Abstract
A large and growing body of evidence suggests that physical activity (PA) may hold therapeutic promise in the management of mental health disorders. Most evidence linking PA to mental health outcomes has focused on the effects of aerobic exercise training on depression, although a growing body of work supports the efficacy of both aerobic and resistance exercise paradigms in the treatment of anxiety and post-traumatic stress disorder. Despite abundant evidence linking PA and mental health, use of exercise training as a mental health treatment remains limited due to three important sources of uncertainty: (a) large individual differences in response to exercise treatment within multiple mental health domains; (b) the critical importance of sustained PA engagement, not always achieved, for therapeutic benefit; and (c) disagreement regarding the relative importance of putative therapeutic mechanisms. Our review of treatment data on exercise interventions and mental health outcomes focuses primarily on depression and anxiety within a health neuroscience framework. Within this conceptual framework, neurobiological and behavioral mechanisms may have additive or synergistic influences on key cognitive and behavioral processes that influence mental health outcomes. We therefore highlight sources of treatment heterogeneity by integrating the critical influences of (a) neurobiological mechanisms enhancing neuroplasticity and (b) behavioral learning of self-regulatory skills. Understanding the interrelationships between dynamic neurobiological and behavioral mechanisms may help inform personalized mental health treatments and clarify why, and for whom, exercise improves mental health outcomes. The review concludes with recommendations for future studies leveraging individual differences to refine treatment approaches to optimize mental health benefits.
Keywords: exercise, physical activity, self-regulation, cognitive control, executive function, affect regulation, behavioral activation
EPIDEMIOLOGICAL EVIDENCE LINKING PHYSICAL ACTIVITY AND MENTAL HEALTH
Numerous epidemiological studies have demonstrated that lower amounts of physical activity (PA) or greater amounts of time spent in sedentary behaviors are associated with greater risk of poor mental health. In a recent study of 1.2 million US adults, in which participants were matched across numerous background and demographic factors, individuals who exercised reported better mental health functioning compared to nonexercisers (1). Prospective studies focusing on specific mental health conditions have reported similar findings, suggesting that greater habitual PA may protect against the development of various mental health conditions. For example, a recent meta-analysis of 49 prospective studies across nearly 267,000 individuals demonstrated that higher levels of PA associated with reduced odds of developing depression [odds ratio (OR) = 0.83, 95% confidence interval (CI) 0.79–0.88] across age groups (2). PA also prospectively associated with lower odds of developing elevated anxiety symptoms (OR = 0.87, 95% CI 0.77–0.99) and anxiety disorders (OR = 0.66, 95% CI 0.53–0.83) in a recent meta-analysis of more than 80,000 individuals (3).
The concept of habitual PA serving a protective role against the development of mental health conditions is further bolstered by data suggesting that greater amounts of PA are associated with incrementally lower risk of mental health problems. Across various exercise modalities, there appears to be a dose-response association between greater PA and mental health functioning (4), even after accounting for numerous social, medical, and behavioral confounds, such as smoking (OR = 0.67, 95% CI 0.61–0.75) (5). Individuals with moderate or low levels of fitness exhibit a 23% and 47% greater risk of developing a mental health problem when compared to their highly fit counterparts, with additive benefits of aerobic and resistance exercise (6). Taken together, epidemiological evidence provides robust evidence that greater habitual PA associates with better mental health functioning. For the purposes of the present review, exercise is defined as planned or intentional training activities, whereas PA also includes leisure-time activities.
Exercise Training and Mental Health: Experimental Evidence
Results from randomized trials of exercise, while generally favorable, have been more mixed than observational findings (7–10). Multiple meta-analytic studies have examined the impact of exercise training, primarily aerobic but also resistance training (10), with varying degrees of efficacy depending on methodological considerations, including study quality, duration (11), and choice of comparator group (12). Most evidence suggests that exercise, particularly aerobic training, improves depression- and anxiety-related outcomes compared with attention control conditions, with treatment effect sizes paralleling those for conventional pharmacotherapeutic approaches. Aerobic exercise trials have generally reported moderate to large improvements compared with no intervention [effect size (ES) = 1.24] or conventional control conditions (ES = 0.68), with improvements comparable to standard psychological or pharmacological approaches. Importantly, the effects of exercise training tend to decline over time and are generally not significant at follow-up (ES = 0.22) (8), with therapeutic benefits only sustained among individuals who continue to exercise following cessation of active treatment (13). Although fewer studies have examined resistance training paradigms, existing data suggest that resistance training also appears to improve depressive symptoms (ES = 0.66, 95% CI 0.48–0.83), albeit with significant heterogeneity across trials (10). Exercise training has also been examined as an add-on therapy, enhancing the efficacy of conventional outpatient treatment approaches over the course of 2–3 months (ES = −0.79, 95% CI −1.01 − −0.57) (7).
Interestingly, evidence for dose-response effects of training is less robust than observational findings; relatively few studies link the degree of improvement with the intensity or duration of training (14). In addition, examination of sustained treatment response over time suggests that the most important factor associating with remission from various mental health conditions is the degree of exercise maintenance over time, regardless of initial intervention assignment, with continued exercise participation demonstrating a threshold of benefit corresponding closely to the American Heart Association’s recommended level of activity of 150 min per week (13, 15). In summary, exercise training appears to have a potentially important impact on mental health functioning, comparable to conventional psychotherapeutic and pharmacological approaches; however, the effects of exercise training vary across patient populations and training modalities, and the long-term mental health benefits appear contingent upon sustained PA engagement. Understanding the mechanisms of treatment response and exercise maintenance is therefore of critical importance to the ultimate efficacy of exercise as a clinical treatment.
Transdiagnostic Mechanisms
Recent studies have begun to examine transdiagnostic mechanisms by which exercise may improve mental health outcomes (16), including integration of interrelated behavioral and neurobehavioral domains (17, 18). For example, recent literature syntheses have begun integrating affective and cognitive mechanisms of treatment across samples of patients with neurodegenerative disorders and depression, demonstrating that exercise improves depressive symptoms (ES = 0.78), with weak dose-response effects (standardized beta = 0.007, p = 0.012). Improvements in depression were paralleled by improvements in behavioral markers of cognitive control (ES = 0.24), with a high degree of heterogeneity (16). While methodological variation is frequently and appropriately identified as a source of heterogeneity, the impact of exercise on mental health outcomes also appears to vary based on the presence and type of neurobehavioral impairment.
An illustrative example of diagnostic treatment heterogeneity is the use of exercise among individuals with late-life depression (LLD) (19, 20) and/or vascular depression (21). These individuals demonstrate substantially poorer treatment outcomes to conventional therapeutic approaches compared to younger individuals with depression or individuals with an earlier first depressive episode, despite ostensibly meeting the same diagnostic criteria. Individuals with LLD are more likely to have cardiovascular disease risk factors, white matter hyperintensities, and comorbid cognitive dysfunction typified by executive function impairments than individuals with an earlier onset of depression. Despite these patients’ lower treatment response, psychomotor activation appears to be an important protective factor, predicting subsequent persistence of treatment benefits (22). The effects of exercise training in this population have been heterogeneous; some large, multisite trials have failed to find a benefit of exercise in LLD samples (23). Nevertheless, in a recent meta-analysis of nine trials and 1,308 participants, exercise training was found to be moderately effective in reducing depressive symptoms (ES = 0.64, 95% CI 0.27–1.01), with significant heterogeneity across studies (9). Follow-up analyses suggested that greater age, cognitive impairment, and severity of depressive symptoms all contributed to treatment response heterogeneity, despite evidence for favorable exercise effects both among older adults (24) and in selected trials among individuals with dementia. These analyses suggested that the observed lack of treatment response in some individuals may be due to a greater prevalence of impairments in reward system pathways in individuals with geriatric depression or LLD. Existing evidence linking exercise training to mental health outcomes suggests that exercise has beneficial effects with wide variation, but there is a lack of explanatory studies delineating for whom and why exercise is beneficial.
EXERCISE TRAINING AND MENTAL HEALTH: EXPLANATORY MECHANISMS
Examination of the literature linking exercise to mental health suggests that exercise training is beneficial for a broad array of mental health outcomes, although the strength of treatment benefit appears to vary across populations and training modalities. The present literature base could be characterized as having three overarching mechanistic hypotheses, which are useful in framing hypotheses regarding treatment improvements: (a) Mental health is improved in association with physical/hedonic effects of exercise, (b) exercise improves mental health via neurobiological mechanisms, or (c) exercise is a vehicle for cultivating behavioral mechanisms of change (e.g., self-regulatory skills and self-efficacy). We contend that exercise training likely improves mental health through synergistic influences of both neurobiological and behavioral learning mechanisms (Figure 1). Within this framework (25, 26), training improves neurobiological systems critical for adaptive learning, as well as affective and cognitive control processes, resulting in synergistic improvements in the regulation of both cognitive and affective responses through a “virtuous circle” of reinforcement (27, 28).
Figure 1.
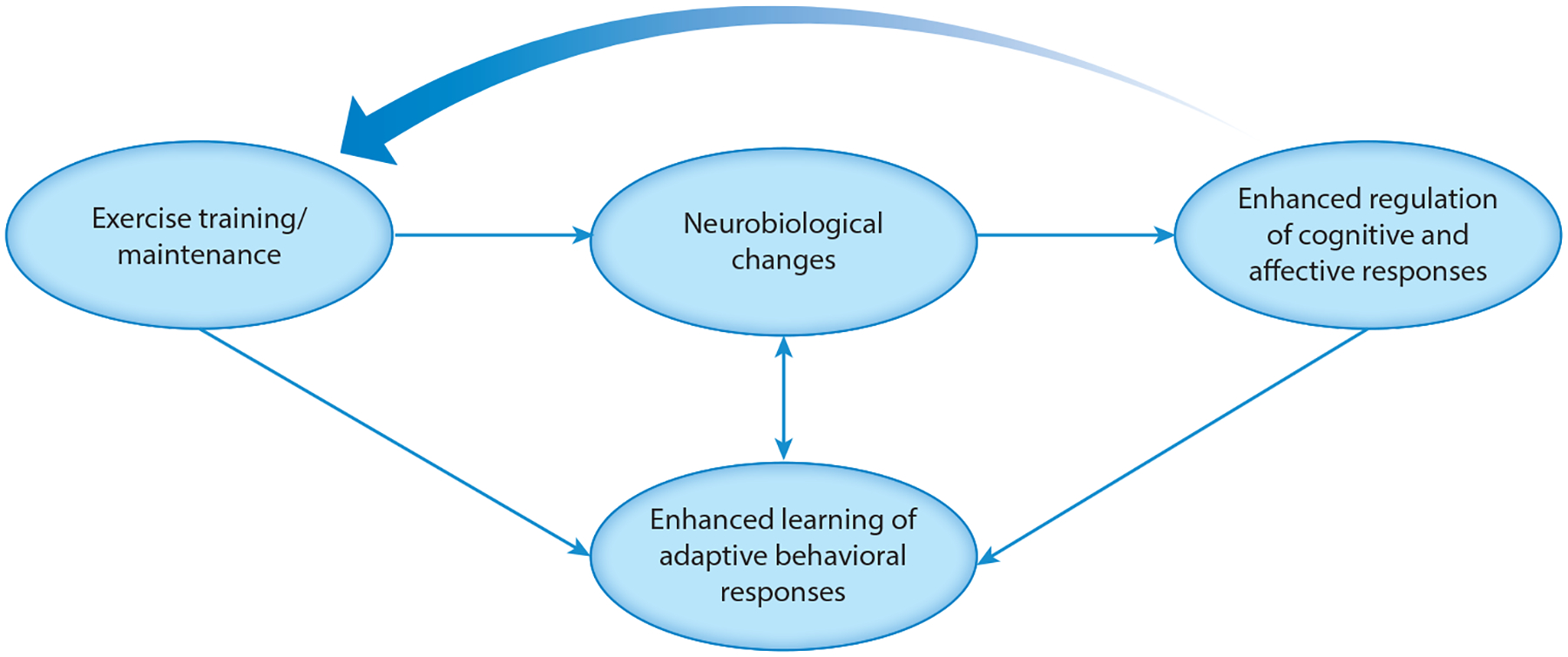
Conceptual model integrating neurobiological and behavioral mechanisms by which exercise training improves mental health outcomes.
Core Influence of Neuroplasticity
Neuroplasticity is increasingly characterized as a central mechanistic component of mental health improvements and is highly influenced by PA. The brain’s capacity for neurobiological remodeling within key neurocircuitry is a critical element of adaptive learning, is impaired across many heterogeneous mental health conditions, declines with age, and may be inhibited by several age-related systemic processes. Adaptive learning, in turn, is a critical element of mental health improvements following behavioral interventions. Increased neuroplastic capacity is one hypothesized mechanism underlying the mental health benefits of several widely used somatic psychiatric treatment modalities, including selective serotonin reuptake inhibitors and transmagnetic stimulation. Exercise is one of the few behavioral processes that appears to increase neuroplasticity.
At the structural level, neuroplasticity has been used to refer to the growth of new neurons and glial cells (neurogenesis), as well as new connections between existing neural networks through dendritic remodeling, synaptic formation and pruning, and axonal augmentation (synaptogenesis). Additional, related changes include the growth of new microvascular pathways (angiogenesis) and potentiation of neurotransmitter systems (monoamines) that are important for reward sensitization. There is now a general consensus that in adult humans, neurogenesis occurs in two specific brain regions, both of which are critical for mental health disorders and treatments: (a) the sub-granular zone of the dentate gyrus (mesial temporal lobe) and (b) the subventricular zone adjacent to the caudate and striatum (subcortex-prefrontal cortex). The dentate gyrus is essential for the creation and consolidation of new memories and regulation of affect, primarily changing through new gray matter cell growth. The subventricular zone and caudate are critical to striatal dopamine function and subcortical white matter pathways (29). Multiple lines of evidence suggest that neuroplastic changes and enhanced learning ability as a behavioral corollary are crucial therapeutic substrates for successful treatment outcomes (Figure 2).
Figure 2.
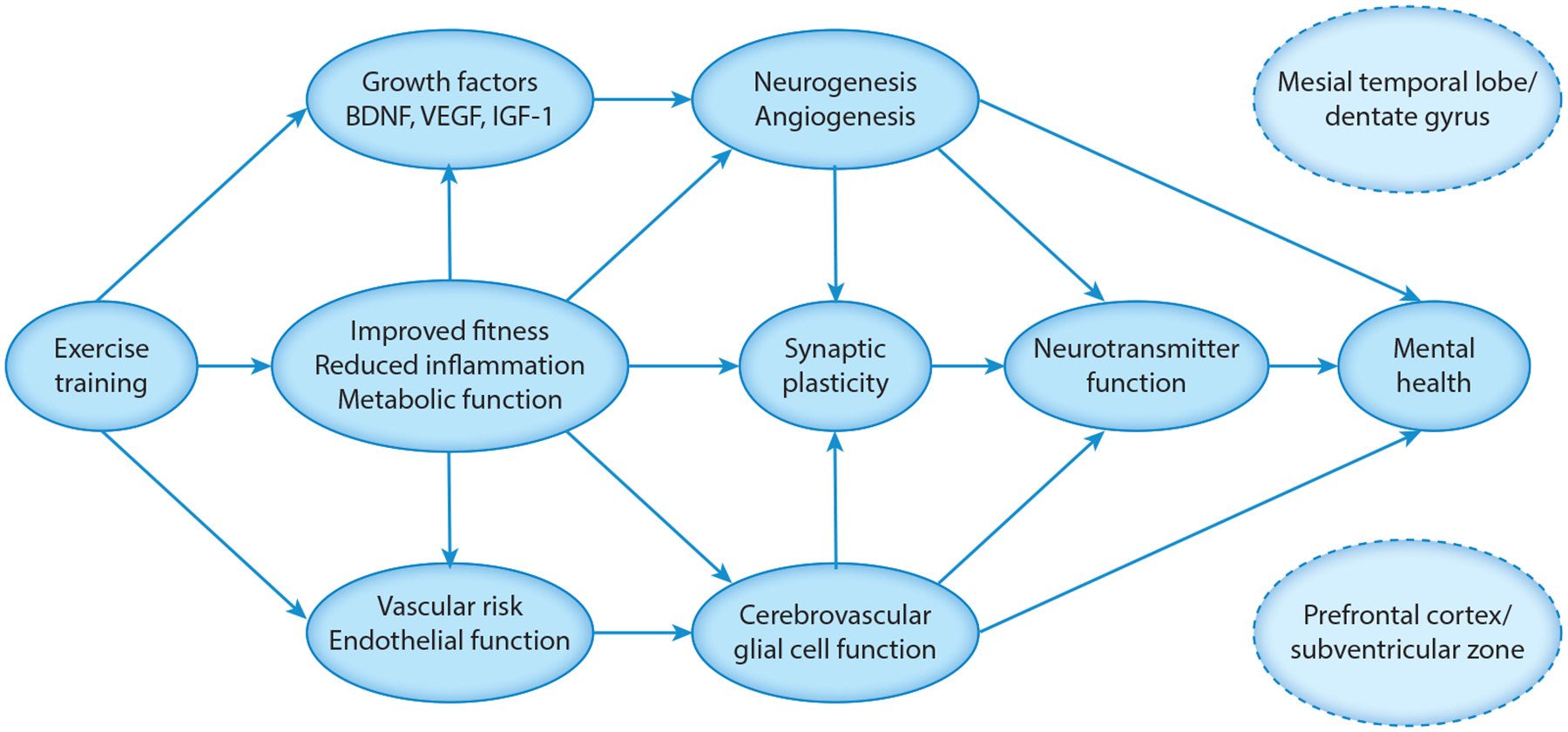
Conceptual model of neurobiological mechanisms by which exercise training improves mental health outcomes. Abbreviations: BDNF, brain-derived neurotrophic factor; IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial growth factor.
Neuroplasticity Facilitators
Several key systemic factors must be present to facilitate neuroplastic changes, all of which are modifiable through exercise training. Briefly, these include neurotrophins, intact cerebral metabolic function, low neuroinflammation, and sleep. Neurotrophic growth factors are enhanced by exercise and linked to neuroplasticity, including modulation of brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and insulin-like growth factor 1 (IGF-1). BDNF is increased following aerobic (30) and resistance exercise training (31) and is critical to neurogenesis in the dentate gyrus. VEGF is more closely tied to cerebrovascular and microglial function, as well as angiogenesis. IGF-1 is more closely tied to cerebral and systemic metabolic changes, conferring structural adaptations similar to those seen with BDNF.
Neuroplasticity Inhibitors
Growth factors, particularly BDNF, also have systemic effects in regulating cellular bioenergetic function (32), which is disrupted in multiple mental health conditions (33), making it more difficult to facilitate affective control, cultivate flexible response patterns, or facilitate synaptic plasticity adaptations within supportive neurocircuitry (34). The bioenergetic system is also highly influenced by inflammation (35), sharing multiple molecular pathways with neurogenesis and depression (36). Elevated levels of inflammation appear to inhibit the effects of aerobic exercise on enhanced neurogenesis (37), partly through increasing kynurenine accumulation in the brain. Exercise may facilitate skeletal muscle clearance of kynurenine, suggesting that the comparable effects of exercise and selective serotonin reuptake inhibitors on mental health outcomes may be due to their parallel, neuroprotective effects through kynurenine pathways (38).
EXERCISE TRAINING: BRAIN NETWORK ALTERATIONS
For the purposes of understanding the effects of exercise on critical brain neurocircuitry, existing evidence can be grouped into three categories. Changes are observed in three functional neuroanatomical circuits characterized in the triple network model of large-scale brain networks (39). As shown in Table 1, these changes occur in the salience network (SN), the executive control network (ECN), and the default mode network (DMN). Both depression and anxiety are associated with lower volume within DMN (hippocampus) and SN (amygdala and anterior cingulate cortex) brain structures (40). Individuals with depression also demonstrate reduced connectivity between the ECN and DMN, whereas individuals with anxiety demonstrate reduced connectivity between the ECN and SN. Improvements following psychiatric treatments with disparate mechanisms also correlate with connectivity alterations between these key networks. For example, treatment-related improvements following emotion regulation (ER)–focused psychotherapy (41) associate with alterations in connectivity between the SN and DMN, with both networks demonstrating increased connectivity to the ECN following successful treatment. A similar pattern of improvements has been demonstrated following pharmacological treatment of depression with aberrant SN and DMN connectivity improving with treatment, as well as differential improvements across symptom clusters (emotional and somatic), type of antidepressant (noradrenergic and serotonergic), and pattern of altered connectivity (42). These findings have been interpreted as demonstrating that enhancing connectivity with the ECN associates with behavioral improvements in cognitive control (for SN connectivity) and reductions in overly self-referential processing (for DMN connectivity).
Table 1.
Large-scale brain networks relevant to exercise training and mental health outcomes
| Salience network (SN) | Executive control network (ECN) | Default mode network (DMN) | |
|---|---|---|---|
| Neurocircuitry (network hub) | Anterior insula, pregenual ACC, amygdala, ventral striatum | Dorsolateral PFC, dorsal ACC, posterior parietal cortex | Posterior cingulate cortex, ventromedial PFC, angular gyrus |
| Neurobehavioral/cognitive processes | Function: goal-directed behavioral engagement, reward sensitization, energization, conflict resolution, motivated learning, interoception Dysfunction: disinhibition, apathy, poor integration of aversive interoceptive cues |
Function: cognitive control, working memory, set maintenance, task monitoring and updating, cognitive flexibility, vigilance, metacognition Dysfunction: dysexecutive function, perseveration, impaired planning and time management, inattention |
Function: self-referential processing, episodic memory and autobiographical retrieval, semantic memory, internal representations, and value-based decision making Dysfunction: impaired social cognition, memory impairment, verbal agnosia |
| Neurotransmitter systems | Norepinephrine, dopamine | Dopamine, GABA | Serotonin, acetylcholine, glutamate |
| Cognitive test analog | Stroop Interference, Go-No Go/Flanker tasks, Iowa Gambling Task, Verbal Fluency | Trail Making Test, Wisconsin Card Sorting Test, Tower of Hanoi, Digit Span, Mental Arithmetic | Logical Memory Test, California Verbal Learning Test, Benton Visual Memory Test, Boston Naming Test |
| Behavioral/psychological processes | Function: approach-oriented coping, task perseverance, empathy Dysfunction: anhedonia, rumination, impulsivity, avoidant behaviors |
Function: cognitive flexibility, adaptation to feedback, flexible attention Dysfunction: inflexible behavioral responses, inattention |
Function: regulated affect, social sensitivity, appropriate self-context Dysfunction: poor affect regulation, generalized/self-referential memory |
| Exercise training relevance (stage) | Critical for initial training engagement, self-regulatory skills acquisition, sensitivity to reinforcement cues, regulation of aversive physical cues | Critical for self-regulatory skills generalization, exercise maintenance, flexible response patterns, adapting self-reinforcement strategies, monitoring progress, contingency management | Critical for relapse prevention, consolidation of new skills, affective control to manage psychosocial stressors, use of self-referential knowledge for behavioral maintenance |
| Psychiatric conditions | Disrupted in GAD, PTSD, OCD, BPD, addiction, anorexia, pain disorders, Parkinson’s, vascular dementia, ADHD, and MDD | Disrupted in most psychiatric conditions including MDD, bipolar, schizophrenia, frontotemporal and vascular dementia, ADHD, autism | Disrupted across multiple conditions including amnestic MCI, Alzheimer’s, MDD, social anxiety disorder, and epilepsy |
ACC, anterior cingulate cortex; ADHD, attention-deficit/hyperactivity disorder; BPD, borderline personality disorder; GABA, gamma-aminobutyric acid; GAD, generalized anxiety disorder; MCI, mild cognitive impairment; MDD, major depressive disorder; OCD, obsessive compulsive disorder; PFC, prefrontal cortex; PTSD, posttraumatic stress disorder.
Volumetric changes following exercise training have been most consistently observed in the prefrontal cortex (PFC), subcortex, and mesial temporal brain structures (43), with the most consistent changes in the dentate gyrus (44, 45). The mesial temporal lobe (MTL) is particularly vulnerable to neurodegenerative disease processes, such as Alzheimer’s disease, although exercise appears to increase volume in this brain region across both middle-aged and older adult samples (46). Moderate-intensity training in older adults, for example, increases hippocampal volume with dose-response improvements from 6 to 12 months, offsetting normative neurodegenerative changes (1–2% annually), without volumetric increases in other brain regions (47). In one of the few exercise trials collecting neuroimaging data from participants with depression, depressive symptoms improved in parallel with increased MTL volume and improved verbal memory, despite a 30% dropout rate and lack of a significant treatment group benefit (48).
While most randomized controlled trials (RCTs) have focused on MTL brain areas, white matter changes have also been reported, particularly in PFC and parietal lobe areas critical for SN and ECN function (43). In a one-year training intervention, Voss and colleagues (49) demonstrated improvements in microstructural parameters of white matter integrity in the PFC, paralleling improvements in MTL brain regions. Similarly, during a 10- to 12-year follow-up examination of participants in the LOOK-AHEAD diabetes trial, the lifestyle intervention group demonstrated an impressive 28% lower white matter hyperintensity volume, compared to only a 9% lower ventricle volume (50). We recently reported parallel results in a pilot study of adults with depression, in which aerobic exercise stabilized white matter hyperintensity progression following a 16-week treatment (51). Notably, preliminary evidence suggests similar improvements following resistance training, with participants exhibiting improvements in PFC white matter volume and executive functioning (52).
The effects of exercise training on connectivity changes extend studies of structural markers by elucidating their functional significance to underlying behavioral changes. Extant connectivity studies suggest critical influences impacting SN and ECN neurocircuitry, independent of volumetric changes in DMN brain structures. Acute exercise, for example, has been shown to enhance efficiency within the DMN and improve functional modulation between networks, as indicated by enhanced ability of SN and ECN brain regions to selectively and adaptively inhibit DMN functioning (53), with parallel improvements in behavioral markers of cognitive control (54). Several RCTs have suggested that exercise training may enhance connectivity in clinically important neurocircuitry (54–56), and some suggest that markers of brain connectivity hold central importance as translational biomarkers (55). Previous randomized trials of exercise training suggest that it may improve ECN and DMN markers of connectivity, with corresponding behavioral improvements. Voss and colleagues, for example, found that a 12-month aerobic exercise program among older adults enhanced ECN and DMN connectivity, with corresponding improvements in executive function (57). The DMN, in particular, has been extensively studied for its responsivity to exercise training (58), as well as being widely implicated in the pathogenesis of depression (59, 60), particularly LLD (59, 60). In addition, a growing body of work suggests that greater baseline evidence of disrupted connectivity is predictive of subsequent behavioral improvements in markers of cognitive control (55, 56). Similar findings have been reported for mood improvements (61), with altered connectivity in DMN and ECN associating with poorer treatment outcomes and greater cognitive dysfunction among adults with depression (62).
Observed changes in connectivity are also likely attributable to enhancements of neurotransmitter systems, particularly neuromodulatory pathways (dopamine, norepinephrine, and (serotonin).
This assertion is supported by large amounts of indirect evidence suggesting that neurobehavioral improvements following exercise are largest in executive functions (17, 18), which are preferentially impacted by dopaminergic disruption. In addition, parallel cognitive improvements are observed across heterogeneous populations with dysfunctional dopaminergic/norepinephrine functioning, including Parkinson’s disease (63), attention-deficit/hyperactivity disorder (64, 65), and bipolar disorder (66), with exercise-related increases in dopamine production (67). Modulation of the serotonin system is also widely hypothesized to play an important mechanistic role in the effects of acute exercise training on mood-related changes (68), with at least one prior trial demonstrating that higher intensity of aerobic training increased plasma 5-HT levels, which associated with improved response inhibition (69). Taken together, these data suggest that enhanced production and synthesis of monoamines is a critical component of improved affect regulation and cognitive control, both of which enhance self-regulation.
EXERCISE TRAINING: BEHAVIORAL MECHANISMS
In addition to direct modulation of cognitive and affective responses, exercise training may also facilitate learning of adaptive behavioral responses that improve mental health (Figure 1), broadly formulated as self-regulatory capacities. While self-regulation is vast, it includes ER and cognitive control, which are transdiagnostic mechanisms of change. Consistent with the Science of Behavior Change initiative (70), these mechanisms should be evaluable and coherent at different levels of analysis (i.e., changes in behavioral mechanisms should correspond to neurobiological changes) and, by extension, could be mutually facilitative, such that changes in one affect the other. Although prior research has examined regulation of both emotion and affect, affect regulation has traditionally been defined as the modulation of emotions or emotional expression in the service of self-regulation. Affect regulation has also traditionally been more closely associated with executive functioning in comparison with ER.
There are few examples that integrate psychological and neurocognitive mechanisms in a single model. One possible exception is self-systems theory (SST) (71–73). According to SST, many psychological disorders result from cumulative or catastrophic failures of goal pursuits (72). SST has most widely been used in depression, where it is superior to conventional therapeutic approaches among depressed adults with self-regulatory deficits (71). However, a critical conceptual element of SST, which parallels mechanistic studies on PA and mental health, is its integration of both psychological function and neurobiological inefficiencies as contributing to potential self-regulatory failures within an individual. Differential impact of exercise on mental health could arise from either source or from a lack of effective synergy between the two. This concept may be critical in understanding for whom interventions work and under what conditions, and in tailoring treatments.
Specific behavioral mechanisms linking exercise interventions to mental health include self-regulatory skills specific to affect regulation (61, 74) (e.g., tolerating and modulating arousal) and cognition (75) (e.g., exerting cognitive control over behavior, sustaining attention, and flexibly shifting attention and behavioral responses to match environmental demands) (76), which could influence mental health directly and through increased self-efficacy (77). Exercise may also potentiate reward salience through increased engagement in personally meaningful or rewarding activities and reward sensitization, or improved fitness (e.g., feeling more fit, improved body image). Behavioral mechanisms broadly correspond with neurological domains of changes, as summarized in Figure 3.
Figure 3.
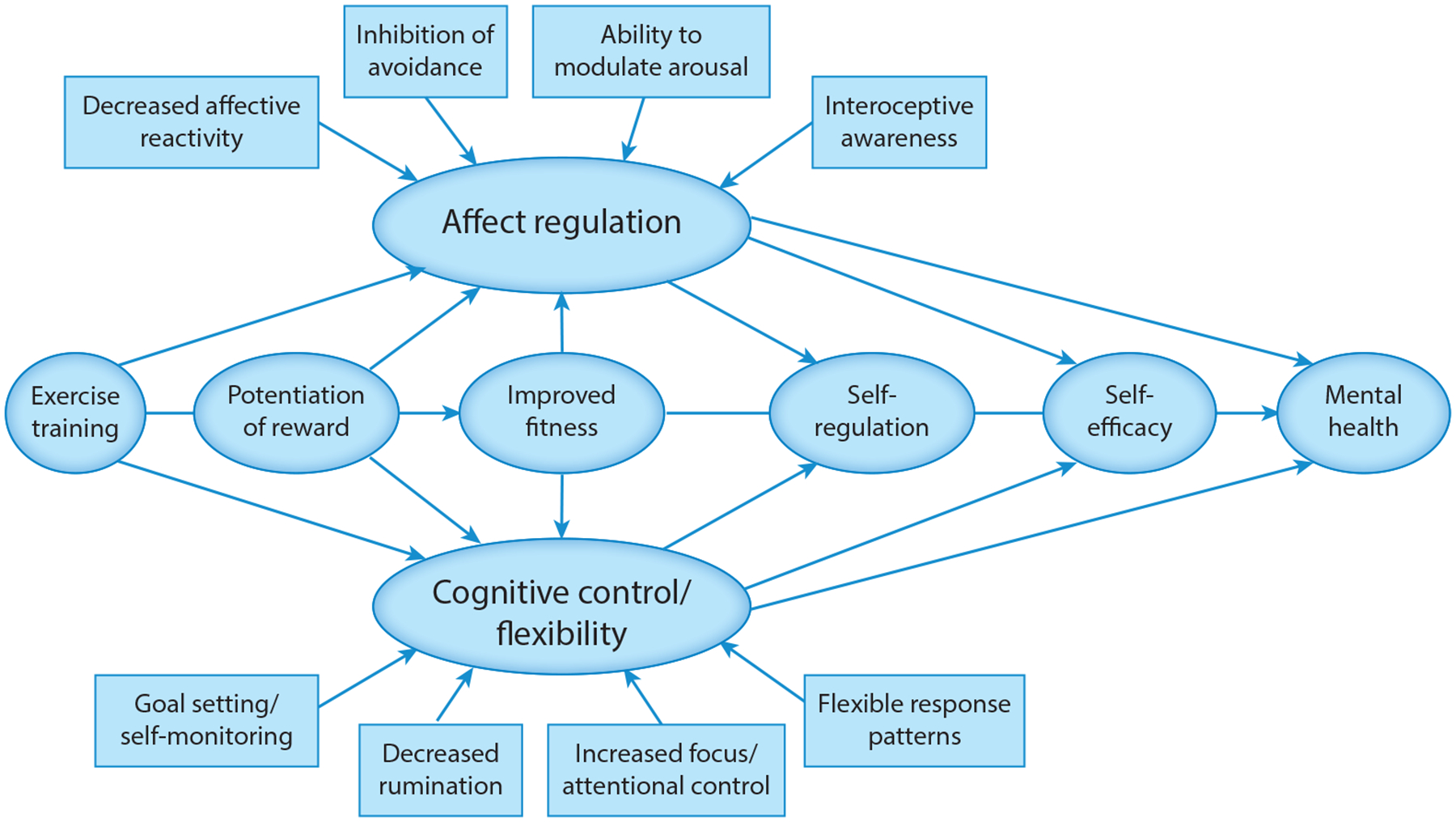
Conceptual model of behavioral mechanisms by which exercise training improves mental health outcomes.
EMOTION REGULATION/EXPERIENTIAL AVOIDANCE
The impact of exercise on mood is one of the most widely studied mechanisms linking exercise to mental health, both through acutely enhancing mood and by improving ER during stressful life circumstances (78). Newer cognitive-behavioral therapies (CBTs), such as Acceptance and Commitment Therapy (77) and Dialectical Behavior Therapy, target difficulties in ER as a key mechanism of change (79). ER is a multidimensional construct that includes awareness and clarity of feelings, as well as acceptance of emotions and the ability to modulate emotional responses. A significant literature indicates that difficulties in ER are associated with mental health problems and that improvements in ER correspond with better mental health. A smaller literature demonstrates improvements in these processes prospectively predicting outcomes. Experiential avoidance and avoidant coping with unwanted thoughts and feelings (i.e., efforts to avoid or suppress thoughts/feelings) are particularly detrimental, contributing to the development and maintenance of anxiety disorders (including panic disorder), depression, substance abuse, and posttraumatic stress disorder (PTSD), among others. Related issues of hypervigilance, reactive or impulsive responding to internal cues, and difficulty labeling emotions (alexithymia or poor interoceptive awareness) are also implicated in psychiatric disorders, most notably PTSD, borderline personality disorder, and eating disorders.
Exercise may improve individuals’ ability to tolerate negative affect or high levels of arousal. For example, high-intensity exercise elicits autonomic arousal that mimics anxiety. During exercise, individuals experience these sensations in a nonthreatening context, in which aversive interoceptive cues are not only expected but may be indicative of effective engagement. This may form new associations with anxiety and increase capacity to tolerate interoceptive sensations while inhibiting avoidance/escape responses. Participants may also learn skills to modulate arousal, such as through paced breathing. Exercise training, particularly high intensity interval training and resistance training, also causes temporary discomfort in pursuit of a more long-term goal (e.g., fitness). The ability to allow short-term discomfort in favor of long-term gain is essential for impulse control. Repeated exercise training over the course of weeks and gradual intensity titration might be particularly effective in helping participants gain mastery over potentially distressing internal experiences. Exercise may therefore incorporate gradual exposure with response prevention in the context of training, similar to conventional psychotherapy paradigms using desensitization techniques through gradual exposure (80–82). Studies with various clinical populations have suggested that aerobic training reduces anxiety and anxiety sensitivity. Indeed, most of the brain regions that have enhanced activation and function following exercise training are well known for their importance in affective reactivity within the DMN pathways, particularly the ventromedial PFC and its regulation of reactivity within subcortical and insular brain regions (83). Decreased affect reactivity would be expected to decrease the need for unhealthy avoidance or suppression of emotional responses.
A separate line of research has examined changes in affective reactivity to provocative stimuli following exercise training in the setting of intentional weight loss. This work suggests that behavioral training exercise may diminish participant SN reactivity to salient food cues, particularly within the ventral striatum and insula (84). In a similar study using a crossover design (85), 60 min of exercise among habitual exercisers reduced activation in the putamen and peri-insular brain regions to high-energy food cues. Similarly, Cornier and colleagues (84) found that reductions in weight were associated with reduced insular activation among overweight/obese adults following a 6-month exercise trial. Taken together, this work suggests that exercise might decrease affective reactivity/saliency of food cues, increasing ability to control impulses or regulate eating behavior. Increased modulation of food responses may also have broader implications for cultivating impulse control and self-regulatory capacity, which holds importance for reduced substance use risk, behavioral impulsivity, and overall improvements in mental health functioning.
COGNITIVE CONTROL/FLEXIBILITY
Exercise training programs often include behavior change strategies in the context of intervention delivery, particularly for home-based training paradigms. Behavior change strategies include behavioral self-regulatory skills such as goal setting, activity planning (including behavioral activation), adaptive problem solving, the provision of feedback, and self-monitoring (86), all of which overlap with key elements of traditional CBT. When these strategies are utilized in behavioral trials among individuals with mental health conditions, they likely play an active therapeutic role and may partially explain treatment improvements (87). As detailed below, effective behavioral engagement and self-monitoring are frequently impaired in individuals with depression and anxiety. Interventions cultivating these skill sets may therefore offer opportunities for experiential practice of clinically relevant skill domains in the service of exercise titration and maintenance.
Conventional exercise training programs teach participants to identify and implement goals that are specific, measurable, achievable, realistic, and timely (SMART goals), in order to optimize exercise participation and maintenance. Through the process of setting SMART goals, striving toward them, and evaluating iterative progress, participants cultivate realistic expectations for themselves and internalize a sense of behavioral control. Realistic goal setting and attainment also contribute to ER by increasing self-mastery, increasing agency, and establishing new, positive associations with self-concept. The process of self-monitoring (tracking behavior and its consequences) may also build a greater capacity to identify and repeat behaviors that are effective, as well as identifying maladaptive behavioral patterns.
Several mental health conditions, including depression and anxiety, are notable for impairments in cognitive flexibility and attentional control. Difficulties include poor set-shifting abilities (i.e., difficulty switching between tasks or altering behavior in response to feedback) (88), selective attention, and distractibility. Sustained attention and vigilance are also impaired across many mental health conditions, particularly in depression, where patients have difficulties sustaining engagement toward goal-directed targets over prolonged periods of time. Competing cognitive processes, such as rumination or excessive worry, may divert attention away from effective task performance and increase emotional suffering.
Impairments in cognitive control domains, including cognitive flexibility and executive function, have been repeatedly tied to behavioral treatment outcomes (89–91). Similarly, behavioral treatments improving cognitive flexibility and executive function also tend to favorably impact depression and anxiety outcomes (89). For example, cognitive flexibility is a putative treatment mechanism of mindfulness-based stress reduction, which has been shown to increase resting-state connectivity within ECN brain areas following training (92). As noted above, exercise training appears to have preferentially favorable effects on executive functions (76) and connectivity within ECN brain areas (57). Notably, poorer cognitive control is also a robust predictor of inadequate PA maintenance (28, 93), suggesting that poorer cognitive control is likely to present barriers both for initial acquisition of self-regulatory skills for PA engagement and utilization of these skills for maintenance over time.
Several newer CBTs teach mindfulness to decrease engagement in unproductive mental activities and improve capacity to be in the present moment. Exercise training programs may increase the ability to direct and sustain attention to the present, particularly if exercises are sufficiently difficult. In this case, effective engagement in the activity requires full attention to the task and awareness of a narrowed set of stimuli (e.g., one’s breathing and ability to pace breathing). These behavioral mechanisms may change in tandem with neurobiological-mediated decreases in the saliency of some stimuli (described above) and potentiation of reward, as detailed below.
Self-efficacy
Individual self-regulatory behaviors are also important for mental health functioning through their overlapping influences on self-efficacy (73). Broadly defined, self-efficacy refers to an individual’s confidence in their ability to achieve specific, personally significant goals. Greater engagement in PA has been shown to increase exercise self-efficacy (73), associated with improved body image and physical quality of life, and increases the likelihood of sustained PA maintenance over time (94). In addition, many of the domains reviewed above align closely with key conceptual elements of improved self-efficacy, including monitoring of performance accomplishments, behavioral modeling, social persuasion, and adaptive interpretation of physiological states. Recent meta-analytic syntheses suggest that exercise interventions confer moderately large improvements in self-efficacy across adolescent and adult cohorts (ES = 0.59) (95). The total number of behavior change strategies demonstrates the strongest associations with improved PA, regardless of the specific strategy used (96).
Reinforcement/Reward
A poverty of reinforcement or adaptive reward may lead to increased negative affect (e.g., depression) or the development of maladaptive behavior patterns to increase positive feelings (e.g., substance abuse). There is an extensive literature on increasing reinforcers/reward as a treatment mechanism of depression through behavioral activation (97). Newer CBTs, such as Acceptance and Commitment Therapy, link behavioral activation specifically to personal values, which may function to augment its reinforcement value or function as an establishing operation, and/or help sustain activity engagement. Behavioral activation often includes exercise or other forms of PA and may therefore both increase opportunities for positive reinforcement and cultivate realistic expectations regarding loss and reward, both of which may improve mental health. In addition, individual differences in reward sensitivity likely explain part of the observed heterogeneity in intermediate, positive consequences of PA (e.g., improved mood or fitness), which have been associated with the self-reinforcing nature of habitual PA and maintenance (74).
Dysfunctional reward sensitivity is a core feature increasing the risk of depression and serves as a key barrier to implementation of many therapeutic interventions. Exercise training has been postulated to improve reward sensitivity by augmenting dopaminergic function. Focusing on goal-directed behaviors has been shown to enhance top-down activation within the ventromedial PFC and ventral striatum, as well as downregulating insular and amygdala activation during stressful activities (98). Individuals engaging in habitual PA demonstrate differential activation of the ventral striatum, a critical dopamine hub, compared to their sedentary counterparts following acute exercise training (99). Individuals accustomed to habitual PA also show improved reward system functioning compared to sedentary counterparts, a relationship that does not appear to differ based on physical fitness (100).
FUTURE DIRECTIONS FOR TAILORED TREATMENTS
Clearly specifying both neurological and behavioral mechanisms that link exercise to mental health and how they might interact synergistically to produce outcomes may have implications for the design of exercise programs to potentiate training engagement and optimize outcomes for individuals or groups of individuals. Pre-existing neurobiological differences might explain the extent to which exercise interventions are effectively engaged and/or suggest indirect mechanisms of benefit in the context of exercise training. Repeated measurement and network analyses may be able to help disentangle the associations among elements that are probably mutually facilitative.
As reviewed above, both neurobiological markers of neuroplasticity and behavioral markers of self-regulatory function hold relevance in the development of future exercise training paradigms. Future studies should integrate phenotypic markers of neuroplasticity into intervention development in order to optimize intervention engagement. For example, individuals with blunted reward sensitivity may have difficulty with initial exercise engagement and therefore be less likely to adequately engage with or complete supervised training programs. These individuals may benefit from more gradual titration of activity, motivational priming prior to training sessions, or enhancement of reward reinforcement cues using external sources. Similarly, individuals with evidence of cognitive inflexibility and/or executive dysfunction may have difficulty with exercise maintenance and could benefit from receiving either greater external regulatory support (e.g., structured classes or exercise with a spouse) or additional self-management training to increase the likelihood of continued PA for optimal ER.
Future studies should also systematically assess and quantify individual differences in self-regulatory skills across participants, both as a baseline predictor of treatment engagement and across time as a mechanism of treatment improvements. Specifying individual differences in self-regulatory skills at baseline might also allow for effective treatment matching (matching individuals to exercise programs that they are more likely to be successful with or to programs that are more likely to address skill deficits and improve overall health and well-being) or tailoring of existing interventions to optimize outcomes. For example, individuals with difficulty regulating affect may also tend to avoid emotional or interoceptive discomfort, and they may experience increased sensitivity to arousal due to either distressing past experiences or inaccurate beliefs that arousal is dangerous. Irrespective of its precise origin, this behavioral tendency would suggest the need for differential titration of activity, greater usage of distraction techniques, or self-monitoring for titration based on external biometric sources (e.g., heart rate) that are not overly dependent on self-referential attention to interoceptive cues. These participants could also be trained to gain mastery by resisting urges to avoid or escape aversive cues or to use strategies to modulate arousal such that it can be sustained during a window of tolerance, which may be gradually expanded over time.
In addition, future RCTs may benefit from the use of optimization designs to determine the relative importance of priming neuroplasticity in order to achieve better mental health outcomes. RCTs utilizing sequential randomization to treat depression, for example, could re-randomize participants contingent upon connectivity or executive functioning changes, suggesting enhanced neuroplasticity as an intermediate marker of treatment responsivity. Similarly, trials examining exercise maintenance could benefit from provision of external support strategies among individuals with poorer self-regulatory capacity, including integration of social support or greater ease of access to exercise training equipment. Because these individuals will be less likely to self-initiate behavioral modifications or flexibly adapt their behavioral responses for exercise maintenance, additional environmental support is critical for outcomes.
CONCLUSIONS
In conclusion, aerobic and resistance exercise training hold promise in the treatment and management of mental health conditions, particularly depression and anxiety. Emerging evidence suggests that changes in underlying neuroplasticity may be an important individual difference explaining heterogeneous treatment benefits. In addition, underlying individual differences in neuroplasticity, either at baseline or in intervention-related changes, likely have significant implications for individuals developing and sustaining behavioral self-regulatory skills essential for mental health. These factors likely work in synergy, creating a “virtuous cycle” to predict long-term effects of exercise on mental health. By clearly specifying both neurobiological and behavioral mechanisms linking exercise to improved mental health, it may be possible to optimize treatment effects and personalize training approaches for maximal benefit.
LLD: late-life depression
SN: salience network
ECN: executive control network
DMN: default mode network
ER: emotion regulation
ACKNOWLEDGMENTS
Dr. Smith thanks Erica Shirts, Jeanne Schwartz, Bryan Feger, and Timothy Strauman for their thoughtful input during the conceptual formulation of this manuscript. This research was supported by funding from the National Institute of Health, NHLBI Grant R01HL130237.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Chekroud SR, Gueorguieva R, Zheutlin AB, et al. 2018. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry 5:739–46 [DOI] [PubMed] [Google Scholar]
- 2.Schuch FB, Vancampfort D, Firth J, et al. 2018. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am. J. Psychiatry 175:631–48 [DOI] [PubMed] [Google Scholar]
- 3.McDowell CP, Dishman RK, Gordon BR, Herring MP. 2019. Physical activity and anxiety: a systematic review and meta-analysis of prospective cohort studies. Am. J. Prev. Med 57:545–56 [DOI] [PubMed] [Google Scholar]
- 4.Kandola A, Ashdown-Franks G, Stubbs B, et al. 2019. The association between cardiorespiratory fitness and the incidence of common mental health disorders: a systematic review and meta-analysis. J. Affect. Disord 257:748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamer M, Stamatakis E, Steptoe A. 2009. Dose-response relationship between physical activity and mental health: the Scottish Health Survey. Br. J. Sports Med 43:1111–14 [DOI] [PubMed] [Google Scholar]
- 6.Bennie JA, Teychenne MJ, De Cocker K, Biddle SJH. 2019. Associations between aerobic and muscle-strengthening exercise with depressive symptom severity among 17,839 U.S. adults. Prev. Med 121:121–27 [DOI] [PubMed] [Google Scholar]
- 7.Morres ID, Hatzigeorgiadis A, Stathi A, et al. 2019. Aerobic exercise for adult patients with major depressive disorder in mental health services: a systematic review and meta-analysis. Depress. Anxiety 36:39–53 [DOI] [PubMed] [Google Scholar]
- 8.Kvam S, Kleppe CL, Nordhus IH, Hovland A. 2016. Exercise as a treatment for depression: a meta-analysis. J. Affect. Disord 202:67–86 [DOI] [PubMed] [Google Scholar]
- 9.Klil-Drori S, Klil-Drori AJ, Pira S, Rej S. 2020. Exercise intervention for late-life depression: a meta-analysis. J. Clin. Psychiatry 81:19r12877. [DOI] [PubMed] [Google Scholar]
- 10.Gordon BR, McDowell CP, Hallgren M, et al. 2018. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry 75:566–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogh J, Nordentoft M, Sterne JA, Lawlor DA. 2011. The effect of exercise in clinically depressed adults: systematic review and meta-analysis of randomized controlled trials. J. Clin. Psychiatry 72:529–38 [DOI] [PubMed] [Google Scholar]
- 12.Schuch FB, Vancampfort D, Rosenbaum S, et al. 2016. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Braz. J. Psychiatry 38:247–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman BM, Babyak MA, Craighead WE, et al. 2011. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom. Med 73:127–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn AL, Trivedi MH, Kampert JB, et al. 2005. Exercise treatment for depression: efficacy and dose response. Am. J. Prev. Med 28:1–8 [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Babyak MA, O’Connor C, et al. 2012. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA 308:465–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dauwan M, Begemann MJH, Slot MIE, et al. 2019. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta-analysis of randomized controlled trials. J. Neurol In press. 10.1007/s00415-019-09493-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith PJ, Blumenthal JA, Hoffman BM, et al. 2010. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med 72:239–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landrigan JF, Bell T, Crowe M, et al. 2019. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol. Res 84:1167–83 [DOI] [PubMed] [Google Scholar]
- 19.Beaudreau SA, Rideaux T, O’Hara R, Arean P. 2015. Does cognition predict treatment response and remission in psychotherapy for late-life depression? Am. J. Geriatr. Psychiatry 23:215–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor WD, Aizenstein HJ, Alexopoulos GS. 2013. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol. Psychiatry 18:963–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheline YI, Pieper CF, Barch DM, et al. 2010. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch. Gen. Psychiatry 67:277–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, O’Neil R, Banerjee S, et al. 2017. “Engage” therapy: prediction of change of late-life major depression. J. Affect. Disord 221:192–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Underwood M, Lamb SE, Eldridge S, et al. 2013. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 382:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridle C, Spanjers K, Patel S, et al. 2012. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br. J. Psychiatry 201:180–85 [DOI] [PubMed] [Google Scholar]
- 25.Stillman CM, Erickson KI. 2018. Physical activity as a model for health neuroscience. Ann. N. Y. Acad. Sci 1428:103–11 [DOI] [PubMed] [Google Scholar]
- 26.Erickson KI, Creswell JD, Verstynen TD, Gianaros PJ. 2014. Health neuroscience: defining a new field. Curr. Dir. Psychol. Sci 23:446–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Audiffren M, Andre N. 2019. The exercise-cognition relationship: a virtuous circle. J. Sport Health Sci 8:339–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boat R, Cooper SB. 2019. Self-control and exercise: a review of the bi-directional relationship. Brain Plast 5:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzanehfar P 2018. Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci. Res 134:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Voss MW, Erickson KI, Prakash RS, et al. 2013. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun 28:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai CL, Ukropec J, Ukropcova B, Pai MC. 2018. An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. Neuroimage Clin 17:272–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marosi K, Mattson MP. 2014. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab 25:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D. 2017. The energy metabolism dysfunction in psychiatric disorders postmortem brains: focus on proteomic evidence. Front. Neurosci 11:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. 2016. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med 22:238–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price JB, Bronars C, Erhardt S, et al. 2018. Bioenergetics and synaptic plasticity as potential targets for individualizing treatment for depression. Neurosci. Biobehav. Rev 90:212–20 [DOI] [PubMed] [Google Scholar]
- 36.Maes M, Berk M, Goehler L, et al. 2012. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly AM. 2018. Exercise-induced modulation of neuroinflammation in models of Alzheimer’s disease. Brain Plast 4:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halaris A, Myint AM, Savant V, et al. 2015. Does escitalopram reduce neurotoxicity in major depression? J. Psychiatr. Res 66–67:118–26 [DOI] [PubMed] [Google Scholar]
- 39.Menon V 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci 15:483–506 [DOI] [PubMed] [Google Scholar]
- 40.Kolesar TA, Bilevicius E, Wilson AD, Kornelsen J. 2019. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin 24:102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scult MA, Fresco DM, Gunning FM, et al. 2019. Changes in functional connectivity following treatment with emotion regulation therapy. Front. Behav. Neurosci 13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner G, Kohler S, Bar KJ. 2017. Treatment associated changes of functional connectivity of midbrain/brainstem nuclei in major depressive disorder. Sci. Rep 7:8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haeger A, Costa AS, Schulz JB, Reetz K. 2019. Cerebral changes improved by physical activity during cognitive decline: a systematic review on MRI studies. Neuroimage Clin 23:101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boldrini M, Fulmore CA, Tartt AN, et al. 2018. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22:589–99.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosano C, Guralnik J, Pahor M, et al. 2017. Hippocampal response to a 24-month physical activity intervention in sedentary older adults. Am. J. Geriatr. Psychiatry 25:209–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raichlen DA, Klimentidis YC, Bharadwaj PK, Alexander GE. 2020. Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle-aged to older adults. Brain Imaging Behav In press. 10.1007/s11682-019-00148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erickson KI, Voss MW, Prakash RS, et al. 2011. Exercise training increases size of hippocampus and improves memory. PNAS 108:3017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krogh J, Rostrup E, Thomsen C, et al. 2014. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression—a randomized clinical trial. J. Affect. Disord 165:24–30 [DOI] [PubMed] [Google Scholar]
- 49.Voss MW, Heo S, Prakash RS, et al. 2013. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum. Brain Mapp 34:2972–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espeland MA, Erickson K, Neiberg RH, et al. 2016. Brain and white matter hyperintensity volumes after 10 years of random assignment to lifestyle intervention. Diabetes Care 39:764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith PJ, Sherwood A, Blumenthal JA. 2018. Effects of aerobic exercise on white matter hyperintensities: an exploratory analysis. Gen. Hosp. Psychiatry 53:84–85 [DOI] [PubMed] [Google Scholar]
- 52.Herold F, Torpel A, Schega L, Muller NG. 2019. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—a systematic review. Eur. Rev. Aging Phys. Act 16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng TB, Pierce GL, Darling WG, et al. 2017. The acute effects of aerobic exercise on the functional connectivity of human brain networks. Brain Plast 2:171–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voss MW, Weng TB, Narayana-Kumanan K, et al. 2020. Acute exercise effects predict training change in cognition and connectivity. Med. Sci. Sports Exerc 52:131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallen CL, D’Esposito M. 2019. Brain modularity: a biomarker of intervention-related plasticity. Trends Cogn. Sci 23:293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baniqued PL, Gallen CL, Voss MW, et al. 2017. Brain network modularity predicts exercise-related executive function gains in older adults. Front. Aging Neurosci 9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voss MW, Prakash RS, Erickson KI, et al. 2010. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li MY, Huang MM, Li SZ, et al. 2017. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int. J. Neurosci 127:634–49 [DOI] [PubMed] [Google Scholar]
- 59.Andreescu C, Ajilore O, Aizenstein HJ, et al. 2019. Disruption of neural homeostasis as a model of relapse and recurrence in late-life depression. Am. J. Geriatr. Psychiatry 27:1316–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, et al. 2012. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord 139:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tozzi L, Carballedo A, Lavelle G, et al. 2016. Longitudinal functional connectivity changes correlate with mood improvement after regular exercise in a dose-dependent fashion. Eur. J. Neurosci 43:1089–96 [DOI] [PubMed] [Google Scholar]
- 62.Albert KM, Potter GG, Boyd BD, et al. 2019. Brain network functional connectivity and cognitive performance in major depressive disorder. J. Psychiatr. Res 110:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duchesne C, Gheysen F, Bore A, et al. 2016. Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals. Neuroimage Clin 12:559–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang YK, Liu S, Yu HH, Lee YH. 2012. Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Arch. Clin. Neuropsychol 27:225–37 [DOI] [PubMed] [Google Scholar]
- 65.Kim H, Heo HI, Kim DH, et al. 2011. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci. Lett 504:35–39 [DOI] [PubMed] [Google Scholar]
- 66.Metcalfe AW, MacIntosh BJ, Scavone A, et al. 2016. Effects of acute aerobic exercise on neural correlates of attention and inhibition in adolescents with bipolar disorder. Transl. Psychiatry 6:e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahlskog JE. 2018. Aerobic exercise: evidence for a direct brain effect to slow Parkinson disease progression. Mayo Clin. Proc 93:360–72 [DOI] [PubMed] [Google Scholar]
- 68.Basso JC, Suzuki WA. 2017. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast 2:127–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmer P, Stritt C, Bloch W, et al. 2016. The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with Stroop task performance: a randomized controlled trial. Eur. J. Appl. Physiol 116:2025–34 [DOI] [PubMed] [Google Scholar]
- 70.Nielsen L, Riddle M, King JW, et al. 2018. The NIH Science of Behavior Change Program: transforming the science through a focus on mechanisms of change. Behav. Res. Ther 101:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strauman TJ, Eddington KM. 2017. Treatment of depression from a self-regulation perspective: basic concepts and applied strategies in self-system therapy. Cognit. Ther. Res 41:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strauman TJ, Socolar Y, Kwapil L, et al. 2015. Microinterventions targeting regulatory focus and regulatory fit selectively reduce dysphoric and anxious mood. Behav. Res. Ther 72:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olson EA, McAuley E. 2015. Impact of a brief intervention on self-regulation, self-efficacy and physical activity in older adults with type 2 diabetes. J. Behav. Med 38:886–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartman ME, Ekkekakis P, Dicks ND, Pettitt RW. 2019. Dynamics of pleasure-displeasure at the limit of exercise tolerance: conceptualizing the sense of exertional physical fatigue as an affective response. J. Exp. Biol 222(Pt. 3):jeb186585. [DOI] [PubMed] [Google Scholar]
- 75.Mehren A, Ozyurt J, Thiel CM, et al. 2019. Effects of acute aerobic exercise on response inhibition in adult patients with ADHD. Sci. Rep 9:19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sprague BN, Freed SA, Webb CE, et al. 2019. The impact of behavioral interventions on cognitive function in healthy older adults: a systematic review. Ageing Res. Rev 52:32–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Casey MB, Smart K, Segurado R, et al. 2018. Exercise combined with Acceptance and Commitment Therapy (ExACT) compared to a supervised exercise programme for adults with chronic pain: study protocol for a randomised controlled trial. Trials 19:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Fu R, Sun L, et al. 2019. How does exercise improve implicit emotion regulation ability: preliminary evidence of mind-body exercise intervention combined with aerobic jogging and mindfulness-based yoga. Front. Psychol 10:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schafer JO, Naumann E, Holmes EA, et al. 2017. Emotion regulation strategies in depressive and anxiety symptoms in youth: a meta-analytic review. J. Youth Adolesc 46:261–76 [DOI] [PubMed] [Google Scholar]
- 80.Hegberg NJ, Hayes JP, Hayes SM. 2019. Exercise intervention in PTSD: a narrative review and rationale for implementation. Front. Psychiatry 10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aylett E, Small N, Bower P. 2018. Exercise in the treatment of clinical anxiety in general practice—a systematic review and meta-analysis. BMC Health Serv. Res 18:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stonerock GL, Hoffman BM, Smith PJ, Blumenthal JA. 2015. Exercise as treatment for anxiety: systematic review and analysis. Ann. Behav. Med 49:542–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gujral S, Aizenstein H, Reynolds CF, et al. 2017. Exercise effects on depression: possible neural mechanisms. Gen. Hosp. Psychiatry 49:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cornier MA, Melanson EL, Salzberg AK, et al. 2012. The effects of exercise on the neuronal response to food cues. Physiol. Behav 105:1028–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evero N, Hackett LC, Clark RD, et al. 2012. Aerobic exercise reduces neuronal responses in food reward brain regions. J. Appl. Physiol 112:1612–19 [DOI] [PubMed] [Google Scholar]
- 86.Suls J, Mogavero JN, Falzon L, et al. 2020. Health behaviour change in cardiovascular disease prevention and management: meta-review of behaviour change techniques to affect self-regulation. Health Psychol. Rev 14:43–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young CL, Trapani K, Dawson S, et al. 2018. Efficacy of online lifestyle interventions targeting lifestyle behaviour change in depressed populations: a systematic review. Aust. N. Z. J. Psychiatry 52:834–46 [DOI] [PubMed] [Google Scholar]
- 88.Keller AS, Leikauf JE, Holt-Gosselin B, et al. 2019. Paying attention to attention in depression. Transl. Psychiatry 9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manning KJ, Steffens DC. 2018. State of the science of neural systems in late-life depression: impact on clinical presentation and treatment outcome. J. Am. Geriatr. Soc 66(Suppl. 1):S17–S23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manning KJ, Alexopoulos GS, Banerjee S, et al. 2015. Executive functioning complaints and escitalopram treatment response in late-life depression. Am. J. Geriatr. Psychiatry 23:440–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barch DM, D’Angelo G, Pieper C, et al. 2012. Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. Am. J. Geriatr. Psychiatry 20:682–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taren AA, Gianaros PJ, Greco CM, et al. 2017. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom. Med 79:674–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Best JR, Nagamatsu LS, Liu-Ambrose T. 2014. Improvements to executive function during exercise training predict maintenance of physical activity over the following year. Front. Hum. Neurosci 8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berli C, Stadler G, Shrout PE, et al. 2018. Mediators of physical activity adherence: results from an action control intervention in couples. Ann. Behav. Med 52:65–76 [DOI] [PubMed] [Google Scholar]
- 95.Jacquart J, Dutcher CD, Freeman SZ, et al. 2019. The effects of exercise on transdiagnostic treatment targets: a meta-analytic review. Behav. Res. Ther 115:19–37 [DOI] [PubMed] [Google Scholar]
- 96.Tang MY, Smith DM, Mc Sharry J, et al. 2019. Behavior change techniques associated with changes in postintervention and maintained changes in self-efficacy for physical activity: a systematic review with meta-analysis. Ann. Behav. Med 53:801–15 [DOI] [PubMed] [Google Scholar]
- 97.Euteneuer F, Dannehl K, Del Rey A, et al. 2017. Immunological effects of behavioral activation with exercise in major depression: an exploratory randomized controlled trial. Transl. Psychiatry 7:e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dutcher JM, Creswell JD. 2018. The role of brain reward pathways in stress resilience and health. Neurosci. Biobehav. Rev 95:559–67 [DOI] [PubMed] [Google Scholar]
- 99.Bothe N, Zschucke E, Dimeo F, et al. 2013. Acute exercise influences reward processing in highly trained and untrained men. Med. Sci. Sports Exerc 45:583–91 [DOI] [PubMed] [Google Scholar]
- 100.Wardle MC, Lopez-Gamundi P, LaVoy EC. 2018. Effects of an acute bout of physical exercise on reward functioning in healthy adults. Physiol. Behav 194:552–59 [DOI] [PMC free article] [PubMed] [Google Scholar]


