Abstract
Introduction:
All major international guidelines for the management of infective endocarditis (IE) have undergone major revisions, recommending antibiotic prophylaxis (AP) restriction to high-risk patients or foregoing AP completely. We performed a systematic review to investigate the effect of these guideline changes on the global incidence of IE.
Methods:
Electronic database searches were performed using Ovid Medline, EMBASE and Web of Science. Studies were included if they compared the incidence of IE prior to and following any change in international guideline recommendations. Relevant studies fulfilling the predefined search criteria were categorized according to their inclusion of either adult or pediatric patients. Incidence of IE, causative microorganisms and AP prescription rates were compared following international guideline updates.
Results:
Sixteen studies were included, reporting over 1.3 million cases of IE. The crude incidence of IE following guideline updates has increased globally. Adjusted incidence increased in one study after European guideline updates, while North American rates did not increase. Cases of IE with a causative pathogen identified ranged from 62% to 91%. Rates of streptococcal IE varied across adult and pediatric populations, while the relative proportion of staphylococcal IE increased (range pre-guidelines 16–24.8%, range post-guidelines 26–43%). AP prescription trends were reduced in both moderate and high-risk patients following guideline updates.
Discussion:
The restriction of AP to only high-risk patients has not resulted in an increase in the incidence of streptococcal IE in North American populations. The evidence of the impact of AP restriction on IE incidence is still unclear for other populations. Future population-based studies with adjusted incidence of IE, AP prescription rates and accurate pathogen identification are required to delineate findings further in these other regions.
Keywords: antibiotic prophylaxis, guideline, infective endocarditis, systematic review
Introduction
Infective endocarditis (IE) remains a sinister disease in modern medicine, despite being first described more than 450 years ago by French physician Jean François Fernel.1 Sir William Osler’s lectures, over 100 years ago, provided a detailed explanation on this disease (initially coined ‘malignant endocarditis’);2 however, it still remains a significant diagnostic and therapeutic dilemma. IE remains a rare disease in western countries, with an incidence of native-valve endocarditis between two and seven cases per 100,000 person-years.3 Despite therapeutic advances, the mortality rate remains high with 14–22% in-hospital mortality rates4,5 and up to 51% mortality at 10 years.6 It is also associated with significant morbidity including prolonged hospital stay, reduced quality of life and a high risk of re-infection.7,8
While antibiotic treatment remains the cornerstone of medical therapy, the microbiological profile of causative organisms has changed over time. Streptococcal species, particularly from oral cavity flora, have historically been the predominant bacterial pathogen.9 However, staphylococcal species, including methicillin resistant strains, now represent an increasingly significant proportion of IE cases.10,11 Patients with advanced age, renal failure, intra-cardiac devices and prosthetic heart valves have the highest incidence of IE in the modern era,7,10,12 while indwelling intravenous catheters and intravenous drug use are routes of infection that now also contribute to a significant number of IE cases.13–16
International societies have previously recommended antibiotic prophylaxis (AP) for patients with uncorrected structural heart disease prior to undergoing invasive dental, respiratory, gastrointestinal (GI) or genitourinary (GU) procedures.17,18 These recommendations targeted streptococcal species found in the oral cavity as the predominant organism responsible for IE. However, recent updates of all major international IE guidelines have undergone significant revision due to a lack of evidence to support the effectiveness of AP to prevent IE and a growing concern surrounding antibiotic resistance. During the years 2002, 2007 and 2009, French, American and European guidelines, respectively, restricted AP to only high-risk patients; that is, those with prosthetic valves, uncorrected cyanotic heart disease or previous IE.19–21 In 2008, the United Kingdom (UK) (National Institute for Health and Care Excellence; NICE) guidelines recommended no AP was required for any patients undergoing invasive dental procedures, including those at high risk, due to a lack of evidence supporting the efficacy of AP.22
Since the implementation of the guideline updates, multiple analyses from population and registry data have sought to determine the impact these guideline changes have had on IE rates, particularly in populations no longer recommended to receive AP prior to invasive procedures. The most recent studies have focused on adjusted incidence, causative organism identification and correlating rates of AP prescriptions across all risk groups.23,24 These studies demonstrate the ongoing international interest in ensuring the safety of a restrictive approach to AP. Considering the high morbidity and mortality associated with IE and therefore the potential significant impact of restricting IE prophylaxis, we performed an updated systematic review to evaluate the impact that changes in AP recommendations from international guidelines have had on the incidence and microbiology of IE in adult and pediatric populations.
Methods
Search strategy and study selection
Electronic searches were performed using Ovid Medline, EMBASE and Web of Science from 2007 until July 2019. The terms ‘endocarditis’ or ‘IE’ or ‘infective endocarditis’ were combined with ‘prophylaxis’ or ‘prevention’ or ‘antibiotic’ and ‘guideline’ or ‘recommendation’ or ‘trend’ as both keywords and MeSH terms. Two reviewers (NM and DT) independently screened the title and abstract of records identified in the search. Full-text publications were subsequently reviewed separately if either reviewer considered the manuscript to be potentially eligible for inclusion. Data were extracted from the included studies independently by two reviewers (NM and DT). Discrepancies were resolved by consensus with a third reviewer (MD). The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. Only studies published in English were included. The systematic review was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline.25 Studies were categorized according to whether studies reported the incidence of IE in adults or children.
Eligibility criteria
Studies were included if they compared the incidence of IE prior to and following any change in international guideline recommendations. In particular, the French (2002), American (2007), NICE (2008) and European (2009) guidelines on the prevention of IE. A summary of the AP indications recommended by each guideline is presented in Table 1. Studies were excluded if their analysis did not specifically compare the incidence of IE prior to and following changes in one of the afore-mentioned international IE guidelines.
Table 1.
Summary of international infective endocarditis guideline recommendation changes for antibiotic prophylaxis.
| Intermediate risk |
High-risk |
Prosthetic valve |
Previous IE |
CHD |
Transplant and VHD |
Uncorrected VHD |
Respiratory |
GI or GU |
Skin and soft tissue |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guideline | Year | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| French | 2002 | ✓ | O | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ † | NS | NS | ✓ | ✗ | ✓ | ✓ ‡ | ✓ | ✓ ‡ | ✓ | ✓ ‡ |
| AHA | 2007 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ * | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| NICE | 2008 | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| ESC | 2009 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ * | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
, Antibiotic prophylaxis recommended; ✗, Antibiotic prophylaxis not recommended.
Limited to the following conditions: (1) unrepaired cyanotic CHD; (2) completely repaired CHD with prosthetic material or device during the first 6 months; (3) repaired CHD with residual defects that inhibit endothelialisation.
Non-operated cyanotic CHD and pulmonary systemic shunts; not for non-cyanotic CHD.
Recommended only in high-risk individuals undergoing high-risk procedures, optional in lesser risk individuals undergoing high-risk procedures (high-risk individuals are those with prosthetic valves, cyanotic heart disease or a history of infective endocarditis. Lesser risk individuals are those with uncorrected valvular heart disease and non-cyanotic heart disease. High-risk procedures are those in which there is instrumentation of infected tissues or when instrumentation of a hollow viscus may result in mucosal injury).
AHA, American Heart Association; CHD, congenital heart disease; ESC, European Society of Cardiology; GI, gastrointestinal; GU, genitourinary; IE, infective endocarditis; NICE, National Institute for Health and Care Excellence; NS, not specified; O, antibiotic prophylaxis optional; Pre, pre-guideline recommendation; Post, post-guideline recommendation; VHD, valvular heart disease.
Quality of evidence
Risk of bias assessment of all included studies was performed using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool, which considers different level of biases across seven different domains.26
Data synthesis and analysis
No formal quantitative pooling or analysis of results was able to be performed due to the inconsistency in reporting metrics across included studies. A qualitative synthesis is presented, and clinical implications have been drawn from the summation of individual study results.
Results
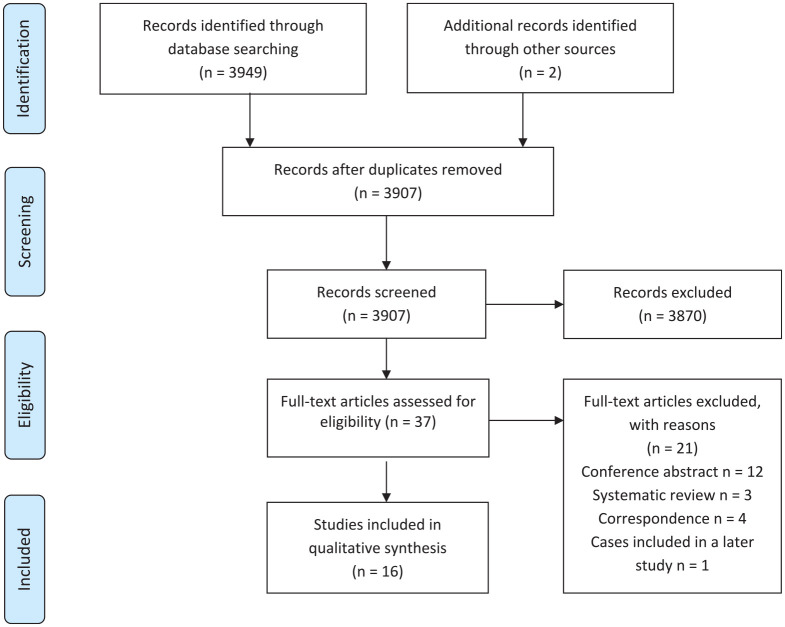
The initial search yielded 3949 studies. After exclusion of duplicates and other irrelevant publications via review of titles and abstracts, 37 studies underwent full-text review. A further 21 studies were excluded because they were either conference abstracts, correspondence, or did not directly compare incidence pre and post-guideline changes. One study was excluded as an extended evaluation was performed 3 years later, which is included in this review. A further study was identified from references and a final study was identified using a new publication alerts function of a large database. A total of 16 studies fulfilled all inclusion criteria and were included in the review.23,24,27–40 The search strategy and results are summarized in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow chart summarizing the search strategy for relevant publications.
The overall risk of bias within individual studies varied from moderate to critical. All studies were at moderate risk for selective reporting as none provided a pre-registered protocol. Level of bias for the confounding domain varied across the included studies from low to critical depending on the level of adjustment to the included patient population (i.e. population factors and/or IE risk). A summary of the risk of bias assessment using the ROBINS-I tool is presented in Table 2.
Table 2.
Risk of bias assessment of included studies (ROBINS-I).
| Study (ref no.) | Guideline | Confounding | Selection | Classification of interventions | Deviation from intended intervention | Missing data | Outcomes | Selective reporting | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Adults | |||||||||
| Thornhill et al.27 | 2008 (NICE) | Critical | Low | Low | NI | Low | Low | Moderate | Critical |
| Duval et al.28 | 2002 (French) | Moderate | Moderate | Low | NI | Low | Low | Moderate | Moderate |
| Bikdeli et al.29 | 2007 (AHA) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Bor et al.30 | 2007 (AHA) | Serious | Low | Low | NI | Low | Low | Moderate | Serious |
| Dayer et al.31 | 2008 (NICE) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| DeSimone et al.32 | 2007 (AHA) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Pant et al.33 | 2007 (AHA) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Mackie et al.34 | 2007 (AHA) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Keller et al.35 | 2009 (ESC) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Toyoda et al.36 | 2007 (AHA) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Van Den Brink et al.37 | 2009 (ESC) | Moderate | Low | Low | NI | Low | Low | Moderate | Moderate |
| Thornhill et al.24 | 2007 (AHA) | Low | Low | Low | NI | Low | Low | Moderate | Moderate |
| Garg et al.23 | 2007 (AHA) | Low | Low | Low | NI | Low | Low | Moderate | Moderate |
| Children | |||||||||
| Pasquali et al.38 | 2007 (AHA) | Critical | Low | Low | NI | Low | Low | Moderate | Critical |
| Bates et al.39 | 2007 (AHA) | Critical | Low | Low | NI | Low | Low | Moderate | Critical |
| Sakai Bizmark et al.40 | 2007 (AHA) | Low | Low | Low | NI | Low | Low | Moderate | Moderate |
AHA, American Heart Association; ESC, European Society of Cardiology; NI, no information; NICE, The National Institute for Health and Care Excellence.
Study characteristics
Over 1.3 million cases of IE were included between the years 1991 and 2015 across six countries. All studies were retrospective, reporting on either crude or adjusted rates of IE within defined populations. A total of 13 studies analysed adult patients with IE23,24,27–37 and three studies assessed the incidence of pediatric IE.38–40 Thirteen studies used International Classification of Disease (ICD) codes to identify cases of IE, with two studies using additional diagnostic criteria28,30 and one used a unique identifier.37
Eight studies23,24,29,30,32–34,36 compared the incidence of adult IE following the introduction of the American Heart Association (AHA) guidelines in 2007. Two studies35,37 reported on the incidence of IE following changes in the European Society of Cardiology (ESC) guidelines in 2009 and a further two studies27,31 reported on the incidence of IE in UK populations following changes in NICE guidelines in 2008. One study reported incidence of IE following changes in French guidelines in 2002.28 All three studies reporting on pediatric IE examined United States (US) populations following changes in the AHA guidelines in 2007.38–40 A summary of study characteristics is presented in Table 3.
Table 3.
Study characteristics.
| Study | Country | Guideline | Study period | N | Data source | Population | Diagnostic definition |
|---|---|---|---|---|---|---|---|
| Adults | |||||||
| Thornhill et al.27 | UK | 2008 (NICE) | 2000–2010 | NR | Secondary Uses Service database | Adults with IE | ICD-10 codes |
| Duval et al.28 | France | 2002 (French) | 1991–2008 | 993 | Survey of medical participants involved in the treatment of IE in three French regions | Adults with IE | Modified von Reyn and Duke criteria |
| Bikdeli et al.29 | USA | 2007 (AHA) | 1999–2010 | 262,658 | Centers for Medicare and Medicaid Services Medicare Inpatient Standard Analytic Files (Medicare) | >65 years patients with IE | ICD-9 codes |
| Bor et al.30 | USA | 2007 (AHA) | 1998–2009 | 382,153 | Nationwide Inpatient Sample (NIS) database | All patients with IE | ICD-9 codes* |
| Dayer et al.31 | UK | 2008 (NICE) | 2000–2013 | 19,804 | National Hospital Episode Statistics (HES) | All patients with IE | ICD-10 codes |
| DeSimone et al.32 | USA | 2007 (AHA) | 1999–2013 | 27 | Rochester Epidemiology Project of Olmsted County; and Nationwide Inpatient Sample (NIS) Database | Adults with VGS IE | Modified Duke criteria |
| Pant et al.33 | USA | 2007 (AHA) | 2000–2011 | 457,052 | Nationwide Inpatient Sample (NIS) database | Adults with IE | ICD-10 codes |
| Mackie et al.34 | Canada | 2007 (AHA) | 2003–2013 | 9431 | Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD) | All patients with IE | ICD-9 + ICD-10 codes |
| Keller et al.35 | Germany | 2009 (ESC) | 2005–2014 | 94,364 | Nationwide inpatient statistic (DRG statistic) of Germany | Adults with IE | ICD-10 codes |
| Toyoda et al.36 | USA | 2007 (AHA) | 1998–2013 | 75,829 | Statewide Planning and Research Cooperative System Database (NY) and Office of Statewide Health Planning and Development database (California) | Adults with IE | ICD-9 codes |
| Van den Brink et al.37 | Netherlands | 2009 (ESC) | 2005–2011 | 5213 | Dutch Healthcare Authority database | Adults with IE | Independent |
| Thornhill et al.24 | USA | 2007 (AHA) | 2003–2015 | 20,340 | MarketScan database (collection of Health Insurance Portability and Accountability Act-compliant databases) | Adults with IE | ICD-9 codes |
| Garg et al.23 | Canada | 2007 (AHA) | 2002–2014 | 7551 | Multiple databases from Institute for Clinical Evaluative Sciences | Adults with IE | ICD-9 + ICD-10 codes |
| TOTAL | 1,335,415 | ||||||
| Children | |||||||
| Pasquali et al.38 | USA | 2007 (AHA) | 2003–2010 | 1157 | Pediatric Health Information System (PHIS) Database | Children with IE | ICD-9 codes |
| Bates et al.39 | USA | 2007 (AHA) | 2003–2014 | 841 | Pediatric Health Information System (PHIS) Database | Children with oral strep IE | ICD-9 codes |
| Sakai Bizmark et al.40 | USA | 2007 (AHA) | 2001–2012 | 3748 | Nationwide Inpatient Sample (NIS) database | Children with IE | ICD-9 codes |
| TOTAL | 5746 | ||||||
Additional codes used for 2009.
AHA, American Heart Association; ESC, European Society of Cardiology; ICD, International Classification of Diseases; IE, infective endocarditis; N, number of patients; NICE, National Institute for Health and Care Excellence; NR, not reported; UK, United Kingdom; USA, United States of America; VGS, viridans group streptococci.
Patient characteristics
Adults
Ten studies reported characteristics of patients diagnosed with IE prior to and following the change in international guidelines.23,28–32,34–37 The mean age of patients included in these studies ranged from 58 to 80 years old, with 42–72% of patients being men. Rates of intravenous drug use, chronic renal failure and prosthetic cardiac valve in patients with IE ranged from 5.5% to 12%, 2.7 to 29.4% and 12.9% to 30.1%, respectively. Mortality occurring up to 90 days following IE diagnosis ranged from 15.5% to 38%.
Children
Three studies examined the incidence of IE in a pediatric population.38–40 All three studies included index hospital admissions for IE in patients aged younger than 18 years. Gender distribution was similar across all three studies. The mean age ranged from 2.9 to 13 years. The proportion of patients with congenital heart disease ranged from 34.2% to 68%. In-hospital mortality ranged from 1.1% to 4% across all three studies.
A summary of study patient characteristics is presented in Table 4.
Table 4.
Patient characteristics.
| Study | Guideline timing | Age ± SD (years) | Male (%) | Mortality* (%) | IVDU (%) | Renal failure (%) | Prosthetic valve (%) | ICD (%) | CHD (%) | Mod risk (%) | High risk (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | |||||||||||
| Thornhill et al.27 | NICE 2008 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Duval et al.28 | French 2002 | 59.8 ± 16.5 | 70.1 | 19.1 | 5.5 | 2.7 | 21.7 | 8.2 | NR | NR | NR |
| Bikdeli et al.29 | AHA 2007 | 79.3 ± 8.3 | 42.3 | 10.1/15.5† | NR | 19.2 | NR | NR | NR | NR | NR |
| Bor et al.30 | AHA 2007 | 59.1 | 57.7 | 14.5 | 7.8 | 4.4 | NR | 13.3–18.9 | NR | NR | NR |
| Dayer et al.31 | NICE 2008 | 59.0 ± 20.3 | 68.5 | NR | NR | NR | NR | NR | NR | NR | NR |
| DeSimone et al.32 | AHA 2007 | NR | 77.7 | NR | NR | NR | NR | NR | NR | NR | NR |
| Pant et al.33 | AHA 2007 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Mackie et al.34 | AHA 2007 | 55 ± 23.7 | 62.8 | NR | 8.90 | NR | 12.80 | 3.8 | 7.2 | NR | NR |
| Keller et al.35 | ESC 2009 | NR | NR | 17.0 | NR | NR | NR | NR | NR | NR | NR |
| Toyoda et al.36 | AHA 2007 | 62.3 ± 18.9 | 59.1 | 23.9 | 12.5 | 29.4 | 12.9 | 12.9 | 4.5 | NR | NR |
| Van den Brink et al.37 | ESC 2009 | 67.5 (22–97)‡ | 62.5 | 36.1 | NR | NR | 30.1 | 7.9 | NR | NR | NR |
| Thornhill et al.24 | AHA 2007 | NR | NR | NR | NR | NR | NR | NR | NR | 5.91 | 0.64 |
| Garg et al.23 | AHA 2007 | 63 (48–75)‡ | 63.7 | 18.0 (18–64 years)/36–38 (>65 years) | NR | 18.9 | 18.6 | NR | 4.7 | 6.6 | 19.2 |
| Children | |||||||||||
| Pasquali et al.38 | AHA 2007 | 2.9 ± 8.8 | 58.0 | 1.1 | NR | NR | NR | NR | 68 | NR | NR |
| Bates et al.39 | AHA 2007 | 13.0 ± 4.4 | 56.6 | 3.6 | NR | NR | NR | NR | 34.2 | NR | NR |
| Sakai Bizmark et al.40 | AHA 2007 | 8.5 ± 0.5 | 57.1 | 3 | 1.5 | <1 | 6 | 2.6 | 44.1 | NR | 63.1 |
90-Day mortality.
In-hospital and 30-day mortality.
Median (range) or (inter-quartile range).
CHD, congenital heart disease; ICD, intra-cardiac device; IVDU, intravenous drug usage; N, number of patients; NR, not reported; SD, standard deviation.
IE incidence
Adults
Infective endocarditis incidence reporting methodology and results varied significantly across all studies. Some studies identified true incidence with rates of IE reported as new cases of IE per population, while others identified prevalence by reporting the total number of IE cases identified.
Three studies found the incidence of IE was declining across the entirety of the study periods, with no significant change in the downward trend following guideline changes.28,29,32 Two of these studies used focused databases in their analysis; isolated to either viridans group streptococcus32 or veterans aged 65 years and older.29 In contrast, five studies demonstrated an increase in IE incidence during their respective study periods with no significant change in the rate of increase following the introduction of the antibiotic-restricting guidelines.27,30,33,34,36 The overall range of IE cases prior to guideline updates was 5.0 to 12.4 cases per 100,000 people, and 7.0 to 14.3 cases per 100,000 people after guideline updates. While the crude incidence increased from 7.6 to 9.3 cases per 100,000 people in one study, the adjusted incidence was not significantly different when sex, age and race were accounted for.36
Five studies showed a significant increase in IE incidence after guideline changes above what would have been expected had previous trends continued.23,24,31,35,37 Two of these studies analysed changes in IE incidence following the implementation of the European guideline update in 2009 and both found increasing rates of IE. The prevalence of IE rose from 9.5 to 10.6 IE diagnoses per 100,000 citizens from 2006 to 2010 in Germany. From 2010 to 2014, a larger increase in prevalence was recorded from 11.1 to 14.4 IE diagnoses per 100,000 citizens (linear regression β=2.9; 95% confidence interval (CI) 1.1–4.6; p = 0.006).35 The incidence of IE in The Netherlands increased significantly above the projected historical trend following publication of updated IE guidelines, with the incidence increasing from 30.2 to 62.9 cases per million (p ⩽ 0.001).37 An increase in the upward trend of IE incidence was demonstrated by an additional 35 cases per month (p ⩽ 0.001) after changes in NICE guidelines in England.31 The crude incidence of IE in Canadian patients over 65 years old increased from 32 patients per million prior to the AHA guideline update, to 47 patients per million in the 7 years following guideline release,23 with a significant increase in IE incidence in high and moderate-risk patients of all ages. Similarly, the incidence of IE in American adults with high risk increased from 872 to 1385 cases per million people in patients over 65 years old, and 1061–1754 cases per million in adults aged 18–64 years. The incidence of IE in patients at moderate risk increased from 229 to 283 cases per million in adults aged over 65 years, and 308 to 423 cases per million in adults from 18 to 64 years old.23 However, these increases occurred several years following the guideline update, while AP for moderate-risk populations decreased in the immediate years following the guideline release.
Change point analysis was performed in three studies. One study identified a change point 3 months after the guideline update (NICE),31 while two studies identified a change point 3 and 4 years after AHA guideline changes.23,34
Children
Two studies reported on IE incidence indexed to hospital admissions38,39 and one reported incidence indexed to the greater population.40 Two studies reported an increasing trend in IE incidence across the study period; however, there was no significant difference in this trend following guideline updates.39,40 One study found a significant decline in IE incidence across the study period with no significant difference following guideline updates.38
A summary of IE incidence and the impact of IE guideline updates is presented in Table 5.
Table 5.
Summary of infective endocarditis incidence, causative pathogens and antibiotic prophylaxis.
| Study | Guideline | Population | Analysis | IE incidence and guideline impact | Pathogens | Antibiotic prophylaxis | Mortality |
|---|---|---|---|---|---|---|---|
| Thornhill et al.27 | 2008 (NICE) | All UK patients with acute/subacute IE | Monthly IE incidence, monthly prescribing data. Estimated APC in IE cases before and after NICE guideline updates | Continued upward trend after 2008 guideline update, not statistically significant [pre-guideline IE rates increased by 3.82 (95% CI 3.04–4.61) cases/year, Post-guideline IE rates increased by 2.72 (95% CI −0.94 to 6.52) cases/year, difference −1.1 (95% CI −3.98 to 1.91, p = 0.61)] | Oral strep IE APC 8.41 (95% CI 6.66–10.19) cases/year prior to guideline updates, 10.38 (95% CI 2.93–18.36) cases/year after guideline updates, difference 1.97 (95% CI −3.73 to 8.17, p = 0.66). Pre-guideline APC staph 9.24 (95% CI 7.45–11.06) cases/year. Post-guideline APC staph 1.49 (95% CI −5.66 to 9.19) cases/year, difference −7.75 (95% CI −13.11 to −1.87, p = 0.008) | Rapid decline (78.6% reduction) in AP rate for IE prophylaxis following guideline update (mean prescriptions decreased from 10727 +/−1068 to 2292 +/−176) | Non-significant increase in IE mortality following guideline update (pre-guideline mortality APC 2.55 (95% CI 0.65–4.48) cases/year. Post-guideline mortality APC 6.64 (95% CI −2.5 to 16.64). difference 4.09 (95% CI −3.15 to 12.16, p = 0.45) |
| Duval et al.28 | 2002 (French) | Three French regions representing 24% of the population aged ⩾20 years. All patients aged ⩾20 years with first hospitalisation of IE in 1991, 1999, and 2008 | Sex and age-adjusted incidence | Non-significant decrease in IE incidence trend over the three time periods. Guideline updates had no influence on IE incidence | No change in IE incidence due to oral strep over the three time periods. Increased in proportion of group D strep between 1991 and 1999 (17–25%) followed by a decline in 2008 to below 1991 rates (12%, p < 0.001). Proportion of staph aureus increased significantly (16%, 21%, 26%, p < 0.011) | NR | In-hospital mortality was not significantly different between the three time periods. (20.7%, 15.4%, 21.2%; p = 0.11) |
| Bikdeli et al.29 | 2007 (AHA) | US patients ⩾65 years with staph or strep IE | Adjusted hospitalisation and mortality rates (in-hospital, 20-day, 6 month and 1 year) | Adjusted hospitalisation rate increased from 1999 to 2005 then declined to 2010 (peaked in 2005: 83.5/100,000 person-years). Rate of hospitalisations declined consistently after guideline updates [incidence rate ratios: 0.97 (95% CI: 0.94–0.99), 0.91 (0.89–0.93), and 0.86 (0.84–0.88) for 2008, 2009, 2010 compared to 2007]. Decreasing trend in IE incidence, no change in the slope following guideline updates | NR | NR | In-hospital mortality declined significantly from 1999 to 2010 11.1% in 1999 to 9.1% in 2010 (p < 0.001). 30-day and 1-year mortality increased significantly during 2008 becoming non-significant in 2009 |
| Bor et al.30 | 2007 (AHA) | Adults hospitalised with IE identified from 20% weighted representative sample of acute-care hospitalisations | IE rates using Census Bureau figures and direct method Chi square test for proportional difference, Cochran-Armitage tests to evaluate time trends | 2.4% annual increase in hospitalisations due to IE over study period. No inflection in hospitalization rates after guideline updates | 62.3% of all IE cases had microbiological data recorded. 35.4% all cases staph, 24.7% strep | NR | In-hospital mortality 14.5%, unchanged over study period. S. aureus higher mortality than other organisms (17.4% versus 11.3%). Independent predictors of mortality: age, CVA, ARF, MI |
| Dayer et al.31 | 2008 (NICE) | Adults hospitalised with acute or subacute IE | Interrupted time series analysis | Consistent upward trend over study period, increase in the slope of this trend following guideline updates | NR | Mean number of AP prescriptions/ month fell significantly after guideline updates, from 10900 to 2236 (p < 0·0001) | NR |
| DeSimone et al.32 | 2007 (AHA) | All Olmsted County (Minnesota) adults with IE caused by VGS | Age- and sex- adjusted incidence of IE in the US Caucasian population. Temporal trend analysis of incidence of IE due to VGS | Decrease in the annual incidence rate of IE (age- and sex- adjusted). No change to declining trajectory following guideline updates | All VGS IE | NR | NR |
| Pant et al.33 | 2007 (AHA) | Adults hospitalised with IE identified from 20% weighted representative sample of acute-care hospitalisations | segmented regression using Interrupted time series analysis | Steady increase in incidence throughout the study period. Change in IE rate for 2000–2007: 0.54/100,000 (95% CI 0.32–0.75; p < 0.001), change in IE rate for 2007–2011: 0.6/100,000 (95% CI 0.23–0.97; p = 0.005). Overall trends in IE rates from 2000–2007 and 2008–2011 not significantly different (p = 0.74) | No significant change in slope of staph IE incidence following guideline update [change in slope = 1.00 (95% CI −0.4 to 2.5, p = 0.13)]. Significant change in slope of strep IE incidence following guideline update [change in slope = 1.37 (95% CI 0.65–2.05, p = 0.002)]. Proportion of staph IE cases of total IE cases increased from 33% to 40% across study period (p < 0.001), proportion of strep IE cases of total IE cases increased from 24.8% to 27% across study period (p < 0.001) | NR | NR |
| Mackie et al.34 | 2007 (AHA) | All Canadian patients with IE excluding the Canadian provinces of Quebec and the Northern Territories | Interrupted time series analysis | Steady increase in incidence over study period (pre-guideline incidence 0.05/10M/month, 95% CI 0.005–0.09; post-guideline incidence 0.07/10M/month, 95% CI −0.05 to −0.09; difference 0.02/10M/month, difference p = 0.5213). Significant increase in IE incidence slope 4 years after guideline updates | Staph aureus most common organism (29.4% of hospitalizations). Strep species second most common (25.3% of hospitalizations) | NR | NR |
| Keller et al.35 | 2009 (ESC) | German patients with staph or strep IE | Time-trend analysis of annual prevalence of IE. Absolute and relative increase in IE events following guideline update (Guideline publication year excluded) | Crude IE prevalence increased over study period, from 8283 cases/year prior to guideline updates to 10,455 cases/year after guideline updates. Annual IE prevalence increased from 2006 until 2010 (9.5–10.6 IE diagnoses/100,000 citizens) with larger increase from 2011 to 2014 (11.1–14.4 IE diagnoses/100,000 citizens, (95% CI 1.1–4.6, p = 0.006). Relative increase of 26% of the annual IE diagnosis | 20.8% (95% CI 18.2–22.8) of IE were due to streptococcus and 21.9% (95% CI 18.1–24.7) due to Staph infections. Prevalence increased continuously from 2005 to 2014 (mean prevalence: 2.4 and 2.5 per 100,000 citizens per year) | NR | Mortality higher for staph IE versus strep IE (21.6% versus 13.2%) |
| Toyoda et al.36 | 2007 (AHA) | Adults with first episode IE identified from Statewide Planning and Research Cooperative System database in New York and the Office of Statewide Health Planning and Development database in California | Segmented regression using Interrupted time series analysis. Age-, sex- and risk-adjusted incidence of IE and rates of AP with poisson regression | Crude incidence increased from 7.6 to 9.3 cases/100,000 annually. Standardised IE incidence remained stable from 7.8 to 7.8 cases/100,000 annually (95% CI −0.3% to 0.2%, p = 0.59) | Crude incidence of Strep IE remained stable (0.84–0.88 cases/100,000 annually, 95% CI −0.8% to 0.6%, p = 0.77), while adjusted incidence decreased (0.84–0.73 cases/100000 annually, 95% CI −1.8% to 0.7%, p < 0.001). Standardised incidence of Staph aureus IE increased from 2.1 to 2.9 cases/100000 annually (95% CI 0.6–1.4%, p < 0.001) | NR | Crude 90-day mortality unchanged over study period (23.9–24.2%, 95% CI −1.0% to 0.4%, p = 0.44). Adjusted mortality rates decreased 2%/year. Healthcare-associated IE significantly higher mortality than community-aquired (HR 1.52, 95% CI 1.48–1.56, p < 0.01) |
| Van Den Brink et al.37 | 2009 (ESC) | Patients with IE in one of three hospitals over study period used as sample of all IE cases nationwide | Segmented regression using Interrupted time series analysis | Incidence of IE increased significantly above the projected historical trend [30.2 new cases per million in 2005 to 62.9 cases per million in 2011 (p < 0.001)] following guideline update | blood-culture positive in 90.7% IE cases. Strep (37.4%) and Staph (36.1%) most prevalent. BC positive strep IE increased after guideline introduction from 31.1% to 53.2% (p = 0.003) | NR | Mortality ranged 37.1% pre-guidelines, to 32.2% post guidelines (p = 0.53). Women higher mortality than men following guideline update (49.3% versus 28.2%, p = 0.002). Age, female sex and conservative treatment all independent prognostic factors for mortality (p < 0.005) |
| Thornhill et al.24 | 2007 (AHA) | Adults >18 years with Medicare or employer-provided health insurance and linked prescription data | Age-, sex- and risk-adjusted incidence of IE and rates of AP with Poisson regression | Declining incidence over study period, rate of decline slowed following guideline update. In mod-high risk populations, 177% relative increase above pre-guideline predicted IE incidence rate (high risk patients to 30.57 cases/month/100,000; 75% increase in mod-risk to 3.41 cases/month/100,000, no sig increase in low risk) | NR | Following guideline update 20% reduction in AP prescriptions for high risk patients (reduction of 186 prescriptions/month/100,000), 64% reduction in mod-risk (reduction of 297 prescriptions/month/100,000), 52% reduction in low-risk (reduction of 45 prescriptions/month/100,000) | NR |
| Garg et al.23 | 2007 (AHA) | IE related hospitalisations in adults at moderate and high-risk of IE in Ontario. AP prescriptions from Ontario Drug Benefit database for >65 years | population-based, cross-sectional time series analysis of IE incidence and AP prescriptions. Changepoint analysis of IE incidence | Significant increase in IE rate 3 years after guideline introduction (mean IE rate increased from 872 ± 195 to 1385 ± 221 per million in high risk; 229 ± 45 to 283 ± 70 in mod risk; 32 ± 4 to 47 ± 5 in all risk >65 years). | 73.8% of IE cases had causative organism identified. Staph (30.3%) and strep (26.4%) most common organisms. No change in causative organism over time for >65 years; 18–64 years had increase in staph IE from 20% to 43% (p < 0.001) while decrease in strep (31–20%, p = 0.007) | Decrease in AP rates in both high risk (level of change −3889 ppm, p = 0.006) and mod-risk patients (level of change −6481 ppm, p < 0.001) post-guideline updates | crude 90-day mortality unchanged: 18–64 years: 18% to 18% (95% CI −1.26% to 2.61%, p = 0.50); >65 years: 38% to 36% (95% CI −2.31 to 0.74%, p = 0.31) |
| Pasquali et al.38 | 2007 (AHA) | All children (<18 years) hospitalised with IE from 37 pediatric hospitals across US. | Poisson regression analysis. Analysis conducted in two different high-risk subsets (CHD and age 5–18 years) | No change in IE incidence over study period. Decrease in incidence of oral strep IE. Estimated annual change in IE incidence prior to guideline updates: 1.4% (95% CI −2.9% to 5.8% + 1.6%), after guideline updates 3.0% (95% CI −1.9% to 8.1%), difference 1.6% (95% CI −6.4% to 10.3%, p = 0.70) | 52% of IE cases had strep or staph. Trend toward decrease over time in IE cases associated with oral strep. APC of oral strep IE prior to guideline update: −2.5% (95% CI −14.2% to 10.9%) and after guideline update: 19.1% (95% CI −32.4% to 3.1%). Difference 17.1% (95% CI −36.9% to 9.0%), p = 0.18 | NR | Mortality rate over study period 1.1% |
| Bates et al.39 | 2007 (AHA) | Children aged <18 years hospitalised with IE after receiving IV AP within 7 days preceding admission, across 27 hospitals in the US. Primary cohort 5–18 years with IV AP targeting strep | Segmented regression analysis of interrupted time series (analysed high-risk subgroup of CHD) | Increase in IE incidence over study period, no significant difference in the rate of change following guideline updates (p = 0.895). Mean rate of IE 4.6/10000 hospitalisations per semi-annual period prior to guideline updates, no change following guideline updates. Rate increase by 0.13 cases/10,000 hospitalisations per semi-annual period prior to guideline updates, 0.12 cases/10,000 hospitalisations post guideline updates (p = 0.895) | NR | NR | In-hospital mortality rate over study period 3.6% |
| Sakai Bizmarket al.40 | 2007 (AHA) | All children (<18 years) with IE in all US hospitals | Descriptive statistics of continuous variables, 2-sample t-test (continuous variables) and chi-square (categorical variables) | No significant difference in IE rates post guideline updates. Significant trend increases for IE due to VGS for ages >10 years. No changes on overall incidence of pediatric IE | Staphylococcus and VGS | NR | No change in in-hospital mortality or length of stay |
AP, antibiotic prophylaxis; APC, annual percentage change; CHD, congenital heart disease; CI, confidence Interval; IE, infective endocarditis; NR, not reported; PPM, prescriptions per million; Staph, staphylococcus; strep, streptococcus; VGS, viridans group streptococcus.
Pathogen characteristics
Adults
Twelve studies reported data on causative organisms.23,27,28,30,32–38,40 All studies reporting rates of pathogens identified rates of staphylococcal and streptococcal IE. The total number of IE cases with a causative pathogen identified data ranging from 62% to 91%. Streptococcal and staphylococcal species were the most commonly identified organisms.
Six studies reported increasing rates of IE due to staphylococcal species following IE guideline updates.23,28,30,34,36,37 The proportion of staphylococcal IE of total IE cases ranged from 21% to 43%. No studies reported a decrease in staphylococcal IE incidence over time, while four studies reported a significant increase. One study reported an increase in the adjusted incidence of staphylococcal IE36 from 2.1 to 2.9 cases per 100,000 persons annually (annual percentage change (APC) 1.0%; 95% CI 0.6–1.4%; p < 0.001). The other three studies reported an increase in the relative proportion of staphylococcus species IE 23,28,33 (range pre-guidelines 16–24.8%, range post-guidelines 26–43%).
Incidence trends of IE due to streptococci, however, were more heterogeneous. Two studies found no significant change27,28 and two North American studies reported a significant decline in rates of streptococcal IE.23,36 Three studies, from North America, Germany and The Netherlands, reported an increase in rates of streptococcal IE over the time periods studied.33,35,37 Two of these studies reported a significant increase in the trend of streptococcal IE following the guideline updates (change in slope pre and post-guideline 1.37; 95% CI 0.69–2.05; p = 0.002)33 and percentage of streptococcal species IE (31.1–53.2%; p = 0.003);37 however, both studies only adjusted incidence for population and did not adjust for patient demographics or risk stratification.
Children
Two studies reported on causative organisms, with conflicting results.38,40 The proportion of IE due to streptococci increased after guideline updates in one study from 23.8% to 32% (p = 0.01).40 In particular, viridans group streptococcus increased from 17.6% to 24.2% (p = 0.02). Conversely, a decrease in IE incidence due to oral streptococci was found by another study, with no change in this trend post-guideline changes.38
A summary of pathogen characteristics is presented in Table 5.
Antibiotic prescribing
Four studies including only adult patients reported AP prescribing patterns prior and subsequent to guideline changes. Three studies report a rapid and significant decline in AP prescription following changes in guidelines.24,27,31 Two studies report this decrease in AP prescription following NICE guideline updates,27,31 and one group following changes to the AHA guidelines.24 Subgroup analysis identified a smaller reduction in AP prescription in high-risk groups compared with moderate and low-risk groups.23,24 A summary of AP prescription rates is presented in Table 5.
Discussion
We have performed, to our knowledge, the largest analysis to date examining how the population incidence of IE has been effected by changes to international AP guidelines in both pediatric and adult patients. Furthermore, this review includes over 500,000 new cases of IE not previously analysed. This study has shown that the restriction of AP to only high-risk patients does not result in an increase in the incidence of streptococcal IE in North American populations. Our results also show that there has been a notable change in the microbiological profile of IE from fewer oral cavity organisms, to a higher percentage of staphylococcal species. However, the large number of publications within the past 4 years signifies an ongoing international apprehension towards AP restriction with the concern of increasing the incidence of IE. This is reiterated in observational studies that express similar concerns regarding AP restriction and increasing incidence of IE.10,41
The most recent studies included in this review have attempted to address some of the methodological, clinical and practical issues identified in earlier reviews. Time-trend analysis, causative organism identification and rates of AP prescriptions have been performed according to IE risk groups. Time-trend analysis alone has demonstrated varying results previously. In this review, crude incidence rates of IE following international guideline updates have uniformly continued to increase gradually, with no significant increase in the crude incidence rate after guideline introduction.34,36,39,40 Only one new study reported that the rate of IE increased significantly more than expected, after the change to European guidelines in 2009.37 This Dutch study used three hospitals as a national representation to extrapolate overall national incidence. Bias in hospital referral patterns and local community demographics may have influenced these results. The inclusion of other recent studies with more robust methodological quality provides reassurance that while the overall global incidence of IE is increasing, the rate of this increase has not changed significantly since the implementation of updated, guideline-directed AP restriction.23,24
The use of crude incidence has been criticised when reporting IE incidence changes. Adjustments for demographics such as age, sex and race as well as clinically based risk stratification has demonstrated differences in IE incidence when compared to crude incidence in several recent studies.23,24,36 Toyoda and colleagues demonstrate the importance of adjusting crude IE rates, as the significant increase in IE incidence seen when crude rates are analysed disappears with the adjustment of incidence for age, sex and race.36 Two other recent studies, both from North America, found an increase in the crude incidence of IE following guideline updates, while subgroup analysis stratified according to risk demonstrated that IE incidence in high-risk patients contributed the majority to the overall rise in incidence.23,24 The restriction of AP should not be a significant contributor to this increased incidence in high-risk populations, providing these high-risk patients continue to receive AP as those specific guidelines recommend. This hypothesis may explain the finding of a UK study, in which the incidence of IE increased significantly just 3 months after the NICE guidelines advised a cessation of AP for all risk groups, including high risk.31 Further analysis from that study to identify if the majority of IE incidence increase was in high-risk patients would be beneficial.
Accurate correlation of AP prescriptions with IE has been scarcely investigated until recent years. Prior to 2015, a single study had attempted to correlate rates of AP prescriptions in relation to IE incidence.27 Over the past 4 years, three further studies have analysed the incidence of IE and AP prescription rates.23,24,31 All three studies reported a significant decrease in AP prescriptions following guideline updates. The two North American studies found AP fell in moderate-risk patients (as advised) as well as a small, but significant decrease in AP prescription for high-risk patients.23,24 Furthermore, an increase in IE incidence was recorded in moderate-risk patients in both studies. Both of these studies concluded in support of recent guideline updates despite the increasing incidence of IE as no causal relationship could be identified. These conclusions were based on two common key findings. First, the increased IE incidence occurred 3–4 years after the guideline updates, not within the immediate months as may be expected. Second, the AP prescription rate fell in both high and moderate-risk patients. This suggests that the increase in IE incidence may be due to a lack of practitioner and patient adherence to recommendations rather than the specific guidelines themselves. Other studies have similarly found that AP prescription rates decreased for high-risk patients after the introduction of guideline updates.31 This decrease in AP for high-risk patients is a cause for concern and reasons for these trends warrant further investigation. Clearly identifying patients at high risk of IE is critical for appropriate AP. Furthermore, delineation of significant risk differences between groups also needs clarification as risk similarities have been shown between moderate-risk and high-risk conditions.42 Finally, practitioners responsible for prescribing AP require access to clear and specific recommendations in order clearly to identify patients at high risk of IE and provide appropriate AP. Consideration of expanding these indications to patients with other unrecognised high-risk features may be required.
The identification of causative organisms, particularly streptococcal species, has been a focus of recent studies investigating incidence trends following guideline updates. This is based on the hypothesis that an increase in IE incidence following AP restriction should be due, at least in part, to an increasing rate of streptococcal infections. Results varied across included studies for the incidence of streptococcal IE. Increases and decreases in streptococcal IE incidence were reported in multiple continents over multiple timeframes in both adults and children. However, in the most recent North American studies with the most thorough analysis (adjusted incidence rates for patient demographics), the incidence of streptococcal IE has been observed either to be stable or decreasing after changes to AP guideline recommendations.23,36 These findings are consistent with a recent South American observational study of 167 patients which reported streptococci as being only the fourth most prevalent causative organism for IE behind Staphylococcus aureus, enterococcus and coagulase negative staphylococci.43 The explanation for the discrepancies in streptococcal IE incidence throughout the included studies is likely to be multifactorial. Most studies were not able to ascertain a causative organism for all IE cases and several studies recognised difficulties in accurately coding microbiological data. Despite these limitations, any report of an increase in streptococcal IE following the restriction of AP that specifically targets streptococcal infection should not be ignored. Further investigation using risk-stratified data in those countries reporting an increase in streptococcal IE and whose timing coincides with the guideline updates may further delineate these results.
In contrast to the discrepancies of streptococcal IE incidence, the incidence of enterococcus (group D streptococci) and staphylococcal IE was almost unanimously reported to be increasing. This is most clear in high-risk patients with prosthetic valves and intra-cardiac devices.30,36,37,44 There has been a precipitous increase in both implanted cardiac devices and prosthetic valve implantation due to improvements in device implantability and expanding indications for these devices.45–47 The rapid expansion of bioprosthetic valve implantation via transcatheter routes, particularly transcatheter aortic valve replacement (TAVR), invariably exposes another cohort of patients to an increased risk of IE who previously may not have been eligible for surgical valve replacement. Bioprosthetic valves implanted via transcatheter routes have shown similar rates of IE to traditional surgical valves48 and aortic valve implantation rates are increasing worldwide. While the surgical management of IE has remained relatively constant over the course of studies included in this review (data not shown), the increasing complexity of high-risk patients and therefore IE patients makes the decision for and timing of surgery in IE difficult. The changing clinical parameters, patient demographics and causative IE organisms may require reconsideration in future AP recommendations.
The main limitation surrounding this review is the inconsistency in reporting metrics across the included studies and lack of quantitative synthesis. Another limitation which needs to be considered when interpreting the qualitative synthesis presented in this study is that the included studies report population level and registry data. Another important consideration is the possibility of overlapping or duplicated patients within the included studies, as a number of these studies are from the same country utilizing national registry data over similar time periods. In addition, due to the inclusion criteria of only studies published in English being included in this review, it is important to consider the possible selection bias that may be present using this search strategy/inclusion criteria.
Conclusion
The restriction of AP to only high-risk patients does not result in an increase in the incidence of streptococcal IE in North American populations, while further investigation is required to clarify the IE incidence in the UK and some European countries. Clarification about risk stratification of patients is required and the importance of AP for appropriate patients must be emphasized for clinicians in prescribing positions. There has been a notable change in the microbiological profile of IE from fewer oral cavity organisms to a higher percentage of staphylococcal species. This change reflects the changing demographic and clinical profile of patients with IE. Future studies should focus on countries where the impact of AP restriction remains unclear, as well as further investigate AP prescription rates for high-risk patients. Accurate identification of causative organisms is also vital in future analyses.
Supplemental Material
Supplemental material, sj-pdf-1-tak-10.1177_17539447211002687 for Epidemiology of infective endocarditis before versus after change of international guidelines: a systematic review by Michael L. Williams, Mathew P. Doyle, Nicholas McNamara, Daniel Tardo, Manish Mathew and Benjamin Robinson in Therapeutic Advances in Cardiovascular Disease
Footnotes
Authors’ Note: Benjamin Robinson is also affiliated with “The Baird Institute of Applied Heart & Lung Surgical Research, Sydney, Australia.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The Baird Institue of Applied Heart & Lung Surgical Research provided financial support for the publication of this article.
ORCID iD: Michael L. Williams  https://orcid.org/0000-0002-7002-9728
https://orcid.org/0000-0002-7002-9728
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Michael L. Williams, Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, 50 Missenden Road, Camperdown, NSW 2050, Australia.
Mathew P. Doyle, Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, Sydney, NSW, Australia Centre for Human and Applied Physiology, School of Medicine, University of Wollongong, Keiraville, Australia.
Nicholas McNamara, Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, Sydney, Australia.
Daniel Tardo, Department of Medicine, St Vincents Hospital, Sydney, NSW, Australia; School of Medicine, University of Notre Dame, Sydney, NSW, Australia.
Manish Mathew, Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, Sydney, Australia.
Benjamin Robinson, Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, Sydney, Australia.
References
- 1. Fye WB. Jean Francois Fernel. Clin Cardiol 1997; 20: 1037–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osler W. The Gulstonian lectures, on malignant endocarditis. BMJ 1885; 1: 467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001; 345: 1318–1330. [DOI] [PubMed] [Google Scholar]
- 4. Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J 2010; 31: 1890–1897. [DOI] [PubMed] [Google Scholar]
- 5. Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012; 54: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 6. Heiro M, Helenius H, Hurme S, et al. Long-term outcome of infective endocarditis: a study on patients surviving over one year after the initial episode treated in a Finnish teaching hospital during 25 years. BMC Infect Dis 2008; 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart 2014; 100: 524–527. [DOI] [PubMed] [Google Scholar]
- 8. Dayer M, Thornhill M. Is antibiotic prophylaxis to prevent infective endocarditis worthwhile? J Infect Chemother 2018; 24: 18–24. [DOI] [PubMed] [Google Scholar]
- 9. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013; 8: e82665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vogkou CT, Vlachogiannis NI, Palaiodimos L, et al. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis 2016; 35: 1227–1245. [DOI] [PubMed] [Google Scholar]
- 12. Cahill TJ, Harrison JL, Jewell P, et al. Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart 2017; 103: 937–944. [DOI] [PubMed] [Google Scholar]
- 13. Meel R, Essop MR. Striking increase in the incidence of infective endocarditis associated with recreational drug abuse in urban South Africa. S Afr Med J 2018; 108: 585–589. [DOI] [PubMed] [Google Scholar]
- 14. Østergaard L, Valeur N, Ihlemann N, et al. Incidence and factors associated with infective endocarditis in patients undergoing left-sided heart valve replacement. Eur Heart J 2018; 39: 2668–2675. [DOI] [PubMed] [Google Scholar]
- 15. Østergaard L, Valeur N, Wang A, et al. Incidence of infective endocarditis in patients considered at moderate risk. Eur Heart J 2019; 40: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 16. Jordal S, Kittang BR, Salminen PR, et al. Infective endocarditis in Western Norway: a 20-year retrospective survey. Infect Dis (Lond) 2018; 50: 757–763. [DOI] [PubMed] [Google Scholar]
- 17. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation 1997; 96: 358–366. [DOI] [PubMed] [Google Scholar]
- 18. Horstkotte D, Follath F, Gutschik E, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the Task Force on Infective Endocarditis of the European Society of Cardiology. Eur Heart J 2004; 25: 267–276. [DOI] [PubMed] [Google Scholar]
- 19. Danchin N, Duval X, Leport C. Prophylaxis of infective endocarditis: French recommendations 2002. Heart 2005; 91: 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for infection and cancer. Eur Heart J 2009; 30: 2369–2413. [DOI] [PubMed] [Google Scholar]
- 21. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116: 1736–1754. [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence. Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. London: National Institute for Health and Clinical Excellence, 2008. [PubMed] [Google Scholar]
- 23. Garg P, Ko DT, Bray Jenkyn KM, et al. Infective endocarditis hospitalizations and antibiotic prophylaxis rates before and after the 2007 American Heart Association guideline revision. Circulation 2019; 140: 170–180. [DOI] [PubMed] [Google Scholar]
- 24. Thornhill MH, Gibson TB, Cutler E, et al. Antibiotic prophylaxis and incidence of endocarditis before and after the 2007 AHA recommendations. J Am Coll Cardiol 2018; 72: 2443–2454. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ 2011; 342: d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol 2012; 59: 1968–1976. [DOI] [PubMed] [Google Scholar]
- 29. Bikdeli B, Wang Y, Kim N, et al. Trends in hospitalization rates and outcomes of endocarditis among medicare beneficiaries. J Am Coll Cardiol 2013; 62: 2217–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bor DH, Woolhandler S, Nardin R, et al. Infective endocarditis in the US, 1998–2009: a nationwide study. PLoS One 2013; 8: e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet 2015; 385: 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeSimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of infective endocarditis due to viridans group streptococci before and after the 2007 American Heart Association’s prevention guidelines: an extended evaluation of the Olmsted County, Minnesota, population and nationwide inpatient sample. Mayo Clin Proc 2015; 90: 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65: 2070–2076. [DOI] [PubMed] [Google Scholar]
- 34. Mackie AS, Liu W, Savu A, et al. Infective endocarditis hospitalizations before and after the 2007 American Heart Association prophylaxis guidelines. Can J Cardiol 2016; 32: 942–948. [DOI] [PubMed] [Google Scholar]
- 35. Keller K, von Bardeleben RS, Ostad MA, et al. Temporal trends in the prevalence of infective endocarditis in Germany between 2005 and 2014. Am J Cardiol 2017; 119: 317–322. [DOI] [PubMed] [Google Scholar]
- 36. Toyoda N, Chikwe J, Itagaki S, et al. Trends in infective endocarditis in California and New York State, 1998–2013. JAMA 2017; 317: 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van den Brink FS, Swaans MJ, Hoogendijk MG, et al. Increased incidence of infective endocarditis after the 2009 European Society of Cardiology guideline update: a nationwide study in the Netherlands. Eur Heart J Qual Care Clin Outcomes 2017; 3: 141–147. [DOI] [PubMed] [Google Scholar]
- 38. Pasquali SK, He X, Mohamad Z, et al. Trends in endocarditis hospitalizations at US children’s hospitals: impact of the 2007 American Heart Association antibiotic prophylaxis guidelines. Am Heart J 2012; 163: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bates KE, Hall M, Shah SS, et al. Trends in infective endocarditis hospitalisations at United States children’s hospitals from 2003 to 2014: impact of the 2007 American Heart Association antibiotic prophylaxis guidelines. Cardiol Young 2017; 27: 686–690. [DOI] [PubMed] [Google Scholar]
- 40. Sakai Bizmark R, Chang RR, Tsugawa Y, et al. Impact of AHA’s 2007 guideline change on incidence of infective endocarditis in infants and children. Am Heart J 2017; 189: 110–119. [DOI] [PubMed] [Google Scholar]
- 41. Fedeli U, Schievano E, Buonfrate D, et al. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis 2011; 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thornhill MH, Jones S, Prendergast B, et al. Quantifying infective endocarditis risk in patients with predisposing cardiac conditions. Eur Heart J 2018; 39: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tagliari AP, Steckert GV, da Silveira LMV, et al. Infective endocarditis profile, prognostic factors and in-hospital mortality: 6-year trends from a tertiary university center in South America. J Card Surg 2020; 35: 1905–1911. [DOI] [PubMed] [Google Scholar]
- 44. Ambrosioni J, Hernandez-Meneses M, Téllez A, et al. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep 2017; 19: 21. [DOI] [PubMed] [Google Scholar]
- 45. Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121: 458–477. [DOI] [PubMed] [Google Scholar]
- 46. Bhatia N, El-Chami M. Leadless pacemakers: a contemporary review. J Geriatr Cardiol 2018; 15: 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neylon A, Ahmed K, Mercanti F, et al. Transcatheter aortic valve implantation: status update. J Thorac Dis 2018; 10 (Suppl. 30): S3637–S3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ando T, Ashraf S, Villablanca PA, et al. Meta-analysis comparing the incidence of infective endocarditis following transcatheter aortic valve implantation versus surgical aortic valve replacement. Am J Cardiol 2019; 123: 827–832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tak-10.1177_17539447211002687 for Epidemiology of infective endocarditis before versus after change of international guidelines: a systematic review by Michael L. Williams, Mathew P. Doyle, Nicholas McNamara, Daniel Tardo, Manish Mathew and Benjamin Robinson in Therapeutic Advances in Cardiovascular Disease