Abstract
Traditional bone tissue engineering (BTE) strategies induce direct bone-like matrix formation by mimicking the embryological process of intramembranous ossification. However, the clinical translation of these clinical strategies for bone repair is hampered by limited vascularization and poor bone regeneration after implantation in vivo. An alternative strategy for overcoming these drawbacks is engineering cartilaginous constructs by recapitulating the embryonic processes of endochondral ossification (ECO); these constructs have shown a unique ability to survive under hypoxic conditions as well as induce neovascularization and ossification. Such developmentally engineered constructs can act as transient biomimetic templates to facilitate bone regeneration in critical-sized defects. This review introduces the concept and mechanism of developmental BTE, explores the routes of endochondral bone graft engineering, highlights the current state of the art in large bone defect reconstruction via ECO-based strategies, and offers perspectives on the challenges and future directions of translating current knowledge from the bench to the bedside.
Keywords: Developmental engineering, bone defect reconstruction, endochondral ossification, hypertrophic cartilage, bone tissue engineering
Introduction
Bone defects resulting from trauma, congenital defects, cancer, infection, and arthritis have become a global healthcare problem. The reconstruction of extensive bone defects continues to be a great challenge medically and socioeconomically. Currently available approaches for bone defect reconstruction, such as the sue of autografts, allografts, xenografts, and synthetic grafts, all have specific indications and limitations.1–3 Thus, extensive research has been performed to identify substitutes approaches for the reconstruction of bone defects. BTE, which aims to regenerate new biological bone tissue with structures, mechanical properties, and functions similar to those of naturally occurring bone tissue, has become a valid approach for bone defect reconstruction.
The classic BTE paradigm frequently relies on several key elements: (1) an osteoconductive scaffold that closely mimics the natural bone extracellular matrix (ECM) niche;4,5 (2) osteogenic cells capable of initiating bone formation at the graft site or, at least, creating an osteoinductive microenvironment to induce resident cells to do so;6 (3) osteoinductive molecules to trigger implanted or resident cells to form bone tissue;7 and (4) sufficient vascularization to meet the needs of the growing tissue for a supply of nutrients and the clearance of waste.8,9 In the past three decades, BTE has traditionally focused on mimicking an intramembranous ossification (IMO) pathway, by which mesenchymal stem cells (MSCs) are induced to undergo osteogenic differentiation with subsequent formation of a bone-like matrix. This approach has achieved major progress in terms of bioreactor design, scaffold engineering, and long-term tissue construct maintenance,10,11 but there have also been encountered setbacks and delays in clinical translation. An emerging paradigm of developmental BTE, which advocates engineering cartilage constructs by replicating certain aspects of ECO for bone defect reconstruction, has been gradually adopted by researchers to address the limitations of traditional BTE strategies. However, the basis of ECO strategies is not widely understood. Furthermore, several key design criteria regarding the choice of cell sources, biomaterials, and endochondral priming protocols remain to be elucidated. Thus, this review introduces the concept of developmental bone engineering, explores the routes of endochondral bone engineering, and summarizes the current experimental data on large bone defect reconstruction via ECO-based strategies. Within this framework, emphasis is placed on the superiority of ECO-based strategies, and future trends in the clinical translation of these strategies are discussed.
Engineering endochondral bone: A developmental engineering strategy
Embryonic bone development and fracture healing
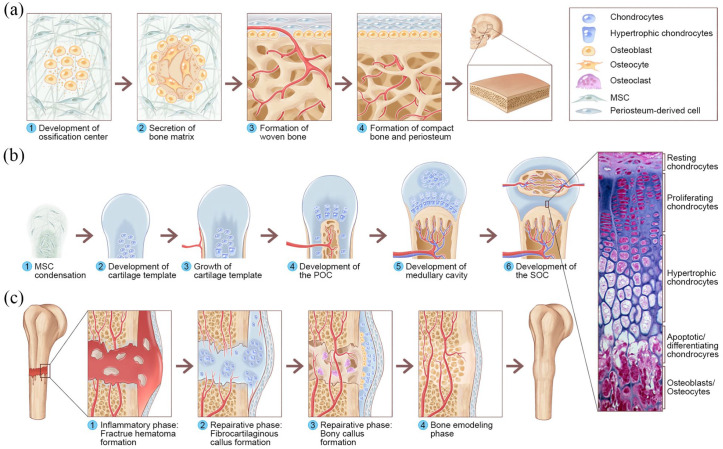
All bone is formed through two mechanisms: IMO and ECO. Both mechanisms begin with MSC migration to sites of future bone. Here, MSCs form condensations of high cellular density that outline the shape and size of the future bone. Within these condensations, the MSCs either differentiate into osteoblasts to directly form flat bone (intramembranous bone formation, Figure 1(a)),12 or differentiate into chondrocytes and form a cartilaginous template that is responsible for long bone formation (Figure 1(b)) as well as bone fracture healing (Figure 1(c)) (endochondral bone formation).13
Figure 1.
Overview of IMO and ECO during embryonic bone development and fracture healing: (a) IMO follows four steps. Step 1: MSCs undergo condensation and form ossification centers. MSCs within the areas of condensation lead to the development of capillaries and osteoblasts. Step 2: Osteoblasts secrete osteoid, which then entraps the osteoblasts, and the osteoblasts transform into osteocytes. Step 3: Osteoid secreted around the capillaries result in trabecular matrix formation, while osteoblasts on the surface of the spongy bone become the periosteum. Step 4: The periosteum then creates compact bone superficial to the trabecular bone. The trabecular bone crowds blood vessels, which eventually condense into red marrow. (b) ECO follows six steps during embryonic bone development. Step 1: MSC condensation. Step 2: MSCs within the areas of condensation differentiate into chondrocytes to form the cartilage template of the future long bone, and MSCs in the periphery form the perichondrium. Step 3: Chondrocytes in the center of the template undergo hypertrophy, while cells in the periphery undergo direct osteogenic differentiation to form a periosteal collar of compact bone around the cartilage template. Step 4: Hypertrophic chondrocytes secrete osteogenic and angiogenic factors that initiate cartilage matrix mineralization and blood vessel invasion, resulting in POC formation. Step 5: The diaphysis elongates, and a medullary cavity forms as ossification continues. Step 6: After this initial bone formation, the same sequence of events occurs in the epiphyseal regions, leading to SOC formation, and (c) The healing of fractures follows three consecutive and overlapping phases. Inflammatory phase: Approximately 6–8 h after the fracture, a hematoma is formed at the fracture site. Reparative phase: Within approximately 48 h after the fracture, chondrocytes from the periosteum and marrow create an internal callus between the two ends of the broken bone and an external callus around the outside of the break. MSCs from the periosteum directly differentiate into osteoblasts, thereby stimulating appositional bone growth and enveloping the defect. Over the next several weeks, the cartilage in the calli is replaced by woven bone via ECO. Remodeling phase: The woven bone remodels into lamellar bone through osteoclast-osteoblast coupling, and the healing process is complete. The histological image of the epiphyseal plate of a growing long bone was adapted from Human Anatomy, sixth edition (Copyright © 2011 Pearson Education, Inc., Figure 6.12).
The replacement of cartilage with mineralized bone in endochondral bone is a complex process. Osteogenesis begins when proliferating chondrocytes within the template enter a non-proliferating, hypertrophic state. These hypertrophic chondrocytes secrete osteogenic and angiogenic factors, such as vascular growth factor (VEGF) and alkaline phosphatase (ALP). Concurrently, the tissue is invaded by vasculature, which delivers MSCs, osteoclasts, endothelial cells, and hematopoietic cells to the diaphysis, thereby forming a primary ossification center (POC).14 Traditionally, hypertrophic chondrocytes undergoing programmed cell death are believed to be removed by osteoclasts from the template.14 MSCs then differentiate into osteoblasts to produce bone matrix.15 However, recent works have highlighted an alternative fate of hypertrophic chondrocytes, consisting of transdifferentiation into osteoblasts and osteocytes in the final stages of endochondral bone formation.16–18 Additional vasculature near the ends of the bone will establish one or more secondary ossification centers (SOCs), which contribute to the growth of the bony epiphyses and articular cartilage (Figure 1(b)).12,19
Bone fracture healing differs from natural bone development. In general, bone fracture healing consists of three overlapping phases: the inflammatory, reparative, and remodeling phases;20 ECO and IMO occur concurrently during the reparative phase.11 When a bone fracture occurs, the inflammatory phase begins immediately. The damaged vasculature and bone marrow create a hypoxic microenvironment that recruits MSCs, fibroblasts, and endothelial cells to the fracture site.21 The reparative phase consists of two subphases: the soft callus and the hard callus phases.20 In the soft callus phase, the recruited MSCs start differentiating in two different ways according to their microenvironment.13 IMO primarily occurs along the periosteal surface of the bone adjacent to the fracture site as MSCs differentiate into osteoblasts that lay down woven bone.22 However, ECO occurs predominantly at the center of the fracture site, where MSCs differentiate into chondrocytes.22 These chondrocytes then form a cartilaginous soft callus, and the synthesized cartilage ECM mineralizes through chondrocyte apoptosis. In the hard callus phase, osteoblasts migrate to where blood vessels invade the calcified cartilage to produce a hard callus.20,23 Finally, after the fracture has been filled with new woven bone, osteoclastic activity occurs at the outer surface to initiate periosteal callus resorption and the remodeling phase (Figure 1(c)).
Endochondral bone engineering: A promising solution for large bone defect reconstruction
Inspired by the process of bone development and fracture healing, current BTE strategies strive to generate bone substitutes by emulating the body’s biochemical and physical environment. Traditional BTE strategies mimic the embryological process of IMO, by which MSCs are induced to undergo osteogenic differentiation with the subsequent formation of a bone-like matrix in vitro. This strategy has clear potential and has made major progress.10,11 A major drawback such strategies for engineering constructs is the limited size of the construct. In vitro osteogenic induction results in extensive matrix deposition on the surface of the construct, which hampers nutrient delivery and makes it difficult to scale up the size.24 Furthermore, extensive bone matrix on the surface hinders the invasion of blood vessels upon construct implantation. Thus, such traditional BTE approaches often fail due to poor perfusion, leading to avascular necrosis and core degradation.9 Consequently, attention has shifted toward an alternative route of “developmental engineering,” which strives to stimulate in vivo developmental processes and imitates natural factors governing cell differentiation and matrix production.25,26
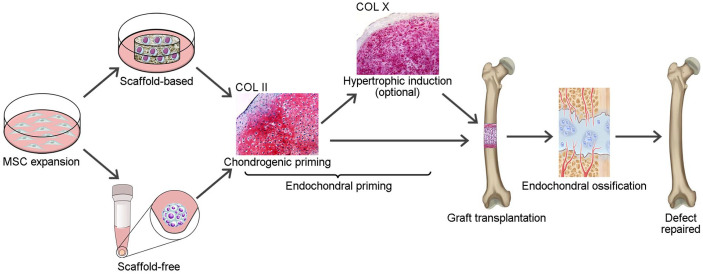
In contrast to IMO-based approaches, approaches based on developmental engineering involve engineering cartilaginous constructs by replicating certain aspects of ECO for bone defect reconstruction. Briefly, MSCs are induced to differentiate into chondrocytes in vitro to form a hypertrophic cartilage construct, which contains essential “biological instructions” to initiate the ECO process after implantation, and the defect is subsequently repaired by endochondral bone formation (Figure 2).27–29 This strategy offers a solution to overcome problems associated with poor vascularization after implantation: (1) chondrocytes that are born in an avascular environment can intrinsically resist hypoxia,30 while MSCs and osteoblasts are less tolerant to a hypoxic environment;31,32 (2) hypertrophic chondrocytes can induce neovascularization and ossification through the release of VEGF, vesicles containing hydroxyapatite, and bone morphogenic proteins (BMPs);33,34 and (3) endochondrally primed grafts allow for faster host integration and bone formation after implantation in vivo.35,36 Therefore, ECO-based strategies have brighter prospects and interest in ECO has gradually increased in recent years.
Figure 2.
Schematic illustration of ECO-based strategies for large bone defect reconstruction.
Engineering strategies for the in vitro recapitulation of ECO
Developmental engineering strategies for recapitulating endochondral bone formation typically involve two steps: (1) engineering a cartilaginous intermediate in vitro and (2) implanting the cartilaginous template in the defect site to induce bone regeneration. As these are tissue engineering approaches, some fundamental factors related to cell sources, bioscaffolds, biochemical factors, and priming protocols have been studied extensively to mimic natural bone development.
Cell sources
Cell from an ideal cell source for endochondral bone engineering must have the capacity to undergo hypertrophic chondrocyte differentiation and synthesize hypertrophic cartilage-specific ECM. Cells derived from numerous sources, including bone marrow, adipose tissue, embryonic tissue, and the nasal septum, have been successfully applied in endochondral bone engineering.
Adult MSCs
Bone marrow-derived mesenchymal stem cells (BMSCs) are currently the most frequently used cells in endochondral bone engineering. These cells are favored for their potential to differentiate into chondrocytes and subsequently develop a hypertrophic phenotype in vitro.37 Generally, the chondrogenic differentiation of BMSCs in vitro shows similarities to the process of ECO: the cells progressively produce collagen type II (COL II) and COL X; when a phosphate donor is added to the culture medium, mineralization can occur in the engineered constructs;38 and when the chondrogenically primed constructs are implanted in vivo, bone formation, mineralized matrix deposition, and blood vessel ingrowth can be observed.39 Furthermore, BMSCs have shown greater chondrogenic potential40,41 and 15-fold greater COL X expression42 than adipose-derived stem cells (ASCs) under the same culture conditions. In donor-matched comparison studies, BMSCs have shown a significantly higher chondrogenic capacity than ASCs after 21 days of culture in chondrogenic differentiation medium (CHM).43 Cartilaginous constructs engineered from BMSCs follow a process similar to that of ECO, with greater COL X expression and mineralization than constructs engineered from joint tissue-derived stem cells under the same conditions.37 The use of allogeneic MSCs, which have shown the ability to elicit endochondral bone regeneration in critical-sized femoral defects in immunocompetent rats,44 represents an interesting alternative to overcome these limitations. However, further research on achieving robust bone formation with allogeneic MSCs is needed. Although BMSCs have shown excellent performance in endochondral bone engineering, limitations of BMSCs, including donor variability, invasive harvesting protocols, and difficulty expanding cells in vitro, have also been reported.
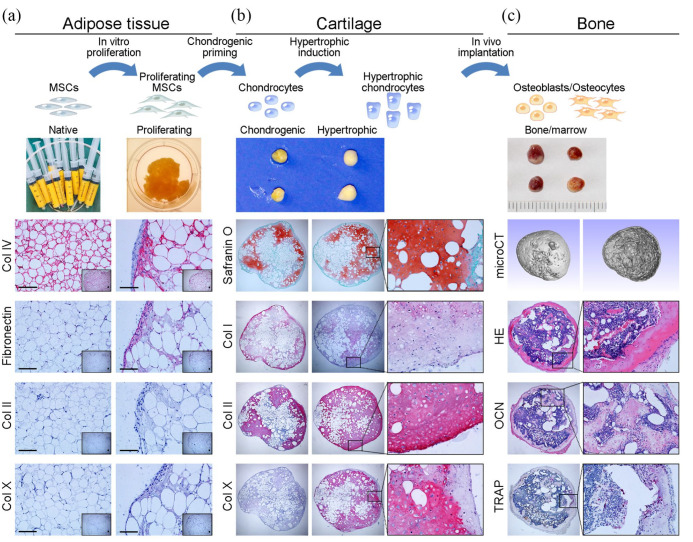
ASCs are a heterogeneous population of cells that are harvested from subcutaneous adipose tissue and exhibit several distinct translational advantages over MSCs from other tissues.45 ASCs have multilineage potential comparable to that of BMSCs,46,47 but they are easily accessible in abundant quantities by a minimally invasive procedure48,49 and have a higher proliferative capacity.50 ASCs have shown the ability to recapitulate ECO in vitro and in vivo. ASCs, either cultured as micromass pellets51,52 or spheroids53 or seeded onto collagen sponges51,54 in CHM, can mature into hypertrophic cartilage tissue. Upon subcutaneous implantation, these hypertrophic cartilage constructs are able to undergo an ECO process and develop into bone containing bone marrow elements.51,52 However, the clinical translation of this approach may still be hampered by the complex ASC processing protocol and long-term in vitro expansion procedures, which impair their multilineage differentiation potential.55 Therefore, our team innovatively fractionated human lipoaspirates into small adipose tissue particles and used them as numerous ASC niches and native scaffolds for hypertrophic cartilage engineering. The resulting constructs develop a hypertrophic cartilage phenotype and even show higher efficacy in endochondral bone formation than ASC-seeded collagen sponges (Figure 3).54,56 These studies provide a clinically translatable approach for bone defect repair.
Figure 3.
Engineering hypertrophic cartilaginous tissue directly from human adipose tissue: (a) Human adipose tissue was harvested during liposuction surgery; the human adipose tissue was positive for COL IV but negative for fibronectin, COL II, and COL X. After 3 weeks of culture in proliferation medium, the adipose tissue was positive for COL IV and fibronectin but negative for COL II and COL X, indicating that proliferative culture results in more stromal cells in the adipose tissue. (b) Then, the cultured adipose tissue was subjected to endochondral priming. After 4 weeks of chondrogenic priming, the engineered constructs showed a cartilaginous phenotype, which was characterized by positive safranin O staining for GAG, weakly positive staining for COL I and COL X, and strongly positive staining for COL II. The chondrogenically primed constructs were cultured in HYM for 2 weeks, which resulted in strong positive staining for COL X, (c) The endochondrally primed constructs were subcutaneously implanted into nude mice for 12 weeks. MicroCT scanning of the retrieved constructs showed a bony shell around bone trabeculae inside. Bone tissue formation and morphological evidence of bone marrow in the retrieved constructs were identified histologically by hematoxylin and eosin (H&E) staining and osteocalcin (OCN) staining. Intensive bone resorption by osteoclasts, characterized as tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells, was observed in the inner margins of the bone marrow-like cavity surrounded by newly formed bone tissue.
Another clinically relevant cell type for recapitulating ECO is periosteum-derived cells (PDCs), which are found in the inner cambium of the periosteum and play a prominent role during fracture healing.57 In the context of BTE, PDCs exhibit strong MSC-like multipotency characteristics at the single-cell level58 but greater clonogenicity, differentiation potential, and bone regeneration capacity.13 Furthermore, PDCs acquire the capacity for endochondral bone formation in response to injury,58 which has made them an attractive cell type for endochondral bone engineering.59 Callus organoids engineered by the chondrogenic priming of human PDC (hPDC) microspheroids in vitro can form bone microorganoids in an ectopic environment and heal murine critical-sized long bone defects.60 In another study, the preconditioning of hPDC microaggregates in vitro resulted in the formation of intermediate cartilage tissue, which could ectopically develop into bone tissue via ECO and facilitate bone defect healing.61
Embryonic stem cells (ESCs)
ESCs possess the potential to differentiate into any cell type and have been shown to achieve bone formation via ECO. The chondrogenic induction of mouse ESCs in vitro results the deposition of cartilage matrix on ceramic scaffolds, which in turn demonstrate robust endochondral bone formation upon implantation in subcutaneous sites or in critical-sized cranial defects.62 The chondrogenesis of differentiated ESCs is typically characterized by five overlapping stages, which are similar to the stages of the embryonic developmental processes of ECO: (1) condensation of differentiated ESCs; (2) differentiation and fibril scaffold formation; (3) ECM deposition and cartilage formation; (4) hypertrophy and degradation of cartilage; and (5) bone replacement with membranous calcified tissues.63
However, concerns regarding ethical objections, immune rejection, and teratoma formation continue to impede the clinical implementation of ESCs. To produce functional bone tissue without these risks, MSCs derived from ESCs (ESC-MSCs), which possess the lineage-specific differentiation potential of MSCs but enhanced proliferative and immunosuppressive capabilities,64 have also shown the capacity to recapitulate the ECO process and repair bone defects semiautonomously without preimplantation differentiation into osteo- or chondroprogenitors.65–67 Furthermore, embryonic limb-derived progenitor cells can also form bone via the ECO pathway in an ectopic environment, which has been harnessed to bridge parietal bone defects in a mouse model.68
Chondrocytes
Chondrocytes, which constitute mature and functional cartilage, are a logical cell type for endochondral bone engineering. As mentioned before, hypertrophic chondrocytes within the fracture callus stimulate osteogenesis and vasculogenesis during bone fracture healing, and hypertrophic chondrocytes isolated from fracture calluses, even from callus tissue directly, have demonstrated the capacity to promote bone regeneration.69,70 These inherent biological features suggest that the use of hypertrophic chondrocytes is a logical therapeutic strategy for bone regeneration.
Articular cartilage is normally a permanent tissue that resists hypertrophy, vascularization, and ossification.71 Under physiological conditions, articular chondrocytes remain in a resting state and refrain from proliferation or terminal differentiation. However, under the conditions of osteoarthritis, articular chondrocytes enter ECO-like cascades of proliferation and phenotypical dysregulation, becoming hypertrophic and abnormally expressing genes such as COL X, matrix metalloproteinase (MMP)-13, and ALP.72 Therefore, by leveraging these inherent biological characteristics, human osteoarthritic articular chondrocytes have been used to engineer endochondral constructs that subsequently undergo ECO after implantation either subcutaneously or orthotopically.72 Interestingly, ectopic bone formation has also been observed in healthy articular chondrocyte-engineered constructs, indicating the bone-forming potential of healthy articular chondrocytes.73 Indeed, healthy articular cartilage lesions often undergo progressive degeneration toward osteoarthritis under pathological conditions, and continuous efforts have been directed to optimize conditions for redirecting articular chondrocytes toward hypertrophy. A recent study has demonstrated that upon transforming growth factor (TGF)-β1 administration during ex vivo expansion, human articular chondrocytes are redirected toward a hypertrophic phenotype,74 which is an undesirable effect for cell culture but could be useful in endochondral bone engineering. Similarly, with the help of BMP-2, primary porcine articular chondrocytes can produce hypertrophic cartilage matrix on poly(ε-caprolactone) (PCL) scaffolds in vitro and even result in bone and bone marrow formation upon implantation.75 Furthermore, ECO with marrow cavity formation can be observed in the long term after the ectopic implantation of primary human articular chondrocytes isolated from the epiphyseal cartilage of infants in nude mice, even without in vitro expansion, induction, and scaffold seeding.76 Although promising results have been reported, the clinical translation of articular chondrocyte-engineered constructs is still limited by phenotype stability, donor site morbidity, and in vitro expansion.
Chondrocytes derived from other tissues have also shown potential as a cell source for endochondral bone engineering. Nasal septal cartilage derives from the same multipotent embryological segment that gives rise to the majority of maxillofacial bones and has been considered an autologous cell source for cartilage and BTE.77 The hypertrophic induction of adult human nasal chondrocyte-based micromass pellets in vitro results in the formation of mineralized cartilaginous tissue rich in COL X, but the tissue remains avascular and reverts to stable hyaline cartilage upon subcutaneous implantation in nude mice.78 In contrast, chondrogenically primed rat nasal chondrocyte-seeded constructs are not only rich in COL X but also show angiogenesis, mineralization, and bone formation upon subcutaneous and orthotopic implantation.79 These results demonstrate the plasticity of nasal chondrocytes in engineering endochondral bone. In addition, chondrocyte-like progenitors possessing a transient phenotype in vitro have been effectively induced toward endochondral bone formation in vivo.80–82
Biomaterials
Generally, an ideal scaffold for endochondral bone engineering should have mechanical characteristics that resemble those of hypertrophic ECM, possess appropriate cues to enhance cellular attachment and subsequent differentiation, and provide an architecture for allowing cellular adhesion, matrix production, and vessel ingrowth in vivo. Typically, four types of biomaterials, natural polymers, synthetic polymers, bioceramics, and decellularized/devitalized ECM (dECM), are used in endochondral bone engineering.
Natural polymers
Natural polymers, such as hyaluronic acid (HyA), collagen, gelatin, and fibrin, are widely used in endochondral bone engineering. Collagen and HyA are both key components of the native cartilage matrix. Highly porous collagen-based scaffolds have been extensively used to support the in vitro growth and differentiation of MSCs toward both osteogenic and chondrogenic lineages due to their biocompatible and biodegradable nature.83–86 Porous collagen sponges were first used to generate cartilage in vitro and have recently also been used to generate endochondral bone.29,51,87 HyA is an important physiological component of the cartilaginous ECM that provides a favorable environment for endochondral bone engineering and has achieved promising results.88 HyA-based scaffolds have been shown to support cell migration and differentiation. MSCs seeded into HyA hydrogel have shown greater COL II and chondroitin sulfate production but less COL I deposition than other materials.89 Therefore, composite scaffolds developed using collagen and HyA have shown greater hypertrophic cartilage formation in vitro and endochondral bone formation in vivo,33,90 but a high HyA concentration in such composite scaffolds may result in a low proportion of cells with a hypertrophic phenotype in the engineered constructs.91
Gelatin is a naturally derived protein obtained by collagen hydrolysis. Due to its biocompatibility, biodegradability, and ability to form hydrogels, gelatin plays a significant role in 3D cell culture models and has been used alone and as a basic material for improving endochondral bone regeneration.92 Porous gelatin sponge scaffolds have been shown to support chondrogenesis in vitro and calvarial healing via ECO.93 Gelatin-methacrylamide (GelMA) hydrogels produced by the chemical modification of gelatin retain some properties of collagen and gelatin, such as cell adhesion domains, thermosensitivity, and biodegradability.94,95 Thus, this material can be used as an embedding material and in the fabrication of bioprintable scaffolds for endochondral bone engineering. For example, GelMA hydrogels have been loaded with MSCs and chondroinductive particles, and the resulting composite constructs stimulated endochondral bone formation in a subcutaneous rat model.96 Additionally, MSC-loaded GelMA hydrogels are used to print constructs with an interconnected microchannel network which are subsequently used for engineering cartilaginous constructs. The 3D-printed microchannels within the cartilage template can promote osteoclast/immune cell invasion, hydrogel degradation, and vascularization during endochondral bone formation.97
Fibrin is a biopolymer of the monomer fibrinogen that plays critical roles in blood clotting, cell-matrix interactions, inflammation, and wound healing.98 Fibrin hydrogels facilitate MSC-mediated vascularization, endochondral bone formation, and bone marrow development.99 Fibrin hydrogels reduce the standard chondrogenic priming duration from 28 days to 7 days but yield comparable endochondral bone formation.100 Fibrin supports cell attachment, condensation, and proliferation but is mechanically weak and degrades rapidly. Hyaluronan is mechanically stronger and degrades much more slowly than fibrin. Therefore, a novel hybrid system composed of 70% hyaluronan and 30% fibrin has been developed to closely mimic the ECM microenvironment. This combination supports cell microaggregation and differentiation and demonstrates the healthy development of chondrogenic and hypertrophic stages with abundant stage-specific ECM components.101 Similarly, fibrin glue combined with MSCs and β-tricalcium phosphate (β-TCP) particles can enhance heterotopic endochondral bone formation.102
Other naturally derived polymers, such as alginate, chitosan, and agarose, have also been used as biocompatible hydrogel materials in endochondral bone engineering. Alginate hydrogels have been used to engineer endochondral bone tissue in subcutaneous spaces or bone defects.39,103 Furthermore, similar to GelMA hydrogels, alginate hydrogels are also used as beads for cell encapsulation52 or as bioinks for 3D printing104 for endochondral bone engineering. Chitosan is structurally similar to various glycosaminoglycans (GAGs) and has been shown to support hypertrophic cartilage matrix deposition and endochondral bone formation.80,81 However, compared with alginate and fibrin hydrogels, chitosan hydrogels are resistant to vascularization and bone remodeling.99 Agarose is a polysaccharide that has been used to encapsulate MSCs, MSC pellets, or other materials in endochondral bone engineering.37,52,105 By leveraging the good mechanical and thermoreversible properties of agarose hydrogels, agarose hydrogels containing an array of microchannel structures have also been shown to support vascularization and conversion to endochondral bone.106
Synthetic polymers
Natural polymers are sometimes limited by their poor mechanical strength and rapid degradation. Synthetic polymers including polylactic acid (PLA), poly(L-lactic acid) (PLLA), polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), PCL, and their copolymers have been extensively utilized for endochondral bone engineering due to their good chondrogenic conduction, biocompatibility, slow degradation, and easily altered mechanical properties.107
Synthetic polymers have demonstrated good chondrogenic conduction both in vitro and in vivo. PLGA scaffolds loaded with chondrogenically predifferentiated rat BMSCs have been shown to heal large bone defects in rats by mimicking ECO.108 Similarly, nasal chondrocyte-seeded PGA scaffolds can promote endochondral bone formation in rat cranial defects.79 The biochemical cues provided by synthetic polymer scaffolds control cellular proliferation and differentiation. PLGA/PLLA copolymer scaffolds can induce human ESC differentiation via an ECO pathway, while hydroxyapatite (HAp)-based PLGA/PLLA composite scaffolds result in bone formation via an IMO pathway.66 However, cell adhesion and proliferation are limited due to the hydrophobicity of these synthetic polymer scaffolds.109,110
Synthetic polymers have better mechanical properties and flexibility than natural polymers. Porous PLGA scaffolds have been added to hyaluronan-fibrin hydrogels to enhance their mechanical strength and fabricate a load-bearing scaffold system. In this polymer-gel hybrid scaffold, the gel phase provides a microenvironment to guide the endochondral process, while the polymer phase is expected to provide mechanical strength to the overall polymer-gel structure.111 PCL possesses an appropriately high bulk stiffness to facilitate MSC differentiation toward skeletal lineages and has been selected as a scaffold material to support chondrogenesis and hypertrophic mineralization.112 In addition, bioprinted PCL microfiber networks have been used to reinforce the mechanical strength of engineered cartilaginous templates, which support the development of a vascularized bone organ containing trabecular-like bone and a supporting hematopoietic marrow structure.104
Synthetic polymer scaffolds with nanofibers similar in size to natural cartilage matrix fibers facilitate tissue regeneration. 3D PLGA/PCL scaffolds fabricated by electrospinning have been demonstrated to support the chondrogenic differentiation of rat BMSCs in vitro and endochondral bone formation in vivo.113,114 Furthermore, the pore size and architecture of nanofibrous polymer scaffolds affect the conversion of cartilage to bone tissue. Under the same in vitro priming and in vivo implantation conditions, nanofibrous PLLA scaffolds with large pores (425–600 μm) have been shown to support endochondral bone formation by allowing blood vessel ingrowth, whereas scaffolds with very small pores (60–125 μm) have been shown to allow cartilage formation but inhibit ECO.115 In another study, an electrospun PCL 3D nanofibrous scaffold with interconnected and hierarchically structured pores morphologically similar to natural ECM could lead to high cell viability. More importantly, it could promote the BMP-2-induced chondrogenic differentiation of mouse BMSCs in vitro and act as a favorable synthetic ECM for endochondral bone regeneration in vivo.116
Bioceramics
Known for their good biocompatibility, bone bioactivity, and osteoconductivity, bioceramics, such as HAp, β-TCP, and biphasic calcium phosphate (BCP), have been utilized as scaffolds for endochondral bone engineering. However, due to their high brittleness, bioceramics are typically combined with various natural or synthetic polymers to create highly porous biocomposite materials with improved mechanical properties.
HAp is one of the main inorganic components of the natural bone matrix. HAp-based scaffolds have been used extensively to promote bone regeneration because of their good biocompatibility and high osteoconductivity.117,118 Recently, nano-HAp particles, when used to coat titanium scaffolds, have been shown to support the chondrogenic differentiation of MSCs and enhance endochondral bone regeneration in mandibular defects in rats.119 Furthermore, when incorporated into biodegradable polymers, such as collagen90 and poly(vinyl alcohol),120 HAp particles enhance the mechanical properties of scaffolds, resulting in mechanical strength very similar to that of bone or cartilage. Moreover, the osteoinductivity of HAp can affect the chondrogenesis, hypertrophy, and ECO of MSCs. In a bioprintable HyA-based hydrogel system, a small number of HAp particles has been shown to promote both the chondrogenic and the hypertrophic differentiation of ASCs, whereas larger numbers of HAp particles promote hypertrophic conversion and early osteogenic differentiation of ASCs.121 In another study, CaP-coated HAp mineral particles have been shown to promote the gene expression of chondrogenic markers and enhance the hypertrophic phenotype of ESC aggregates in vitro in a dose-dependent manner.67
β-TCP has been shown to promote cartilage regeneration and biomineralization122 and support endochondral bone formation123 because of its biodegradability, biocompatibility, and bioactivity. β-TCP is often combined with other materials to enhance the chondrogenic and endochondral potential of scaffolds. It has been shown that the addition of β-TCP particles to a 3D biomimetic hydrogel scaffold not only induces abundant expression of the chondrogenic markers COL II and aggrecan but also, most notably, results in overexpression of the hypertrophic marker COL X and the osteogenic marker ALP.124
BCP consists of two CaP phases, namely, a stable HAp phase and a soluble β-TCP phase, in distinct proportions. These two materials are proportionally combined to obtain a suitable balance between the predictable biodegradability offered by β-TCP and the improved resiliency provided by HAp. The chemical and structural cues provided by porous HAp/TCP scaffolds have been shown to support progressive lamellar-like bone formation and mature bone marrow development.125 Our previous study showed that porous HAp/β-TCP granules support endochondral bone formation at ectopic sites by enhancing biomineralization.56 Furthermore, porous HAp/TCP scaffolds are more suitable for implantation in loadbearing areas than other scaffolds, including those made of polyurethane foam, electrospun PLGA/PCL fibers, and COL I gel, due to their excellent mechanical properties.114
dECM
dECM not only retains the original 3D morphological architecture of the tissue but also retains a complex mixture of proteins and macromolecules that facilitate the proliferation and differentiation of endogenous or exogenous cells.126 Importantly, the structural and functional proteins of the ECM are highly conserved across species, which allows xenogeneic dECM to be implanted in recipients of different species without an immune reaction.127,128 dECM derived from natural bone and cartilage tissues has been used as scaffolds for endochondral bone engineering. Recent works have shown that endochondral constructs can be engineered by culturing ASC-seeded decellularized bone matrix (dBM) sequentially in CHM and then hypertrophic medium (HYM). These engineered constructs have been shown to enhance bone deposition, bone remodeling, and bone marrow formation in critical-sized femoral defects in rats.35 In addition, decellularized cartilage matrix (dCM), which consists predominantly of COL II but lacks GAGs and cells, is a promising scaffold material for endochondral bone engineering.129 The biological integrity of dCM can stimulate endochondral bone regeneration by enhancing the chondrogenesis of MSCs, PDCs, or chondrocytes in vitro and eliciting a regenerative response upon implantation in vivo96,128 but has no significant effect on construct mineralization96 and a lower potential for endochondral bone formation.130 Therefore, the use of dCM for endochondral bone engineering remains controversial, and studies have demonstrated that dCM promotes the chondrogenic differentiation but inhibits the hypertrophic differentiation of MSCs in vitro and subsequent ECO in vivo.131–133 It has long been realized that viable hypertrophic cartilage will form bone in vivo; decellularized hypertrophic cartilage matrix (dHCM), which is manufactured from native epiphyseal plates or fractured callus tissue, can also trigger the natural ECO process upon implantation.134
A major drawback of these types of naturally derived dECMs is the limited donor source, which hinders their clinical application. Recent works have sought to engineer off-the-shelf decellularized/devitalized tissue engineered-cartilaginous matrix (dTECM) for endochondral bone engineering, which is expected to induce regenerative processes not only through specific “organomorphic” structures but also through the physiological presentation of different cocktails of regulatory molecules in a suitable environment.135,136 Bourgine et al. engineered a novel hypertrophic cartilage construct using immortalized human MSCs, in which decellularization was achieved by the induction of apoptosis with efficient ECM preservation. The resulting dHCM could efficiently remodel to form endochondral bone tissue of host origin, including a mature vasculature and a hematopoietic compartment.137–139 Furthermore, dTECM engineered from various cell sources, such as ATDC5 cells,105 adipose-derived stromal vascular fraction (SVF) cells,140 and BMSCs,141,142 has shown the ability to be activated by MSCs to initiate ECO and induce new bone formation upon implantation without long-term endochondral priming in vitro. These promising results offer an alternative solution to overcome the drawbacks of naturally derived dECM and the extensive production of dECM using allogenic MSCs.
Optimal endochondral priming medium and duration
Chondrogenesis is the first stage of the ECO process, involving with differentiation and maturation of chondrocytes. Different endochondral priming protocols have been used to replicate the initial stages of ECO by driving progenitors toward a hypertrophic phenotype.
Endochondral priming medium
Endochondral priming largely relies on the chondrogenic differentiation stage, as chondrocytes possess specific metabolic features and secrete diverse biochemical cues when they are in different developmental stages. Generally, the engineering of a cartilage template from MSCs in vitro is necessary to recapitulate the process of ECO because chondrogenically differentiated MSCs tend to simultaneously acquire a certain degree of hypertrophic properties upon persistent exposure to CHM.143,144 However, chondrogenic induction alone usually results in “early hypertrophic” tissues with low and localized COL X expression,51,87 which is specifically produced by hypertrophic chondrocytes.145 Due to the critical roles of hypertrophic chondrocytes during ECO, an additional in vitro hypertrophic priming step is often applied to the chondrogenically primed constructs to elicit the most extensive ECO.146,147 The differentiation of MSCs In vitro into mature and hypertrophic chondrocytes requires a precise combination of growth and differentiation factors. However, standard medium formulations for chondrogenic differentiation and hypertrophic induction are lacking. CHM is typically defined as Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 U/mL penicillin/streptomycin, 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, 50 μg/mL L-ascorbic acid 2-phosphate, 4.7 μg/mL linoleic acid, 1.5 mg/mL bovine serum albumin (BSA), 1× insulin-transferrin-selenium (ITS) premix, and 100 nM dexamethasone, along with various growth factors. Serum is sometimes used, but serum-free media are more common because serum-free cultures closely mimic the serum-free physiological environment in which chondrocytes reside. Growth factors play a dominant role in the differentiation of MSCs toward chondrogenic phenotypes in vitro; these growth factors include TGF-βs and BMPs, which play fundamental roles in early-stage MSC differentiation and chondrogenic phenotype maintenance. Some of these factors are also involved in terminal differentiation.
TGF-β superfamily members including TGF-β1, TGF-β2, and TGF-β3 are the core components of most chondrogenic differentiation protocols.148,149 In vitro, TGF-βs induce chondrogenesis, proliferation, and matrix deposition in MSCs, with eventual progression toward hypertrophy. TGF-β1 and TGF-β2 initiate MSC condensation during the early stage of endochondral bone development. TGF-β3 has stronger effects than TGF-β1150 or TGF-β2151 on the chondrogenic differentiation of MSCs. Furthermore, BMPs, which are morphogens in the TGF-β superfamily, have been shown to regulate chondrogenesis and osteogenesis during skeletal development. BMP-2, BMP-4, and BMP-6 are the most commonly used BMPs for chondrogenic differentiation. BMP-2 has been reported to induce chondrogenic differentiation by upregulating the levels of COL II and aggrecan in various types of stem cells in vitro152,153 and to promote chondrocyte hypertrophy by increasing COL X and ALP expression as well as upregulating of Indian hedgehog expression.154,155 BMP-4 plays a fundamental role in early-stage MSC chondrogenic differentiation and chondrogenic phenotype maintenance156 and is also involved in regulating the terminal differentiation of chondrocytes.152 BMP-6 participates in mediating chondrocyte hypertrophy. The treatment of chondrocytes with BMP-6 has been widely shown to stimulate COL X gene expression.157
Various combinations of TGF-βs and BMPs have shown a synergistic ability to enhance chondrogenic differentiation and hypertrophy and have been widely used in endochondral bone engineering. For example, a combination of TGF-β1 and BMP-2 has been shown to increase the GAG and collagen content and initiate robust endochondral lineage commitment.158–160 The dual delivery of TGF-β1 and BMP-2 within BMSC aggregates has been shown to result in enhanced chondrogenesis and an enhanced osteogenic phenotype, as well as a greater degree of mineralization and COL X expression.161,162 Additionally, compared to TGF-β3 alone, combinations of TGF-β3 and BMP-6 can enhance the chondrogenic potential of BMSCs and ASCs.163,164 Among numerous growth factor combinations, TGF-β3 in conjunction with BMP-6 appears to be the most effective for chondrogenic induction and ECO in ASCs51,54,56 because TGF-β3 alone is a potent inducer of chondrogenic differentiation, whereas BMP-6 acts synergically with TGF-β3 by inducing the expression of TGF-β receptor I, which is usually not expressed by ASCs.165
Additional culture in HYM has been applied to promote chondrocyte hypertrophy and ossification in vitro. HYM is typically defined as CHM without growth factors, with a reduced dexamethasone concentration (1–10 nM), and with β-glycerophosphate (β-GP, 10 mM) and thyroxine (1–50 nM) or triiodothyronine (T3, 1 nM). The reduced dexamethasone concentration can induce Runx2 upregulation, followed by COL I upregulation. β-GP can act as a source of phosphate for HAp.166 In addition to these basic supplements in HYM, other molecules or growth factors have been added to improve the efficiency of hypertrophic induction. Thyroid hormone and T3 have been shown to induce morphological and hypertrophic marker expression without inducing proliferation.167 Furthermore, inflammatory cytokines, such as interleukin-1β, have been used to induce inflammation to improve hypertrophic cartilaginous construct remodeling into bone tissue without hampering mineralization.29,168
Endochondral priming duration
Endochondral priming protocols typically aim to reach a stage with a certain degree of hypertrophy before implantation. For chondrogenic priming, the in vitro induction duration varies from 1 to 5 weeks for different cells, media, and culture systems but usually lasts for 3 to 4 weeks. For clinical translation, prolonged in vitro culture is associated with high treatment costs and a large regulatory burden, which are not ideal. Fine coordination between the progression of chondrogenesis and endochondral transformation needs to be achieved by choosing the optimal duration of in vitro chondrogenic and hypertrophic priming. Typically, mineralization occurs in constructs cultured for 3 weeks in CHM followed by 2 weeks in HYM.169 It has been shown that the priming of BMSC pellets in vitro for 3 weeks in CHM followed by 4 weeks in HYM optimized GAG production and mineralization, resulting in a construct with mineralization throughout the core.170 However, a longer chondrogenic priming duration resulted in a significant increase in the homogeneous deposition of cartilage matrix, whereas the bone volume was not affected by the priming duration. Two weeks of chondrogenic priming in vitro is sufficient to generate a substantial amount of vascularized endochondral bone in vivo.125
Bone defect reconstruction via the endochondral route: current state of the art
Cartilaginous constructs engineered via endochondral priming tend to undergo hypertrophy and generate endochondral bone tissue at ectopic sites, which opens the possibility of using endochondrally primed constructs for bone defect repair. Such an ectopic model is useful for investigating vascular invasion and mineralization but does not offer an ideal environment for assessing the efficacy of bone defect reconstruction. In an orthotopic environment, bone regeneration starts with a low-grade inflammatory phase,76 low oxygen tension,171 and continuous biomechanical stimuli, which are all known to affect bone regeneration. To analyze the current state of bone defect reconstruction using ECO-based strategies, we searched the PubMed databases for articles published between January 1, 2000, and March 1, 2021, using the search terms “endochondral ossification,” “bone defect reconstruction,” “bone regeneration,” and “bone tissue engineering.” Here, we include 25 publications related to bone defect reconstruction using tissue-engineered endochondral grafts. A flow diagram of the initial identification, exclusion, and final selection of studies is shown in Figure 4. The included publications are categorized according to whether the endochondral grafts were engineered by chondrogenic priming alone (Table 1) or a combination of chondrogenic and hypertrophic priming (Table 2) to analyze the efficacy of two different endochondral bone engineering strategies.
Figure 4.
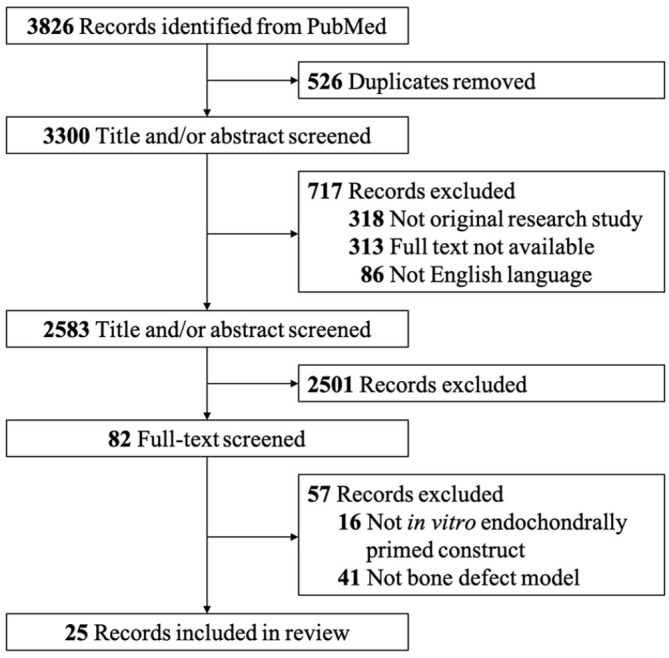
Flow diagram for study selection.
The inclusion criteria were as follows: (1) the constructs were engineered via chondrogenic and/or hypertrophic priming in vitro; (2) bone defect reconstruction in animal model. The included studies must meet all the above criteria at the same time. The excluded criteria were: (1) not an original article; (2) full text was not available; (3) not English language; (4) duplicate publications. Reports meet any of the above criteria were excluded.
Table 1.
Cartilaginous constructs engineered by chondrogenic priming alone for bone defect reconstruction.
| Reference | Cell source | Biomaterial | In vitro priming condition | Bone defect model | Highlighted results |
|---|---|---|---|---|---|
| Iimori et al. (2021)172 | hiPSC line | / | Scaffoldless suspension culture; 10, 12, or 17 weeks in CHM containing TGF-β1, BMP-2, and GDF-5 | 3.5-mm femoral defect in SCID mice | The hiPSC-derived cartilage produced new bone via reminiscent of SOC-ECO process in the defects. |
| Less time for chondrogenic differentiation of hiPSCs resulting in faster bone formation. | |||||
| Longoni et al. (2020)44 | hBMSCs; rat BMSCs | COL I gel | Spheroid culture; 4 weeks in CHM containing TGF-β1 and BMP-2 | 6-mm femoral defect in Brown Norway rats | The amount of endochondral bone formation was proportional to the degree of host-donor relatedness. |
| No full bridging of the defect was observed in the hBMSCs group, whereas 2/8 and 7/7 bridges formed in allogeneic and syngeneic group, respectively. | |||||
| Nilsson Hall et al. (2020)60 | hPDCs | / | Microspheroid culture; 4 weeks in chemically defined CHM containing BMP-2, TGF-β1, GDF-5, BMP-6, and FGF-2 | 4-mm tibial defect in NMRInu/nu mice | Engineered callus organoids spontaneously bioassembled in vitro into large, engineered tissues able to heal murine critical-sized long bone defects via ECO. |
| Freeman et al. (2020)36 | hBMSCs; hUVECs | PCL scaffold | Endochondral priming: 3 weeks in CHM containing TGF-β3; Osteogenic priming: 3 weeks in osteogenic medium |
4-mm calvarial defect in immunocompromised mice | The addition of hUVECs alone or a coculture of hUVECs and hBMSCs did not benefit for either the vascularization or mineralization potential of the scaffolds. |
| Endochondral priming alone was sufficient to induce vascularization and subsequent mineralization. | |||||
| Wang et al. (2018)119 | Rat BMSCs | HAp-coated porous Ti6Al4V scaffolds | 4 weeks in CHM | 5-mm full-thickness circular mandibular defect in SD rats | The HAp-coated Ti6Al4V scaffolds improved the chondrogenic differentiation of BMSCs in vitro and increased new bone formation via ECO in vivo. |
| Daly et al. (2018)97 | Rat BMSCs | GelMA hydrogel with 3D printed microchannels | 4 weeks in CHM containing TGF-β3 and BMP-2 | 5-mm femoral defect in Fischer rats | 3D-printed hypertrophic cartilage grafts with microchannels promoted osteoclast/immune cell invasion, hydrogel degradation, and vascularization following implantation. |
| Bolander et al. (2017)61 | hPDCs | COL I gel | Cell aggregate culture; 6 days of preconditioning in serum-free CDM or growth medium followed by 6 days of stimulation by BMP-2, BMP-4, BMP-6, BMP-7, BMP-9, and GDF-5 in CDM | 4-mm tibial defect in NMRInu/nu mice | Serum-free preconditioning in CDM enhanced BMP-2-induced osteochondrogenic differentiation of PDCs. |
| Combined in vitro priming by BMP-2 treatment and aggregation led to endochondral bone formation and critical-size bone defect healing in vivo. | |||||
| Bardsley et al. (2017)79 | Rat nasal chondrocytes | PGA scaffold | Constructs were cultured in basic medium containing insulin and ascorbic acid for 5 weeks | 4-mm full-thickness calvarial defect in Wistar rats | Constructs derived from nasal chondrocytes had the capacity to express features of hypertrophic chondrocytes. |
| Nasal chondrocytes can be used to engineer hypertrophic cartilage and repair bone defects. | |||||
| van der Stok et al. (2014)173 | hBMSCs | / | Pellet culture; undifferentiated pellets: 3 days in CHM containing TGF-β1; Chondrogenically differentiated pellets: 3 weeks in CHM containing TGF-β1 |
6-mm femoral defect in RUN rats | Chondrogenically differentiated pellets resulted in significantly more bone and vascularization in critical bone defects through ECO than undifferentiated pellets. |
| Harada et al. (2014)108 | Rat BMSCs | PLGA scaffold | 3 weeks in CHM containing TGF-β3 and BMP-2 | 5-mm or 15-mm femoral defect in Fischer rats | The large 15-mm implants reached 75% of the strength of the normal rat femur, while there was no significant difference in the strength of the 5-mm implants. |
| Mikael et al. (2014)174 | hBMSCs | Donut-shaped Healos scaffold disc | Pellet culture, 16 days in CHM containing TGF-β1 | 3.5-mm calvarial defect in NSG mice | Precartilage template formed in vitro induced mineralized tissue formation via a cartilage-mediated process. |
| Bahney et al. (2014)70 | hBMSCs; hACs |
PEGDA scaffolds | Pellet culture, 3 weeks in CHM containing TGF-β1; | 2-mm segmental tibial defect in Nude mice | Cartilage grafts from fracture callus produced well-vascularized and integrated bone regeneration via ECO in bone defects. |
| Scaffold culture, 6 weeks in CHM containing TGF-β1 | hBMSC-derived cartilage pellets promoted bone regeneration via ECO in bone defects. | ||||
| Both hBMSC and hAC-encapsulated PEGDA scaffolds synthesized COL II and sulfated proteoglycans, but only hBMSC-encapsulated PEGDA scaffolds elaborated COL I and X proteins. | |||||
| Jukes et al. (2008)62 | Mouse ESC line IB10 | Ceramic scaffolds | 3 weeks in serum-free CHM containing TGF-β3 | 8-mm calvarial defect in immunodeficient rats | Significantly more bone ingrowth was observed in the inner circle of the tissue-engineered cartilaginous constructs. |
| Huang et al. (2006)175 | Rabbit BMSCs | Composite sponge of 70% esterified hyaluronan and 30% gelatin | 3 weeks in serum-free CHM containing TGF-β1 | Lunate excision in adult New Zealand white rabbits | Cartilaginous implants formed abundant bone tissue and blood vessels through ECO. |
BMSCs, bone marrow-derived mesenchymal stem cells; CDM, chemically defined medium; CHM, chondrogenic medium; ESCs, embryonic stem cells; GDF-5, growth/differentiation factor 5; GelMA, gelatin-methacrylamide; hACs, human articular chondrocytes; HAp; hydroxyapatite; hiPSCs, human induced pluripotent stem cells; hPDCs, human periosteum-derived cells; hUVECs, human umbilical vein endothelial cells; PCL, poly(ε-caprolactone); PEGDA, poly(ethylene glycol) diacrylate; PGA, polyglycolic acid; PLGA, poly(lactic-co-glycolic acid).
CHM is typically defined as DMEM supplemented with 100 U/mL penicillin/streptomycin, 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, 50 μg/mL L-ascorbic acid 2-phosphate, 4.7 μg/mL linoleic acid, 1.5 mg/mL BSA, 1× ITS, 100 nM dexamethasone, and 10 ng/mL human TGF-β1 or TGF-β3.
Table 2.
Cartilaginous constructs engineered by chondrogenic priming and hypertrophic priming for bone defect reconstruction.
| Reference | Cell source | Biomaterial | In vitro priming condition | Bone defect model | Highlighted results |
|---|---|---|---|---|---|
| Mikael et al. (2020)111 | hBMSCs | Hybrid matrix scaffolds composed of PLGA microspheres and HyA-fibrin hydrogel | 2 weeks in CHM containing TGF-β1 followed by 2 weeks in HYM | 3.5-mm calvarial defect in NSG mice | Hybrid matrix recruited host cells, leading to new bone formation and remodeling through ECO. |
| Zhang et al. (2020)176 | Mouse gingiva-derived iPSCs line |
/ | 3D rotary suspension culture pellet culture; 2 weeks in CHM containing TGF-β3 and BMP-4 followed by 2 weeks in HYM | 5-mm circular calvarial defect in SD rats | The hypertrophic cartilage pellets derived from iPSCs were capable of vascularized bone regeneration via ECO in the bone defects. |
| Li et al. (2019)177 | Mouse BMSCs | Ceria nanoparticle modified cancellous bones | 2 weeks in CHM containing TGF-β3 followed by 2 weeks in HYM | 3-mm femoral defect in FVB/N mice | Ceria nanoparticles significantly promoted |
| ECO-based bone regeneration by ensuring sufficient hypertrophic differentiation via DHX15 activation. | |||||
| Petersen et al. (2018)178 | hBMSCs | Collagen scaffold with a channel-like pore architecture | 3 weeks in CHM containing TGF-β1 followed by 2 weeks in HYM | 5-mm femoral defect in SD rats | Channel-like macroporous architecture had the potential to induce the ECO process for bone healing. |
| Matsiko et al. (2018)33 | MSCs | Collagen-HyA scaffolds | 3 weeks in CHM followed by 2 weeks in HYM | 5-mm femoral defect in Fischer rats | Collagen-based scaffolds acted as suitable templates for the development of ECO constructs capable of supporting early-stage bone repair. |
| Bai et al. (2018)179 | Murine BMSCs | / | Pellet culture; 2 weeks in CHM containing TGF-β3 and mangiferin followed by 2 weeks in HYM | 2-mm femoral defect in BALB/c mice | Mangiferin promoted the chondrogenic and hypertrophic differentiation of BMSCs in vitro and enhanced ECO-based bone repair in vivo. |
| Dang et al. (2017)180 | hBMSCs | Gelatin microparticles loaded with TGF-β1; mineral-coated HAp microparticles loaded with BMP-2 |
Microparticle-loaded hBMSC sheets; 2 weeks in serum-free CHM followed by 3 weeks in serum-free osteogenic medium | 5-mm circular calvarial defect in athymic rats | Constructs containing microparticles loaded with TGF-β1 and BMP-2 promoted the greatest degree of healing with bony bridging via ECO. |
| Bernhard et al. (2017)35 | hASCs | Decellularized bovine trabecular bone matrix | Hypertrophic chondrocyte graft: 2 weeks in CHM containing TGF-β3 and BMP-6 followed by 3 weeks in HYM; osteoblast graft: 5 weeks in osteogenic medium |
5-mm femoral defect in RUN nude rats | Hypertrophic chondrocyte grafts enhanced bone regeneration by recapitulating ECO in critical-sized orthotopic long bone defects. |
| Hypertrophic chondrocyte grafts bridged 7/8 defects compared to only 1/8 for osteoblast grafts, and 3/8 for acellular scaffolds. | |||||
| Thompson et al. (2016)90 | Rat BMSCs | Collagen-HyA scaffolds; | ECO constructs: 3 weeks in CHM containing TGF-β3 followed by 2 weeks in HYM; | 7-mm circular calvarial defect in Fischer rats | ECO-based constructs yielded more new bone formation within the defects than IMO-based constructs, which may be associated with VEGF secretion in the ECO-based constructs. |
| collagen-HAp scaffolds | IMO constructs: 5 weeks in osteogenic medium | Collagen-HyA hypertrophic constructs supported the greatest new bone formation within the defects. | |||
| Bahney et al. (2016)72 | Human OA chondrocytes | / | Pellet culture; 1 week in CHM containing TGF-β1 and BMP-4 followed by 3 weeks in CHM without growth factors | 3-mm tibial defect in immunocompromised mice | Endochondrally primed cartilage grafts generated from passaged OA chondrocytes underwent ECO, variably remodeled into woven bone, and integrated with host bone at 15/16 junctions. |
| Cartilage grafts formed from primary OA chondrocytes without endochondral priming did not undergo ECO in vivo. | |||||
| Cunniffe et al. (2015)181 | Rat BMSCs | Alginate hydrogels | 4 weeks in CHM containing TGF-β3 followed by 3 weeks in HYM | 5-mm femoral defect and 7-mm circular calvarial defect in Fischer rats | Chondrogenically primed BMSC-alginate constructs acted as templates to treat critical-sized defects within bones formed through either IMO or ECO. |
BMSCs, bone marrow-derived mesenchymal stem cells; CHM, chondrogenic medium; hASCs, human adipose-derived stem cells; iPSCs, induced pluripotent stem cells; HYM, hypertrophic medium; HAp, hydroxyapatite; HyA, hyaluronic acid; PEGDA, poly(ethylene glycol) diacrylate; PLGA, poly(lactide-co-glycolic) acid; GelMA, gelatin-methacrylamide; OA, osteoarthritis.
HYM is typically defined as CHM with no growth factors, with a reduced dexamethasone concentration (1–10 nM), and with β-GP (10 mM) and thyroxine (1–50 nM) or triiodothyronine (1 nM).
Chondrogenically primed cartilaginous grafts act as logical templates for bone repair at orthotopic sites
As shown in Table 1, a wide range of distinct approaches have been adopted to engineer cartilaginous grafts by chondrogenic priming. In 2006, a cartilaginous construct engineered via the chondrogenic priming of autologous BMSC-seeded biodegradable scaffolds for 3 weeks effectively prevented carpal collapse in a New Zealand white rabbit model.175 This is the first report demonstrating that tissue-engineered cartilaginous grafts could recapitulate the ECO process and support bone formation at orthotopic sites. Inspired by this study, attempts have been made to engineer cartilaginous grafts in vitro for critical-sized bone defect reconstruction and have yielded promising results. Tissue-engineered cartilaginous grafts have been observed to mature and form bone tissue in critical-sized calvarial defect models, although they are not the most logical model for endochondral bone formation because craniofacial bones form through IMO.182 For example, compared with sham implants, cartilaginous constructs engineered via the chondrogenic priming of mouse ESC-seeded ceramic scaffolds could transform into bone tissue in the inner circle of the constructs in 8-mm rat cranial defects.62 Moreover, cartilaginous grafts engineered via the chondrogenic priming of other cell sources, such as nasal chondrocytes79 and PDCs,60 have shown promising results in critical-sized bone defect reconstruction. To further translate the use of chondrogenically primed cartilaginous constructs for therapeutic applications, cartilaginous grafts engineered by the chondrogenic priming of BMSCs-seeded PLGA scaffolds have been used to heal both critical-sized (5-mm) and massive (15-mm) full-thickness femoral defects in rats. After 8 weeks, the mean biomechanical strength of femora with 15-mm implants reached 75% of that of the normal rat femur, while there was no significant difference in the strength of femora with 5-mm implants.108 Collectively, this evidence suggests that engineering of cartilaginous grafts via chondrogenic priming alone is a viable, underexplored strategy for critical-sized bone defect reconstruction.
Additional hypertrophic priming promotes the efficiency of endochondral bone formation
Although chondrogenically primed constructs have shown a certain degree of hypertrophy and can recapitulate ECO upon implantation in vivo, insufficient construct ossification and vascularization have also been observed in several studies.51,87 Additional hypertrophic priming of chondrogenically primed constructs not only maintains the chondrogenic features but also promotes the abundant expression of hypertrophic markers and ultimately results in abundant vascularization and mature bone matrix formation.87 Similar results have also been observed in adipose tissue-derived SVF-based cartilaginous constructs.51 These results from ectopic bone formation models suggest that the hypertrophic priming of cartilaginous grafts provides a valuable solution for enhancing endochondral bone regeneration and accelerating vascularization. Unfortunately, to date, no studies have compared the efficiency of bone formation between cartilage constructs engineered via chondrogenic priming alone and those engineered via a combination of chondrogenic and hypertrophic priming in orthotopic bone formation models.
However, several studies have revealed the superiority of hypertrophically primed constructs over traditional IMO-based constructs in bone defect reconstruction. Thompson et al. compared the orthotopic bone formation efficiency of constructs engineered via chondrogenic and hypertrophic priming with that of constructs engineered by osteogenic priming. Because hypertrophic chondrocytes can secrete osteogenic and angiogenic signals, the endochondral constructs showed better results than the osteogenic constructs in terms of bone regeneration, vascularization, and remodeling.90 Similar results have been observed in a rat model of critical-sized femoral defects repaired by hASC-based grafts. The hypertrophic grafts engineered by 3 weeks of chondrogenic priming followed by 2 weeks of hypertrophic priming substantially enhanced bone regeneration associated with extensive bone remodeling and hematopoietic marrow formation. Furthermore, the hypertrophic cartilaginous grafts resulted in significantly greater bone volume in the defect space than the osteoblast grafts and acellular scaffolds.35 However, the additional in vitro hypertrophic priming step prolongs the endochondral bone engineering period. A delicate balance between chondrogenic differentiation and hypertrophic induction should be further investigated to improve the efficiency of ECO-based strategies in the future.
Future perspectives
To date, numerous studies have shown promising results in bone defect reconstruction using endochondrally primed constructs in animal models. However, the translation of these ECO-based strategies from the bench to the bedside is still ongoing and will face many challenges.
Integration of “top-down” tissue engineering and developmental engineering approaches provides a new solution for repairing large bone defects
Generally, tissue-engineered grafts for bone defect reconstruction should ensure osteogenesis, angiogenesis, and survivability after implantation. To date, successes in large bone defect reconstruction using ECO-based strategies have been achieved in small animal models, but no studies have verified the practicability of such a strategies in a large animal model, which is closer to the actual clinical situation. For repairing large bone defects in large animal models or under clinical conditions, scaled-up endochondral constructs are needed to fit the defects, which poses a new challenge.
Classic approaches for recapitulating ECO adopt a “top-down” strategy that relies on seeding progenitor cells onto scaffolds and then guiding the ECO process with growth factors. Such strategies are limited in the fabrication of large tissue constructs in vitro. Conversely, an emerging “bottom-up” strategy for engineering large endochondral constructs is the scaffold-free culture technique, which aims to precondition cells to form modular tissue units represented by spheroid culture techniques, including cell pellets, cell sheets, and cell aggregates.183,184 These high-density cell cultures provide a more homogeneous 3D culture format than other cultures to allow cell-cell interactions that are similar to the precartilage condensation process during embryonic bone development. Importantly, stem cell condensation has been shown to enhance chondrogenic differentiation.185 By integrating the principles of “bottom-up” tissue engineering and “developmental engineering” approaches, endochondrally primed spheroids derived from MSCs or ESCs can spontaneously fuse with each other and recapitulate ECO events, making them ideal building blocks for engineering large-scale bone grafts.49 This integrated approach has several advantages that may support its clinical translation: (1) the possibility of scaling up tissue-engineered bone grafts to a clinically relevant size; (2) the ability to create endochondral bone tissues with high cellular densities without scaffolds; and (3) the potential to model an endochondral bone graft with a complex geometric shape. The engineering of endochondral constructs via cell aggregates60,61 and pellets72,173 has been reported in the studies regarding critical-sized bone defect reconstruction and large bone graft prefabrication.186
dTECM: An off-the-shelf material capable of recapitulating ECO
A major obstacle to the clinical translation of ECO-based strategies is the long-term in vitro endochondral priming period. Furthermore, other issues, such as cost-effectiveness, engineering process complexity, the need for two surgical procedures, and tissue engineering-associated regulatory hurdles, also need to be overcome. These limitations have driven the development of dTECM as an off-the-shelf and immune-compatible alternative to living grafts with the capability of recapitulating the ECO process for bone defect reconstruction. The dTECMs could be used to directly attract endogenous MSCs toward the scaffold by leveraging bioactive cues embedded within the dTECM137,138 or activated by living cells prior to implantation, with the assumption that the dTECM is capable of directing these cells to differentiate into hypertrophic chondrocytes.105,135,178 To date, various chemical, enzymatic, and physical procedures have been developed to eliminate the cellular components of tissue-engineered cartilaginous tissue while minimally disrupting the ECM.187,188 Cunniffe et al. created porous scaffolds by freeze-drying hypertrophic cartilage constructs engineered from allogeneic BMSCs. The resulting scaffolds retained their proangiogenic ability and capacity to direct host-mediated orthotopic bone regeneration in critical-sized femoral defects.142 Bourgine et al. developed a decellularization methodology that induces the apoptosis of cells within tissue-engineered hypertrophic cartilage. Compared to standard production and freeze/thaw treatment, the resulting dHCM showed superior ECM preservation, leading to enhanced bone formation upon implantation.137–139 Therefore, developing a reproducible and cost-effective technique to manufacture a large amount of human tissue-derived dTECM may have good potential for clinical translation. Overall, the “off-the-shelf” availability and immune-compatible properties of dTECM may determine the extent of its clinical use.
Conclusion
Comprehensively, these initial results demonstrated that ECO-based strategies can be considered highly promising approaches for large bone defect reconstruction. Although limited success has been observed in clinical cases, these strategies have shown tremendously promising results in critical-sized bone defect reconstruction in animal models and have provided new insights into the fabrication of large, vascularized bone grafts. Nevertheless, research in this field is ongoing, and extensive research is undoubtedly needed to further improve bone output, scale up constructs, and enhance graft vascularization in the future.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (81871571) and the Shanghai Pujiang Program (2019PJD023).
ORCID iD: Ru-Lin Huang  https://orcid.org/0000-0003-3305-6740
https://orcid.org/0000-0003-3305-6740
References
- 1. Fernandez de Grado G, Keller L, Idoux-Gillet Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng 2018; 9: 2041731418776819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017; 2: 224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buser Z, Brodke DS, Youssef JA, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine 2016; 25: 509–516. [DOI] [PubMed] [Google Scholar]
- 4. Roseti L, Parisi V, Petretta M, et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl 2017; 78: 1246–1262. [DOI] [PubMed] [Google Scholar]
- 5. Wubneh A, Tsekoura EK, Ayranci C, et al. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater 2018; 80: 1–30. [DOI] [PubMed] [Google Scholar]
- 6. Tevlin R, Walmsley GG, Marecic O, et al. Stem and progenitor cells: advancing bone tissue engineering. Drug Deliv Transl Res 2016; 6: 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visser R, Rico-Llanos GA, Pulkkinen H, et al. Peptides for bone tissue engineering. J Control Release 2016; 244: 122–135. [DOI] [PubMed] [Google Scholar]
- 8. Shahabipour F, Ashammakhi N, Oskuee RK, et al. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res 2020; 216: 57–76. [DOI] [PubMed] [Google Scholar]
- 9. Mercado-Pagan AE, Stahl AM, Shanjani Y, et al. Vascularization in bone tissue engineering constructs. Ann Biomed Eng 2015; 43: 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nantavisai S, Egusa H, Osathanon T, et al. Mesenchymal stem cell-based bone tissue engineering for veterinary practice. Heliyon 2019; 5: e02808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perez JR, Kouroupis D, Li DJ, et al. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol 2018; 6: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berendsen AD, Olsen BR. Bone development. Bone 2015; 80: 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duchamp de Lageneste O, Julien A, Abou-Khalil R, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun 2018; 9: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramasamy SK, Kusumbe AP, Schiller M, et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun 2016; 7: 13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Florencio-Silva R, Sasso GR, Sasso-Cerri E, et al. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015; 2015: 421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cervantes-Diaz F, Contreras P, Marcellini S. Evolutionary origin of endochondral ossification: the transdifferentiation hypothesis. Dev Genes Evol 2017; 227: 121–127. [DOI] [PubMed] [Google Scholar]
- 17. Aghajanian P, Mohan S. The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res 2018; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolff LI, Hartmann C. A Second career for chondrocytes-transformation into osteoblasts. Curr Osteoporos Rep 2019; 17: 129–137. [DOI] [PubMed] [Google Scholar]
- 19. Rolian C. Endochondral ossification and the evolution of limb proportions. Wiley Interdiscip Rev Dev Biol 2020; 9: e373. [DOI] [PubMed] [Google Scholar]
- 20. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 2015; 11: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutter S, Todorov A, Ismail T, et al. Contrast-enhanced microtomographic characterisation of vessels in native bone and engineered vascularised grafts using ink-gelatin perfusion and phosphotungstic acid. Contrast Media Mol Imaging 2017; 2017: 4035160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grcevic D, Pejda S, Matthews BG, et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells 2012; 30: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Keefe RJ. Fibrinolysis as a target to enhance fracture healing. N Engl J Med 2015; 373: 1776–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma J, Both SK, Yang F, et al. Concise review: cell-based strategies in bone tissue engineering and regenerative medicine. Stem Cells Trans Med 2014; 3: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B Rev 2009; 15: 395–422. [DOI] [PubMed] [Google Scholar]
- 26. Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B-Re 2009; 15: 381–394. [DOI] [PubMed] [Google Scholar]
- 27. Studle C, Vallmajo-Martin Q, Haumer A, et al. Spatially confined induction of endochondral ossification by functionalized hydrogels for ectopic engineering of osteochondral tissues. Biomater 2018; 171: 219–229. [DOI] [PubMed] [Google Scholar]
- 28. Occhetta P, Pigeot S, Rasponi M, et al. Developmentally inspired programming of adult human mesenchymal stromal cells toward stable chondrogenesis. Proc Natl Acad Sci USA 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scotti C, Piccinini E, Takizawa H, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA 2013; 110: 3997–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Browe DC, Coleman CM, Barry FP, et al. Hypoxia activates the PTHrP -MEF2C pathway to attenuate hypertrophy in mesenchymal stem cell derived cartilage. Sci Rep 2019; 9: 13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyette LB, Creasey OA, Guzik L, et al. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med 2014; 3: 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang M, Liu H, Wang Y, et al. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect Tissue Res 2019; 60: 583–596. [DOI] [PubMed] [Google Scholar]
- 33. Matsiko A, Thompson EM, Lloyd-Griffith C, et al. An endochondral ossification approach to early stage bone repair: Use of tissue-engineered hypertrophic cartilage constructs as primordial templates for weight-bearing bone repair. J Tissue Eng Regen Med 2018; 12: e2147–e2150. [DOI] [PubMed] [Google Scholar]
- 34. Sun MM, Beier F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res C Embryo Today 2014; 102: 74–82. [DOI] [PubMed] [Google Scholar]
- 35. Bernhard J, Ferguson J, Rieder B, et al. Tissue-engineered hypertrophic chondrocyte grafts enhanced long bone repair. Biomaterials 2017; 139: 202–212. [DOI] [PubMed] [Google Scholar]
- 36. Freeman FE, Brennan MÁ, Browe DC, et al. A Developmental engineering-based approach to bone repair: endochondral priming enhances vascularization and new bone formation in a critical size defect. Front Bioeng Biotechnol 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinardell T, Sheehy EJ, Buckley CT, et al. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 2012; 18: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cals FL, Hellingman CA, Koevoet W, et al. Effects of transforming growth factor-beta subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J Tissue Eng Regen Med 2012; 6: 68–76. [DOI] [PubMed] [Google Scholar]
- 39. Freeman FE, Allen AB, Stevens HY, et al. Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo. Stem Cell Res Ther 2015; 6: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li CS, Zhang X, Peault B, et al. Accelerated chondrogenic differentiation of human perivascular stem cells with NELL-1. Tissue Eng Part A 2016; 22: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lam J, Lee EJ, Clark EC, et al. Honing cell and tissue culture conditions for bone and cartilage tissue engineering. Cold Spring Harb Perspect Med 2017; 7: a025734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang X, Zhong L, Hendriks J, et al. The Effects of the WNT-signaling modulators BIO and PKF118-310 on the chondrogenic differentiation of human mesenchymal stem cells. Int J Mol Sci 2018; 19: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohamed-Ahmed S, Fristad I, Lie SA, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther 2018; 9: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Longoni A, Pennings I, Cuenca Lopera M, et al. Endochondral Bone Regeneration by Non-autologous Mesenchymal Stem Cells. Front Bioeng Biotechnol 2020; 8: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kamat P, Frueh FS, McLuckie M, et al. Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat—a review. Cytotherapy 2020; 22: 400–411. [DOI] [PubMed] [Google Scholar]
- 46. Zhou W, Lin J, Zhao K, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med 2019; 47: 1722–1733. [DOI] [PubMed] [Google Scholar]
- 47. Li X, Wang M, Jing X, et al. Bone marrow- and adipose tissue-derived mesenchymal stem cells: characterization, differentiation, and applications in cartilage tissue engineering. Crit Rev Eukaryot Gene Expr 2018; 28: 285–310. [DOI] [PubMed] [Google Scholar]
- 48. Mazini L, Rochette L, Amine M, et al. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 2019; 20: 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kronemberger GS, Matsui RAM, Miranda G, et al. Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J Stem Cells 2020; 12: 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lotfy A, Salama M, Zahran F, et al. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. International Journal of Stem Cells 2014; 7: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osinga R, Di Maggio N, Todorov A, et al. Generation of a bone organ by human adipose-derived stromal cells through endochondral ossification. Stem Cells Transl Med 2016; 5: 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weiss-Bilka HE, McGann ME, Meagher MJ, et al. Ectopic models for endochondral ossification: comparing pellet and alginate bead culture methods. Journal of Tissue Engineering and Regenerative Medicine 2018; 12: e541–e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kronemberger GS, Dalmonico GML, Rossi AL, et al. Scaffold- and serum-free hypertrophic cartilage tissue engineering as an alternative approach for bone repair. Artif Organs 2020; 44: E288–E299. [DOI] [PubMed] [Google Scholar]
- 54. Guerrero J, Pigeot S, Muller J, et al. Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification. Acta Biomater 2018; 77: 142–154. [DOI] [PubMed] [Google Scholar]
- 55. Di Maggio N, Martella E, Frismantiene A, et al. Extracellular matrix and alpha5beta1 integrin signaling control the maintenance of bone formation capacity by human adipose-derived stromal cells. Sci Rep 2017; 7: 44398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang RL, Guerrero J, Senn AS, et al. Dispersion of ceramic granules within human fractionated adipose tissue to enhance endochondral bone formation. Acta Biomater 2020; 102: 458–467. [DOI] [PubMed] [Google Scholar]
- 57. Moore SR, Heu C, Yu NY, et al. Translating periosteum’s regenerative power: insights from quantitative analysis of tissue genesis with a periosteum substitute implant. Stem Cells Transl Med 2016; 5: 1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Debnath S, Yallowitz AR, McCormick J, et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018; 562: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Gastel N, Stegen S, Stockmans I, et al. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cells 2014; 32: 2407–2418. [DOI] [PubMed] [Google Scholar]
- 60. Nilsson Hall G, Mendes LF, Gklava C, et al. Developmentally engineered callus organoid bioassemblies exhibit predictive in vivo long bone healing. Adv Sci (Weinh) 2020; 7: 1902295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bolander J, Ji W, Leijten J, et al. Healing of a large long-bone defect through serum-free in vitro priming of human periosteum-derived cells. Stem Cell Reports 2017; 8: 758–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jukes JM, Both SK, Leusink A, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA 2008; 105: 6840–6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamashita A, Nishikawa S, Rancourt DE. Identification of five developmental processes during chondrogenic differentiation of embryonic stem cells. PLoS One 2010; 5: e10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gadkari R, Zhao L, Teklemariam T, et al. Human embryonic stem cell derived-mesenchymal stem cells: an alternative mesenchymal stem cell source for regenerative medicine therapy. Regen Med 2014; 9: 453–465. [DOI] [PubMed] [Google Scholar]
- 65. Kuhn LT, Liu Y, Boyd NL, et al. Developmental-like bone regeneration by human embryonic stem cell-derived mesenchymal cells. Tissue Eng Part A 2014; 20: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hwang NS, Varghese S, Lee HJ, et al. Biomaterials directed in vivo osteogenic differentiation of mesenchymal cells derived from human embryonic stem cells. Tissue Eng Part A 2013; 19: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y, Yu X, Baker C, et al. Mineral particles modulate osteo-chondrogenic differentiation of embryonic stem cell aggregates. Acta Biomater 2016; 29: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verbeeck L, Geris L, Tylzanowski P, et al. Uncoupling of in-vitro identity of embryonic limb derived skeletal progenitors and their in-vivo bone forming potential. Sci Rep 2019; 9: 5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sielatycki JA, Saito M, Yuasa M, et al. Autologous chondrocyte grafting promotes bone formation in the posterolateral spine. JOR Spine 2018; 1: e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bahney CS, Hu DP, Taylor AJ, et al. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res 2014; 29: 1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ng JJ, Wei Y, Zhou B, et al. Recapitulation of physiological spatiotemporal signals promotes in vitro formation of phenotypically stable human articular cartilage. Proc Natl Acad Sci USA 2017; 114: 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bahney CS, Jacobs L, Tamai R, et al. Promoting endochondral bone repair using human osteoarthritic articular chondrocytes. Tissue Eng Part A 2016; 22: 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robla Costales D, Junquera L, Garcia Perez E, et al. Ectopic bone formation during tissue-engineered cartilage repair using autologous chondrocytes and novel plasma-derived albumin scaffolds. J Craniomaxillofac Surg 2016; 44: 1743–1749. [DOI] [PubMed] [Google Scholar]
- 74. Narcisi R, Quarto R, Ulivi V, et al. TGF beta-1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J Cell Physiol 2012; 227: 3282–3290. [DOI] [PubMed] [Google Scholar]
- 75. Jeong CG, Zhang H, Hollister SJ. Three-dimensional polycaprolactone scaffold-conjugated bone morphogenetic protein-2 promotes cartilage regeneration from primary chondrocytes in vitro and in vivo without accelerated endochondral ossification. J Biomed Mater Res A 2012; 100A: 2088–2096. [DOI] [PubMed] [Google Scholar]
- 76. Nasu M, Takayama S, Umezawa A. Endochondral ossification model system: designed cell fate of human epiphyseal chondrocytes during long-term implantation. J Cell Physiol 2015; 230: 1376–1388. [DOI] [PubMed] [Google Scholar]
- 77. Elsaesser AF, Schwarz S, Joos H, et al. Characterization of a migrative subpopulation of adult human nasoseptal chondrocytes with progenitor cell features and their potential for in vivo cartilage regeneration strategies. Cell Biosci 2016; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pippenger BE, Ventura M, Pelttari K, et al. Bone-forming capacity of adult human nasal chondrocytes. J Cell Mol Med 2015; 19: 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bardsley K, Kwarciak A, Freeman C, et al. Repair of bone defects in vivo using tissue engineered hypertrophic cartilage grafts produced from nasal chondrocytes. Biomater 2017; 112: 313–323. [DOI] [PubMed] [Google Scholar]
- 80. Oliveira SM, Mijares DQ, Turner G, et al. Engineering endochondral bone: in vivo studies. Tissue Eng Part A 2009; 15: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oliveira SM, Amaral IF, Barbosa MA, et al. Engineering endochondral bone: in vitro studies. Tissue Eng Part A 2009; 15: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weiss HE, Roberts SJ, Schrooten J, et al. A semi-autonomous model of endochondral ossification for developmental tissue engineering. Tissue Eng Part A 2012; 18: 1334–1343. [DOI] [PubMed] [Google Scholar]
- 83. Flaig I, Radenkovic M, Najman S, et al. In Vivo Analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Han L, Zhang ZW, Wang BH, et al. Construction and biocompatibility of a thin type I/II collagen composite scaffold. Cell Tissue Bank 2018; 19: 47–59. [DOI] [PubMed] [Google Scholar]
- 85. Shekhter AB, Fayzullin AL, Vukolova MN, et al. Medical Applications of collagen and collagen-based materials. Curr Med Chem 2019; 26: 506–516. [DOI] [PubMed] [Google Scholar]
- 86. Gao L, Orth P, Cucchiarini M, et al. Effects of solid acellular type-I/III collagen biomaterials on in vitro and in vivo chondrogenesis of mesenchymal stem cells. Expert Rev Med Devices 2017; 14: 717–732. [DOI] [PubMed] [Google Scholar]
- 87. Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA 2010; 107: 7251–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chircov C, Grumezescu AM, Bejenaru LE. Hyaluronic acid-based scaffolds for tissue engineering. Rom J Morphol Embryol 2018; 59: 71–76. [PubMed] [Google Scholar]
- 89. Erickson IE, Kestle SR, Zellars KH, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater 2012; 8: 3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thompson EM, Matsiko A, Kelly DJ, et al. An endochondral ossification-based approach to bone repair: chondrogenically primed mesenchymal stem cell-laden scaffolds support greater repair of critical-sized cranial defects than osteogenically stimulated constructs in vivo. Tissue Eng Part A 2016; 22: 556–567. [DOI] [PubMed] [Google Scholar]
- 91. Amann E, Wolff P, Breel E, et al. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater 2017; 52: 130–144. [DOI] [PubMed] [Google Scholar]
- 92. Echave MC, Saenz del Burgo L, Pedraz JL, et al. Gelatin as biomaterial for tissue engineering. Curr Pharm Des 2017; 23: 3567–3584. [DOI] [PubMed] [Google Scholar]
- 93. Lin CY, Chang YH, Li KC, et al. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013; 34: 9401–9412. [DOI] [PubMed] [Google Scholar]
- 94. Klotz BJ, Gawlitta D, Rosenberg AJWP, et al. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trend Biotechnol 2016; 34: 394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015; 73: 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Visser J, Gawlitta D, Benders KE, et al. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015; 37: 174–182. [DOI] [PubMed] [Google Scholar]
- 97. Daly AC, Pitacco P, Nulty J, et al. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018; 162: 34–46. [DOI] [PubMed] [Google Scholar]
- 98. Noori A, Ashrafi SJ, Vaez-Ghaemi R, et al. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomedicine 2017; 12: 4937–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sheehy EJ, Mesallati T, Vinardell T, et al. Engineering cartilage or endochondral bone: a comparison of different naturally derived hydrogels. Acta Biomater 2015; 13: 245–253. [DOI] [PubMed] [Google Scholar]
- 100. Knuth CA, Witte-Bouma J, Ridwan Y, et al. Mesenchymal stem cell-mediated endochondral ossification utilising micropellets and brief chondrogenic priming. Eur Cell Mater 2017; 34: 142–161. [DOI] [PubMed] [Google Scholar]
- 101. Mikael PE, Kim HS, Nukavarapu SP. Hybrid extracellular matrix design for cartilage-mediated bone regeneration. J Biomed Mater Res B Appl Biomater 2018; 106: 300–309. [DOI] [PubMed] [Google Scholar]
- 102. Janicki P, Kasten P, Kleinschmidt K, et al. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater 2010; 6: 3292–3301. [DOI] [PubMed] [Google Scholar]
- 103. Sheehy EJ, Mesallati T, Kelly L, et al. Tissue engineering whole bones through endochondral ossification: regenerating the distal phalanx. Biores Open Access 2015; 4: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Daly AC, Cunniffe GM, Sathy BN, et al. 3D Bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater 2016; 5: 2353–2362. [DOI] [PubMed] [Google Scholar]
- 105. Quang Le B, Van Blitterswijk C, De Boer J. An approach to in vitro manufacturing of hypertrophic cartilage matrix for bone repair. Bioengineering (Basel) 2017; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sheehy EJ, Vinardell T, Toner ME, et al. Altering the architecture of tissue engineered hypertrophic cartilaginous grafts facilitates vascularisation and accelerates mineralisation. PLoS One 2014; 9: e90716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bhardwaj N, Devi D, Mandal BB. Tissue-engineered cartilage: the crossroads of biomaterials, cells and stimulating factors. Macromol Biosci 2015; 15: 153–182. [DOI] [PubMed] [Google Scholar]
- 108. Harada N, Watanabe Y, Sato K, et al. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 2014; 35: 7800–7810. [DOI] [PubMed] [Google Scholar]
- 109. Rai V, Dilisio MF, Dietz NE, et al. Recent strategies in cartilage repair: A systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A 2017; 105: 2343–2354. [DOI] [PubMed] [Google Scholar]
- 110. Yao Q, Wei B, Liu N, et al. Chondrogenic regeneration using bone marrow clots and a porous polycaprolactone-hydroxyapatite scaffold by three-dimensional printing. Tissue Eng Part A 2015; 21: 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mikael PE, Golebiowska AA, Xin X, et al. Evaluation of an Engineered Hybrid Matrix for Bone Regeneration via Endochondral Ossification. Ann Biomed Eng 2020; 48: 992–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Abrahamsson CK, Yang F, Park H, et al. Chondrogenesis and mineralization during in vitro culture of human mesenchymal stem cells on three-dimensional woven scaffolds. Tissue Eng Part A 2010; 16: 3709–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yang W, Yang F, Wang Y, et al. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater 2013; 9: 4505–4512. [DOI] [PubMed] [Google Scholar]
- 114. Yang W, Both SK, van Osch G, et al. Performance of different three-dimensional scaffolds for in vivo endochondral bone generation. Eur Cells Mater 2014; 27: 350–364. [DOI] [PubMed] [Google Scholar]
- 115. Gupte MJ, Swanson WB, Hu J, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater 2018; 82: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xu T, Miszuk JM, Zhao Y, et al. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv Healthc Mater 2015; 4: 2238–2246. [DOI] [PubMed] [Google Scholar]
- 117. Lowe B, Hardy JG, Walsh LJ. Optimizing nanohydroxyapatite nanocomposites for bone tissue engineering. ACS Omega 2020; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Siddiqui HA, Pickering KL, Mucalo MR. A review on the use of hydroxyapatite-carbonaceous structure composites in bone replacement materials for strengthening purposes. Materials (Basel) 2018; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang Y, Cai X, Huang J, et al. Bone regeneration in critically sized rat mandible defects through the endochondral pathway using hydroxyapatite-coated 3D-printed Ti6Al4V scaffolds. RSC Advances 2018; 8: 31745–31754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Li W, Wang D, Yang W, et al. Compressive mechanical properties and microstructure of PVA–HA hydrogels for cartilage repair. RSC Advances 2016; 6: 20166–20172. [Google Scholar]
- 121. Wang Y, Wu S, Kuss MA, et al. Effects of hydroxyapatite and hypoxia on chondrogenesis and hypertrophy in 3D bioprinted ADMSC laden constructs. ACS Biomaterials Science & Engineering 2017; 3: 826–835. [DOI] [PubMed] [Google Scholar]
- 122. Wu S, Kai Z, Wang D, et al. Allogenic chondrocyte/osteoblast-loaded beta-tricalcium phosphate bioceramic scaffolds for articular cartilage defect treatment. Artif Cells Nanomed Biotechnol 2019; 47: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 123. Freeman FE, Haugh MG, McNamara LM. An in vitro bone tissue regeneration strategy combining chondrogenic and vascular priming enhances the mineralization potential of mesenchymal stem cells in vitro while also allowing for vessel formation. Tissue Eng Part A 2015; 21: 1320–1332. [DOI] [PubMed] [Google Scholar]
- 124. Kosik-Koziol A, Costantini M, Mroz A, et al. 3D bioprinted hydrogel model incorporating beta-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication 2019; 11: 035016. [DOI] [PubMed] [Google Scholar]
- 125. Yang W, Both SK, van Osch GJ, et al. Effects of in vitro chondrogenic priming time of bone-marrow-derived mesenchymal stromal cells on in vivo endochondral bone formation. Acta Biomater 2015; 13: 254–265. [DOI] [PubMed] [Google Scholar]
- 126. Sun Y, Yan L, Chen S, et al. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater 2018; 74: 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zheng CX, Sui BD, Hu CH, et al. Reconstruction of structure and function in tissue engineering of solid organs: Toward simulation of natural development based on decellularization. J Tissue Eng Regen Med 2018; 12: 1432–1447. [DOI] [PubMed] [Google Scholar]
- 128. Vas WJ, Shah M, Blacker TS, et al. Decellularized cartilage directs chondrogenic differentiation: creation of a fracture callus mimetic. Tissue Eng Part A 2018; 24: 1364–1376. [DOI] [PubMed] [Google Scholar]
- 129. Gawlitta D, Benders KE, Visser J, et al. Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration. Tissue Eng Part A 2015; 21: 694–703. [DOI] [PubMed] [Google Scholar]
- 130. Zhang Y, Li J, Davis ME, et al. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater 2015; 20: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ghosh P, Gruber SMS, Lin C-Y, et al. Microspheres containing decellularized cartilage induce chondrogenesis in vitro and remain functional after incorporation within a poly(caprolactone) filament useful for fabricating a 3D scaffold. Biofabrication 2018; 10: 025007. [DOI] [PubMed] [Google Scholar]
- 132. Bordbar S, Lotfi Bakhshaiesh N, Khanmohammadi M, et al. Production and evaluation of decellularized extracellular matrix hydrogel for cartilage regeneration derived from knee cartilage. J Biomed Mater Res A 2020; 108: 938–946. [DOI] [PubMed] [Google Scholar]
- 133. Rowland CR, Colucci LA, Guilak F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials 2016; 91: 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Urist MR, Adams T. Cartilage or bone induction by articular cartilage. Observations with radioisotope labelling techniques. J Bone Joint Surg Br 1968; 50: 198–215. [PubMed] [Google Scholar]
- 135. Bourgine PE, Pippenger BE, Todorov A, Jr., et al. Tissue decellularization by activation of programmed cell death. Biomaterials 2013; 34: 6099–6108. [DOI] [PubMed] [Google Scholar]
- 136. Tang C, Jin C, Xu Y, et al. Chondrogenic differentiation could be induced by autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds without exogenous growth factor. Tissue Eng Part A 2016; 22: 222–232. [DOI] [PubMed] [Google Scholar]
- 137. Bourgine PE, Gaudiello E, Pippenger B, et al. Engineered extracellular matrices as biomaterials of tunable composition and function. Adv Functional Mater 2017; 27. [Google Scholar]
- 138. Bourgine PE, Scotti C, Pigeot S, et al. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc Natl Acad Sci USA 2014; 111: 17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bourgine P, Le Magnen C, Pigeot S, et al. Combination of immortalization and inducible death strategies to generate a human mesenchymal stromal cell line with controlled survival. Stem Cell Res 2014; 12: 584–598. [DOI] [PubMed] [Google Scholar]
- 140. Todorov A, Kreutz M, Haumer A, et al. Fat-derived stromal vascular fraction cells enhance the bone-forming capacity of devitalized engineered hypertrophic cartilage matrix. Stem Cells Transl Med 2016; 5: 1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Feng X, Li Z, Wei J, et al. Injectable cartilaginous template transformed BMSCs into vascularized bone. Sci Rep 2018; 8: 8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cunniffe GM, Vinardell T, Murphy JM, et al. Porous decellularized tissue engineered hypertrophic cartilage as a scaffold for large bone defect healing. Acta Biomater 2015; 23: 82–90. [DOI] [PubMed] [Google Scholar]
- 143. Chen S, Fu P, Cong R, et al. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis 2015; 2: 76–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Meretoja VV, Dahlin RL, Wright S, et al. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 2013; 34: 4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Knuth CA, Andres Sastre E, Fahy NB, et al. Collagen type X is essential for successful mesenchymal stem cell-mediated cartilage formation and subsequent endochondral ossification. Eur Cell Mater 2019; 38: 106–122. [DOI] [PubMed] [Google Scholar]
- 146. Dennis SC, Berkland CJ, Bonewald LF, et al. Endochondral ossification for enhancing bone regeneration: converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng Part B Rev 2015; 21: 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Fu JY, Lim SY, He PF, et al. Osteogenic treatment initiating a tissue-engineered cartilage template hypertrophic transition. Ann Biomed Eng 2016; 44: 2957–2970. [DOI] [PubMed] [Google Scholar]
- 148. Grafe I, Alexander S, Peterson JR, et al. TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol 2018; 10: a022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tang X, Fan L, Pei M, et al. Evolving concepts of chondrogenic differentiation: history, state-of-the-art and future perspectives. Eur Cells Mater 2015; 30: 12–27. [DOI] [PubMed] [Google Scholar]
- 150. Eslaminejad MB, Karimi N, Shahhoseini M. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochem Cell Biol 2013; 140: 623–633. [DOI] [PubMed] [Google Scholar]
- 151. Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 2001; 268: 189–200. [DOI] [PubMed] [Google Scholar]
- 152. Wang T, Nimkingratana P, Smith CA, et al. Enhanced chondrogenesis from human embryonic stem cells. Stem Cell Res 2019; 39: 101497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yamashita A, Morioka M, Yahara Y, et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports 2015; 4: 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhou N, Li Q, Lin X, et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res 2016; 366: 101–111. [DOI] [PubMed] [Google Scholar]
- 155. Caron MM, Emans PJ, Cremers A, et al. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis Cartilage 2013; 21: 604–613. [DOI] [PubMed] [Google Scholar]
- 156. Craft AM, Rockel JS, Nartiss Y, et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol 2015; 33: 638–645. [DOI] [PubMed] [Google Scholar]
- 157. Dvorakova J, Kucera L, Kucera J, et al. Chondrogenic differentiation of mesenchymal stem cells in a hydrogel system based on an enzymatically crosslinked tyramine derivative of hyaluronan. J Biomed Mater Res A 2014; 102: 3523–3530. [DOI] [PubMed] [Google Scholar]
- 158. Nasrabadi D, Rezaeiani S, Eslaminejad MB, et al. Improved protocol for chondrogenic differentiation of bone marrow derived mesenchymal stem cells -effect of PTHrP and FGF-2 on TGFbeta1/BMP2-induced chondrocytes hypertrophy. Stem Cell Rev Rep 2018; 14: 755–766. [DOI] [PubMed] [Google Scholar]
- 159. Legendre F, Ollitrault D, Gomez-Leduc T, et al. Enhanced chondrogenesis of bone marrow-derived stem cells by using a combinatory cell therapy strategy with BMP-2/TGF-beta1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci Rep 2017; 7: 3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Herberg S, McDermott AM, Dang PN, et al. Combinatorial morphogenetic and mechanical cues to mimic bone development for defect repair. Sci Adv 2019; 5: eaax2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dang PN, Dwivedi N, Phillips LM, et al. Controlled dual growth factor delivery from microparticles incorporated within human bone marrow-derived mesenchymal stem cell aggregates for enhanced bone tissue engineering via endochondral ossification. Stem Cells Transl Med 2016; 5: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. McMillan A, Nguyen MK, Gonzalez-Fernandez T, et al. Dual non-viral gene delivery from microparticles within 3D high-density stem cell constructs for enhanced bone tissue engineering. Biomaterials 2018; 161: 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ude CC, Chen HC, Norhamdan MY, et al. The evaluation of cartilage differentiations using transforming growth factor beta3 alone and with combination of bone morphogenetic protein-6 on adult stem cells. Cell Tissue Bank 2017; 18: 355–367. [DOI] [PubMed] [Google Scholar]
- 164. Moradian Tehrani R, Mirzaei H, Verdi J, et al. Chondrogenic differentiation of human scalp adipose-derived stem cells in Polycaprolactone scaffold and using Freeze Thaw Freeze method. J Cell Physiol 2018; 233: 6705–6713. [DOI] [PubMed] [Google Scholar]
- 165. Hennig T, Lorenz H, Thiel A, et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 2007; 211: 682–691. [DOI] [PubMed] [Google Scholar]
- 166. Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 2013; 4: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jia PT, Zhang XL, Zuo HN, et al. A study on role of triiodothyronine (T3) hormone on the improvement of articular cartilage surface architecture. Exp Toxicol Pathol 2017; 69: 625–629. [DOI] [PubMed] [Google Scholar]
- 168. Mumme M, Scotti C, Papadimitropoulos A, et al. Interleukin-1beta modulates endochondral ossification by human adult bone marrow stromal cells. Eur Cell Mater 2012; 24: 224–236. [DOI] [PubMed] [Google Scholar]
- 169. Farrell E, Both SK, Odorfer KI, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 2011; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Freeman FE, Haugh MG, McNamara LM. Investigation of the optimal timing for chondrogenic priming of MSCs to enhance osteogenic differentiation in vitro as a bone tissue engineering strategy. J Tissue Eng Regen Med 2016; 10: E250–262. [DOI] [PubMed] [Google Scholar]
- 171. Walters G, Pountos I, Giannoudis PV. The cytokines and micro-environment of fracture haematoma: Current evidence. J Tissue Eng Regen Med 2018; 12: e1662–e1677. [DOI] [PubMed] [Google Scholar]
- 172. Iimori Y, Morioka M, Koyamatsu S, et al. Implantation of human iPS cell-derived cartilage in bone defects of mice. Tissue Eng Part A 2021. doi: 10.1089/ten.TEA.2020.0346. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 173. van der Stok J, Koolen MK, Jahr H, et al. Chondrogenically differentiated mesenchymal stromal cell pellets stimulate endochondral bone regeneration in critical-sized bone defects. Eur Cell Mater 2014; 27: 137–148; discussion 148. [DOI] [PubMed] [Google Scholar]
- 174. Mikael PE, Xin X, Urso M, et al. A potential translational approach for bone tissue engineering through endochondral ossification. Conf Proc IEEE Eng Med Biol Soc 2014; 2014: 3925–3928. [DOI] [PubMed] [Google Scholar]
- 175. Huang JI, Durbhakula MM, Angele P, et al. Lunate arthroplasty with autologous mesenchymal stem cells in a rabbit model. J Bone Joint Surg Am 2006; 88: 744–752. [DOI] [PubMed] [Google Scholar]
- 176. Zhang M, Shi J, Xie M, et al. Recapitulation of cartilage/bone formation using iPSCs via biomimetic 3D rotary culture approach for developmental engineering. Biomaterials 2020; 260: 120334. [DOI] [PubMed] [Google Scholar]
- 177. Li J, Kang F, Gong X, et al. Ceria nanoparticles enhance endochondral ossification-based critical-sized bone defect regeneration by promoting the hypertrophic differentiation of BMSCs via DHX15 activation. FASEB J 2019; 33: 6378–6389. [DOI] [PubMed] [Google Scholar]
- 178. Petersen A, Princ A, Korus G, et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat Commun 2018; 9: 4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Bai Y, Liu C, Fu L, et al. Mangiferin enhances endochondral ossification-based bone repair in massive bone defect by inducing autophagy through activating AMP-activated protein kinase signaling pathway. FASEB J 2018; 32: 4573–4584. [DOI] [PubMed] [Google Scholar]
- 180. Deng C, He Y, Feng J, et al. Extracellular matrix/stromal vascular fraction gel conditioned medium accelerates wound healing in a murine model. Wound Repair Regen 2017; 25: 923–932. [DOI] [PubMed] [Google Scholar]
- 181. Cunniffe GM, Vinardell T, Thompson EM, et al. Chondrogenically primed mesenchymal stem cell-seeded alginate hydrogels promote early bone formation in critically-sized defects. European Polymer Journal 2015; 72: 464–472. [Google Scholar]
- 182. Kruijt Spanjer EC, Bittermann GKP, van Hooijdonk IEM, et al. Taking the endochondral route to craniomaxillofacial bone regeneration: A logical approach? J Craniomaxillofac Surg 2017; 45: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 183. Baptista LS, Kronemberger GS, Silva KR, et al. Spheroids of stem cells as endochondral templates for improved bone engineering. Front Biosci (Landmark Ed) 2018; 23: 1969–1986. [DOI] [PubMed] [Google Scholar]
- 184. Baptista LS, Kronemberger GS, Cortes I, et al. Adult stem cells spheroids to optimize cell colonization in scaffolds for cartilage and bone tissue engineering. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yoon HH, Bhang SH, Shin JY, et al. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng Part A 2012; 18: 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Epple C, Haumer A, Ismail T, et al. Prefabrication of a large pedicled bone graft by engineering the germ for de novo vascularization and osteoinduction. Biomaterials 2019; 192: 118–127. [DOI] [PubMed] [Google Scholar]
- 187. Benders KEM, Terpstra ML, Levato R, et al. Fabrication of decellularized cartilage-derived matrix scaffolds. J Vis Exp 2019. [DOI] [PubMed] [Google Scholar]
- 188. Mansour A, Mezour MA, Badran Z, et al. (*) Extracellular matrices for bone regeneration: a literature review. Tissue Eng Part A 2017; 23: 1436–1451. [DOI] [PubMed] [Google Scholar]