Abstract
Background:
Disease-modifying therapies (DMTs) for multiple sclerosis (MS) are approved for their ability to reduce disease activity, namely clinical relapses and signal changes on magnetic resonance imaging (MRI). Disease activity appears age dependent. Thus, the greatest benefit would be expected in younger people with MS (PwMS) whereas benefits in the elderly are uncertain.
Methods:
Real-world data were obtained from PwMS from the North American Research Committee on Multiple Sclerosis (NARCOMS) registry and the US Department of Veterans Affairs Multiple Sclerosis Surveillance Registry (MSSR).
Results:
6948 PwMS were surveyed from NARCOMS, and the MSSR had 1719 participants. In younger adult PwMS 40-years old or less, 183 (61.4%) in NARCOMS and 179 (70.5%) in the MSSR were prescribed DMTs. Among PwMS over age 60, 1575 (40.1%) in NARCOMS and 239 (36.3%) in the MSSR were prescribed DMTs. More PwMS in the age group of 31–40 (p = 0.035) and 41–50 (p = 0.001) in the MSSR were using DMTs compared with PwMS of the same age groups in NARCOMS.
Conclusion:
These findings suggest that DMTs are under-utilized in the younger population and continue to be commonly prescribed in the elderly. Broader access may explain the higher prescription rate of DMTs in US veterans.
Keywords: multiple sclerosis, age, disease-modifying therapies, clinical practice
Introduction
Treatment options for multiple sclerosis (MS) have greatly expanded in recent years, and there are currently at least 18 approved disease-modifying therapies (DMTs) that have shown efficacy in reducing disease activity.1,2 DMTs are most effective in reducing clinical relapses and the formation of new MRI lesions, both of which are age-dependent processes. With increasing age, people with MS (PwMS) are less likely to have clinical relapses or radiological disease activity.3,4 As a result, younger PwMS are more likely to derive the most documented or apparent benefits from using DMTs due to a higher likelihood of disease activity.5–7
In clinical practice, there are a lack of data showing DMT prescribing patterns in real-world populations by age. This information is highly relevant to ascertain if PwMS in different age groups receive adequate care. The objective of this study was to determine the frequencies of DMT prescriptions in relation to age in the real-world setting using two large MS registries. We hypothesize that DMTs are prescribed for the majority of younger PwMS and less consistently in the elderly.
Methods
NARCOMS registry
The North American Research Committee on Multiple Sclerosis (NARCOMS) registry has collected voluntarily reported demographic and clinical information from PwMS since 1996. After enrolling in the registry, participants were asked to update their information on a semi-annual basis. The fall 2018 semi-annual update survey was used to identify current DMT use (including interferon beta-1a, interferon beta-1b, peginterferon beta-1a, glatiramer acetate, teriflunomide, fingolimod, dimethyl fumarate, natalizumab, and ocrelizumab). The age of the participants was their age at the survey date. The frequency and proportion of DMT prescriptions for each 10-year age group were reported.
Multiple sclerosis surveillance registry
The Multiple Sclerosis Surveillance Registry (MSSR) is an MS database established by the US Veterans Health Administration (VHA) in 2013.8 Data on key demographic and clinical variables related to MS were collected by clinicians and directly entered into the database during live or telehealth visits or telephone calls. The MSSR has approximately 2000 PwMS at present and features an interactive tool to pull data based on pre-specified queries. We obtained de-identified information on the number of PwMS being prescribed a US Food and Drug Administration (FDA)-approved DMT by each 10-year age group.
Ethical approval and informed consent
The NARCOMS registry was approved by the institutional review board (IRB) at Washington University in St. Louis (IRB#: 201610132), and participants consent to the use of de-identified information for research. An IRB-approved protocol within the US Department of VHA MS Centers of Excellence (MSCoE) has been approved by the University of Maryland IRB [HP-00043983 MSCoE Epidemiology Core (H-28293)] and covers the analysis of data in the MSSR.
Statistical analysis
Analyses were conducted using SAS V9.4 statistical software (SAS Institute Inc., Cary, NC, US). Chi-square tests were used to assess differences in DMT use between PwMS of each age group in the two registries, and nominal two-sided p values of 0.05 or less were considered statistically significant.
Results
DMT use by age in NARCOMS
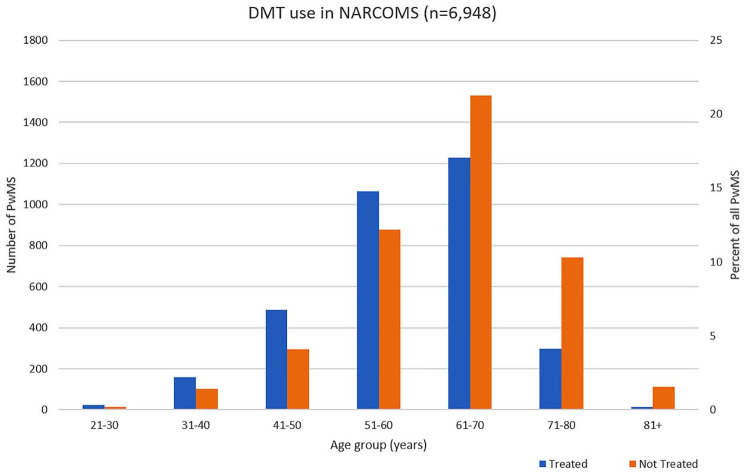
Of the 6948 respondents to the NARCOMS fall 2018 update survey, 3325 (47.9%) of participants report using DMTs, and 3623 (52.1%) participants report not using DMTs at the time of the survey. With increasing age, the proportion of DMT use decreased (Table 1, Figure 1). In the age 71–80 group, less than a third (29.5%) of participants reported DMT use. Conversely, the majority (61.4%) of younger PwMS age 40 or less reported DMT use.
Table 1.
Current use of disease-modifying therapies (DMTs) according to age groups based on data from the North American Research Committee on Multiple Sclerosis (NARCOMS) registry and the Multiple Sclerosis Surveillance Registry (MSSR).
| Age group | NARCOMS | MSSR | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 6948) | Female (%) | Not taking DMT (n = 3623) (%) | Taking DMT (n = 3325) (%) | Total (n = 1719) (%) | Female (%) | Not taking DMT (n = 783) (%) | Taking DMT (n = 936) (%) | ||
| 21–30 | 36 | 33 (91.7) | 14 (38.9) | 22 (61.1) | 54 | 17 (31.5) | 16 (29.6) | 38 (70.4) | 0.361 |
| 31–40 | 262 | 226 (86.3) | 101 (38.5) | 161 (61.5) | 200 | 78 (39.0) | 59 (29.5) | 141 (70.5) | 0.035 |
| 41–50 | 781 | 669 (85.7) | 291 (37.3) | 490 (62.7) | 342 | 132 (38.6) | 94 (27.5) | 248 (72.5) | 0.001 |
| 51–60 | 1943 | 1612 (83.0) | 866 (44.6) | 1077 (55.4) | 464 | 140 (30.2) | 194 (41.8) | 270 (58.2) | 0.188 |
| 61–70 | 2760 | 2188 (79.3) | 1508 (54.6) | 1252 (45.4) | 469 | 78 (16.7) | 276 (58.9) | 193 (41.1) | 0.178 |
| 71–80 | 1039 | 763 (73.4) | 732 (70.5) | 307 (29.5) | 162 | 13 (8.0) | 118 (72.8) | 44 (27.2) | 0.708 |
| 81+ | 127 | 95 (74.8) | 111 (87.4) | 16 (12.6) | 28 | 3 (10.7) | 26 (92.9) | 2 (7.1) | 0.541 |
The p values are reported from Chi-square tests used to assess differences in DMT use between PwMS of each age group in the two registries.
PwMS, people with multiple sclerosis.
Figure 1.
Current use of disease-modifying therapies (DMTs) in people with multiple sclerosis (PwMS) by age groups based on data from the North American Research Committee on Multiple Sclerosis (NARCOMS) registry.
DMT use by age in MSSR
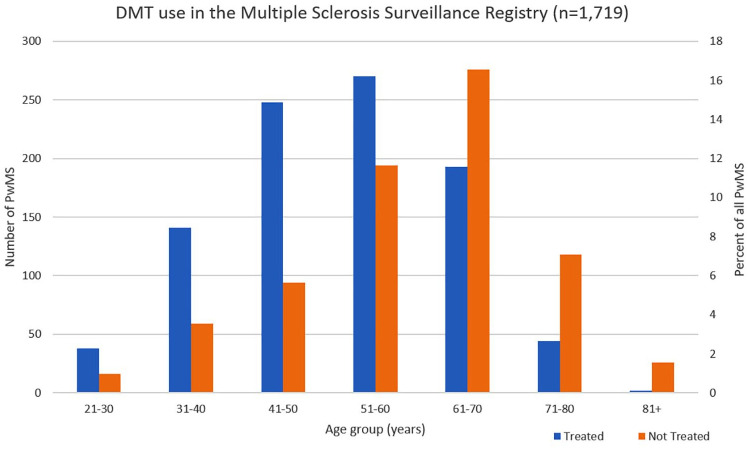
The MSSR presented information on DMT use for 1719 PwMS as of July 2019, of whom 936 (54.5%) were currently prescribed DMTs, and 783 (45.5%) were currently not prescribed DMTs. The proportion of PwMS prescribed DMTs decreased with age, with over a quarter (27.2%) of the individuals in the 71–80 age group prescribed DMTs and over two thirds (70.5%) of younger PwMS age 40 or less prescribed DMTs (Table 1, Figure 2).
Figure 2.
Current use of disease-modifying therapies (DMTs) in people with multiple sclerosis (PwMS) by age groups based on data from the US Department of Veterans Affairs (VA) Multiple Sclerosis Surveillance Registry (MSSR).
More PwMS in the age group of 31–40 (p = 0.035) and 41–50 (p = 0.001) in the MSSR were prescribed DMTs (70.5% and 72.5% respectively) compared with PwMS of the same age groups in NARCOMS (61.5% and 62.7% respectively). There were no differences in the frequency of DMT use between all other age groups in the two databases.
Discussion
Real-world data from NARCOMS and the MSSR showed decreasing frequencies of DMT use with age. Nevertheless, almost a third of younger PwMS below age 40 appear not to be using DMTs despite widespread recognition of the importance of early treatment in MS.9,10 Other studies have shown that early treatment delays time to reach diagnosis of clinically definite MS in people with clinically isolated syndrome, as well as disability accumulation in PwMS.11,12 In a study that surveyed 507 relapsing–remitting MS patients in the US, the most frequently reported barriers to DMT use included insurance authorization requirements and high out-of-pocket costs.13 Our own observations suggest more consistent access to health care and DMTs as a potential explanation for the slightly higher rate in US veterans compared with PwMS in NARCOMS, which is a more general population. In addition, it is likely that more young PwMS in NARCOMS are not on treatment due to family planning and pregnancy compared with participants in MSSR who are predominantly male. Nevertheless, this does not explain the almost 30% of younger PwMS who are not being prescribed DMTs. Still, the overall similarity in rates and patterns by age suggest the two databases are reasonably consistent assessments of DMT use in PwMS by age. Costs of DMTs are uniformly lower in the integrated VA health care system and similar to rates in Canada due in part to the ability to negotiate prices with the pharmaceutical industry.14 An observational study of 10,698 Canadian PwMS identified an association between increasing number of comorbidities and lower likelihood of DMT use.15 The investigators attributed the findings to hesitancy by PwMS and providers towards multidrug use and the perception of decreased treatment benefits in the setting of other chronic health conditions. Additional reasons for not using DMTs including side effects, disease-related stress, and the perception of a mild disease course have also been identified in studies.16,17 Addressing the undertreatment of younger PwMS who are more likely to benefit from DMT use requires ongoing efforts to improve DMT access and better understanding of treatment expectations.
In contrast to young PwMS, over a third of older PwMS above age 60 in both databases were treated with DMTs. With earlier diagnosis of MS and improved care, PwMS are growing both in number and age. In North America, the peak prevalence of MS is in the late 50s.18,19 With prolonged DMT use, there is an increased risk of adverse reactions especially in older PwMS who are more susceptible to established side effects of DMTs, including infections and lymphopenia as well as multi-drug interactions.20–22 In addition, disease activity in MS diminishes due to immune senescence, and relapses are less frequent in PwMS over age 60 compared with those who are younger.23 The evidence for DMT efficacy in PwMS is largely from clinical trials of MS DMTs, but these trials tend to exclude individuals over age 55. Subgroup analyses of clinical trial data have shown diminished DMT efficacy in PwMS older than age 40 compared with those younger,6 and a meta-analysis of clinical trials of DMTs showed decreased inhibition of disease progression with aging.24 While these studies suggests decreased efficacy of DMTs in elderly PwMS, there is a need for randomized controlled trials to definitively address the topic.
The continued use of DMTs in the elderly may be the result of the perceived notion that disease inactivity is due to treatment rather than the natural disease course with aging despite lower projected benefits in the elderly.25 Further contributing to the hesitancy to discontinue DMT may be a concern of rebound disease activity with some agents.26,27 The safety of DMT use in the elderly remains unclear and deserves more rigorous study. While recent retrospective data suggest relative safety in discontinuing DMTs in older patients without disease activity,28–30 these findings were not universal, and cases of disease worsening after stopping DMTs have been reported.31 The currently ongoing DISCOMS trial is expected to address whether DMTs can be safely discontinued in stable MS patients over age 55.32 Nevertheless, PwMS who are doing well may be very reluctant to stop therapy, and this too can have detrimental effects if mandated by payors or clinicians without taking patient perceptions into account.
Some limitations are present in this study. Participation in the NARCOMS registry is voluntary, which may be subject to responder bias resulting in differences between the registry’s MS population and the general MS population. However, validation of diagnoses in NARCOMS has been previously established,33 and reports and findings from NARCOMS have mirrored clinical and trial data on smaller cohorts repeatedly. Finally, the consistency of responses between NARCOMS and the MSSR increase our confidence that these age-related findings are indeed valid. While the MSSR comprises a US veteran population with different characteristics than the general population, several relevant characteristics, including age of MS diagnosis and disease phenotype distribution, are similar to the general MS population.34 Lastly, the present data do not include reasons for prescribing or not prescribing DMTs to PwMS. Despite differences in methodology of data collection in NARCOMS and the MSSR as well as their population differences, there were no significant differences in percentage of PwMS using DMTs by age among older PwMS in both large databases that would have ample power to see meaningful differences. A higher percentage of younger PwMS in the MSSR were treated compared with NARCOMS, but this might be the more standardized treatment approach with the VA compared with general practice neurologists and specialists.
DMTs for MS are more frequently used at younger ages when there is likely higher disease activity, yet a substantial proportion of younger PwMS remains untreated. As the probability of active disease declines with age and susceptibility to side effects increases, the risk versus benefit ratio of continuing DMTs in the elderly very likely diminishes. Further studies are needed to understand and address lack of treatment in young adults with MS as well as the reasons for persistent DMT use in the elderly.
Footnotes
Author contributions: YZ, AS, MW, and OS contributed to the study design. AS, SJ, WC, and MW participated in data collection. YZ, AS, SJ, WC, MW participated in data analysis. The first draft was written by YZ with input from all authors. All authors contributed to the review and approval of the final manuscript version for submission.
Conflict of interest statement: Y Zhang, A Salter, S Jin, W Culpepper, and M Wallin declare no competing interests related to this study. G Cutter has participated on data-monitoring and safety-monitoring boards for Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals,Hisun Pharmaceuticals, Mapi Pharmaceuticals, Merck, Merck/Pfizer, Opko Biologics, Neurim, Novartis, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva pharmaceuticals, Vivus, NHLBI (Protocol Review Committee), NICHD (OPRU oversight committee); participated in consulting or advisory boards for Biogen, Click Therapeutics, Genzyme, Genentech, Gilgamesh Pharmaceuticals, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion Pharmaceuticals, Roche, Somahlution, TG Therapeutics; is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc. a private consulting company located in Birmingham AL. O Stuve serves on the editorial boards of Therapeutic Advances in Neurological Disorders and on data-monitoring committees for Genentech-Roche, Pfizer, and TG Therapeutics without monetary compensation, advised EMD Serono, Celgene, Genentech, TG Therapeutics, and Genzyme, and receives grant support from Sanofi Genzyme and EMD Serono.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Yinan Zhang  https://orcid.org/0000-0001-9934-4564
https://orcid.org/0000-0001-9934-4564
Olaf Stuve  https://orcid.org/0000-0002-0469-6872
https://orcid.org/0000-0002-0469-6872
Contributor Information
Yinan Zhang, Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Amber Salter, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO, USA.
Shan Jin, VA Maryland Health Care System, Baltimore, MD, USA; VA Multiple Sclerosis Center of Excellence-East, Baltimore, MD, USA.
William J. Culpepper, II, VA Multiple Sclerosis Center of Excellence-East, Baltimore, MD, USA; Department of Neurology, University of Maryland School of Medicine, Baltimore, MD, USA.
Gary R. Cutter, Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA
Mitchell Wallin, VA Multiple Sclerosis Center of Excellence-East, Baltimore, MD, USA; Department of Neurology, University of Maryland School of Medicine, Baltimore, MD, USA; Department of Neurology, George Washington University School of Medicine, Washington, DC, USA.
Olaf Stuve, Neurology Section, VA North Texas Health Care System, Medical Service Dallas, VA Medical Center, 4500 South Lancaster Rd, Dallas, TX 75216, USA; Department of Neurology and Neurotherapeutics, The University of Texas Southwestern Medical Center, Dallas, TX, USA.
References
- 1. Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis - success from bench to bedside. Nat Rev Neurol 2019; 15: 53–58. [DOI] [PubMed] [Google Scholar]
- 2. Li H, Hu F, Zhang Y, et al. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. Epub ahead of print 25 May 2019. DOI: 10.1007/s00415-019-09395-w. [DOI] [PubMed] [Google Scholar]
- 3. Scalfari A, Lederer C, Daumer M, et al. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler 2016; 22: 1750–1758. [DOI] [PubMed] [Google Scholar]
- 4. Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 5. Zeydan B, Kantarci OH. Impact of age on multiple sclerosis disease activity and progression. Curr Neurol Neurosci Rep 2020; 20: 24. [DOI] [PubMed] [Google Scholar]
- 6. Signori A, Schiavetti I, Gallo F, et al. Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol 2015; 22: 960–966. [DOI] [PubMed] [Google Scholar]
- 7. Gartner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: a post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin 2018; 4: 2055217318778610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallin MT, Whitham R, Maloni H, et al. The Multiple Sclerosis Surveillance Registry: a novel interactive database within the Veterans Health Administration. Fed Pract 2020; 37: S18–S23. [PMC free article] [PubMed] [Google Scholar]
- 9. Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry 2018; 89: 844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord 2016; 9(Suppl. 1): S5–S48. [DOI] [PubMed] [Google Scholar]
- 11. Armoiry X, Kan A, Melendez-Torres GJ, et al. Short- and long-term clinical outcomes of use of beta-interferon or glatiramer acetate for people with clinically isolated syndrome: a systematic review of randomised controlled trials and network meta-analysis. J Neurol 2018; 265: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalmer TA, Baggesen LM, Nørgaard M, et al.; Danish Multiple Sclerosis Group. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol 2018; 25: 1262-e110. [DOI] [PubMed] [Google Scholar]
- 13. Simacek KF, Ko JJ, Moreton D, et al. The impact of disease-modifying therapy access barriers on people with multiple sclerosis: mixed-methods study. J Med Internet Res 2018; 20: e11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartung DM, Bourdette DN, Ahmed SM, et al. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology 2015; 84: 2185–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang T, Tremlett H, Leung S, et al.; CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology 2016; 86: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schoor R, Bruce A, Bruce J, et al. Reasons for nonadherence and response to treatment in an adherence intervention trial for relapsing-remitting multiple sclerosis patients. J Clin Psychol 2019; 75: 380–391. [DOI] [PubMed] [Google Scholar]
- 17. Grytten N, Aarseth JH, Espeset K, et al. Stoppers and non-starters of disease-modifying treatment in multiple sclerosis. Acta Neurol Scand 2013; 127: 133–140. [DOI] [PubMed] [Google Scholar]
- 18. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991–2010). J Neurol 2015; 262: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallin MT, Culpepper WJ, Campbell JD, et al.; US Multiple Sclerosis Prevalence Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 2019; 92: e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020; 77: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grebenciucova E, Berger JR. Immunosenescence: the role of aging in the predisposition to neuro-infectious complications arising from the treatment of multiple sclerosis. Curr Neurol Neurosci Rep 2017; 17: 61. [DOI] [PubMed] [Google Scholar]
- 22. Goldman MD, Dwyer L, Coleman R, et al. Patient-specific factors modulate leukocyte response in dimethyl fumarate treated MS patients. PLoS One 2020; 15: e0228617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaughn CB, Jakimovski D, Kavak KS, et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol 2019; 15: 329–342. [DOI] [PubMed] [Google Scholar]
- 24. Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol 2017; 8: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwehr NA, Kuntz KM, Enns EA, et al.; BeAMS Study group. Informing medication discontinuation decisions among older adults with relapsing-onset multiple sclerosis. Drugs Aging 2020; 37: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011; 76: 1858–1865. [DOI] [PubMed] [Google Scholar]
- 27. Hatcher SE, Waubant E, Nourbakhsh B, et al. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 2016; 73: 790–794. [DOI] [PubMed] [Google Scholar]
- 28. Kaminsky A-L, Omorou AY, Soudant M, et al. Discontinuation of disease-modifying treatments for multiple sclerosis in patients aged over 50 with disease inactivity. J Neurol 2020; 267: 3518–3527. [DOI] [PubMed] [Google Scholar]
- 29. Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler 2019; 25: 699–708. [DOI] [PubMed] [Google Scholar]
- 30. Yano H, Gonzalez C, Healy BC, et al. Discontinuation of disease-modifying therapy for patients with relapsing-remitting multiple sclerosis: effect on clinical and MRI outcomes. Mult Scler Relat Disord 2019; 35: 119–127. [DOI] [PubMed] [Google Scholar]
- 31. Berkovich R. Clinical and MRI outcomes after stopping or switching disease-modifying therapy in stable MS patients: a case series report. Mult Scler Relat Disord 2017; 17: 123–127. [DOI] [PubMed] [Google Scholar]
- 32. ClinicalTrials.gov. Discontinuation of disease modifying therapies (DMTs) in multiple sclerosis (MS) (DISCOMS). Identifier NCT03073603. Bethesda, MD: National Library of Medicine (US), 2017. [Google Scholar]
- 33. Marrie RA, Cutter G, Tyry T, et al. Validation of the NARCOMS registry: diagnosis. Mult Scler 2007; 13: 770–775. [DOI] [PubMed] [Google Scholar]
- 34. Culpepper WJ, Wallin MT, Magder LS, et al. VHA multiple sclerosis surveillance registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev 2015; 52: 263–272. [DOI] [PubMed] [Google Scholar]