider nitrate as a resource that is constantly supplied by either diet or NO/nitrite oxidation and unused excess is excreted with only minimal nitrate retention in the body, mainly in the bloodstream [43]; for review see [44]. The question rarely considered was a possibility of some intermittent larger nitrate reservoir in the body that would supply necessary nitrate when its dietary supply falls or perhaps the NOS pathway becomes nonfunctional (such as in hypoxia).
Thus, for us the question had become: does the mammalian body store nitrate or nitrite and if it does, where? To find a tissue/organ which would function as a possible nitrate reservoir, we previously concentrated our efforts on a simple question of the distribution of nitrate and nitrite ions through the body. To our surprise, we and others found that there is a particular mammalian tissue that contains high nitrate levels and appears to serve as a temporary nitrate reservoir – skeletal muscle tissue [17; 45]. As the current study confirms again, our previous reports of the existence of nitrate concentration gradients from muscle to blood to liver (which is in this study 9.2- and 3.9- fold of liver values in gluteus muscle and blood, respectively) was confirmed for the baseline values before treatment by high nitrate diet.
Considerable amounts of published data in exercise physiology research during the past few years leads to the hypothesis that nitrate/nitrite/NO pathway differs for different type of muscle fiber types [25; 26; 46]. In all our previously published studies [17; 18; 20] we only collected gluteus (type II), as a representative muscle, which provided a comfortable amount of material for all of our experiments. In an attempt to clarify if there are indeed differences in nitrate/nitrite/NO pathway that are due to different muscle fiber types or other factors (such as muscle size and function), we also collected four smaller additional muscles from the rat hind leg – EDL (type II fibers), TA and gastrocnemius (both predominantly type II fibers) and soleus (predominantly type I fibers) in addition to gluteus (type II fibers). When comparing the baseline nitrate values in these five different muscles, there is a spread over an interval of values, ranging from 12.1±3.5nmol/g tissue for TA, up to gluteus with 43.4±14.6 nmol/g tissue, with EDL (14.4±5.2 nmol/g tissue), soleus (22.8±4 nmol/g tissue) and gastrocnemius (18.8±9.2nmol/g tissue) spreading in this interval. Levels of nitrate in gluteus and soleus are higher than in blood and liver and follow the previously described muscle-blood-liver gradient, when their nitrate values are compared to ones in blood and liver. EDL and gastrocnemius nitrate levels are similar to levels found in blood and still significantly higher than that in liver. TA nitrate levels are lower than those in blood, but higher than nitrate levels in liver. As follows from these observations, there is definitely diversity in the nitrate levels among different muscles, an observation that deserves further study to find a possible pattern. It is possible that only some muscles function as the real nitrate reservoir and supply the whole body, while other muscles might only store limited amounts of nitrate for their own local use and/or some might rely on other mechanisms, such as NOS-derived NO synthesis. The distinction among these possibilities is an important one and can be only made by carefully mapping different muscle groups and comparing the measured values, possibly with and without exercise.
In all of our previous animal studies we only collected gluteus (type II), as a representative muscle, which provided adequate amounts of material for all our experiments. However, a recent study [46] also reports significant differences in the levels of nitrate in soleus (mostly type I) and vastus lateralis (mostly type II) in rat, with nitrate and nitrite concentrations 3.4- and 1.8-fold higher in soleus than vastus lateralis. In our study, similar differences were less pronounced but were in the same direction, with 1.4-fold increase of nitrate and 1.2-fold increase of nitrite in soleus when compared to EDL (type II). Interestingly, both the Jones and Verdijk’s groups recently proposed that nitrate would be more beneficial for improving performances of type 2, fast twitch muscle, which includes EDL and vastus lateralis [25; 45; 47]. At this point, due to the lack of enough experimental data, it is probably too early to formulate any more detailed hypotheses about this phenomenon, but certainly, one might reasonably expect significant differences in how different muscle and, in general, different organs, handle storage and use of nitrate.
Nitrite levels at baseline conditions in liver, blood and reported muscle samples range from 0.20±0.07, to 0.44±0.06 nmol/g tissue for liver and soleus, respectively, and with similar ranges in all five muscle specimens (from 0.28±0.05 to 0.44±0.06nmol/g tissue for gluteus and soleus, respectively). Such relatively uniform distributions of nitrite levels through various organs is in agreement with our previous observations for sedentary rats, where we found that nitrite concentrations among tissues change only when nitrite is in physiological need, such as during the exercise-caused hypoxia and functional hyperemia in skeletal muscle [20; 21].
Five days of consumption of high nitrate water led to a significant increase of nitrate and nitrite levels in all collected rat organs and tissues, followed by rapid decrease within 12 hours after withdrawal of high nitrate supply. Such observations are naturally expected and are not surprising. It is also unsurprising that nitrate and nitrite decrease at different rates from different organs and tissues. Unsurprisingly, blood is the tissue with fastest decay of both values, reaching pre-supplementation nitrate baseline level at day 4 and nitrite levels already only 12 hours after returning to regular tap water. However, to our surprise, in liver and all skeletal muscles nitrate and nitrite decreased further after reaching their pre-supplementation values and only returned to original values after reaching a lower “dip” values below the previously observed baseline. This suggests that some other changes at the biochemical level take place in these tissues. Both, nitrate and nitrite levels in gluteus decrease below the baseline levels at day 1, declining to only 60% (nitrate) and 50%(nitrite) of values at baseline. Interestingly, nitrate decreases slower in both the EDL and soleus one day following the removal of high nitrate intake (D1); however, nitrate is still well above the original baseline level. Nitrate levels in all three skeletal muscles decline to their minima at 30%, 60% and 40% of their baseline value for gluteus, EDL and soleus, respectively, at day 7. Interestingly, the absolute minimal value of nitrate reached at this point, around 10.3 nmol/g tissue, are almost identical in all three muscles (within 25%). A similar observation was made for the respective minimum reached for nitrite at day 7, where the minimal value hovers around 0.17 nmol/g tissue (within 20%). From these observations one might assume the existence of a minimal basal amount of nitrate and nitrite that are retained in the muscle and are likely crucial for its proper functionality. At this point, we do not have a definitive hypothesis of a mechanism to explain either the differences in nitrate and nitrite decline rates observed in different muscle groups or the similarities in minimum values reached by different muscle tissues nor can we explain the differences in nitrate values for different muscles observed at the baseline.
The complex behavior of these nitrate and nitrite dynamics in rat muscle tissue levels naturally requires further study of the underlying biochemical changes in tissues, such as altered expression of proteins involved in nitrate-nitrite-NO cycle that are induced by and persist after dramatic dietary changes. In our previous work, we reported that introducing high nitrate diet after seven days of a very low nitrate diet leads to significant accumulation of nitrate in the gluteus muscle, well over the value observed without the period of nitrate starvation included [18]. The capability of muscle tissue to “super-store” this ion was clear evidence of the importance of nitrate for metabolism and gave a first insight into the complex dynamics which govern regulation of its levels. The current study shows the opposite: how the body handles (presumably) “excess” of stored nitrate when the dietary supply returns to the usual baseline levels. Again, we see the tissue “over-reacting”, in this case, likely trying to dispose of the excess of accumulated nitrate by altering either the amount or activities (or both) of proteins involved in nitrate metabolism. We also suggest that nitrate-handling proteins respond slightly differently in different types of muscle, depending on ability of muscle and, presumably, its local needs.
This hypothesis about a local needs-driven response of individual muscles is further supported by several pieces of information available to us at this current time. In the fast-twitch, predominantly the type II fiber muscle, such as EDL, we saw that the amount of newly synthetized nitrite by XOR-related nitrate reduction doubled at day 7 and day 21 when compared with the amount of nitrite found at baseline. When we measured levels of XOR expressed in EDL at baseline, day 0, 7 and 21, we saw a dramatic increase of the amounts of protein over time, which doubled by day 0 and tripled at day 21 when compared to pre-supplementation period, which is likely the cause of increased observed nitrate reduction. Clearly, when given access to nitrate, EDL muscle (and, possibly also other fast-twitch type II fiber muscles) can use it. Nitrate reduction was significantly inhibited in all cases by oxypurinol, an XOR inhibitor, with only 11 – 26% newly synthetized nitrite detected, comparing with the samples without oxypurinol. This confirms the importance of XOR for nitrate reduction, as well as pointing to the existence of some additional non XOR-related nitrate reduction or nitrite transport responsible for the remaining portion of detected nitrite after XOR inhibition. In slow-twitch, predominantly type I fiber muscle, such as soleus, the increase of XOR levels was rather modest, not exceeding 40% above control values at day 7 and 21. We do not have direct data showing nitrate reduction in soleus, but it is likely that a modest increase of XOR levels would be reflected by a similarly modest increase of nitrate reduction when compared with controls. However, the kinetics of XOR-governed nitrate and nitrite reductions is also complicated by the fact that these two reactions are with each other and, as reported by Tanus-Santos’ group, presence of nitrate decreased nitrite reduction [48]. Interestingly, expression of NOS1, which is responsible for NO and nitrate synthesis from L-arginine [49], was also affected differently in EDL and soleus. In EDL, we observed modest, about 40%, increases of NOS1 levels at day 21, while in soleus NOS1 levels more than doubled at the same time point. In our opinion, these almost exact mirror changes observed for XOR and NOS1 in EDL and soleus clearly illustrate different roles of nitrate in different types of muscles, with some handling their NO needs mainly through NOS-related pathways (slow-twitch, type I) and others being able to rely more on nitrate/nitrite reduction pathways (fast-twitch, type II). To our knowledge, this is the first report of such a comparison based on protein level changes.
Expression of sialin, a nitrate (and possibly nitrite) transporter, shows almost identical patterns in EDL and soleus, with 50% increased amounts of protein at day 21 for both muscles when compared to control levels. Similarly, expression of myoglobin, which has a dual role as NO/nitrite oxygenase (oxyMb) or nitrite reductase (deoxyMb) to produce NO, was mostly unaffected by nitrate changes in the diet.
In summary, we observed an unexpected dynamic of stored nitrate levels in rat skeletal muscle, not dissimilar to a “withdrawal syndrome”. These dynamics are summarized in Figure 5. We believe that 5 days of high dietary nitrate led to full “acclimatization” of the rodent body to a high nitrate supply and caused a gradual switch to the nitrate reduction pathway as a main NO supplying pathway in type II, fast-twitch muscles - with a progressive increase of XOR levels over the whole period of observation, as seen by Western blots. This was not as pronounced in type I, slow-twitch. However, because most muscles contain both types of fibers (type I and II), significant changes in expression of any protein in one type of fiber will necessarily have some effects on the other fiber type in the same muscle, as strongly suggested in mirror-like changes in expression of XOR and NOS1 proteins observed over time in EDL and soleus, respectively. When abrupt diet changes (lowering the nitrate content back to baseline) disturbed this adaptation, levels of XOR in type II muscle continued to rise because the amount of substrate (nitrate) declined and, albeit with a delay, levels of sialin (nitrate transporter) and NOS1 (which is able to synthetize nitrate directly during futile cycle) eventually increased. In contrast, in type I muscle, decreased nitrate supply led to an increasing reliance on the NOS-related pathway. At this point, it is not clear if NOS1 in slow-twitch muscle supplied “missing” nitrate or if the enzyme was used as a source of NO, and its myoglobin was used to oxidize NO to nitrate. More research needs to be done to further support or refute these several hypotheses, but we consider this unusual nitrate handling by the mammalian body, especially in muscle tissue, very intriguing.
Figure 5.
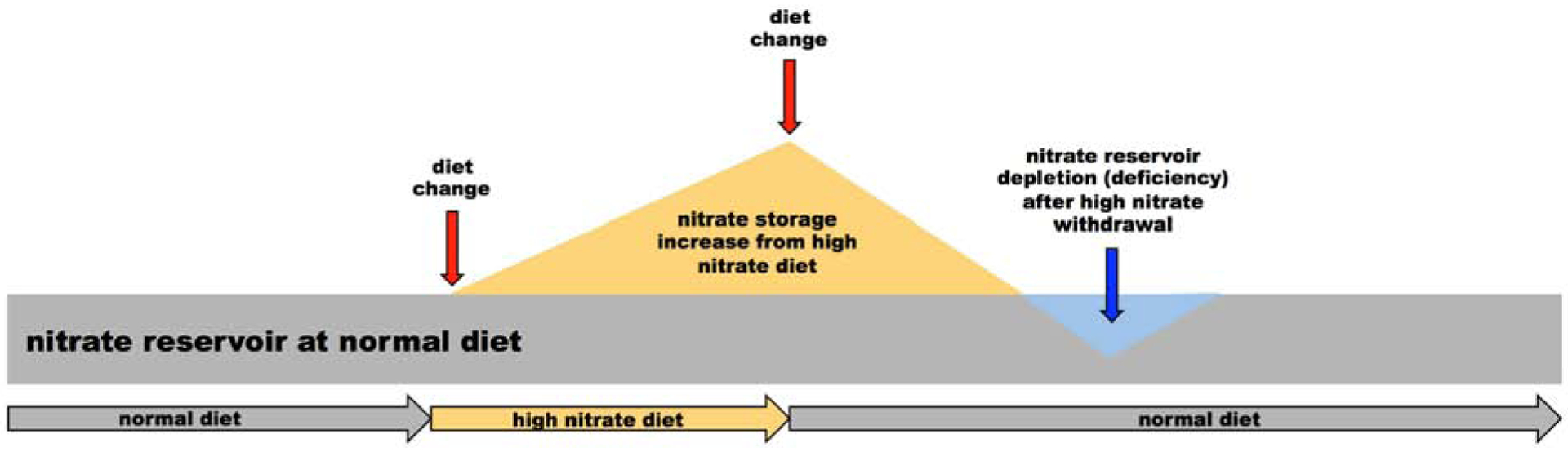
Schematic representation of the effect of switching diets with different nitrate content on the nitrate levels in skeletal muscle. When rats are switched from normal diet (gray area) to high nitrate diet (yellow area), increase nitrate content in muscle is observed. When high nitrate diet is abruptly switched back to its normal nitrate content, nitrate content in skeletal muscle rapidly decreases and depletion of nitrate from skeletal muscle (blue area) is observed. It takes significant time for nitrate to reach back up to values observed before dietary manipulation started.
Conclusions.
In the present study in rat, we further support the idea that nitrate and nitrite distributions in muscle, blood and liver and fluxes of these ions into and from different compartments are highly dynamic processes, with many still unknown factors. In conjunction with our previous studies, we showed that these fluxes and states reached during the equilibration processes are highly dependent on the previous nutritional history, which is a fact that had not been previously considered for most small molecules, such as nitrate or nitrite ions, with the exception of carbohydrates in the well-known “carb-loading” routine used by long-distance runners.
We believe that understanding nitrate and nitrite fluxes in rat muscle tissue and possible differences among different muscle types is an important step in studying overall NO physiology in mammals. It will provide useful, basic information not only about so far little known metabolism and physiological pathways involving nitrate and nitrite but, if confirmed in humans, also it may further open the door to therapeutic uses of these ions, possibly in neuromuscular diseases (such as dystrophies), age-related muscular changes (sarcopenia) or for simple increase of athletic performance. Also, in general, better understanding of the physiology of these processes for nitrate could lead to discoveries of similar processes/reservoirs that might exist in mammalian bodies for other ion substances, not considered so far.
Highlights.
Nitrate/nitrite levels time course in rat after high nitrate withdrawal was studied
Nitrate levels returned to baseline in 2-phase nonlinear manner
Variable nitrate levels in different muscle groups were found
Nitrate withdrawal was accompanied by changes in several protein expression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest.
Alan N. Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. These arrangements do not affect his adherence to NO journal policies. The authors declare that they have no conflicts of interest.
References.
- [1].Jin RC, Loscalzo J, Vascular Nitric Oxide: Formation and Function, J Blood Med 2010 (2010) 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu VW, Huang PL, Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice, Cardiovasc Res 77 (2008) 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Poderoso JJ, Helfenberger K, Poderoso C, The effect of nitric oxide on mitochondrial respiration, Nitric Oxide 88 (2019) 61–72. [DOI] [PubMed] [Google Scholar]
- [4].Philippu A, Nitric Oxide: A Universal Modulator of Brain Function, Curr Med Chem 23 (2016) 2643–2652. [DOI] [PubMed] [Google Scholar]
- [5].Bogdan C, Nitric oxide and the immune response, Nat Immunol 2 (2001) 907–16. [DOI] [PubMed] [Google Scholar]
- [6].Knowles RG, Moncada S, Nitric oxide synthases in mammals, Biochem J 298 (Pt 2) (1994) 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr., The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2, Proc Natl Acad Sci U S A 98 (2001) 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thomas DD, Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide, Redox Biol 5 (2015) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gladwin MT, Crawford JH, Patel RP, The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation, Free Radic Biol Med 36 (2004) 707–17. [DOI] [PubMed] [Google Scholar]
- [10].Kim-Shapiro DB, Schechter AN, Gladwin MT, Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics, Arterioscler Thromb Vasc Biol 26 (2006) 697–705. [DOI] [PubMed] [Google Scholar]
- [11].Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD Jr., Kraus D, Ho C, Gladwin MT, Patel RP, Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation, Blood 107 (2006) 566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT, Nitrite as regulator of hypoxic signaling in mammalian physiology, Med Res Rev 29 (2009) 683–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hendgen-Cotta UB, Kelm M, Rassaf T, Myoglobin’s novel role in nitrite-induced hypoxic vasodilation, Trends Cardiovasc Med 24 (2014) 69–74. [DOI] [PubMed] [Google Scholar]
- [14].Li H, Samouilov A, Liu X, Zweier JL, Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues, J Biol Chem 276 (2001) 24482–9. [DOI] [PubMed] [Google Scholar]
- [15].Li H, Samouilov A, Liu X, Zweier JL, Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues, Biochemistry 42 (2003) 1150–9. [DOI] [PubMed] [Google Scholar]
- [16].Maia LB, Moura JJG, Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: Nitrite reduction by molybdoenzymes, Redox Biol 19 (2018) 274–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT, Schechter AN, Skeletal muscle as an endogenous nitrate reservoir, Nitric Oxide 47 (2015) 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gilliard CN, Lam JK, Cassel KS, Park JW, Schechter AN, Piknova B, Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver, Nitric Oxide 75 (2018) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Srihirun S, Park JW, Teng R, Sawaengdee W, Piknova B, Schechter AN, Nitrate uptake and metabolism in human skeletal muscle cell cultures, Nitric Oxide 94 (2020) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Piknova B, Park JW, Kwan Jeff Lam K, Schechter AN, Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle, Nitric Oxide 55–56 (2016) 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wylie LJ, Park JW, Vanhatalo A, Kadach S, Black MI, Stoyanov Z, Schechter AN, Jones AM, Piknova B, Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise, J Physiol 597 (2019) 5565–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL, Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase, J Biol Chem 283 (2008) 17855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO, A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis, Nat Chem Biol 4 (2008) 411–7. [DOI] [PubMed] [Google Scholar]
- [24].Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS, Wang S, Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane, Proc Natl Acad Sci U S A 109 (2012) 13434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC, Fiber Type-Specific Effects of Dietary Nitrate, Exerc Sport Sci Rev 44 (2016) 53–60. [DOI] [PubMed] [Google Scholar]
- [26].Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI, Poole DC, Microvascular oxygen pressures in muscles comprised of different fiber types: Impact of dietary nitrate supplementation, Nitric Oxide 48 (2015) 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Piknova B, Schechter AN, Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay, Methods Mol Biol 704 (2011) 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eng CM, Smallwood LH, Rainiero MP, Lahey M, Ward SR, Lieber RL, Scaling of muscle architecture and fiber types in the rat hindlimb, J Exp Biol 211 (2008) 2336–45. [DOI] [PubMed] [Google Scholar]
- [29].Piknova B, Park JW, Cassel KS, Gilliard CN, Schechter AN, Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method, J Vis Exp (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kina-Tanada M, Sakanashi M, Tanimoto A, Kaname T, Matsuzaki T, Noguchi K, Uchida T, Nakasone J, Kozuka C, Ishida M, Kubota H, Taira Y, Totsuka Y, Kina SI, Sunakawa H, Omura J, Satoh K, Shimokawa H, Yanagihara N, Maeda S, Ohya Y, Matsushita M, Masuzaki H, Arasaki A, Tsutsui M, Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice, Diabetologia 60 (2017) 1138–1151. [DOI] [PubMed] [Google Scholar]
- [31].Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T, Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk, J Am Coll Cardiol 63 (2014) 1584–5. [DOI] [PubMed] [Google Scholar]
- [32].Liu AH, Bondonno CP, Russell J, Flood VM, Lewis JR, Croft KD, Woodman RJ, Lim WH, Kifley A, Wong G, Mitchell P, Hodgson JM, Blekkenhorst LC, Relationship of dietary nitrate intake from vegetables with cardiovascular disease mortality: a prospective study in a cohort of older Australians, Eur J Nutr 58 (2019) 2741–2753. [DOI] [PubMed] [Google Scholar]
- [33].Lundberg JO, Weitzberg E, Gladwin MT, The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics, Nat Rev Drug Discov 7 (2008) 156–67. [DOI] [PubMed] [Google Scholar]
- [34].Williams JK, Smallwood MJ, Benjamin N, D’Souza RJ, Shore AC, Winyard PG, Gilchrist M, Renal nitrate clearance in chronic kidney disease, Nitric Oxide 97 (2020) 16–19. [DOI] [PubMed] [Google Scholar]
- [35].Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO 3rd, Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans, Proc Natl Acad Sci U S A 97 (2000) 11482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moretti C, Zhuge Z, Zhang G, Haworth SM, Paulo LL, Guimaraes DD, Cruz JC, Montenegro MF, Cordero-Herrera I, Braga VA, Weitzberg E, Carlstrom M, Lundberg JO, The obligatory role of host microbiota in bioactivation of dietary nitrate, Free Radic Biol Med 145 (2019) 342–348. [DOI] [PubMed] [Google Scholar]
- [37].Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A, Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite, Hypertension 51 (2008) 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, Hodgson JM, Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women, Am J Hypertens 28 (2015) 572–5. [DOI] [PubMed] [Google Scholar]
- [39].Govoni M, Jansson EA, Weitzberg E, Lundberg JO, The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash, Nitric Oxide 19 (2008) 333–7. [DOI] [PubMed] [Google Scholar]
- [40].Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A, Physiological role for nitrate-reducing oral bacteria in blood pressure control, Free Radic Biol Med 55 (2013) 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kapil V, Khambata RS, Jones DA, Rathod K, Primus C, Massimo G, Fukuto JM, Ahluwalia A, The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway, Pharmacol Rev 72 (2020) 692–766. [DOI] [PubMed] [Google Scholar]
- [42].Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT, Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis, Nat Chem Biol 2 (2006) 486–93. [DOI] [PubMed] [Google Scholar]
- [43].Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N, Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate, Nat Med 1 (1995) 546–51. [DOI] [PubMed] [Google Scholar]
- [44].Carlstrom M, Lundberg JO, Weitzberg E, Mechanisms underlying blood pressure reduction by dietary inorganic nitrate, Acta Physiol (Oxf) 224 (2018) e13080. [DOI] [PubMed] [Google Scholar]
- [45].Nyakayiru J, Kouw IWK, Cermak NM, Senden JM, van Loon LJC, Verdijk LB, Sodium nitrate ingestion increases skeletal muscle nitrate content in humans, J Appl Physiol (1985) 123 (2017) 637–644. [DOI] [PubMed] [Google Scholar]
- [46].G. DA Long Gary M., Troutman Ashley D., Fisher Amanda, Brown Mary Beth, Coggan Andrew R., Muscle fiber type differences in nitrate and nitrite storage and nitric oxide signaling in rats, bioRxiv 2020. [Google Scholar]
- [47].Jones AM, Thompson C, Wylie LJ, Vanhatalo A, Dietary Nitrate and Physical Performance, Annu Rev Nutr 38 (2018) 303–328. [DOI] [PubMed] [Google Scholar]
- [48].Damacena-Angelis C, Oliveira-Paula GH, Pinheiro LC, Crevelin EJ, Portella RL, Moraes LAB, Tanus-Santos JE, Nitrate decreases xanthine oxidoreductase-mediated nitrite reductase activity and attenuates vascular and blood pressure responses to nitrite, Redox Biol 12 (2017) 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S, Update on mechanism and catalytic regulation in the NO synthases, J Biol Chem 279 (2004) 36167–70. [DOI] [PubMed] [Google Scholar]


