Significance
Interactions between the nervous system and immune system are central regulators of chronic itch, a key feature of pathologies like atopic dermatitis and allergic contact dermatitis. Cysteinyl leukotrienes (LTC4, LTD4, and LTE4) are eicosanoid lipids known for mediating inflammation, bronchoconstriction, and vascular leakage. We demonstrate here that CysLTs are potent itch inducers and that this effect depends on the specific coupling of LTC4 with its receptor CysLT2R, which is expressed in a population of peripheral sensory neurons in the mouse and in human. We show that the LTC4/CysLT2R pathway contributes to a model of chronic itch, suggesting that CysLT2R could be a new therapeutic target for intractable chronic itch.
Keywords: itch, neuroimmune, atopic dermatitis, skin, inflammation
Abstract
Acute and chronic itch are burdensome manifestations of skin pathologies including allergic skin diseases and atopic dermatitis, but the underlying molecular mechanisms are not well understood. Cysteinyl leukotrienes (CysLTs), comprising LTC4, LTD4, and LTE4, are produced by immune cells during type 2 inflammation. Here, we uncover a role for LTC4 and its signaling through the CysLT receptor 2 (CysLT2R) in itch. Cysltr2 transcript is highly expressed in dorsal root ganglia (DRG) neurons linked to itch in mice. We also detected CYSLTR2 in a broad population of human DRG neurons. Injection of leukotriene C4 (LTC4) or its nonhydrolyzable form NMLTC4, but neither LTD4 nor LTE4, induced dose-dependent itch but not pain behaviors in mice. LTC4-mediated itch differed in bout duration and kinetics from pruritogens histamine, compound 48/80, and chloroquine. NMLTC4-induced itch was abrogated in mice deficient for Cysltr2 or when deficiency was restricted to radioresistant cells. Itch was unaffected in mice deficient for Cysltr1, Trpv1, or mast cells (WSh mice). CysLT2R played a role in itch in the MC903 mouse model of chronic itch and dermatitis, but not in models of dry skin or compound 48/80- or Alternaria-induced itch. In MC903-treated mice, CysLT levels increased in skin over time, and Cysltr2−/− mice showed decreased itch in the chronic phase of inflammation. Collectively, our study reveals that LTC4 acts through CysLT2R as its physiological receptor to induce itch, and CysLT2R contributes to itch in a model of dermatitis. Therefore, targeting CysLT signaling may be a promising approach to treat inflammatory itch.
Itch, or pruriception, is defined as an uncomfortable sensation that triggers the desire to scratch, and is mediated by peripheral sensory neurons termed pruriceptors (1, 2). Increasing evidence indicates that inflammatory mediators are released into the skin by immune cells and other cell types that directly activate or sensitize pruriceptors to produce itch. Chronic itch is a debilitating symptom of many skin pathologies, including atopic dermatitis (AD) and allergic contact dermatitis (ACD), and the roles of individual molecular pathways in chronic itch are not clearly defined. While certain classes of mediators such as histamine, proteases, and cytokines have been investigated more recently, less is known about the roles of lipid mediators in itch. There is a great need for better understanding of the molecular mechanisms leading to itch and to develop novel therapeutic modalities to treat itch.
Leukotrienes (LTs) are eicosanoid lipid mediators generated upon activation of both immune and structural cells. LTs are comprised of LTB4 and the cysteinyl LTs (CysLTs; LTC4, LTD4, and LTE4; Fig. 1A). They were named “leukotrienes” to highlight their originally defined source: leukocytes, including mast cells (MCs), eosinophils, basophils, and macrophages (3). More recently, platelet–neutrophil aggregates (4) and tuft cells (5), which are specialized epithelial cells, were identified as potent producers of CysLTs, highlighting the ubiquitous and versatile sources of CysLTs during inflammation. The biosynthesis of LTs begins when arachidonic acid is liberated from membrane phospholipids and is converted into LTA4 by the enzyme 5-lipoxygenase (5-LO) in the presence of the 5-LO–associated protein (FLAP; Fig. 1A). LTA4 hydrolase processes LTA4 into LTB4, which binds to the LTB4 receptors. LTC4 synthase (LTC4S), at the outer nuclear membrane, conjugates LTA4 with reduced glutathione to produce LTC4, the first and only intracellular CysLT (6). LTC4 is rapidly transported extracellularly (within minutes) and converted sequentially by membrane-bound γ-glutamyl transferases and dipeptidases to LTD4 and LTE4. CysLTs exert their effects through three G protein-coupled receptors (GPCRs)—CysLT1R, CysLT2R, and CysLT3R—with different affinities. CysLT1R and CysLT2R, the receptors for the short-lived LTC4 and LTD4, are widely expressed in hematopoietic and structural cells. The stable end metabolite LTE4 binds to the epithelial CysLT3R and mediates mucin release in response to the airborne fungus Alternaria (7, 8). Pharmacologic studies using heterologous transfectants indicated that CysLT1R is the high-affinity receptor for LTD4 and binds LTC4 with lesser affinity. By contrast, CysLT2R binds LTC4 and LTD4 at equimolar concentrations (9, 10). However, in vivo selectivity for LTC4 and LTD4 differ depending on the tissue distribution, frequency of CysLT receptor-expressing cells, and proximity to the ligand source (11–14). The brief half-life of LTC4 within the tissue likely requires close proximity between the LTC4 source and the target cell. CysLTs are potent inducers of airway smooth muscle constriction, vascular permeability, leukocyte recruitment, and chemokine production (3). Classically, the proinflammatory effects of CysLTs are attributed to the CysLT1R receptor, which participates in the recruitment of eosinophils, the activation of ILC2s, and Th2 response during airway inflammation (12, 15, 16). The CysLT1R-specific antagonist montelukast is widely used to treat bronchoconstriction and inflammation in asthmatic patients (17, 18). CysLT2R is resistant to montelukast, and its role is not understood as well as CysLT1R. CysLT2R signaling in platelets is required for type 2 lung inflammation and MC activation (19). Additionally, CysLT2R has a newly defined role in lung metastasis through an effect on angiogenesis (20). Eosinophil-derived LTC4 also mediates skin fibrosis and inflammation through CysLT2R in an ovalbumin-induced mouse model of AD (14). Several groups have worked to elucidate the role of LTB4 and its receptors in pain and itch (21–24). However, the role of CysLTs and their associated receptors in itch has not been well studied. Recent transcriptome studies suggest Cysltr2 to be expressed in sensory neurons, but how CysLT2R regulates neural responses and itch behavior in vivo has not been clarified.
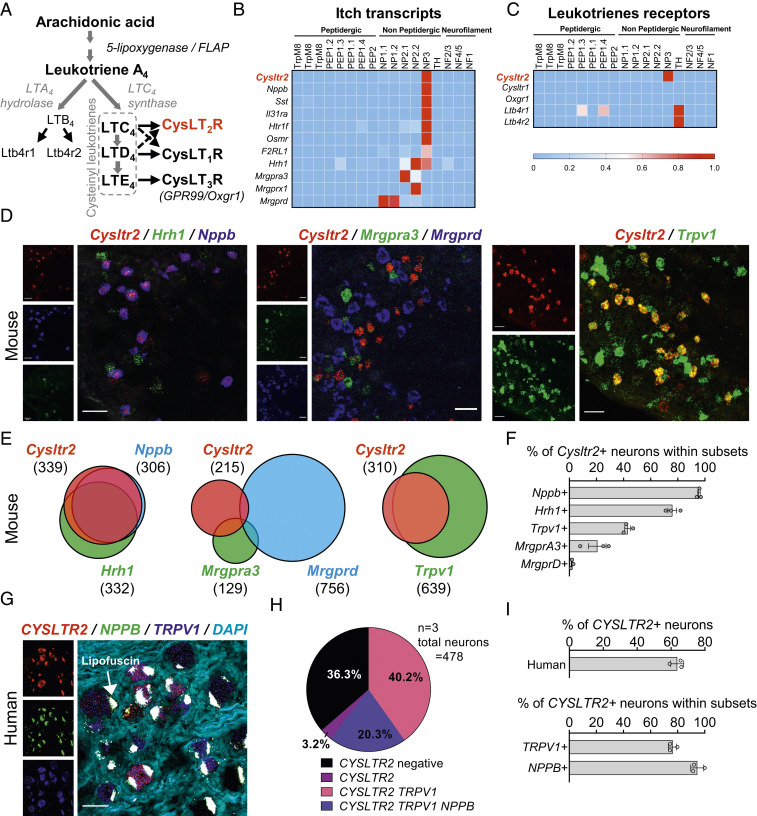
Fig. 1.
Cysltr2 is expressed on a subset of DRG sensory neurons. (A) Diagram of the LT pathway. (B and C) Expression of selected transcripts (B, related to itch; C, LT receptors) in mouse DRG neuron populations clustered into functional subsets based on single-cell RNA-seq data. Full dataset and methods are available in a previous study (32). (D) Triple/double-label ISH done with RNAscope in DRG (Left, Cysltr2, red; Nppb, blue; Hrh1, green; Middle, Cysltr2, red; MrgprA3, green; Mrgprd, blue; Right, Cysltr2, red; Trpv1, green). (Scale bar: 50 µm.) (E and F) ISH quantification: (E) Venn diagram representing overlap between markers and (F) percentages of Cysltr2+ neurons within subsets defined by other markers (n = 3 mice). (G) Representative images of human DRG labeled with RNAscope ISH for CYSLTR2 (red), NPPB (green), and TRPV1 (blue) and costained with DAPI (cyan). Lipofuscin (globular structures) that autofluoresced in all three channels and appear white in the overlay image were not analyzed, as this is background signal that is present in all human nervous tissue. (Scale bar: 50 μm.) (H) Pie chart displaying the distribution of CYSLTR2 neuronal subpopulations in human DRG. (I) Percentage of all human sensory neurons expressing CYSLTR2 transcript (Top) and of TRPV1 neurons and NPPB neurons coexpressing CYSLTR2 (Bottom). Values presented as mean ± SEM.
Pruriceptors are primary sensory neurons that mediate itch, whose cell bodies reside in the dorsal root ganglia (DRG) and trigeminal ganglia (25). Pruriceptors express molecular receptors at their peripheral nerve terminals in epidermal layers of the skin that respond to pruritogens including histamine, cytokines, and proteases. HRH1 and HRH4 are the major histamine receptors linked to pruriceptor activation and itch (26, 27). IL-4, IL-13, and IL-31 are cytokines that mediate itch via their cognate receptors expressed by pruriceptors (28, 29). Chloroquine (CQ) and BAM8-22, an endogenous peptide derived from proenkephalin, act via the Mas-related G protein receptors MrgprA3 and MrgprC11, respectively, to produce itch (30). Recent transcriptomic analyses have revealed distinct subsets of DRG neurons that may correspond to distinct pruriceptor subtypes and function, known as NP1, NP2, and NP3 neurons (31, 32). NP1 neurons express Mrgprd that responds to β-alanine to produce itch (33); NP2 neurons express Mrgpra3 and Mrgprc11 (30); NP3 neurons express Il31ra and Osmr (Oncostatin M receptor), which bind the pruritogenic cytokine IL-31 (28). NP3 neurons are also characterized by expression of neuropeptides Nppb and Sst, which have both been functionally linked to neurotransmission in itch (34–36).
In our molecular analysis as well in published datasets from other groups, we find that Cysltr2 is highly enriched in the NP3 subset of DRG neurons (31, 32). However, the functional role of CysLT2R in pruriception, and the role of specific CysLTs (LTC4, LTD4, LTE4) in itch, are unknown. Given that CysLTs are characteristic of type 2 immune cell activation in tissues during allergies, neuronal CysLT2R could allow the immediate response and induction of itch coupled to allergic-type inflammation.
In this study, our goal was to determine the functional role of specific CysLTs and CysLT2R in itch induction and skin inflammation. We find that Cysltr2 is enriched in pruriceptor-lineage DRG sensory neurons in mouse and is also expressed in human DRG neurons. LTC4, but not LTD4 or LTE4, induces acute itch behaviors in mice that differ in duration and quality from other pruritogens. We demonstrate that LTC4-induced itch is dependent on the CysLT2R receptor. Bone-marrow chimeras show that radioresistant cells expressing CysLT2R are necessary for LTC4-induced itch. Levels of LTC4 are elevated in the late stage of a mouse model of chronic dermatitis and itch, and Cysltr2−/− mice have a decreased scratching phenotype at this time point. By contrast, CysLT2R did not mediate itch in other inflammatory skin models that we tested. Overall, our findings show that CysLTs play a role in itch in acute and chronic situations by acting through the CysLT2R receptor.
Results
Expression of Cysltr2 Receptor in a Pruriceptive Subset of DRG Neurons.
Molecular and genetic analysis of DRG neurons has recently identified distinct neurons linked to itch and other somatosensory functions (31, 37, 38). We previously performed single-cell profiling of FACS-sorted Nav1.8-lineage and Parvalbumin-lineage mouse DRG neurons (39). We observed a population cluster (VI) from Nav1.8-lineage neurons with enriched levels of itch-associated transcripts including Il31ra, the receptor for the cytokine IL-31 that drives pruritus (28, 40), and Nppb, a neuropeptide that mediates neurotransmission of itch signaling from DRG to the spinal cord (36). We found that Cysltr2, the receptor for LTC4, was highly expressed in the same neuronal subset (SI Appendix, Fig. S1A). Other recently published single-cell RNA sequencing datasets of mouse DRG neurons revealed similar expression of Cysltr2 in a population of neurons expressing Il31ra, Nppb, Hrh1, and Sst, termed NP3 neurons (31, 32, 38, 41). The Cysltr2+ population is distinct from neurons expressing Mrgpra3 or Mrgprd (Fig. 1B), markers of NP2 and NP1 neurons, respectively (42).
CysLTs are synthesized as part of arachidonic acid metabolism, where LTA4 is processed by LTC4S into LTC4, which is subsequently metabolized to LTD4 and LTE4 (Fig. 1A). These ligands bind with different affinities to the receptors CysLT1R, CysLT2R, or CysLT3R (Oxgr1). Our analysis of the publicly available mouse RNA-sequencing (RNA-seq) dataset showed that Cysltr2 was the only CysLT receptor expressed in sensory neurons, as transcripts for Cysltr1 and Oxgr1 were not detected (Fig. 1C). Ltb4r1 and Ltb4r2, the receptors for LTB4, were absent from pruriceptors but highly expressed in tyrosine hydroxylase (TH) neurons, a population of unmyelinated low-threshold mechanoreceptors (C-LTMRs) characterized by the expression of TH and associated with pleasant touch; Ltb4r1 was additionally expressed in peptidergic subsets (PEP1.3 and PEP1.4; Fig. 1C). These data led us to focus on analysis of the role of CysLT2R in itch signaling.
To confirm the presence of Cysltr2 transcript in mouse DRG neurons, we performed RNAscope in situ hybridization analysis. Cysltr2 was expressed in 9.4 ± 1.9% of neurons marked by neuronal markers Tubb3 (β-3 tubulin) and in 11.6 ± 2.2% of neurons marked by Scn10a (Nav1.8; SI Appendix, Fig. S1 C and D). Cysltr2 overlapped extensively with Nppb: 95.7 ± 0.9% of Nppb+ neurons were Cysltr2+, whereas 86.0 ± 3.1% of Cysltr2+ neurons are also Nppb+ (Fig. 1 D–F). While still overlapping, 76.0 ± 3.2% of Cysltr2+ neurons were Hrh1+ and 74.7 ± 3.3% of Hrh1+ neurons were Cysltr2+, which confirms that the histamine receptor H1 is not completely restricted to the NP3 population (41). RNAscope analysis confirmed that Mrgpra3, which marks NP2 neurons, and Mrgprd, which marks NP1 neurons, had very little overlap with Cysltr2+ neurons (Fig. 1 D–F), with 10.3 ± 3.2% and 7.0 ± 1.6% of Cysltr2+ neurons being, respectively, Mrgpra3+ and Mrgprd+, and, inversely, 20.7 ± 6.4% of Mrgpra3+ neurons and 2.0 ± 0.6% of Mrgprd+ neurons being Cysltr2+. The transient receptor potential (TRP) channel TrpV1 has been shown to play a role in histamine-dependent itch (26). RNA-seq data show that this ion channel is expressed in NP3 neurons (SI Appendix, Fig. S1B). Using RNAscope, we confirmed that the majority of Cysltr2+ neurons (87.3 ± 2.2%) were Trpv1+, while 43.0 ± 2.1% of Trpv1+ neurons are Cysltr2+ (Fig. 1 D–F).
A recent study performed developmental analysis of DRG neurons at the single-cell level to examine the evolution of transcript expression in sensory neurons at different stages of mouse development (SI Appendix, Fig. S1 E and F) (38). Our analysis of this database showed that DRG neurons started expressing Cysltr2 after postnatal day 0 (SI Appendix, Fig. S1 E and F), and that it remained restricted to the same lineage of Sst+ neurons from the moment it was expressed into adulthood (SI Appendix, Fig. S1F).
We next characterized the expression of CYSLTR2 in human DRGs using RNAscope analysis (Fig. 1G and SI Appendix, Fig. S1G). We found that 63.6 ± 2.2% of human DRG neurons expressed CYSLTR2 (Fig. 1 G–I), which is broader in expression than in mouse, and these neurons ranged in size from 30 to 122 µm (SI Appendix, Fig. S1H). As in mouse, the majority of NPPB+ neurons (94.5 ± 2.8%) coexpressed CYSLTR2, as well as a large proportion of TRPV1+ neurons (76.3 ± 1.8%; Fig. 1 H and I). Overall, we found that Cysltr2 was expressed in sensory neurons from both mice and humans that overlapped with NPPB, with a broader CYSLTR2 expression in humans.
LTC4 Specifically Induces Dose-Dependent Acute Itch.
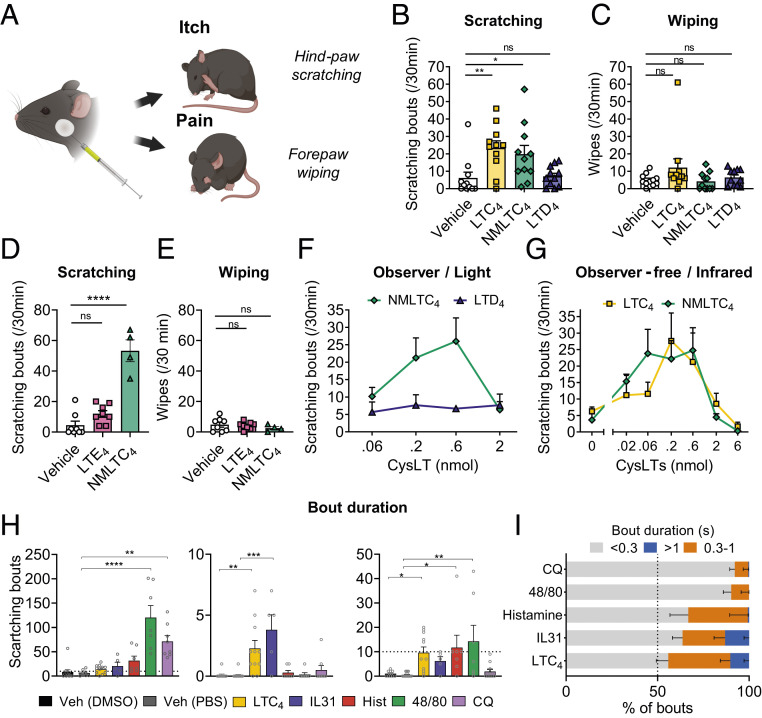
We next determined whether specific CysLTs induced itch when injected in vivo. Cheek injection of ligands into mice allows the distinguishing of pruritogens vs. algogens based on whether they trigger hind-paw scratching vs. nocifensive forepaw wiping behaviors, respectively (43) (Fig. 2A). For our behavioral analysis, we utilized an infrared behavior observation box (iBOB) in order to analyze scratching and wiping behaviors in the dark using infrared LEDs. We first injected the known ligands of CysLT2R, LTC4, or LTD4. Because LTC4 is rapidly converted to LTD4 at the membrane, we used, in addition, a version of LTC4 conjugated with an N-methyl group (NMLTC4), which is a nonhydrolyzable form resistant to conversion to LTD4. We found that, while LTC4 and NMLTC4 cheek injections induced robust scratching behaviors, neither vehicle nor LTD4 injections induced scratching (Fig. 2B). This was the case when quantified as total scratching bouts or duration of scratching (Fig. 2B and SI Appendix, Fig. S2A). LTC4 and NMLTC4 cheek injections did not induce wiping with the forepaws, indicative of pain (Fig. 2C). We next tested whether LTE4, which is the terminal metabolite of the CysLT pathway, also had the ability to induce itch. We found that, while NMLTC4 induced robust scratching behaviors, LTE4 injections did not induce itch (Fig. 2D). Neither LTD4 nor LTE4 induced significant scratching or wiping behaviors over vehicle controls (Fig. 2 B and D). These data indicate that injections of LTC4 and NMLTC4, but not LTD4 nor LTE4, induced robust itch but not pain behaviors.
Fig. 2.
LTC4 but not LTD4 or LTE4 induces dose-dependent acute itch behaviors. (A) Diagram of experimental design of acute itch/pain induction by intradermal injection of ligands in the cheek of the mice. (B) Scratching bouts in response to vehicle, LTC4, NMLTC4, and LTD4 at 0.6 nmol (n = 8 to 11). (C) Wiping responses, indicative of pain, to vehicle, LTC4, NMLTC4, and LTD4 at 0.6 nmol (n = 8 to 11). (D) Scratching bouts in response to vehicle, NMLTC4, and LTE4 at 0.6 nmol (n = 4 to 8). (E) Wiping responses, indicative of pain, to vehicle, LTE4, and NMLTC4 at 0.6 nmol (n = 4 to 8). (F) Scratching bouts’ dose responses to NMTLC4 and LTD4 were recorded for 30 min at different concentrations and scored live (n = 3 to 12). (G) Scratching bouts’ dose responses to LTC4 and NMLTC4 were recorded for 30 min at different concentrations (n = 6 to 8). (H and I) Analysis of kinetics and scratching bout duration differences in response to various pruritogens: LTC4 (0.6 nmol), IL-31 (0.02 nmol), histamine (100 μg), compound 48/80 (100 μg), CQ (200 μg), and vehicles (phosphate-buffered saline [PBS] and dimethyl sulfoxide [DMSO]). (H) Absolute numbers of bouts shorter than 0.3 s, bouts between 0.3 and 1 s, and bouts longer than 1 s. (I) Distribution of bouts according to their duration. Values presented as mean ± SEM. One-way ANOVA with Dunnett’s posttest. ns, nonsignificant (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
We next determined whether NMLTC4 and LTC4 induced a dose-dependent scratching response. LTC4 and NMLTC4 scratching curves had a bell shape, peaking at 0.2 to 0.6 nmol, whereas higher doses of LTC4 and NMLTC4 did not induce itch (Fig. 2 F and G). As pain can inhibit itch, we checked whether NMLTC4 at higher doses could have a nociceptive effect; however, we detected no wiping indicative of pain (SI Appendix, Fig. S2B). LTD4 did not induce scratching at any of the doses tested (Fig. 2F). The scratching induced by LTC4 and NMLTC4 occurred mainly during the first 30 min and is gone by 45 min (SI Appendix, Fig. S2C). Our phenotype was not affected by the infrared setup: NMLTC4 induced itch that was similar when scored traditionally by observers in the light (Fig. 2F) as when recorded in iBOB (Fig. 2G). Therefore, LTC4, but not LTD4, gives a classic bell-shaped curve in dose dependency, which is similar to some other GPCR ligands, for acute itch induction.
LTC4 Induces Itch that Is Distinct in Quality Compared with Other Itch Ligands.
Most pruritogens have been classified based on their overall ability to induce itch, but the quality of the itch responses has not been well characterized. We next questioned whether scratching bouts induced by injections of different pruritogens could vary in kinetics or duration. We first looked at when itch occurred following intradermal cheek injections of several pruritogenic compounds: LTC4 (0.6 nmol), IL-31 (0.02 nmol), histamine (100 µg), compound 48/80 (100 µg), and CQ (200 µg; Fig. 2 H and I and SI Appendix, Fig. S2D). IL-31, histamine, and CQ induce itch by acting directly on sensory neurons, while compound 48/80 triggers itch by activating MC through Mrgprb2 receptors (44).
We first measured the length of individual scratching bouts induced by pruritogens, and we empirically divided bouts in three categories: short bouts (<0.3 s), medium bouts (0.3 to 1 s), and long bouts (>1 s). By this analysis, LTC4 induced a significant increase in medium and long bouts (Fig. 2H). Overall, 45% of bouts induced by LTC4 were medium to long bouts (>0.3 s; Fig. 2I). By contrast, CQ and compound 48/80 induced a majority of shorter bouts, with less than 10% of longer bouts (>0.3 s; Fig. 2I). IL-31 showed a similar profile of scratching bouts as LTC4, with a significant increase of long bouts and around 40% of medium-long bouts. Histamine produced a significant proportion of medium bouts but did not produce any long bouts (Fig. 2H). We observed that LTC4-, CQ-, and histamine-induced itch started within the first 5 min, beginning at 4.1 min, 4.7 min, and 4.6 min on average, respectively (SI Appendix, Fig. S2E), while IL-31–induced itch started at 8.7 min and 48/80-induced itch started at 11.1 min (SI Appendix, Fig. S2E). LTC4-induced itch is at the highest between 5 and 10 min (SI Appendix, Fig. S2C), while histamine-induced itch started and peaked at 10 min, CQ- and compound 48/80-induced itches started at 10 min and seemingly peaked at 15 to 20 min, whereas IL-31–induced itch was strongest at 25 to 30 min (SI Appendix, Fig. S2F).
Alloknesis is a form of itch that occurs when the skin gets sensitized by inflammatory mediators and responds to innocuous touch stimuli. For example, histamine injection induces strong alloknesis (45). The types of neurons involved and molecular mechanisms of alloknesis are different from acute ligand-induced itch (46). We asked whether LTC4 was able to induce alloknesis following injection. The nape of the neck was injected by ligands, followed by stimulation with a thin Von Frey filament (SI Appendix, Fig. S2G). Histamine injection was able to increase the number of responses to that filament within 20 min (SI Appendix, Fig. S2H), and this alloknesis lasted until 3 h after injection (SI Appendix, Fig. S2J). However, LTC4 did not induce sustained alloknesis during the first 60 min, nor at the 3 h time point (SI Appendix, Fig. S2 H–J). This shows that LTC4 can induce acute scratching and itch but does not mediate alloknesis in naïve animals. These data, taken together, show that LTC4 induces differences in kinetics and quality of itch following injection compared with other pruritogens.
LTC4-Mediated Itch Is Dependent on CysLT2R.
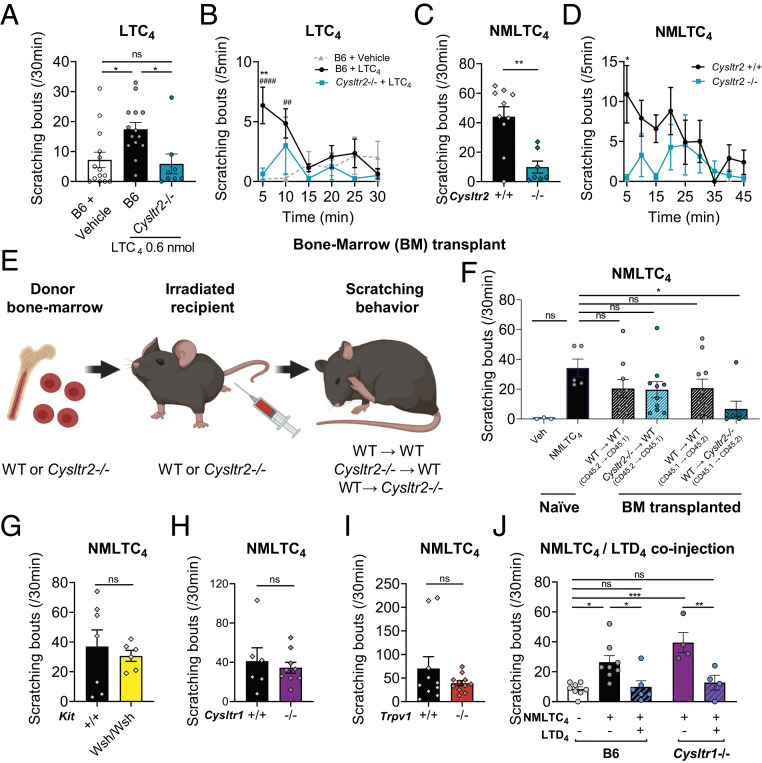
We next determined the functional role of the CysLT2R receptor in CysLT-induced itch. In studies in the lung and also in heterologous systems, LTC4 has been found to bind to both CysLT2R and CysLT1R with different affinities; therefore, it is important to clarify the roles of these receptors in vivo in itch. LTC4-induced itch was significantly decreased in Cysltr2−/− mice compared with Cysltr2+/+ control littermates (SI Appendix, Fig. S3 A and B). Levels of LTC4-induced scratching in Cysltr2−/− mice did not appear to be completely gone (SI Appendix, Fig. S3A), so, to elucidate whether some residual itch was present, we repeated this experiment including a vehicle condition in wild-type (WT) mice as a comparison for baseline itch. We confirmed with this experiment that LTC4-induced scratching was significantly decreased in Cysltr2−/− mice to levels compared with the vehicle condition (Fig. 3 A and B). NMLTC4-induced itch was eliminated in Cysltr2−/− mice compared to Cysltr2+/+ control littermates, indicating a major role for Cysltr2 in mediating this itch (Fig. 3 C and D).
Fig. 3.
LTC4-induced itch is dependent on CysLT2R. (A and B) Scratching bouts induced by intradermal cheek injection of vehicle or LTC4 0.6 nmol in B6 mice or by injection of LTC4 0.6 nmol in Cysltr2−/− mice (7 to 44 wk old; A) and detailed scratching bout kinetics over 30 min (#B6 + LTC4 vs. B6 + vehicle; *B6 + LTC4 vs. Cysltr2−/− + LTC4; B). (C and D) Scratching bouts induced by intradermal cheek injection in Cysltr2+/+ or Cysltr2−/− mice of NMLTC4 (0.6 nmol; C) and detailed scratching bout kinetics over 45 min (D). (E and F) Generation of BM chimeras with WT or Cysltr2−/− mice as donors and WT or Cysltr2−/− mice as recipients. (E) Diagram of BM transplant procedure. (F) Scratching bouts induced by intradermal cheek injection of NMLTC4 0.6 nmol in naïve mice and in BM chimera. Behavior recorded in daylight settings (not in iBOB). (G–I) Scratching bouts induced by intradermal cheek injection of NMLTC4 (0.6 nmol) in (G) KitWsh/Wsh mice, (H) Cysltr1−/− mice, and (I) Trpv1−/− mice. (J) Scratching bouts induced by the coinjection of NMLTC4 (0.6 nmol) and LTD4 (2 nmol) in B6 mice and in Cysltr1−/− mice. Values presented as mean ± SEM. Unpaired t test (C, G, and I), repeated-measures two-way ANOVA with Šidák’s posttest (B and D), one-way ANOVA with Dunnett’s posttest (F), and one-way ANOVA with Tukey’s posttest (A and J). ns, nonsignificant (*P < 0.05; **P < 0.01; ***P < 0.001).
We next generated bone marrow (BM) chimeric mice to determine which cells expressing CysLT2R were involved in CysLT-induced itch, as the receptor is expressed on numerous immune cells in addition to sensory neurons. WT or Cysltr2−/− mice were lethally irradiated to eliminate radiosensitive hematopoietic cells but not somatic cells (including neurons), followed by transplantation with WT or Cysltr2−/− BM, thus reconstituting radiosensitive hematopoietic cells with WT or Cysltr2−/− genotypes (Fig. 3E). Flow cytometry of skin cells from BM-chimera mice showed that nearly 100% of eosinophils were from donor mice (SI Appendix, Fig. S4B), macrophages showed fractions from both donor and recipient origins (SI Appendix, Fig. S4C), and skin T cells were mostly from recipient mice (SI Appendix, Fig. S4D). These differences likely reflect the effect of radiation on proliferating vs. tissue-resident nondividing immune cells. Mice were injected with NMLTC4 and scored for itch behavior. Irradiated Cysltr2−/− mice reconstituted with WT donor BM had lower levels of NMLTC4-induced scratching compared with levels in naïve WT mice (Fig. 3F), whereas irradiated WT mice reconstituted from WT or Cysltr2−/− BM showed levels unchanged from naïve WT mice, indicating that Cysltr2-expressing radioresistant cells mediate NMLTC4 itch (Fig. 3F).
We also investigated whether CysLTs acted through MCs to produce itch. MCs express Cysltr2 (47) and generate LTC4 during inflammation. MCs are major sources of histamine, serotonin, and other pruritogenic mediators that induce itch (41, 44). MCs are radioresistant and would not be affected in BM chimeras (48). We found that NMLTC4 induced equivalent itch in KitWsh/Wsh mice deficient for MCs as their WT littermates (Fig. 3G).
We next asked whether CysLT1R was involved in CysLT-induced itch. When injected with NMLTC4, Cysltr1−/− mice had the same levels of scratching as their Cysltr1+/+ littermates (Fig. 3H). These data indicate that CysLT2R but not CysLT1R is involved in CysLT-induced itch. TRP channels are known to be activated and induce calcium influxes downstream of GPCRs in itch pathways. TrpV1 mediates histamine-dependent neuronal signaling and itch (26), while TrpA1 mediates CQ or BAM8-11 scratching through MrgprA1 and MrgprC11, respectively (49). As most Cysltr2+ neurons expressed Trpv1 (Fig. 1E and SI Appendix, Fig. S1 A and B) and Trpa1 (SI Appendix, Fig. S1B), we next determined whether CysLT responses could also be mediated through those TRP channels. We first found that NMLTC4-induced and LTC4-induced itch was not altered in Trpv1−/− mice (Fig. 3I and SI Appendix, Fig. S3C). We then found that preinjecting the TrpA1 antagonist HC-030031 in the cheek failed to inhibit NMLTC4-induced scratching (SI Appendix, Fig. S3D). These data indicate that targeting TrpV1 or TrpA1 alone is unable to impact LTC4-induced itch. Taken together, these results show that LTC4 can induce itch in mice by acting through CysLT2R in nonhematopoietic cells and in a manner independent from MC, CysLT1R, and TrpV1.
We next asked if there could be interactions between the CysLTs in itch. LTD4, which we found does not induce itch when injected acutely (Fig. 2 B and F), inhibited NMLTC4-induced itch when coinjected with NMLTC4 (Fig. 3J). LTD4 is able to act through both CysLT1R and CysLT2R, so we tested whether this inhibition was dependent on CysLT1R expression. Interestingly, LTD4 inhibition of NMLTC4-induced itch was intact in Cysltr1−/− mice (Fig. 3J), showing that this inhibition is independent of CysLT1R and suggesting a potential competitive antagonism at CysLT2R.
CysLT2R Does Not Mediate Dry Skin or Alternaria-Induced Itch.
We next wished to determine whether CysLT2R plays an endogenous role in physiological models of skin pathology and itch. Cysltr2−/− mice did not have a defect in histamine-induced itch (SI Appendix, Fig. S5A). Similarly, the MC degranulator compound 48/80 induced robust scratching that was not reduced in Cysltr2−/− mice (SI Appendix, Fig. S5B). Alternaria alternata is an environmental airborne fungus involved in allergic diseases like asthma and potentially AD. It has recently been shown to be able to induce acute itch and scratching when injected intradermally (50). We show here that Alternaria-induced itch was CysLT2R-independent (SI Appendix, Fig. S5 C–E). Dry skin is a major cause of itch (51), and dry skin-induced itch can be modeled in mice by repeated application of a mixture of acetone and ether followed by water (AEW) for 5 d (SI Appendix, Fig. S5F). We found that AEW-induced itch and scratching behaviors were equivalent in Cysltr2−/− mice as compared with littermate controls (SI Appendix, Fig. S5H). Taken together, these data show that CysLT2R does not mediate dry skin or Alternaria-induced itch.
CysLT Pathway and CysLT2R in a Model of Chronic Itch.
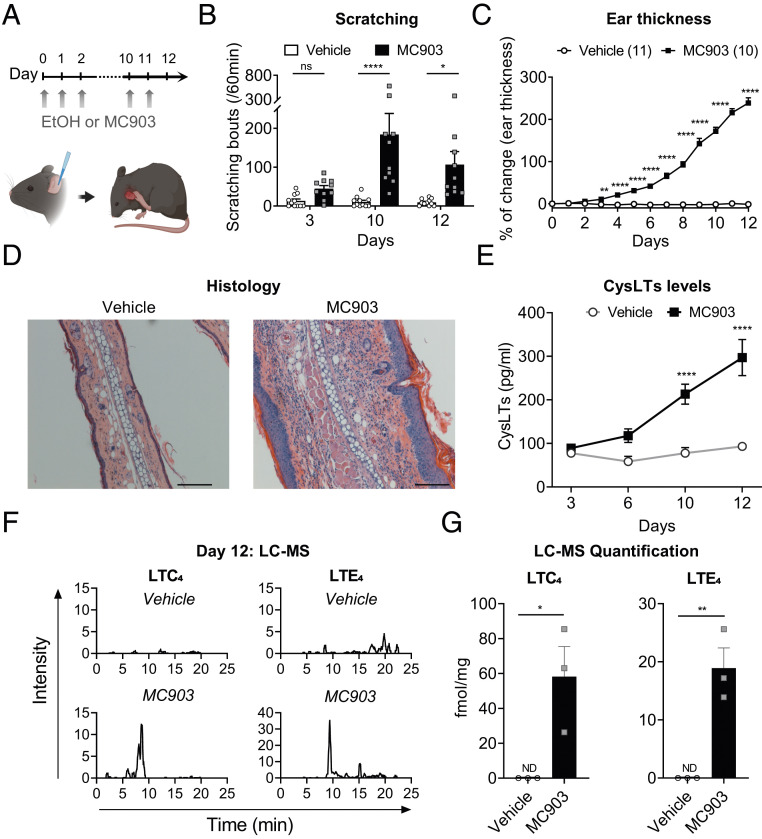
We next investigated the role of the endogenous CysLT pathway in chronic itch and dermatitis and utilized the MC903 model of skin inflammation, which has some characteristics of AD and ACD (52). The vitamin D analog MC903 induces skin thickening, immune cell influx, inflammation, and itch (29, 53). When we applied MC903 to the ears of male and female mice over 12 d (Fig. 4A), we found that this treatment caused spontaneous scratching behaviors (Fig. 4B), thickening of the ear (Fig. 4C), and severe acanthosis (thickening of epidermis), hyperkeratosis (thickening of the stratum corneum), and inflammation (Fig. 4D and SI Appendix, Fig. S6A). Flow cytometry analysis of the ear skin revealed a major influx of immune cells, including eosinophils, neutrophils, dendritic cells, macrophages, and T cells detectable at day 6 and with a peak at day 12 (SI Appendix, Fig. S6 B and C). Staining with toluidine blue at day 12 revealed an increase in the MC number present in the ear (SI Appendix, Fig. S7 A and B). However, we found that MCs were unlikely to be involved in driving chronic itch or skin inflammation in this model: WT controls and KitWsh/Wsh mice deficient in MCs showed similar levels of itch behavior and ear thickening (SI Appendix, Fig. S7 C and D). We next determined the role of Trpv1+ neurons in MC903-induced itch, given that Trpv1 expression encompasses Cysltr2 expression in the DRG. Using resiniferatoxin (RTX) to ablate Trpv1+ neurons, we observed that RTX-treated mice failed to develop any itch/scratching behaviors in response to MC903 treatment (SI Appendix, Fig. S8A) while showing the same levels of ear thickening as control mice (SI Appendix, Fig. S8B), indicating that these neurons do mediate itch but not overall inflammation in this model.
Fig. 4.
CysLT levels are increased in the skin of the MC903 model of dermatitis and itch. (A) Diagram of procedure: daily application of MC903 on the mouse ear for 12 d. (B) Scratching bouts recorded on days 3, 10, and 12 for 60 min before daily application of vehicle (ethanol) or MC903. (C) Percentage of ear thickness change following daily application of vehicle or MC903. (D) H&E staining performed on ear section at day 12 of MC903 model. (Scale bar: 50 µm.) (E) CysLTs (LTC4, LTD4, and LTE4) levels measured by ELISA at days 3, 6, 10, and 12 (n = 5 to 6). (F and G) Liquid chromatography–mass spectrometry. (F) Representative chromatograms for LTC4 (Left) and LTE4 (Right) from ear homogenates at day 12 after daily vehicle treatment (Top) or MC903 treatment (Bottom). (G) LTC4 and LTE4 quantification per milligram of ear collected at day 12 after vehicle or MC903 treatment. Values presented as mean ± SEM. Repeated-measures two-way ANOVA, Šidák’s posttest (B and C), two-way ANOVA with Šidák’s posttest (E), and unpaired t test (G). ns, nonsignificant (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
We next investigated whether CysLTs and CysLT2R played a role in the MC903 model. Given that many immune cells recruited into the skin, including eosinophils, MCs, and dendritic cells, can generate CysLTs, we hypothesized that we could detect an increase in these key lipid mediators. Using enzyme-linked immunoassays (ELISAs), we first measured the overall CysLT levels from skin homogenates. CysLTs steadily increased over time, with significant differences at day 10 and day 12 (Fig. 4E). We next used mass spectrometry to detect individual CysLTs (LTC4, LTD4, and LTE4) in ear homogenates at day 12. While MC903-inflammed ears had significant levels of LTC4 and LTE4, none were detectable in the vehicle conditions. LTC4 in particular was highly elevated in MC903 samples, with LTE4 detected at lower levels and levels of LTD4 too low to be reliably measured (Fig. 4 F and G). Therefore, CysLTs are significantly induced in MC903-inflamed ears, with the highest level of LTC4 at the later stage of inflammation.
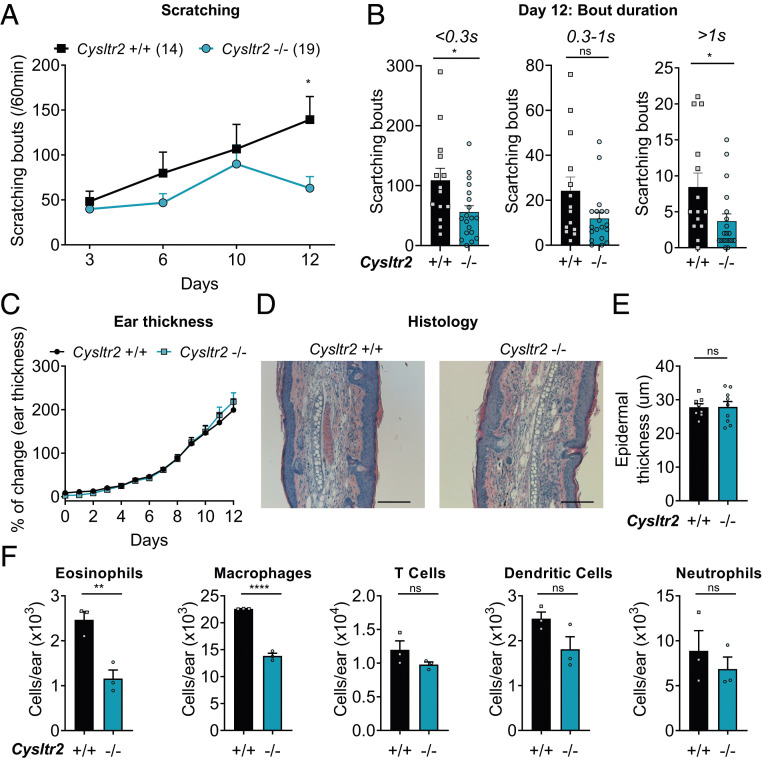
We next ascertained the role for CysLT2R in this model of skin inflammation and itch. We found that MC903-treated Cysltr2−/− mice had similar scratching levels as wild-type control littermates at early time points of the model; however, at day 12, Cysltr2−/− mice showed a significant decrease in scratching compared with WT controls (Fig. 5A). Our findings do indicate, therefore, a specific role for CysLT2R in later phases of itch in this model. Previous work had shown contributions of other major mediators to earlier phases of MC903-induced itch, such as neutrophils and CXCL10, which were more involved before day 10 (54). Analysis of bout duration at the day 12 timepoint further showed that the decrease in scratching in Cysltr2−/− mice was observed in both short bouts (<0.3 s) and longer bouts, with a significant decrease in long bouts (>1 s) in the Cysltr2−/− mice (Fig. 5B).
Fig. 5.
CysLT2R is involved in chronic itch but not in inflammation in MC903. (A) Scratching bouts recorded on days 3, 6, 10, and 12 for 60 min before daily application of MC903 in Cysltr2+/+ or Cysltr2−/− mice. (B) Bout duration analysis for Cysltr2+/+ and Cysltr2−/− mice at day 12 of MC903 (number of bouts: <0.3 s, between 0.3 and 1 s, and >1 s). (C) Percentage of ear thickness change following daily application of MC903 in Cysltr2+/+ or Cysltr2−/− mice. (D and E) H&E staining performed on ear section at day 12 of the MC903 model in Cysltr2+/+ or Cysltr2−/− mice. (D) Representative images of H&E staining. (E) Quantification of epidermal thickness (n = 8 to 9 with 10 to 15 fields quantified per animal). (F) Quantification of immune cell populations by flow cytometry from ear homogenates collected at day 12 from Cysltr2+/+ or Cysltr2−/− mice (n = 3). Values presented as mean ± SEM. Repeated-measures two-way ANOVA, Šidák’s posttest (A and C), and unpaired t test (B, E, and F). ns, nonsignificant (*P < 0.05; **P < 0.01; ****P < 0.0001).
We next determined whether CysLT2R mediates skin inflammation in MC903-treated mice, given its known role in ovalbumin-induced skin thickening and collagen deposition in a different model of dermatitis (14). Interestingly, we found no difference between Cysltr2−/− and littermate controls in ear swelling over time (Fig. 5C) or in epidermal thickening as analyzed by hematoxylin and eosin (H&E) staining (Fig. 5 D and E). However, we did find, by flow cytometry analysis, significant decreases in some specific immune cells, including eosinophils and macrophages, in the skin of Cysltr2−/− mice at day 12, while T cells, dendritic cells, and neutrophils remained unchanged (Fig. 5F).
As a comparison with Cysltr2−/− mice, we next determined that the increase in ear thickness and itch behaviors were not impaired in Cysltr1−/− mice in the MC903 model (SI Appendix, Fig. S9), indicating that CysLT1R is not involved in mediating chronic itch. These data, taken together, indicate that CysLT2R specifically contributes to itch in the late stage of inflammation in MC903-treated mice.
Discussion
Chronic itch negatively impacts the quality of life in patients with AD and ACD. Recent advances in the field have highlighted the importance of molecular receptors for immune mediators and neuroimmune cross-talk in those processes (29, 54, 55). CysLTs have long been suspected to be an important mediator of allergic skin reactions. CysLTs produce wheal and flare reactions when injected into human skin (56). LTC4 is released in great quantities during in vivo allergic cutaneous reactions to ragweed or grass pollen antigen (57), and LTC4 levels are increased in the skin of patients with AD (58). The presence of Cysltr2 expressed by the NP3 subset of pruriceptors strongly hinted at a role for the CysLTs/CysLT2R pathway in itch, but its functional relevance had not been explored prior to this study. Our study definitively shows that LTC4, but no other CysLTs, can specifically and functionally induce itch in vivo through the CysLT2R receptor, and that CysLT2R contributes to itch in a physiological model of dermatitis.
Prior to our work, two previous studies have suggested a potential role of NMLTC4 and LTD4 in inducing itch (31, 41). In a first study, LTD4 was injected intradermally at one dose and found to induce itch similarly to serotonin and IL-31 (31). By contrast, we found that LTD4 does not produce itch in a detailed dose–response analysis. A more recent study found that NMLTC4 induced calcium influx into mouse DRG neurons and induced itch following acute injections in mice at one dose, together with sphingosine 1 phosphate and serotonin (41). While this did show initial phenotypes induced by NMLTC4 with scratching behaviors, without a comparison with endogenous LTC4 or other CysLTs, the physiological relevance of this response in inflammatory itch and the role of CysLT2R in both acute and chronic itch remained unknown.
Here, we show that LTC4, in particular, is a critical mediator of itch, and that CysLT2R mediates this functional response in vivo in acute LTC4-induced itch, and also is relevant in a chronic model of itch. Even though CysLT2R expressed in heterologous cell systems binds to both LTC4 and LTD4 in vitro (10), in vivo datasets show a more context-dependent role of each ligand in how it interacts with the receptor. CysLT2R is preferentially activated by LTC4 in vivo in platelets (13) and during type 2 airway immunopathology (19). Our data on itch fits with LTC4 and not LTD4 in being the major driver of itch in vivo. We found that LTD4 was able to inhibit NMLTC4 itch when it was coinjected with NMLTC4. Previous work has shown that LTC4/CysLT2R and LTD4/CysLT1R pathways can have antagonistic effects: CysLT2R negatively regulates CysLT1R-dependent Th2 pulmonary inflammation to dust mites in mice sensitized and challenged with Dermatophagoides farinae (11) and CysLT1R-induced mitogenic signaling responses of MC (59). However, in our study, LTD4 inhibition of NMLTC4-induced scratching was still present in Cysltr1−/− mice, showing that the mechanism was CysLT1R-independent. A recent study in platelet biology indicates interesting parallels with our data (60). In this study, it was shown that LTD4 inhibits LTC4-driven platelet activation in vitro in a manner independent of CysLT1R, most likely by the ligand competing with LTC4 on the CysLT2R receptor (60).
We found that, while LTC4 and NMLTC4 induce increasing itch at lower doses, this response no longer occurred at high doses. The bell-shaped curve observed with LTC4 and NMLTC4 may be similar to other pruritogen responses, such as the inverted U-shaped itch response observed with CQ injections (61). Given that many of the pruritogen receptors are membrane-bound GPCRs, it could be related to receptor internalization and desensitization. CysLT1R receptors are known to undergo rapid agonist-dependent internalization (62, 63). A recent study found that a high dose of LTC4 induced internalization of CysLT2R in MCs, while a low dose induced its expression at the membrane (64). The downstream signaling of CysLT2R in neurons is yet unknown. Our results show that TrpV1, which mediates histamine-dependent neuronal signaling and itch (26), was unnecessary for LTC4-induced itch. Our data also do not indicate a role for TrpA1, which is involved in itch dependent on MrgprA3 and MrgprC11 (49). Nonetheless, it is possible that double deficiency in both ion channels could show a phenotype, and this remains to be determined with further studies.
Other than sensory neurons, many other cell types express CysLT2R, including myeloid immune cells (3). By using BM chimeras from Cysltr2−/− mice, our results showed that receptor expression in radiosensitive hematopoietic cells such as eosinophils was unnecessary for NMLTC4-induced itch, while Cysltr2 in radioresistant cells was necessary. These data fit with a role for Cysltr2 in sensory neurons, which are radioresistant nonhematopoietic cells. Future studies using conditional knockout mice for Cysltr2 are necessary to determine its exact role in neurons.
We also investigated the kinetics and the quality of itching induced by distinct ligands, finding that LTC4-induced scratching differed from histamine, CQ, and compound 48/80 by having longer bouts. Potentially, the responsive neuronal subset could determine the subsequent itch responses. LTC4 and IL-31, which are ligands for CysLT2R and IL-31ra coexpressed by NP3 neurons, induce the same type of longer scratching bouts. CQ, which activates MrgprA3 expressed on NP2 neurons, produces almost exclusively short bouts. Histamine receptors are known to be spread across NP3 and NP2 subsets (32), which could explain the intermediary phenotype observed with histamine. Furthermore, LTC4 did not induce alloknesis (touch-induced itch). While chemical ligands can be coupled to itch by direct gating of pruriceptors, alloknesis is processed by other sensory neurons, including TLR5-expressing Aβ low-threshold mechanoreceptors (reviewed in ref. 46).
Transcript types and expression levels in DRG neurons can differ significantly between different species (65). We showed here that the expression of the Cysltr2 transcript was broader in human DRG neurons (63%) than in mouse DRG neurons (10%). Most neurons expressing NPPB coexpressed CYLTR2 in human neurons; however, CYLTR2 expression was not limited to NPPB+ neurons. The broader CYLTR2 expression in human DRG neurons suggests that the receptor is relevant to human sensory physiology and might have roles beyond those we have highlighted in the mouse. A recent study highlighted the differences between mouse and human sensory neurons, including Trpv1 expression (∼30% in mice vs. ∼70% in humans) and TSLP receptor expression (∼70% in mice vs. ∼5% in humans) (66). In rats, a previous study found that Cysltr2 was expressed in 36% of DRG neurons and that CysLT2R could be involved in mediating pain in rats (67). Cysltr2+ rat neurons were mainly isolectin B4+ neurons coexpressing ATP receptor P2rx3, and LTC4 injected into the footpad of rats potentiates αβ-me-ATP–induced thermal hyperalgesia (67). This contrasts with mouse DRG neurons, where Cysltr2 is expressed in ∼10% of sensory neurons and P2rx3 is in a much broader population of neurons (31, 32, 41). In our study, we did not find nocifensive phenotypes with LTC4 cheek injections, which does not rule out sensitization of pain, but shows no induction of acute pain phenotypes. Another study found that sensory neurons from the trigeminal ganglion innervating the nasal mucosa of guinea pigs expressed Cysltr1 rather than Cysltr2, and LTD4 could increase the excitability of those neurons (68). By contrast, we do not detect Cysltr1 transcript in mouse DRG neurons and do not find a functional role for CysLT1R in itch. These distinct findings in human, mouse, rat, and guinea pigs highlight a need for species-specific analysis of sensory transcripts, and may have implications for translatability of results from mouse to humans in itch biology.
We demonstrated the involvement of the CysLT pathway and CysLT2R in the MC903 mouse model of chronic itch. In this mouse model of dermatitis, several immune and inflammatory mediators have been found to contribute to chronic itch. The cytokines TSLP, IL-4, and IL-13, as well as serotonin and its receptor 5HTR7, have been found to critically drive itch in this model (29, 52–54). Recent work has shown that neutrophils and the chemokine CXCL10 mediate distinct phases of inflammation and itch in MC903 mice (54). Interestingly, this study showed that Cysltr2, Nppb, and Il31ra are transcriptionally up-regulated in trigeminal neurons following cheek application of MC903 (days 5 to 8). We found that CysLT levels went up in the skin especially in the later stages of the MC903 model. The detection of LTC4 at day 12 indicates that the production of CysLTs is actively ongoing at that stage, since LTC4 is quickly converted to LTD4 (69), and it is at day 12 that Cysltr2−/− mice showed a significant decrease in itch, suggesting that active LTC4 production at that stage is able to induce scratching. Future studies are needed to determine how CysLT2R signaling synergizes with the other cytokine- and immune cell-driven pathways in this model to drive itch signaling.
We found that several immune cell types increased in MC903-treated mice, which could be sources of CysLTs. In the skin and other tissues, CysLTs are produced by eosinophils, MC, macrophages, and monocytes (70, 71). MCs generate CysLTs upon activation (72) and communicate with neurons bidirectionally in inflammation and itch (73). However, we observed that mice lacking MC (KitWsh/Wsh) showed intact itch in MC903-treated mice. Eosinophils are other possible candidates. Eosinophil-derived LTC4 acts on fibroblasts through CysLT2R to induce collagen deposition and skin thickening in ovalbumin-induced allergic sensitization (14). Macrophages and dendritic cells are also high expressers of LTC4S, which is the enzyme that produces LTC4 (Immgen database; https://www.immgen.org/). Of note, we found that both eosinophils and macrophages decreased in Cysltr2−/− mice after MC903 challenge. Therefore, LTC4 might be acting through both immune cells and sensory neurons to drive itch in vivo, and the relative roles of sensory neuron-expressed CysLT2R compared with nonneuronal CysLT2R in the MC903 model remain to be determined. It is possible that there is a positive neuroimmune feedback loop between CysLT-induced itch and immune cell recruitment through CysLT2R.
By contrast with the MC903 model, we did not find a role for CysLT2R in the Alternaria model or compound 48/80-induced itch. CysLT2R also did not mediate AEW model of dry skin itch. One major difference between MC903 and the AEW models is that AEW does not cause significant infiltration of inflammatory cells in the dermis (74). Alternaria and compound 48/80 are both acute inflammatory models that could drive other immune pathways and cellular recruitment distinct from MC903-driven skin immune responses.
Our study may have therapeutic implications for treatment of dermatitis. The 5-LO inhibitor zileuton, which targets upstream conversion of arachidonic acid to LTA4, has demonstrated efficacy in treating pruritus in small clinical trials (75). The CysLT1R-specific antagonist montelukast (Singulair), which is successful in treating bronchoconstriction in asthmatic patients (9, 17), was tested in the treatment of AD patients but showed mixed results (76–79). Our work indicates that montelukast might have failed to show positive results because it targets CysLT1R instead of CysLT2R.
The role of the CysLT pathway and its receptors in skin conditions has been suspected for a long time but has not been previously studied in itch. Here, we have shown that LTC4, acting through CysLT2R, is able to induce scratching in mice and participated in chronic itch in a mouse model of AD. This suggests that drugs targeting CysLT2R could be useful to treat recalcitrant chronic itch.
Materials and Methods
Detailed descriptions of methods and materials are provided in SI Appendix, Materials and Methods.
Mice.
All animal experiments were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
BM Chimera Generation.
BM was collected from tibia, femur, and hips of both sides of either WT (C57BL/6) or Cysltr2−/− mice 8 to 12 wk of age, dissected and cleaned from all soft tissue. Recipient animals (WT or Cysltr2−/−) 6 to 12 wk of age underwent lethal irradiation on the day of the transplantation. At 3 to 5 h after lethal irradiation, mice were anesthetized with isoflurane for retrobulbar injection of 3 × 106 BM donor cells. After 6 to 8 wk, behavioral experiments were performed.
Mouse RNAscope In Situ Hybridization Analysis.
DRGs were dissected from mice and embedded in optimal cutting temperature compound, and cryosections of 16 µm were cut. Multilabeling in situ hybridization (ISH) was performed using the RNAscope technology (ACD) according to the manufacturer’s instructions. Probes against mouse Cysltr2, Trpv1, Nppb, Mrgpra3, Mrgprd, Hrh1, Tubb3, and Scn10a in conjunction with the RNAscope multiplex fluorescent development kit were used.
Human DRG RNAscope Analysis.
Procurement procedures for all human tissue were approved by the institutional review board at the University of Texas at Dallas, and samples were deidentified prior to use in the study. Human lumbar dorsal root ganglions collection and RNAscope in situ hybridization were performed as described previously (66).
Behavioral Analysis.
For all behavior experiments, experimenters were blinded to experimental groups and/or genotypes. Recording of behaviors was performed with an experimental setup that enables us to record the mice in the dark in an experimenter-free environment: iBOB (Crimson Scientific) unless otherwise specified. Acute itch and pain behavior experiments were performed as described previously (43).
Bout duration quantification.
Duration of individual bouts was measured, and bouts were then classified in three categories according to their length: <0.3 s, 0.3 to 1 s, and 1 s.
Touch-induced itch (alloknesis).
For touch-induced itch, the nape of neck was mechanically stimulated using a 0.07-g Von Frey filament for 1 s three times in a row, with this sequence repeated three times, and the scratching responses was recorded out of a total of nine.
Chronic itch models.
The MC903 model of chronic itch was performed as described previously (29, 53), and dry skin-evoked itch behaviors assessment was carried out as previously described (80).
Cysteinyl LT Detection.
Whole ears from mice were collected, and CysLT generation was measured in acetone-precipitated homogenates by a commercially available ELISA according to the manufacturer’s protocol (Cayman).
Mass Spectrometry.
Samples were analyzed on an ultimate 3000 LC coupled with a Q Exactive plus mass spectrometer (Thermo Fisher), with a method based on previous studies (81, 82).
Flow Cytometry.
Ears were mechanically separated and minced, then digested. The preparation was stained with antibodies. Flow cytometry was conducted on an LSRII flow cytometer.
Statistics Analysis.
Data in figures represent mean ± SEM. All significance tests were chosen considering the experimental design, and we assumed normal distribution and variance of data. No data were excluded from statistical analyses unless due to technical errors. Statistical significance was determined by unpaired Student’s t test for two-group comparisons, one-way ANOVA, or ANOVA for multivariate linear models. Statistical analyses were performed using Prism 7 (GraphPad Software).
Supplementary Material
Acknowledgments
We thank members of the I.M.C. laboratory, especially Nicole J. Yang and Kimbria Blake, for helpful discussions. We thank James Searson, Lin Ni, Amélie Bouvier, Noah Gilman, Victoria Flecha Maria, Tsz Man Fion, Lucy Wesemann, and Samantha Choi for strong technical support and watching behavioral videos. We thank Mark A. Hoon, Hans J. Solinski, and Juan-Manuel Leyva-Castillo for sharing their technical expertise and advice on neurobiological and immunological analysis. We are grateful to the Microscopy Resources on the North Quad core at Harvard Medical School and the Harvard Center for Mass Spectrometry for excellent technical assistance. Schematic diagrams were created using BioRender. Research in the I.M.C. laboratory is supported by NIH Grants (DP2AT009499, R01AI130019), the Food Allergy Science Initiative, GlaxoSmithKline and Allergan Pharmaceuticals, the Harvard Stem Cell Institute, and the Burroughs Wellcome Fund. This work was also supported by the Brigham and Women’s Hospital Hypersensitivity Fund (to K.F.A.), National Institutes of Allergy and Infectious Diseases Grant K08 AI132723 (to L.G.B.), and the American Academy of Allergy, Asthma & Immunology Foundation Faculty Development Award (to L.G.B.). Research in the T.J.P. laboratory is supported by the NIH Grant (NS111929).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022087118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Dong X., Dong X., Peripheral and central mechanisms of itch. Neuron 98, 482–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoon M. A., Molecular dissection of itch. Curr. Opin. Neurobiol. 34, 61–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanaoka Y., Austen K. F., Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv. Immunol. 142, 65–84 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Laidlaw T. M., et al., Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 119, 3790–3798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ualiyeva S., et al., Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci. Immunol. 5, eaax7224 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam B. K., Austen K. F., Leukotriene C4 synthase: A pivotal enzyme in cellular biosynthesis of the cysteinyl leukotrienes. Prostaglandins Other Lipid Mediat. 68-69, 511–520 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Kanaoka Y., Maekawa A., Austen K. F., Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J. Biol. Chem. 288, 10967–10972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bankova L. G., et al., Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc. Natl. Acad. Sci. U.S.A. 113, 6242–6247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch K. R., et al., Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399, 789–793 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Heise C. E., et al., Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 275, 30531–30536 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Barrett N. A., et al., Cysteinyl leukotriene 2 receptor on dendritic cells negatively regulates ligand-dependent allergic pulmonary inflammation. J. Immunol. 189, 4556–4565 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett N. A., et al., Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 208, 593–604 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings H. E., et al., Cutting edge: Leukotriene C4 activates mouse platelets in plasma exclusively through the type 2 cysteinyl leukotriene receptor. J. Immunol. 191, 5807–5810 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyoshi M. K., et al., Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc. Natl. Acad. Sci. U.S.A. 109, 4992–4997 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty T. A., et al., Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 132, 205–213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Moltke J., et al., Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J. Exp. Med. 214, 27–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volovitz B., et al., Montelukast, a leukotriene receptor antagonist, reduces the concentration of leukotrienes in the respiratory tract of children with persistent asthma. J. Allergy Clin. Immunol. 104, 1162–1167 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Zhang H. P., Jia C. E., Lv Y., Gibson P. G., Wang G., Montelukast for prevention and treatment of asthma exacerbations in adults: Systematic review and meta-analysis. Allergy Asthma Proc. 35, 278–287 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Liu T., et al., Type 2 cysteinyl leukotriene receptors drive IL-33-dependent type 2 immunopathology and aspirin sensitivity. J. Immunol. 200, 915–927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duah E., et al., Cysteinyl leukotriene 2 receptor promotes endothelial permeability, tumor angiogenesis, and metastasis. Proc. Natl. Acad. Sci. U.S.A. 116, 199–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andoh T., Kuraishi Y., Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur. J. Pharmacol. 353, 93–96 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Andoh T., et al., Involvement of leukotriene B4 in itching in a mouse model of ocular allergy. Exp. Eye Res. 98, 97–103 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Trang T., McNaull B., Quirion R., Jhamandas K., Involvement of spinal lipoxygenase metabolites in hyperalgesia and opioid tolerance. Eur. J. Pharmacol. 491, 21–30 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Zinn S., et al., The leukotriene B4 receptors BLT1 and BLT2 form an antagonistic sensitizing system in peripheral sensory neurons. J. Biol. Chem. 292, 6123–6134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cevikbas F., Lerner E. A., Physiology and pathophysiology of itch. Physiol. Rev. 100, 945–982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim W. S., et al., TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 27, 2331–2337 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunford P. J., et al., Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 119, 176–183 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Cevikbas F., et al., A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133, 448–460 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oetjen L. K., et al., Sensory neurons Co-opt classical immune signaling pathways to mediate chronic itch. Cell 171, 217–228.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L., et al., A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 16, 174–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usoskin D., et al., Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Zeisel A., et al., Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q., et al., Mechanisms of itch evoked by β-alanine. J. Neurosci. 32, 14532–14537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stantcheva K. K., et al., A subpopulation of itch-sensing neurons marked by ret and somatostatin expression. EMBO Rep. 17, 585–600 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J., et al., Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 21, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra S. K., Hoon M. A., The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C. L., et al., Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma N., et al., The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu I. M., et al., Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3, e04660 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonkoly E., et al., IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 117, 411–417 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Solinski H. J., et al., Nppb neurons are sensors of mast cell-induced itch. Cell Rep. 26, 3561–3573.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meixiong J., Dong X., Mas-related G protein-coupled receptors and the biology of itch sensation. Annu. Rev. Genet. 51, 103–121 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Shimada S. G., LaMotte R. H., Behavioral differentiation between itch and pain in mouse. Pain 139, 681–687 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meixiong J., et al., Activation of mast cell-expressed Mas-related G-1 protein coupled receptors drives non histaminergic itch. Immunity 50, 1163–1171.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan H., et al., Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron 103, 1135–1149.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai K., Akiyama T., New insights into the mechanisms behind mechanical itch. Exp. Dermatol. 29, 680–686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mellor E. A., et al., Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: Functional distinction from CysLT1R. Proc. Natl. Acad. Sci. U.S.A. 100, 11589–11593 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oldford S. A., Marshall J. S., Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 63, 113–124 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Wilson S. R., et al., TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 14, 595–602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perner C., et al., Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 53, 1063–1077.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yosipovitch G., et al., Skin barrier damage and itch: Review of mechanisms, topical management and future directions. Acta Derm. Venereol. 99, 1201–1209 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Li M., et al., Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. U.S.A. 103, 11736–11741 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita T., et al., HTR7 mediates serotonergic acute and chronic itch. Neuron 87, 124–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh C. M., et al., Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife 8, e48448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voisin T., Bouvier A., Chiu I. M., Neuro-immune interactions in allergic diseases: Novel targets for therapeutics. Int. Immunol. 29, 247–261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soter N. A., Lewis R. A., Corey E. J., Austen K. F., Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J. Invest. Dermatol. 80, 115–119 (1983). [DOI] [PubMed] [Google Scholar]
- 57.Talbot S. F., Atkins P. C., Goetzl E. J., Zweiman B., Accumulation of leukotriene C4 and histamine in human allergic skin reactions. J. Clin. Invest. 76, 650–656 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hua Z., Fei H., Mingming X., Evaluation and interference of serum and skin lesion levels of leukotrienes in patients with eczema. Prostaglandins Leukot. Essent. Fatty Acids 75, 51–55 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Jiang Y., Borrelli L. A., Kanaoka Y., Bacskai B. J., Boyce J. A., CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood 110, 3263–3270 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu T., et al., Leukotriene D(4) paradoxically limits LTC(4) driven platelet activation and lung immunopathology. J. Allergy Clin. Immunol. 10.1016/j.jaci.2020.10.041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green A. D., Young K. K., Lehto S. G., Smith S. B., Mogil J. S., Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 124, 50–58 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Naik S., et al., Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J. Biol. Chem. 280, 8722–8732 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Deshpande D. A., et al., PKC-dependent regulation of the receptor locus dominates functional consequences of cysteinyl leukotriene type 1 receptor activation. FASEB J. 21, 2335–2342 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Agier J., Różalska S., Wódz K., Brzezińska-Błaszczyk E., Leukotriene receptor expression in mast cells is affected by their agonists. Cell. Immunol. 317, 37–47 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Price T. J., Flores C. M., Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J. Pain 8, 263–272 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiers S., Klein R. M., Price T. J., Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 161, 2410–2424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okubo M., et al., Expression of leukotriene receptors in the rat dorsal root ganglion and the effects on pain behaviors. Mol. Pain 6, 57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor-Clark T. E., Nassenstein C., Undem B. J., Leukotriene D4 increases the excitability of capsaicin-sensitive nasal sensory nerves to electrical and chemical stimuli. Br. J. Pharmacol. 154, 1359–1368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keppler D., Huber M., Baumert T., Guhlmann A., Metabolic inactivation of leukotrienes. Adv. Enzyme Regul. 28, 307–319 (1989). [DOI] [PubMed] [Google Scholar]
- 70.Haeggström J. Z., Leukotriene biosynthetic enzymes as therapeutic targets. J. Clin. Invest. 128, 2680–2690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soberman R. J., Christmas P., The organization and consequences of eicosanoid signaling. J. Clin. Invest. 111, 1107–1113 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moon T. C., Befus A. D., Kulka M., Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 5, 569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serhan N., et al., House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 20, 1435–1443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyamoto T., Nojima H., Shinkado T., Nakahashi T., Kuraishi Y., Itch-associated response induced by experimental dry skin in mice. Jpn. J. Pharmacol. 88, 285–292 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Woodmansee D. P., Simon R. A., A pilot study examining the role of zileuton in atopic dermatitis. Ann. Allergy Asthma Immunol. 83, 548–552 (1999). [DOI] [PubMed] [Google Scholar]
- 76.Pei A. Y., Chan H. H., Leung T. F., Montelukast in the treatment of children with moderate-to-severe atopic dermatitis: a pilot study. Pediatr. Allergy Immunol. 12, 154–158 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Friedmann P. S., et al., A double-blind, placebo-controlled trial of montelukast in adult atopic eczema. Clin. Exp. Allergy 37, 1536–1540 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Nettis E., Pannofino A., Fanelli M., Ferrannini A., Tursi A., Efficacy and tolerability of montelukast as a therapeutic agent for severe atopic dermatitis in adults. Acta Derm. Venereol. 82, 297–298 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Jeon Y. H., Min T. K., Yang H. J., Pyun B. Y., A double-blind, randomized, crossover study to compare the effectiveness of montelukast on atopic dermatitis in Korean children. Allergy Asthma Immunol. Res. 8, 305–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson S. R., et al., The ion channel TRPA1 is required for chronic itch. J. Neurosci. 33, 9283–9294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gijón M. A., Zarini S., Murphy R. C., Biosynthesis of eicosanoids and transcellular metabolism of leukotrienes in murine bone marrow cells. J. Lipid Res. 48, 716–725 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Zarini S., Gijón M. A., Ransome A. E., Murphy R. C., Sala A., Transcellular biosynthesis of cysteinyl leukotrienes in vivo during mouse peritoneal inflammation. Proc. Natl. Acad. Sci. U.S.A. 106, 8296–8301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.