Abstract
Since its conception as an applied biomedical technology nearly 30 years ago, nanopore is emerging as a promising, high-throughput, biomarker-targeted diagnostic tool for clinicians. The attraction of a nanopore-based detection system is its simple, inexpensive, robust, user-friendly, high-throughput blueprint with minimal sample preparation needed prior to analysis. The goal of clinical-based nanopore biosensing is to go from sample acquisition to a meaningful readout quickly. The most extensive work in nanopore applications has been targeted at DNA, RNA, and peptide identification. Although, biosensing of pathological biomarkers, which is covered in this review, is on the rise. This review is broken into two major sections: (i) the current state of existing biological, solid state, and hybrid nanopore systems and (ii) the applications of nanopore biosensors toward detecting neurodegenerative biomarkers.
Keywords: biomarker, detection, hemolysin, amyloid protein, Alzheimer’s disease, analytical chemistry
I. INTRODUCTION
Nanopore biosensing is a competitive alternative to traditional pathological detection techniques, offering itself as a high-throughput, ultrasensitive single molecule (biomarker) detection device capable of quantifying and discriminating from a range of systemically circulating ultralow concentration biopolymers (DNA, RNA, and protein) and metabolites. Nanopore biosensing has attracted work attention in the fields of genomics, proteomics, polymers, and metabolomics—to list a few—and offers special potential in early neurodegenerative (ND) biomarker detection due to its ultrasensitive, single molecule discriminative nature. ND disorders are becoming more prevalent as living standards rise and people live longer, worldwide. It is believed senility due to ND pathologies can be avoided if preventative action can be encouraged early in life. Herein, we will review the three types of nanopore biosensors and their applications in the most commonly studied ND disorders. This review is by no means exhaustive and intended to both inform and guide the reader to available nanopore systems, their basic characteristics, and potential applications in the biomedical setting of ND disease.
Nanopore sensors are categorized based on the chemical composition of the pore and its support (membrane). Three types of nanopore systems exist: biological, solid state, and hybrid.1 Biological nanopores are formed by the spontaneous assembly of reproducibly shaped, amino acid–based biopolymers within a planar lipid bilayer (PLB).2 The most commonly researched biological nanopore is α-hemolysin (αHL), a toxic heptameric protein pore secreted in monomer form by pathogenic Staphylococcus aureus.3 This toxic protein pore preferentially lyses red blood cells in nature4 and, in situ, αHL can be used as a stochastic single molecule sensor.5 Excellent experiment-to-experiment signal reproducibility with a fully assembled αHL pore is observed. Solid state nanopores, on the other hand, involve milling nanometer diameter holes into membranes of solid state materials (inorganic/nonlife associated materials), such as silicon6 or hafnium oxide,7 with high-powered electron beams or chemical etching protocols. The challenge in forming solid state nanopore sensors is yielding consistent pore shape in batch-to-batch millings. Reproducible hole circularity in solid state systems is less than optimal, routinely obtaining irregularly sided pore circles.8 This inconsistency in pore shape naturally influences experiment-to-experiment reproducibility and renders difficulties in data analysis. Hybrid nanopores involve integrating biological nanopores into solid state membranes,1 which is easier said than done. Producing hybrid systems typically involves milling a larger-than-usual, hourglass-shaped, nanometer diameter hole followed by a corking process, which involves electrophoretically wedging the biological nanopore into the milled, tapered hole.9 The result is a biological nanopore embedded within a nonbiological membrane. The implication is greater pore life, fine control over membrane properties, and batch-to-batch signal reproducibility.9 Oxford Nanopore Technologies minION DNA sequencing device is a handheld unit consisting of αHL protein pore embedded in a synthetic membrane chip,10 signifying a potentially large commercial value for the fully developed, stable hybrid pore. Biological nanopores assembled in PLB yield excellent reproducibility yet low stability due to PLB nature; whereas, solid state nanopores yield less-than-optimal reproducibility but heightened stability. Hybrid nanopores combine the best of both.1
The scope for nanopores as biosensors continues to rapidly expand. Initially imagined as DNA sequencing technology only,11 nanopore biosensing has expanded to RNA sequencing,12 protein identification,13 biosensing pathological biomarkers,14 and even probing the molecular conformations peptides in solution.15 ND diseases, such as Parkinson’s Disease (PD), Alzheimer’s Disease (AD), and Huntington’s Disease (HD)—afflicting the aged population—could be counteracted by detecting disease indicators early in a patient’s life. Early sensing of systemically circulating ND biomarkers, such as AD associated β-amyloid (Aβ) peptides, could lead to informed preventative action. As of now, ND disorders are best monitored through studying a patient’s family history and genealogy, cerebrospinal fluid (CSF) analysis via invasive lumbar punctures, and neuroimaging of biomarkers through positron emission tomography (PET) and magnetic resonance imaging (MRI).16
MRI and PET scanning machines are large, expensive, require a skilled technician to operate and a doctor to interpret images, and costly upkeep, while lumbar punctures are painful and dangerously invasive. Blood biomarker detection tests consisting of immunoprecipitation followed with mass spectroscopy analysis (IP-MS) targeted at AD detection have proven robust though MS equipment is similar to PET and MRI in regard to expense, upkeep, and skilled technical workers required to operate. Nanopore biosensors have demonstrated a potential for fast, inexpensive, point-of-care applications in early detection cancer through monitoring tumor-induced structural variants of a patient’s DNA sequence.17 This point-of-care device can be applied similarly to studying ND pathologies by monitoring minimal invasively acquired body fluid (i.e., plasma, saliva, and urine) for circulating DNA, peptide, and small molecule biomarkers, early in a patient’s life. AD is a leading cause of death in populations > 65 because the effects of accumulated amyloid plaques and neurofibrillary tangles, which is believed to begin early in life, compounds senescence.18 Literature suggests detection of Aβ levels in blood favorably correlates with brain PET imaging and CSF analysis.19 This lays a foundation for the possibility of high-throughput nanopore applied analysis of processed patient blood—a less expensive, more user friendly alternative to existing technologies.19
II. NANOPORE SYSTEMS
A. Biological Nanopore
A variety of wild-type (WT) and experimentally mutated biological nanopores exist. As nanopore technology sophisticates, exceedingly more broad applications are being developed than initially intended. In this section, we highlight key characteristics of these channel-forming proteins. Because of the availability of information, the most commonly studied pores will be discussed individually and the remaining will be concisely discussed and grouped as one large section. Table 1 corresponds to biological nanopores, Table 2 to solid state, and Table 3 to hybrid. Tables 4-6 summarize their potential ND applications.
TABLE 1:
Biological nanopores
| Biological | Protein pore | Oligomer no. | Constriction diameter (nm) |
Ideal biomarker/polymer class |
|---|---|---|---|---|
 |
αHL | 7 | 1.4 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
MspA | 8 | 1.2 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
Phi29 | 12 | 3.6 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Aerolysin | 7 | 1.0–1.7 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
ClyA - I, II, III | 12, 13, 14 | 2.2, 2.7, 4.2 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
FhuA | 1 | 3.1 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
SP1 | 12 | 3.0 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
FraC - I, II, III | 8, 7, 6 | 1.6, 1.1, 0.8 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
OmpG | 1 | 2.0–2.9 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
Lysenin | 9 | 3.0 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
CsgG | 9 | 1.2 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
NfpAB | 8 | Undisclosed | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
Anthrax Toxin Pore | 7 | 1.5 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
 |
gA | 2 | 4.0 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
VDAC | 1–4 | 3.0–4.0 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Perforin | 4–21 | 3.2–5.3 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Streptolysin O | 50–80 | 25.0–35.0 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
VCC | 7 | 0.8 | ssDNA, ssRNA, peptide, small metabolites, synthetic polyelectrolytes |
TABLE 2:
Solid state nanopore materials
| Solid state | Material | Thickness range (nm) |
Pore diameter (nm) |
Ideal biomarker/ polymer class |
|---|---|---|---|---|
| (See reference (9) for image) | Silicon | 18.0–75.0 | > 5.0 | dsDNA, dsRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Gaphene | 1.0–5.0 | > 2.0 | dsDNA, ssDNA, dsRNA, ssRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Aluminum | 5 × 104 to 10 × 104 | > 10.0 | dsDNA, dsRNA, protein, peptide, antibody capture |
 |
Boron nitride | 1.0–1.3 | > 12.3 | dsDNA, dsRNA, protein, peptide, antibody capture |
 |
Molybdeneum disulfide | 6.5 | > 5.0 | dsDNA, ssDNA, dsRNA, ssRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Hafnium oxide | 2.0–7.0 | > 1.4 | dsDNA, ssDNA, dsRNA, ssRNA, protein, peptide, antibody capture, synthetic polyelectrolyte |
 |
Glass (Borosilicate) | 20.0–75.0 | > 14.0 | dsDNA, dsRNA, protein, peptide, antibody capture |
 |
Lipid coated | Depends on membrane material | Depends on membrane material | Depends on membrane material |
TABLE 3:
Hybrid nanopores
| Hybrid | Novelty | Membrane | Pore | Ideal biomarker/ polymer class |
|---|---|---|---|---|
| Traditional hybrid: any biological pore embedded within solid state membrane | Biological pore in solid state membrane | Solid state material | Biological | Depends on protein pore |
| DNA origami | DNA coated solid state membrane | Solid state material | Solid state | Depends on membrane material |
TABLE 4:
Hybrid nanopores paired with detectable analytes
| Novelty | Potential disease and related biomarker applications |
|---|---|
| Biopore in solid state membrane |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| DNA origami |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
TABLE 6:
Paired solid state materials with detectable analytes
| Material | Potential disease and related biomarker applications |
|---|---|
| Silicon |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Gaphene |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Aluminum |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Boron nitride |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Molybdeneum disulfide |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Hafnium oxide |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Glass (Borosilicate) |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJI HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Lipid coated |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
1. α-Hemolysin (αHL)
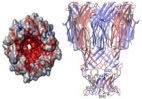
αHL is a toxic protein pore secreted in monomer form by S. aureus and the most widely studied biological nanopore. In nature, αHL preferentially oligomerizes on the membranes of erythrocytes, causing leaching of valuable ions and small molecules into the extracellular environment, ultimately resulting in cell death.20 This transmembrane toxin is spontaneously assembled from seven identical monomer subunits forming a mushroom-shaped, water-filled, ion-conducting homoheptameric channel on PLB surfaces. The fully assembled pore can be morphologically divided into two regions: a portion resembling a Basidiomycota mushroom cap, known as the vestibule, and the narrowed, barrel-like portion anchored within the PLB, known as the stem. The vestibule opening has a diameter of 4.6 nm and narrows in the stem region to a constriction diameter of 1.4 nm.21 The structure of αHL is depicted in Table 1.
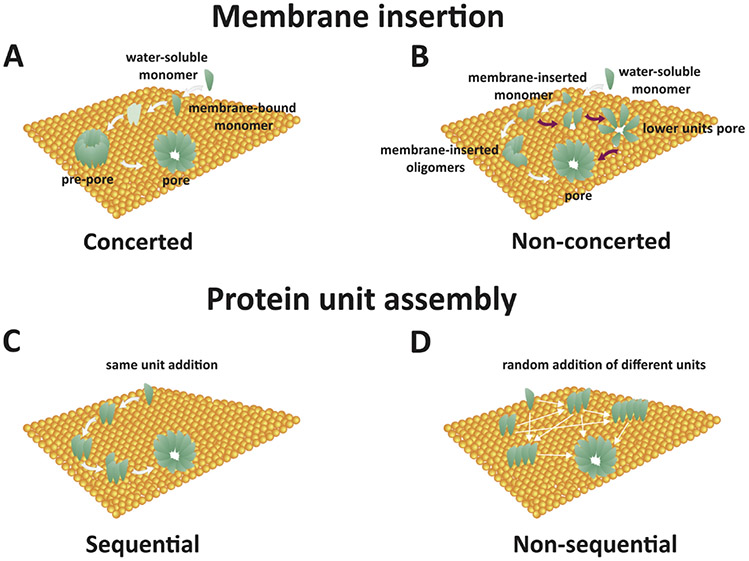
Each monomer consists of 293 amino acid residues with an isoelectric point (pI) of 8.5, consequently bearing a net + 1.5 charge at neutral pH.22 The net charge of a fully assembled αHL channel at neutral pH is approximately +10, based on Innovagen’s Peptide Property Calculator. On the basis of molecular dynamic (MD) simulation approximations at neutral pH, Roux et al. estimates a net charge of αHL to be +14.20 Pore assembly is not a perfectly understood phenomenon, as four possible mechanisms of the pore forming protein (PFP) assembly have been suggested (Fig. 1).23 These mechanisms suggestively represent all possible ways a PFP can be constructed on the PLB surface. The concerted mechanism [Fig. 1(a)] involves binding of water-soluble monomers to the PLB surface, followed by unordered heptameric oligomerization, and finally insertion of the fully assembled pore complex (known as a pre-pore when assembled but not inserted) into the PLB. The nonconcerted mechanism [Fig. 1(b)] involves water-soluble monomeric binding to the PLB followed by a possible immediate insertion of the bound monomer, dimer, timer, etc. These smaller complexes have been observed to conduct less ions and consequently have a detectable lower open-pore current than their fully assembled counterpart. The sequential mechanism [Fig. 1(c)] involves binding of a water-soluble monomer to the PLB followed by the income, orientation, and attachment of stoichiometrically fixed subunits of dimers or trimers, for example, to form a fully assembled pore. The nonsequential assembly [Fig. 1(d)], the final proposed mechanism of the pore forming phenomenon, follows an initial random assembling nature of the nonconcerted mechanism and an insertion phenomenon of the sequential mechanism.23
FIG. 1:
Four proposed mechanisms of pore forming protein assembly: (a) Concerted: Water-soluble monomers bind to the membrane with subsequent oligomerization yielding a pre-pore ensemble that then penetrates the membrane; (b) Nonconcerted: Water-soluble monomers bind to the membrane surface and either insert into the membrane immediately as detectable partial pore complexes or assemble entirely and then insert; (c) Sequential: Water-soluble monomers bind to the membrane surface and combine in units of fixed molecularity followed with insertion; and (d) Nonsequential: Similar to all other steps, oligomerization is seeded by an individual water-soluble monomer adsorbing to the membrane surface followed by random addition of molecular units (monomers, dimers, trimers, etc.) to form a stable pore for insertion. (Reprinted with permission from Elsevier, Copyright 2016.23)
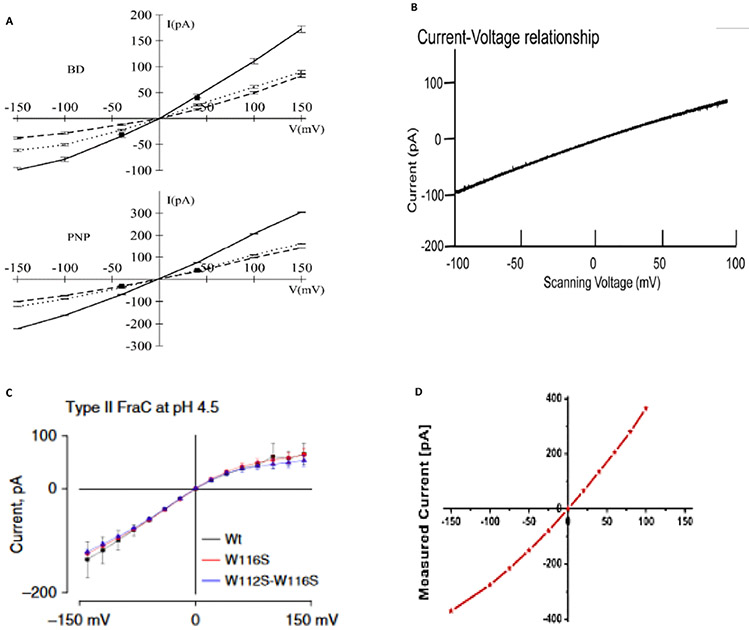
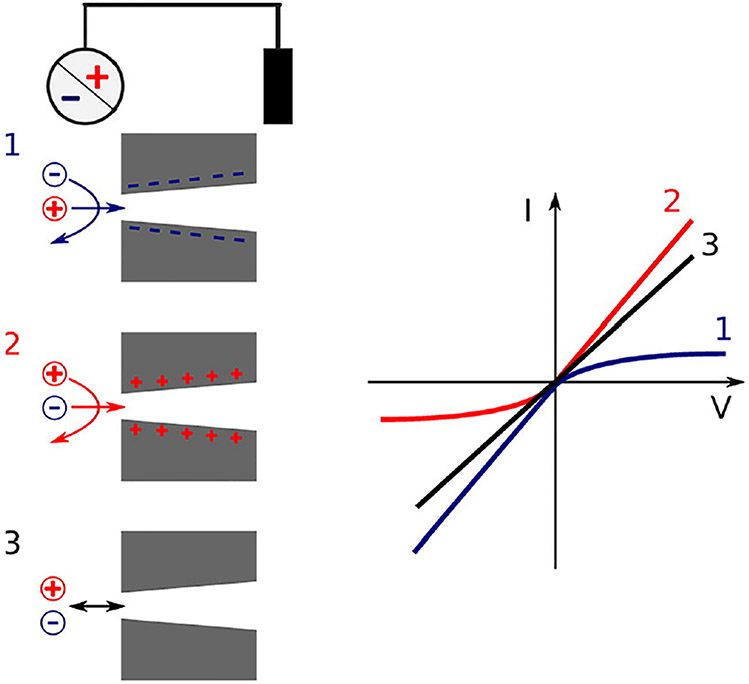
Fully assembled αHL, which consists of seven subunits, has been observed to follow all four mechanisms of assembly, which is mentioned little in the literature.24,25 In 1M KCl, under 100 mV bias, fully assembled αHL conveniently conducts 100 pA of charge, where the vestibule is pointing to the cis chamber and the bias source is the trans chamber.26 If the bias is flipped to −100 mv, however, the αHLpore conducts −80 pA of charge. This disparity in conductance reflects the asymmetric charge conducting nature of the αHL protein pore, depicted by current-voltage (I-V) curves in Fig. 2(a). This can be explained through the conically shaped, anion selective nature of αHL at pH lower than its pI, whereby basic residues at the narrowest constriction point of the stem are protonated. This results in the disproportionate selection for anion permeability.20 Figure 3 represents a schematic of the asymmetric rectification phenomenon observed for conically shaped, charged nanopores (like αHL). Though disparity of net ion conductance varies with the direction of electric-force field lines from the input electrode, analyte entering the vestibule end first versus entering the stem end first produce no noticeable distinction in observed translocation signal. The main difference for analyte translocation entering the vestibule versus stem is larger translocation frequency when analytes enter the vestibule first.27
FIG. 2:
(a) Current-voltage relation in a 1.0 M KCl symmetric solution from GCMC/BD and 3d-PNP computations. The total current (solid line) is the sum of K+ (dashed line) and Cl− (dotted line) currents. Experimental data (squares) correspond to total current at V = 40 mV and V = −40 mV, from Menestrina165 and Miles et al.,166 respectively. (Reprinted with permission from Elsevier, Copyright 2004.20). (b) A continuous current-voltage trace under a ramping voltage of −100 to 100 mV for membrane-embedded phi29 connector channel. An asymetry in current conductance of ~0 V is observed, with a greater current conducted at negative voltages. (Reprinted with permission from ACS publications, Copyright 2013.28). (c) Current-potential curves of type II nanopores formed by Wt-FraC, W116S-FraC, and W112s-W116S-FraC at pH 4.5. (Reprinted with permission from Nature Publishing Group, Copyright 2019.45). (d) Current-voltage curve of single-channel N. farcinica channel, which are asymmetric. (Reprinted with permission from ACS publications, Copyright 2012.61)
FIG. 3:
Schematic of asymmetrical nanopores with their surface charges and their corresponding IV curves (Reprinted with permission from Elsevier, Copyright 2017.69)
2. Phi29
The abbreviation Phi29 represents a DNA-packaging polymerase generated by the bacteriophage phi29 composed of a connector core of 12 subunit copies (known as gp10 subunits), which form a dodecameric protein pore (Table 1).28 The bacteriophage uses this protein pore to pass its genome to its host.29 This channel is stable under a wide range pH and displays an asymmetric conductance-voltage rectification observed with other charged, mushroom-shaped biological nanopores [Fig. 2(b)]. One major issue with DNA sequencing via a nanopore is the high translocation speed (low dwell time) of the analyte (noted above for MspA). As DNA length increases so does the net charge of the biopolymer, inducing powerful electrostatic interactions with the nanopore system electric field. Phi29 is an attractive biological nanopore for its capabilities in conducting double-stranded (ds) DNA slow enough for resolution of nucleotide sequence. With a constriction diameter of ~ 3.6 nm, not only can dsDNA easily translocate, but other biopolymers such as peptides and antibodies show analytical promise.29 In experiments performed by Haque et al.,1 lysine in position 234 of each gp10 subunit was replaced with cysteine through site-directed mutagenesis. This yielded reactive sulfhydryl groups in position 234 of the gp10 subunits, which are positioned along the inner channel wall when assembled, allow for the capture and detection of thioreactive analytes.29 These experiments signify the robustness and versatility of the Phi29 polymerase motor pore in biomarker detection and DNA processing and lay the framework for site-specific mutations to be carried out in other protein nanopores.
3. MspA
Mycobacterium smegmatis porin A (MspA) is a V-shaped, octameric protein pore with a constriction diameter of 1.2 nm and constriction length of 0.5 nm.30 A representation of MspA, acquired from the Protein Data Bank, can be found in Table 1. Because of the slightly enhanced ion modulation of translocating analyte offered by the narrower and shorter constriction of the pore, MspA is a more ideal DNA-sequencing channel than the traditionally studied αHL.30 One drawback of WT MspA is the high velocity of translocating DNA making single molecule discrimination difficult. MspA’s robust pore forming ability persists even after many residues have been site-directionally mutated, reflecting ample room for chemical modification and tuning.31 Slower analyte translocation (made possible by site-directed mutation techniques) coupled with MspA’s natural properties of a narrow and short constriction signifies a real potential as a front-runner in third-generation nanopore DNA sequencing technology.
4. Aerolysin
Reigning from Aeromonas hydrophila, aerolysin was first proposed as a stochastic biological nanopore sensor for peptide detection in 2006.32 Shaped much like the well-known αHL nanopore, aerolysin has a mushroom-shaped morphology with a much less pronounced vestibule (Table 1).33 The constriction point of this heptameric, pore-forming toxin is confined to the transmembrane β-barrel with a diameter of approximately 1.0–1.7 nm.34 Aerolysin has been used to study chimeric molecules, proteins, peptides, DNA, and different size linear polyethylene glycols (PEGs) and oligosaccharide.35 Under a wide range of pH conditions, Long et al.36 demonstrates aerolysin’s ability to discriminate between various length nucleic acids, reflecting potential for genetic applications. One drawback is that long, single-stranded (ss) DNA polymers had difficulty being snared by the narrow vestibule and, consequently, a lower analyte event-frequency could be observed.37
5. ClyA
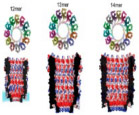
Cytolysin A (ClyA) is a homododecameric, barrel-shaped protein channel that is 13 nm in length, approximately 2.0–4.0 nm in diameter, and is secreted in monomer form by Escherichia coli.38,39 Compared to other biological nanopores, the wide pore diameter of ClyA allows for the translocation studies of dsDNA, peptides, and proteins; although, the negatively charged pore lumen requires high ionic strength solutions for DNA translocation.39 Folded proteins as large as 40 kDa have been successfully detected with ClyA.38 Interestingly, different oligomeric variants of ClyA exist (i.e., types I, II, and III). These three pore types have been successfully isolated and applied for capturing different size analytes. Type I consists of a homo-12mer; type II a homo-13mer; and type III, a homo-14mer (Table 1).40 The diameter of the pore depends on the number of subunits involved. The pore also displays asymmetrical ion conductance rectification similar to that of αHL and other charged mushroom-shaped pores. Soskine et al. showed that a ~ 10% variation in pore diameter (caused by different numbers of subunits involved) can greatly influence accurate recognition of target analytes.40
6. FhuA
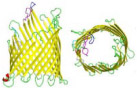
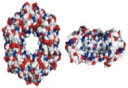
Ferric hydroxamate uptake component A (FhuA) is a monomeric outer membrane protein pore endemic to E. coli and consists of a 714 amino acid sequence. The pore assumes a β-barrel protein sheet conformation—produced from one long peptide that folds back-and-forth on itself to produce 22 anti-parallel β-strands—yielding a flat sheet and wraps end-to-end to form one large cylindrical pore.41 FhuA as a biosensor has an ovular shape with a narrow diameter of 3.1 nm, width of 4.4 nm, height of ~ 6.5 nm and is almost entirely embedded within the PLB (Table 1).42 In nature, FhuA partakes as a dual function iron receptor and antibiotic transporter.41 Not much research exists on this pore, but it demonstrates potential for versatile sensing capabilities in genetics, proteomics, and metabolomics.
7. SP1
Stable protein (SP1) is a recently developed biological nanopore lacking the mushroom-shaped conformation commonly contained by others. SP1 is composed of 12 homomonomeric subunits arranged in a ringlike shape with a 3 nm diameter hole, embedded entirely within the PLB (Table 1).43 The experimentation by Long et al. has demonstrated an ability to deposit gold, silver, and copper nanoparticles onto the SP1 surface in lieu of direct amino acid modification for enhanced pore sensitivity and analyte detection.44 The doughnut-shaped SP1 pore is a site-direction engineered protein pore with enhanced thermal stability (temperature of 107°C).2 The enhanced thermostability conflated with resistance to protease activity renders SP1 an ideal biological nanopore for experimentation in harsh chemical and physical environments.
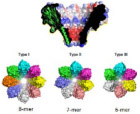
8. FraC
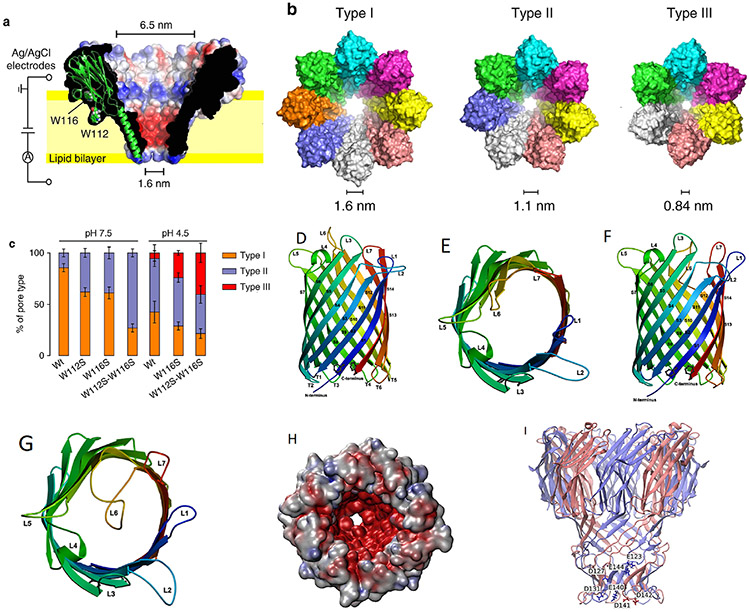
Wild-type Fragaceatoxin C (FraC), a biological transmembrane nanochannel from the sea anemone Actinia fragacea, is a 1.6 nm diameter, flower-shaped homooctameric protein. FraC nanopore has demonstrated the ability to form pH-dependent oligomeric variants, similar to the type I, II, and III oligomeric variants of ClyA protein nanopore.45 Demonstrated in Fig. 4(a), decreasing pH of the FraC working solution decreases its ability to form octameric nanopores; consequently, smaller oligomeric variants are observed, with constrictions narrowing to 1.1 nm for type II variants and 0.84 nm for type III (Table 1). Asymmetric current-voltage (I-V) rectification can also be observed in Fig. 2(c). FraC has demonstrated capabilities in resolving small organic molecules, protein, and peptide biomarkers.14 Interestingly, 1.2 nm diameter genetically engineered FraC has shown potential in processing both ssDNA and dsDNA.46 FraC nanopores as biosensors can be used to study DNA, RNA, protein conformations, and small molecule metabolites.
FIG. 4:
(a) A cut through wild-type FraC (Wt-FraC) oligomer colored according to the vacuum electrostatic potential calculated by PyMOL, (b) Molecular models of the three type FraC nanopores constructed from the FraC crystal structure using the symmetrical docking function of Rosetta, and (c) the percentage of distribution of type I, II, and III for Wt-FraC, W112S-FraC, W116S-FraC, and W112S-W116S-FraC at pH 7.5 and 4.5. (Reprinted with permission from Nature Publishing Group, Copyright 2019.45). (d–g) Overall structure of OmpG showing the 14-stranded, antiparallel β-barrel in the open (d, e) and closed (f, g) conformation viewed along the membrane (d, f) and from the extracellular side (e, g). The β-strands S1–S14 are starting from the N terminus to the C terminus on the periplasmic side, where adjacent strands are connected by short turns T1–T6. Loops L1–L7 extend outward into the extracellular space in the open conformation, but L6 folds across the barrel entrance in the closed conformation. (Reprinted with permission from EMBO Press, Copyright 2006.51). (h, i) The modeled structure of the N. farcinica porin A (NfpA) and N. farcinica porin B (NfpB) subunits based on multiple templates of MspA from M. smegmatis. Negatively charged amino acid residues are clustered in the channel, resulting in high charge density. (Reprinted with permission from ACS publications, Copyright 2006.61)
9. OmpG
Outer membrane protein G (OmpG) is a monomeric biological nanopore, similar to the shape described for FhuA, endemic to the outer membrane of E. coli, and has been demonstrated to toggle between an open and closed state in a pH-dependent manner.47 An appeal of the OmpG nanopore is its simplistic monomeric form (Table 1), not requiring heteromeric assembly into the larger oligomeric variants we see with ClyA and FraC.48 Simultaneously, a drawback to this simple nanopore is the already mentioned flickering between open and closed states, which can be negated through chemical modification by disulfide cross-linkage,47 loop-lipid pinning via alkylation,49 and tertiary structure loop shortening via residue deletion.50 The OmpG nanopore has an observed diameter of 2–2.9 nm,50,51 making it an ideal candidate for ssDNA, peptides, and small proteins. The pore is characterized by forming a flat sheet (paperlike) 14-stranded, transmembrane β-barrel bearing seven distinct, large, periplasmically oriented loops, identified as L1–L7.51 Referring to Figs. 4(d)-4(g), it becomes evident that the pH-dependent flickering between an open and closed state depends on the conformation of L6 occupying the pore lumen or not. When L6 occupies the lumen, analytes are blocked from translocation, defining the closed state.
10. Lysenin
Lysenin is a 3.0 nm diameter, homononameric protein nanochannel secreted in monomer form and extracted from the coelomic fluid of the annelid (segmented worm) Eisenia fetida (Table 1).52 Lysenin monomers have a sphingomyelin (SM) binding domain, which causes monomers to attach to the SM surface moiety of lipid bilayers, specifically, and is then followed by oligomerization and insertion.53 Lysenin has DNA, peptide, and protein resolving potential and has been shown to stochastically sense the hormone peptide biomarker angiotensin II.54 Evidence suggests the insertion of stable lysenin nanochannels depends heavily on pH, where lower pH environments (< 6) favor stable lysenin nanopore formation.55
11. CsgG
CsgG, colloquially “curli.” is a functional outer membrane amyloid commonly secreted by a large variety of bacteria but most commonly studied in E. Coli. The pore contains a 1.2 nm diameter constriction and nine identical (homononameric) subunits (Table 1).56 The Oxford Nanopore Technologies (ONT) revised minION device will supplant one variant of CsgG, identified by ONT as R7, to a more stable variant, extracted from E. coli, known as R9.57 The > 5.0 nm intra- and extracellular domains display potential for catching and trapping large, natively folded proteins and antibodies, but the 1.2 nm constriction prevents their subsequent translocation. Therefore, if translocating signals are desired, ssDNA and small organic biomolecules are ideal for this pore.58
12. NfpAB
Nocardia farcinica is a gram-positive, acid-fast, soil-dwelling, aerobic bacteria reported as the most frequent cause of nocardiosis in the United States.59 N. farcinica secretes NfpAB, the only hetero-oligomeric stochastic biosensor discussed in this paper, consisting of NfpA and NfpB subunits that assemble together within PLBs.60 Table 1 and Figs. 4(h)-4(i) contain a properly constituted NfpAB of ambiguous pore diameter. Figure 2(d) shows a I-V curve representing an asymmetric rectification of ~ 0 V, similar to the pore conductance characteristics of other vestibule bearing-biological nanopores. Peptide translocation has been demonstrated with this novel pore, leaving the door open for investigations of translocating ssDNA, dsDNA, RNA, polyelectrolytes, and small organic molecules and metabolites like antibodies and vitamins.61
13. Anthrax Toxin Pore
Anthrax, caused by Bacillus anthracis (a gram-positive, bacilli-shaped, endospore forming bacteria), primarily affects farm animals and owes its deadly tendencies to a toxic, pore-forming ensemble consisting of three proteins, none of which are toxic alone.62 Modeling has predicted the constriction of this cation-selective pore-forming toxin to be ~ 15 Å (1.5 nm) and morphologically similar to αHL with a cis-oriented vestibule and trans-oriented stem (Table 1).63 The toxin is said to have formed a homoheptameric, membrane-bound pre-pore followed by subsequent assembly of two additional protein components, lethal factor and edema factor.64 The assembly and conductivity of this pore-forming toxin is pH dependent, as the toxin is more effective in acidic environments.64 The pore has demonstrated protein translocating capabilities, and the narrow lumen suggests this pore may also be efficient in resolving ssDNA base sequences in mildly acidic conditions.
14. gA
Gramicidin A (gA) is a 1.9 kDa, ion channel, homodimeric protein secreted by rod-shaped Bacillus brevis.65 gA is an ideal nanopore for the bioanalysis of larger markers like dsDNA, proteins, and antibodies due to the ~ 4.0 nm diameter of the pore. This pore-forming protein consists of 15 hydrophobic amino acids configured into a monovalent, cation selective β-helix structure (Table 1).66 It should be noted that gA activity requires a true lipid bilayer for functional assembly as thicker lipid membranes prevent successful oligomerization.67-69
15. VDAC
The voltage-dependent anion channel (VDAC) is an abundant integral outer membrane protein of mitochondria and displays voltage-dependent gating of its β-helical 4.0 nm diameter pore (Table 1).70 At low to no voltages, the ion channel is open though at absolutely greater potentials (+/−) the channel switches to partially closed conformation, limiting analyte-conducting capabilities.71 VDAC is known to produce oligomeric variants, depending on the presence or absence of certain enzymatic cofactors (like cytochrome c). The variants range from monomer to tetramer channels, with each variant displaying different current and analyte-conducting abilities.72
16. Perforin
Perforin, a pore-forming protein and member of the membrane attack complex form pores with diameters of 3.2–5.3 nm (larger pores imply presence of more subunits) with oligomeric variants ranging in stoichiometry from four to twenty-one subunits.73 It is believed the oligomerization process follows the formation of a membrane bound pre-pore complex, which then behaves as a nucleation site for other monomers to join the growing complex.74 The advantage to these larger pore diameter variant oligomers is the possibility of monitoring larger biopolymers, such as intact proteins and antibodies. The diameter of this pore-forming complex is too wide for accurate base resolution of translocating ssDNA and ssRNA.75
17. SLO
Pore-forming toxin streptolysin O (SLO) is a 63 kDa exotoxin encoded within the SLO gene, bearing a large diameter (25.0–35.0 nm) that has been used to deliver biopolymers and drugs directly to cells through a “Trojan peptide penetration” system (Table 1).76 Counterintuitively, this large pore has been used as a DNA Coulter counter by Yasuga et al.77 Because of the narrow αHL lumen, DNA translocation occurs in a single-file manner, reflecting an application of large pores for DNA utilities other than sequencing.77 Studies suggest the presence of cholesterol influences the possibility of a pore formation.78 It is hypothesized that 50–80 homomonomeric subunits are required for functional assembly.79,80 Not much research of SLO as an applied nanopore for biosensing exists.
18. VCC
Vibrio cholera is a noninvasive pathogen, secreting one of several toxins, that behaves as a pore-forming protein.81 Vibrio cholera cytolysin (VCC), a protein product encoded by the HLYA gene of V. cholera, is an 80 kDa homoheptameric biopore that becomes activated via proteolytic 15 - residue cleavage at any one of a number of pro-toxin sites (Table 1).82,83 The very narrow 0.8 nm constriction of this pore-forming toxin is attractive as a biosensor for small metabolites and molecules whose diameters are less than that of ssDNA and ssRNA (~ 1.2 nm), which cannot be sensed.83
B. Solid State Nanopores
Although biological nanopores are very reproducible, their low stability in physiologically unfavorable conditions limits the full range of nanopore detection applications and consequently motivates researchers to look for alternative materials with which to experiment. For this reason, solid state nanopores (ssNPs) are attractive surrogates to biological nanopores. A diversely available pool of pliable solid state materials and fabrication techniques allow researchers to design biomarker specific pores. Table 2 summarizes the basic characteristics and potential ND biosensing applications of different ssNPs.
1. Silicone-Based Nanopore
Silicon-based nanopores are the most widely studied nanopore applied substrate due to their plasticity, stability, and ease in forming nanoscale pores. Nanopore fabrication techniques range from focused ion-beam (FIB) drilling, focused electron-beam drilling, ion-beam sculpting, controlled dielectric breakdown, and lithography (etching) to even size reduction through deposition. Silicon-on-insulator (SOI) wafer chips allow nanoscale pores to be milled in silicon nitride– or silicon oxide–based membranes with a high degree of pore size and shape control.84 Ion-beam sculpting coupled with low-pressure chemical vapor deposition (CVD), first attempted in 2001, has been shown to have accurate produce 1.8 nm diameter holes with DNA detection capabilities.85 Silicon dioxide nanopores have been milled in SOI wafers through field emission gunned drilling with a high-voltage transmission electron microscope (TEM) all the way down to 2 nm diameter.86 Experiments have supplanted silicon with a polyimide substrate that has been shown to enhance the signal-to-noise (S/N) ratio significantly, effectively increasing detection capabilities.87 Controlled dielectric breakdown for fabricating an array of nano-diameter pores on a silicon nitride substrate shows promise for high-throughput, cost-effective nanopore fabrication.88 Wet chemical etching in alkaline solutions of potassium hydroxide integrated with an electrochemical feedback sensor (to indicate pore formation) have produced 10–30 nm diameter holes.89 TEM images display irregularly sided pore circles as a seeming inevitability in pore mforming processes.90
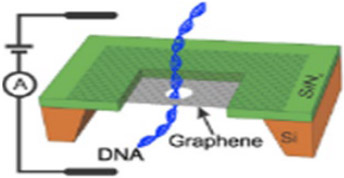
2. Graphene
Carbon is a particularly amazing atom for its ubiquity in composing the molecules of life. Carbon exists in two dominant allotropes under standard conditions: graphite and diamond. Graphene is a form of graphite in which the crystal lattice of the carbon atoms is hexagonally arranged in a honeycomb-like two-dimensional (2D) array.91 Graphene sheets are attractive for their native, atomic-scale thinness, allowing a high degree of base resolution for translocating biopolymers like DNA, which is not observed in thicker nanopore substrates, such as silicon nitride or biological nanopores, with membrane-embedded conducting lumens like αHL and other similarly structured channel forming proteins.21,86,92 Table 2 demonstrates the basic setup of a graphene sheet on a silicon-supported wafer chip. Aside from low-pressure CVD followed by TEM drilling, nanometer diameter tubes of graphene can be obtained through wrapping the sheets end-to-end, forming atomically monolayered, long graphene cylinders. In other words, single-wall carbon nanotubes.93 A review of these cylinders applied as biosensors has been covered.94
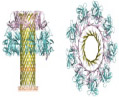
3. Aluminum Oxide
Anodic oxidation is an electrochemical process during which a porous oxide film is systematically precipitated onto the surface of a metal substrate. Aluminum oxide nanoporous materials are produced through this anodic oxidation process, which has been studied extensively.95 Anodic aluminum oxides (AAOs) as separators and sensors have garnered much attention in the past decade for their chemical and mechanical stability, homogeneity in pore size distribution, and resistance to corrosion.96 Critical factors influencing pore size and homogeneity include voltage, electrolyte composition and concentration, and temperature.97 Figure 5 demonstrates how AAO materials are fabricated [Fig. 5(b)] and their potential application as an array of biosensors on a chip [Fig. 5(a)].
FIG. 5:
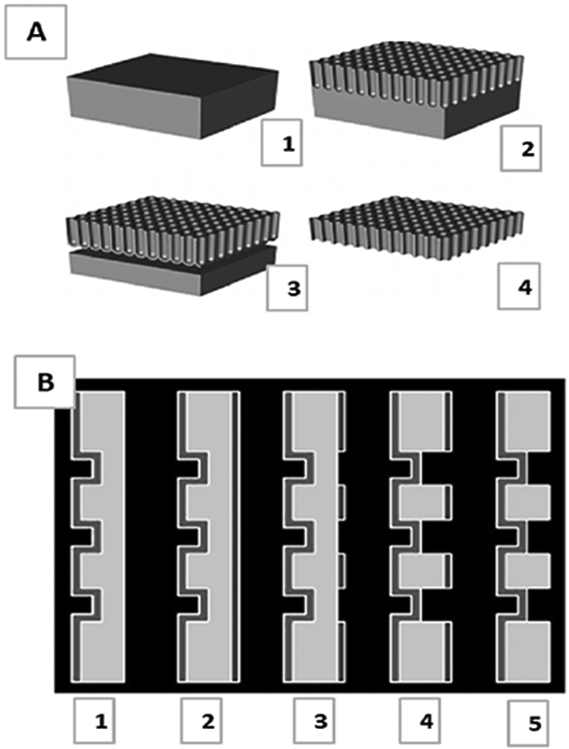
(a) Sketch of the self-supporting aluminum oxide membrane in its fully developed state: (1) Initial aluminum block worked down [(2)–(3)] to its final, free-standing state (4). (Reprinted with permission from Wiley-VCH, Copyright 2007.167); and (b) Process steps for the fabrication of mechanically stabilized aluminum oxide membranes: (1) anodization, (2) photoresist coating on back, (3) resist exposure, (4) selective aluminum etching, and (5) photoresist removal. (Reprinted with permission from Wiley-VCH, Copyright 2007.167).
4. Boron Nitride
Boron nitride (BN) is an attractive alternative to graphene. It bears a similar porous, 2D, hexagonal honeycomb-like atomic distribution of alternating boron and nitrogen atoms. The material has high temperature stability, corrosion resistance, hardy mechanical strength,98 and a lower S/N ratio than graphene.99 Amazingly, sheets of porous boron nitride material have been shown to separate gas phase hydrogen (permeable) and methane (impermeable) effectively.100 BN nanopores are generated in a similar manner to many silicon-based nanopores. First, low-pressure catalytic CVD at high temperature producing ~ 1 nm thick films and is followed with TEM drilling, producing pore diameters of 1–2 nm.101 Table 2 depicts DNA translocation through BN nanopore film.
5. Molybdenum Disulfide
Molybdenum disulfide (MoS2) nanopores have attracted attention for similar reasons as graphene and BN. CVD yields ~ 6 nm thick sheets of MoS2, which is then followed with TEM drilling, yielding ~ 5 nm diameter pores.102 MoS2 applications as solid-state biosensors are largely unexplored, and consequently, little literature exists on the topic. Table 2 depicts a simple schematic of a silicon nitride-supported molybdenum disulfide nanopore system. A greater S/N ratio for MoS2 nanopores is observed when compared to ratios of traditional silicon nitride materials, rendering MoS2 a competitive alternative.103
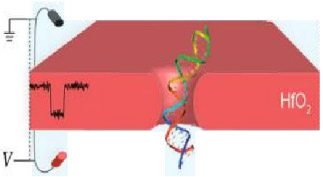
6. Hafnium Oxide
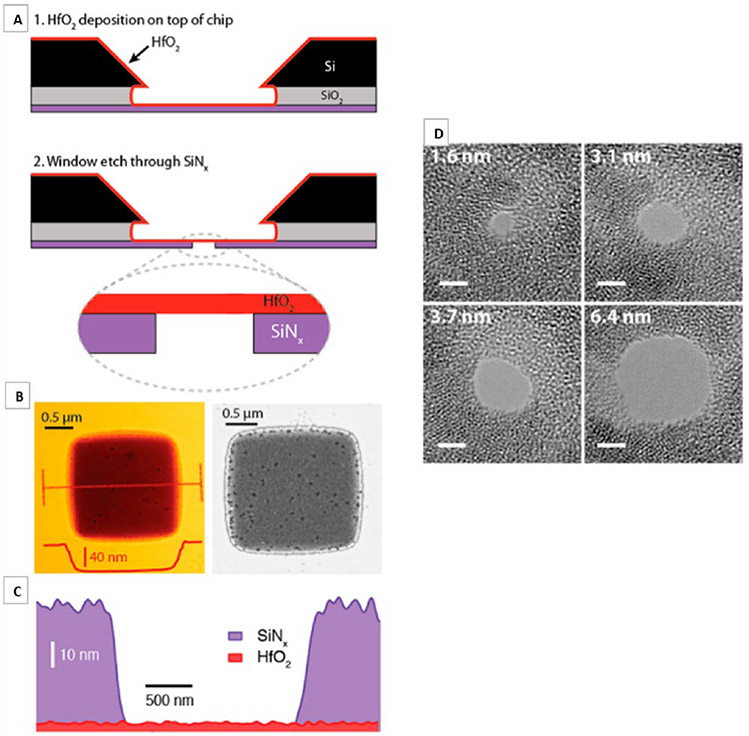
Silicon nitride materials were first studied for solid state applications due to their chemical and mechanical stability. The oxidizing nature of SiNx material in oxygen-rich environments rendered the material less optimal to use as a reliable, reproducible, chemically consistent sensor.7 Efforts in exploring materials that can supplant the oxidative nature of silicon nitride have led researchers to hafnium oxide as an attractive alternative for its simplicity in fabrication and higher resistance to oxidation.104 DNA translocations have been detected in nanopores of co-fabricated hafnium oxide-graphene sheet materials.105 Figures 6(a)-6(d) demonstrate the pore forming procedure and inconsistencies in pore circle edges, respectively, characteristic of solid state systems.
FIG. 6:
Freestanding HfO2 membrane fabrication: (a) 1–Atomic layer deposition is used to deposit a 3–8 nm thick HfO2 layer onto the trench side of a freestanding silicon nitride window, and 2–Reactive ion etching of a predefined window to expose the freestanding HfO2. (b) Atomic force microscopy (AFM) topography (left) and dark-field scanning transmission electron micrograph (right) of a freestanding HfO2 region. (c) Energy dispersive spectroscopy (EDS)-based thickness map of SiNx and HfO2 (thickness estimated from AFM) and (d) bright-field TEM images of nanopores of diameters 1.6–6.4 nm, drilled in a HfO2 membrane. (Reprinted with permission from ACS publications. Copyright 2007.7)
7. Glass (Borosilicate)
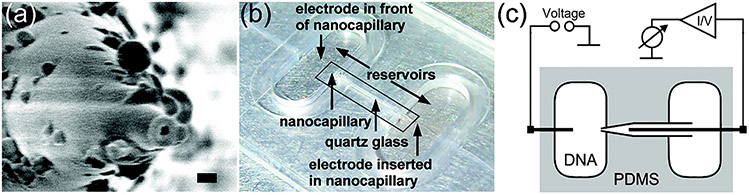
Borosilicate nanocapillaries composed have been explored as possible inexpensive Coulter counter options to the traditional and equipment intense involved fabrication techniques of ssNPs (CVD and TEM drilling). Borosilicate capillaries are pulled to nanometer diameter via a laser-equipped pipette puller and subsequently used as an electrochemical biosensor.106 Figure 7 depicts simple inclusion of the pulled capillary into the sensing chamber. By incorporation of the six-sugar, ringed, porous β-cyclodextrin, researchers were able to resolve differences in translocating enantiomers.107 A major attraction in working with glass pulled capillary tubes is the stability and speed in which the pore is formed.108 Expensive TEM, CVD, and etching equipment can be circumvented with this method. Researchers have demonstrated dsDNA event detection is possible with enhanced S/N ratios when working with borosilicate nanometer pulled capillaries,109 which leaves the door open for studying chemical variations of the pulled borosilicate along with biosensing analytes other than dsDNA.
FIG. 7:
(a) Quartz glass nanocapillary after pulling with a laser-assisted pipet puller. The nanocapillary was coated with a 10 nm thick Pt layer to prevent charge effects. The black scale bar is 100 nm long; the inner diameter of the tip is ~ 45 nm. (b) Photograph of the PDMS cell with two reservoirs that were connected to the nanocapillary. One electrode was placed in the reservoir, facing the nanocapillary. The second electrode was inserted into the capillary from the opposite reservoir. (c) Schematic of the experimental setup with sample cell, nanocapillary and measuring circuits. (Reprinted with permission from ACS publications, Copyright 2013.106)
8. Lipid-Coated ssNP
Lipid-coated substrates—a new class of ssNP—has been developed and applied to many of the abovementioned solid state systems. Fine control of solid state nanopore diameter fabrication is challenging and has potential to be overcome by integrating lipids of different sizes (degrees of saturation and chain length) into the solid state system. These lipids of various chain lengths and saturations have shown to finely influence pore diameter.110 Another advantage to a lipid-coated ssNP is the possibility of chemical modification to the lipid head in aid of analyte specific sensing, a method similar to the olfactory mechanism of lipophilic pheromones in the antennae of insects and depicted in Table 2. Aggregation of chemically sticky molecules, such as the AD associated Aβ peptide, clogs nonlipid-coated solid state pores, can be avoided by layering lipid material onto the substrate, behaving as a sort of anticoagulant toward Aβ.110 In addition to silicon nitride, lipid coatings of solid state nanopores have been applied to borosilicate pulled nanocapillaries111 and aluminum oxide.112
C. Hybrid Nanopore
This final section of nanopore systems covers the blending together of biological and solid state systems, yielding hybrids. Tables 3 and 4 summarize hybrid pore information for this section. Hybrid nanopores are unsurprisingly difficult to generate, and for this reason published research on the topic is scarce. The benefits of a functioning hybrid outperform that of either of its individual (solid state or biological) counterparts. Nanopore research giant and pioneer, Hagan Bayley, has demonstrated direction-specific electrophoretic insertion of αHL into a silicon nitride solid state membrane through attachment of a polyanionic DNA pull string to a site-directed mutation to a cysteine-residue within αHL’s β-barrel.9 Other types of hybrid nanopores have been fabricated on graphene sheets and functionalized with DNA, forming a DNA origami-graphene nanopore-conjugat,e which allows for the discrimination of nucleotide sequence for translocating DNA.113 Oxford Nanopore Technologies has recently developed a commercially available hybrid array of nanopores on a chip, known as the minION, and it has been demonstrated to successfully sequence large sequences of yeast DNA in relatively short amounts of time.114 The minION is a 90 g portable device bearing up to 2048 individually addressable hybrids, requiring an adapter enzyme tethered to one end of the analyte DNA, which ratchets the DNA-enzyme complex into the minION’s biological nanopore and helps facilitate translocation and consequential informative readout of the bound analyte.10 The process involves a DNA-enzyme complex, pore adsorption, analyte translocation, and consequent release of the once adsorbed enzyme-DNA complex, segregated into opposites sides of the partitioned chambers. There is still plenty of room for research in this most complicated area of nanopore work.
III. SUMMARY OF NANOPORES
As time continues, more and more biological pores, solid state substrates, and combinations of the two are being discovered and innovated. Site-directed mutagenesis of biological nanopores and advancing solid state fabrication techniques are expanding the possibilities of a Coulter counter, single molecule detection. Biological nanopore diameter and signal reproducibility reflect biomimetic relevance. No current fabrication technique can yield pores as small and reproducibly shaped as nature. Although nature has advantages in consistent, precise pore diameters, stability of fabricated biological nanopores cannot compare to that of solid state. Solid state nanopores pose the possibility of being easily carried into impoverished, low resource areas to monitor relevant disease prevalence. Combining the robustness of solid state membranes and reproducibility of biological nanopores, Oxford Nanopore Technologies is en route to a commercial monopoly in generating arrays of hybrid systems on-a-chip with the hopes of inventing one that is an inexpensive, sensitive, specific, lightweight biomarker detection medical device for disease monitoring. The most relevant parameter for optimal applications of analytes to nanopore sensors is the relative size of the pore to the analyte diameter. Large pore-to-analyte diameter will reflect in weak signal resolution, because translocating analytes will block a negligible fraction of conducting ions relative to the same analyte translocating a smaller pore. Ideally, pores should be just wide enough for biomarker translocation, producing high-resolution, high-quality signals. However, and although not exhaustive, Tables 1-6 list many known pores and they are paired with their detectable ND biomarker, based on relative sensing volume of the nanopore to the biomarker size – the biomarker volume must be less than the nanopore sensing volume to translocate. Analytes too large or small relative to the sensing volume of the nanopore will not produce strong signals. For example, ClyA type III (4.0 nm diameter) can translocate ssDNA (width of ~ 1.3 nm), albeit with poor signal resolution when compared to the narrower, smaller sensing volume of 1.4 nm diameter αHL.
IV. APPLICATIONS AS BIOSENSORS
We now turn our attention to the second section of this paper: discussion and detection possibilities in commonly studied neurodegenerative diseases. Because of the vastness of ND disorders, this review will be limited to discussing basic ND characteristics and their known biomarkers (genes, mRNA, protein expression, and metabolic products) of Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Huntington’s Disease (HD), Friedreich’s ataxia, Lewy body dementia (LBD), spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). A common sentiment iterated through the ensuing paragraphs is the idea of early disease detection predicated on the idea of preventative action following from the patient to ensure maximum quality of life into senior (> 65) years. Dementia describes a set of slow developing, nondisease-specific, cognitive impairment symptoms, such as memory disorder and personality changes observed in many ND pathologies, and therefore development of sensitive, specific, molecular marker detection devices like nanopores are of high interest in early diagnosis, screening, and progression monitoring. Current clinical methodologies (physical examination, PET, MRI, and CSF sampling) have potential to be complimented through incorporation of nanopore technology in patient processing. Each disorder will be discussed separately.
A. Alzheimer’s Disease
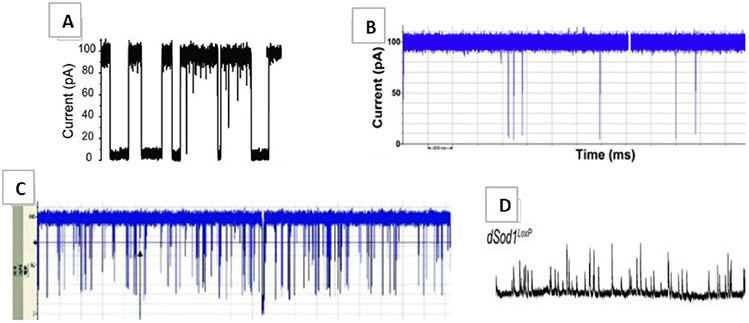
Alzheimer’s Disease, the sixth leading cause of death in the United States,115 is the most heavily studied ND disorder. Thought to be a slow developing illness, onset can be likened to sporadic (age-association) or familial (genetic-association) and is characterized by the amyloid hypothesis,116 which ties AD progression to the accumulation of amyloid plaques and neurofibrillary tangles within the brain. Amyloid precursor protein (APP), a protein thought to help neurons grow and repair, is digested by a series of protease enzymes that cleave APP into small β-amyloid (Aβ) peptide fragments of various size. The number following the abbreviation Aβ represents the number of amino acids constituting that particular Aβ fragment. Aβ isoforms 15–17 and 34–42 are naturally observed; although, Aβ42 is argued as the most abundant and toxic.117 Aggregates of Aβ clumps within the neuronal extracellular spaces and on their surfaces are identified as amyloid plaques, which can interfere with interneuron neurotransmitter communications and also produce amyloid angiopathies in the brain.118 Applications in nanopore detection of small molecule-induced oligomerization of toxic Aβ42 have been successfully demonstrated [Fig. 8(a)].15,119 The amyloid hypothesis explains only one portion of observed molecular pathophysiology; the remaining is thought to be explained by kinase hyperphosphorylation of intra-axonal microtubule-linking tau proteins. This results in tau dissociation from the microtubule followed by intracellular aggregation, disintegration of the supporting microtubule infrastructure, and ultimate cell death, forming the famous neurofibrillary tangles.120 This combination of protein-based amyloid plaques and neurofibrillary tangles makes the highly sensitive nanopore an attractive biosensor in probing for circulating AD disease indicators (biomarkers): tau and Aβ fragments.121
FIG. 8:
(a) Representative traces of Aβ42 added from the cis chamber (Reprinted with permission from ACS publications.15); (b) Typical current traces for α-Syn (Reprinted with permission from Elsevier, Copyright 2010.129); (c) Myelin basic protein translocations (Reprinted with permission from Elsevier, Copyright 2010.160); and (d) Representative traces of dSod1 (Reprinted with permission from the Society for Neuroscience, Copyright 2010.138)
B. Parkinson’s Disease
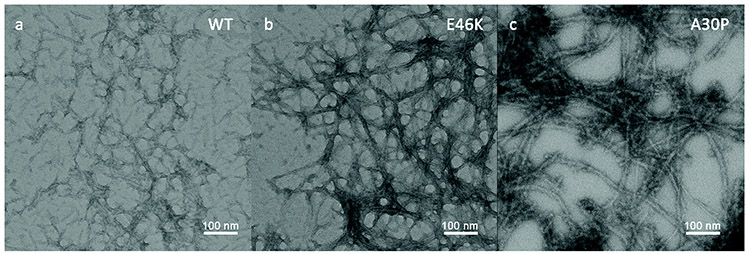
Parkinson’s disease, the second most common ND disorder to AD, is thought to be a lifelong progressing, incurable, fatal movement disorder characterized by the loss of motor dopaminergic (dopamine producing) neurons in the substantia nigra region (midbrain) via α-synuclein (α-syn) aggregation. α-Syn is a naturally occurring protein that aggregates in patients with PD into large, toxic, eosinophilic, intracellular Lewy body masses that affect ~ 1% of people > 60 and can be especially difficult to diagnose early.122 Although the exact mechanism remains ambiguous, genes PARKIN, PINK1, and alpha-synuclein seem to be associated.123 PD symptoms include some combination of rest tremor, muscle rigidity, bradykinesia, hypokinesia, stooped posture, expressionless face, no observed muscle atrophy, and awkward gait.124 Depression, dementia, and sleep disorders are affiliated with the location of brain degeneration.125 Li et al. recently demonstrated the successful label-free, single-molecule, solid state, nanopore-based, aggregation rate detection of various aggregating α-syn isoforms (WT, A30P, and E46K), thus signifying potential for highly discriminatory, biomarker-based, disease monitoring applications.126 Figure 9 reflects a 15-day old TEM image of aggregating α-syn isoforms; A30P amyloid fibrils oligomerize fastest, followed by E46K and WT isoforms, respectively. Hong et al. demonstrated correlations between α-syn and another PD associated protein biomarker DJ-1, in monitoring PD progression of +100 patients.127 Current therapies target the replacement of lost dopamine in the brain and exercise. Recent studies suggest cannabidiol may display neuroprotective propensities in PD patients.128 Nanopore shows promise in applications of high-throughput DNA sequencing of PD patient genome, detection of circulating DJ-1 and α-syn proteins [Fig. 8(b)],129 monitoring aggregation kinetics and intensity, and can act as complimenting contemporary clinical detection and monitoring practices.
FIG. 9:
TEM micrographs of three alpha-Syn samples after 15-day incubation: (a) WT, (b) E46K, and (c) A30P (Reprinted with permission from The Royal Society of Chemistry publishing, Copyright 2009.126)
C. Huntington’s Disease
Huntington’s Disease, first described in 1872 by physician George Huntington, is characterized by the autosomal recessive inheritance of trinucleotide repeat (CAG) of chromosome 4130 and when translated yields a defective, intracellularly aggregating Huntington protein in the basal ganglia (midbrain, similar, in ways, to PD). This defect promotes localized neurodegeneration.131 HD is described by a set of symptoms known as the HD triad: chorea (involuntary body movements), cognition, and behavior changes.132 Patients tend to be disproportionately affected by one symptom or another, making an accurate, early diagnosis through current methods very difficult. The severity of Huntington gene mutation (characterized by quantity of CAG trinucleotide repeats) promotes more rapid aggregation of defective Huntington protein and consequently correlates with the relative onset time in a patient’s life (more severe mutations result in earlier onset). Symptoms of this incurable, fatal disorder begin to emerge, typically, in middle age and are characterized, again, by impaired cognition, mood, balance, muscle rigidity, depression, and sleep disorder.133 The amount of CAG repeats, and its consequential protein product renders studying HD with nanopore-based assays widely applicable. High-throughput DNA sequencing for mutated genes and probing body fluids for quantities of circulating, mutated Huntington protein or its metabolic products are well within the scope of nanopore-based biosensing.
D. Amyotrophic Lateral Sclerosis
Famously known as Lou Gehrig’s (or motor neuron) disease, amyotrophic lateral sclerosis (ALS) is symptomatically characterized by uncontrolled muscle spasm, chorea, weakening and atrophy of muscles, subcutaneous fasciculation, dysarthria (difficulty speaking), and respiratory failure, which commonly leads to death.134 ALS onset observed in adulthood begins with weakening in one place of the body, typically the distal extremities (hands, feet, or muscles of the face), and progresses proximally toward the trunk.135 Because of the ambiguity of the symptoms in early stages, diagnosis takes about one year from observation of the first symptom and is typically fatal three to five years postdiagnosis.136 Upper motor neurons, whose soma reside in the brain, synapse with lower motor neurons, whose soma reside in the spinal cord, synapse with distant effector muscles. Accumulating intracellular mutant superoxide dismutase (SOD1) protein aggregates of upper and lower motor neurons inevitably cause cell death, resulting in effector muscles undergoing atrophy [due to their loss in connection to, and stimulation from, the central nervous system (CNS)].137,138 Other causes have been proposed.139 ALS progresses as a result of continued motor neuron death, consequently resulting in accumulating muscle atrophy and systemic patient weakening. Nanopore applications in ALS require more research and can include DNA sequencing and SOD1 [Fig. 8(d)] conformation and concentration monitoring as well as in vitro monitoring of drug-induced changes.
E. Spinal Muscular Atrophy
Although the ND genetic disorder spinal muscular atrophy bears strikingly similar symptomatic manifestations to ALS, differing genetic etiology and childhood onset sets SMA apart from ALS.140 Different types of SMA have been identified: 1a and 1b onset before birth or right after, respectively, and are fatal, while types 2–4 are progressively milder and onset occurs later in life.141,142 Survival motor neuron (SMN) genes, SMN1 and SMN2, are found and expressed in the cytoplasm of all animal cells and responsible for cellular maintenance via transcription regulation, telomerase regeneration, and intra-extra-cellular trafficking.143 Mutations to the SMN1 and SMN2 genes result in defective SMN protein, which is quickly degraded by intracellular proteases.144 These mutants bear the largest effect on lower motor neurons conducting signals from upper motor neurons. As mutant SMN protein concentrations decrease due to rapid degradation in lower motor neurons, cell death follows, losing connection between effector muscles and CNS, which leads to muscle weakening and the inevitable.145 This sequence of events bears a remarkable resemblance to ALS progression in the middle-aged. Nanopore applications for SMA include high-throughput DNA sequencing and monitoring of SMN1 and SMN2 genes, mRNA expression levels, and detecting circulating SMN1 and 2 mutant proteins.
F. Lewy Body Dementia
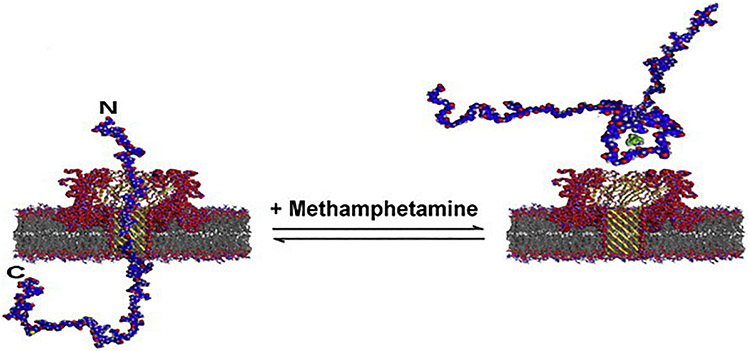
Lewy body dementia represents a spectrum of disorders encapsulating symptoms and treatments synonymous with both AD and PD, listed earlier.146-148 Although the molecular pathophysiology of LBD is not entirely understood, the presence of α-syn protein aggregates, already mentioned, in different brain regions is observed upon autopsy.149,150 Researchers have demonstrated nanopore protein aggregation probing capabilities for aggregating α-syn,151 similar to work done on Aβ42 aggregation by Wang et al.15 Conformational changes of α-syn induced by the presence of small molecule methamphetamine were also detected via nanopore.151 Figure 10 represents two different conformations for α-syn due to the presence or absence of a small molecule (methamphetamine). These conformations yield distinguishable nanopore signals as they interact with the αHL biological channel, α-syn abnormalities are useful nanopore biomarkers to probe for.
FIG. 10:
Full length alphaS can readily translocate the alpha hemolysin pore from the C-terminal end, which carries a net negative charge. In the presence of methamphetamine, a loop is formed that causes bumping events to be more likely. (Reprinted with permission from Elsevier, Copyright 2012.151)
G. Multiple Sclerosis
Multiple sclerosis is known as an autoimmune demyelinating ND disease afflicting the CNS with onset occurring, typically, in midlife.152 Oligodendrocyte glial processes intimately layer concentric rings of fatty insulating material (myelin, white matter) found wrapped around neuronal axons, and participates in protecting and enhancing axon conductivity.153 Gray matter is characterized by the absence of myelin. The demyelinating process occurs through enhanced T-cell degradative activity of myelin and leaves behind sclera (scars, plaque) of gray matter.154 Environmental as well as genetic factors seem to play a role in plaque accumulation over time where symptoms can be characterized by Charcot’s neurologic triad: nystagmus (involuntary eye movement), dysarthria (impaired speech), and chorea (tremor, spasm, ataxia, and in serious cases, paralysis).155 As plaque accumulation broadens to autonomic and sensory neurons, loss in control of bowel and bladder, sexual sensation and arousal, and sensations of pins and needles in the limbs is reported observed.156 HLA-DR2 genes seem to have some correlation with MS onset.157 MS is monitored and detected through CSF analysis for autoantibodies, MRI neuroimaging for plaques, and physical examination. Treatments involve steroids, immunosuppressants, plasmapheresis, physical therapy, and vitamin D.158 Researchers have demonstrated nanopore sensing capabilities of autoantibodies associated with MS proliferation159 and the detection of myelin basic protein—a protein critical for the development and stability of central nervous system myelin [Fig. 8(c)].160 If the antibodies are found to circulate in blood, immunoprecipitation followed by ultrasensitive nanopore analysis can allow doctors to circumvent invasive and dangerous lumbar punctures.
H. Friedreich’s Ataxia
Friedreich’s ataxia is an autosomal recessive genetic disorder inherited on chromosome 9 and codes for translation of the frataxin protein.161 Deficiency in frataxin, a 210 amino acid protein typically expressed in high metabolic tissues like heart, liver, and skeletal muscle,162 leads to mitochondrial impairment and negatively impacts nerves of the spinal cord controlling muscle movements in the arms and legs.163 FRDA presents itself in early childhood as kyphoscoliosis [spine curvature in both sagittal (kyphosis) and frontal (scoliosis) planes], staggering gait, ataxia, nystagmus, muscle weakness, dysarthria, claw-foot, hammertoe, heart palpitations, and hypertrophic cardiomyopathy.162 Nanopore applications in biosensing of FRDA include searching for trinucleotide repeats on chromosome 9 while sequencing DNA164 and monitoring circulating frataxin levels in patient body fluids.
V. SUMMARY OF APPLICATIONS IN NEURODEGENERATIVE DISEASES
Human life expectancy rises with technological advancements, necessitating ND disease detecting equipment. Early detection of ND pathologies has proven to be a monumental task. Most ND disorders are detected in the clinical stage, long after irreversible molecular pathophysiology onset. Undetected, accumulated damage at the cellular and molecular levels renders disease regression nearly impossible. The demand for inexpensive, fast, specific, sensitive, point-of-care diagnostic tools for early biomarker screening is on the rise. It is believed that if at-risk patients are aware of their proclivity toward ND decay early in life, simple preventative action can be taken well before one becomes symptomatic. As of now, with no current cures and few drugs that can halt progression, preventative care in circumventing ND disintegration is a patient’s best chance at avoiding sporadic onset. Advancements in biomedical nanotechnologies are affording clinicians high through-put, ultrasensitive instruments at arm’s reach and pose the potential for supplanting or complimenting larger, more expensive, invasive, uncomfortable, traditionally used PET, MRI, and CSF sampling techniques.
TABLE 5:
Paired biological pores with detectable analytes
| Protein pore | Potential disease and related biomarker applications |
|---|---|
| αHL |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| MspA |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Phi29 |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| Aerolysin |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| ClyA - I, II, III |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| FhuA |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| SP1 |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| FraC - I, II, III |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| OmpG |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Lysenin |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| CsgG |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJI HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| NfpAB |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| Anthrax Toxin Pore |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
| gA |
AD: gene products - β isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| VDAC |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| Perforin |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| Streptolysin O |
AD: gene products - Aβ isoforms, Tau PD: gene products - alpha-syn, DJ1 HD: gene product - mutant Huntingtin protein ALS: gene product - mutant SOD1 protein SMA: gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: CNS targeted autoantibodies FRDA: gene product - Frataxin mutant protein |
| VCC |
AD: gene - APOE chromosome 19; gene products - Aβ isoforms, Tau PD: gene - PARKIN, PINK1, alpha-synuclein; gene product - alpha-syn, DJ1 HD: gene - HTT trinucleotide repeat (CAG) chromosome 4; gene product - mutant HTT protein ALS: gene - mutant superoxide dismutase 1 (SOD1); gene product - SOD1 protein SMA: gene - SMN1 and SMN2 mutants; gene product - SMN1 and SMN2 mutant proteins LBD: similar to PD MS: gene - HLA-DR2; CNS targeted autoantibodies FRDA: gene - Frataxin trinucleotide repeat (GAA); gene product - Frataxin mutant protein |
REFERENCES
- 1.Haque F, Li J, Wu HC, Liang XJ, Guo P. Solid-state and biological nanopore for real-time sensing of single chemical and sequencing of DNA. Nano Today. 2013;8(1):56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ying YL, Cao C, Long YT. Single molecule analysis by biological nanopore sensors. Analyst. 2014;139(16):3826–35. [DOI] [PubMed] [Google Scholar]
- 3.Gouaux JE, Braha O, Hobaugh MR, Song L, Cheley S, Shustak C, Bayley H. Subunit stoichiometry of staphylococcal alpha-hemolysin in crystals and on membranes: A heptameric transmembrane pore. Proc Natl Acad Sci U S A. 2006;91(26):12828–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vesper SJ, Magnuson ML, Dearborn DG, Yike I, Haugland RA. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect Immun. 2001;69(2):912–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aksimentiev A, Schulten K. Imaging α-hemolysin with molecular dynamics: Ionic conductance, osmotic permeability, and the electrostatic potential map. Biophys J. 2005;88:3745–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storm AJ, Zandbergen H, Chen J, Joanny J-F, Storm C, Dekker C. Fast DNA translocation through a solid-state nanopore. Nano Lett. 2005;5(7):1193–7. [DOI] [PubMed] [Google Scholar]
- 7.Henley R, Rosenstein JK, Wanunu M, Larkin J, Cohen-Karni T, Bell DC. Slow DNA transport through nanopores in hafnium oxide membranes. ACS Nano. 2013;7(11):10121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Z, Zhang D, Cui W, Zhang H, Pang W, Duan X. Fabrications, applications and challenges of solid-state nanopores: A mini review. Nanomater Nanotechnol [Internet]. 2016;6(35):1–12. Available from: http://journals.sagepub.com/doi/10.5772/64015. [Google Scholar]
- 9.Hall AR, Scott A, Rotem D, Mehta KK, Bayley H, Dekker C. Hybrid pore formation by directed insertion of α-haemolysin into solid-state nanopores. Nat Nanotechnol [Internet]. 2010;5(12):874–7. Available from: 10.1038/nnano.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain M, Olsen HE, Paten B, Akeson M. The Oxford Nanopore MinION: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016;17(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howorka S, Cheley S, Bayley H. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat Biotechnol. 2001;19(7):636–9. [DOI] [PubMed] [Google Scholar]
- 12.Garalde DR, Snell EA, Jachimowicz D, Sipos B, Lloyd JH, Bruce M, Pantic N, Admassu T, James P, Warland A, Jordan M, Ciccone J, Serra S, Keenan J, Martin S, McNeill L, Wallace EJ, Jayasinghe L, Wright C, Blasco J, Young S, Brocklebank D, Juul S, Clarke J, Heron AJ, Turner D. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods. 2018;15(3):201–6. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J, Sloman L, He Z, Aksimentiev A. Graphene nanopores for protein sequencing. Adv Funct Mater. 2016;26(27):4830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang G, Willems K, Soskine M, Wloka C, Maglia G. Electro-osmotic capture and ionic discrimination of peptide and protein biomarkers with FraC nanopores. Nat Commun. 2017;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HY, Ying YL, Li Y, Kraatz HB, Long YT. Nanopore analysis of β-amyloid peptide aggregation transition induced by small molecules. Anal Chem. 2011;83(5):1746–52. [DOI] [PubMed] [Google Scholar]
- 16.DeKosky Steven T., Marek Kenneth. Looking backward to move forward: Early detection of neurodegenerative disorders. Brain Dis [Internet]. 2003;44(195):1053–982. Available from: http://www.scual.sld.cu/documentos_pdf/Biomarkers-DeKoskyDic2003.pdf. [DOI] [PubMed] [Google Scholar]
- 17.Norris AL, Workman RE, Fan Y, Eshleman JR, Timp W. Nanopore sequencing detects structural variants in cancer. Cancer Biol Ther [Internet]. 2016;17(3):246–53. Available from: 10.1080/15384047.2016.1139236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaugler J, James B, Johnson T, Scholz K, Weuve J. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016;12(4):459–509. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL. Yanagisawa K. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249–54. [DOI] [PubMed] [Google Scholar]
- 20.Noskov SY, Im W, Roux B. Ion permeation through the α-hemolysin channel: Theoretical studies based on Brownian dynamics and Poisson-Nernst-Plank electrodiffusion theory. Biophys J. 2004;87(4):2299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouaux JE, Song L, Cheley S, Bayley H, Hobaugh MR, Shustak C. Structure of Staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274(5294):1859–65. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman GM. The hemolysins of staphylococcus aureus. Bacteriol Rev [Internet]. 1975;39(4):317–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1108866%0A; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC408339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosentino K, Ros U, García-Sáez AJ. Assembling the puzzle: Oligomerization of α-pore forming proteins in membranes. Biochim Biophys Acta - Biomembr. 2016;1858(3):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz N, Kobayashi T. Assemblies of pore-forming toxins visualized by atomic force microscopy. Biochim Biophys Acta - Biomembr. 2016;1858(3):500–11. [DOI] [PubMed] [Google Scholar]
- 25.Rachakonda V, Pan TH, Le WD. Biomarkers of neurodegenerative disorders: How good are they? Cell Res [Internet]. 2004;14(5):347–58. Available from: http://www.embase.com/search/results?subaction=viewrecord&-from=export&id=L39703823. [DOI] [PubMed] [Google Scholar]
- 26.Misakian M, Kasianowicz JJ. Electrostatic influence on ion transport through the αHL channel. J Membr Biol. 2003;195(3):137–46. [DOI] [PubMed] [Google Scholar]
- 27.Gibrat G, Pastoriza-Gallego M, Thiebot B, Breton MF, Auvray L, Pelta J. Polyelectrolyte entry and transport through an asymmetric α-hemolysin channel. J Phys Chem B. 2008;112(47):14687–91. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Haque F, Rychahou PG, Evers MB, Guo P. Engineered nanopore of Phi29 DNA-packaging motor for real-time detection of single colon cancer specific antibody in serum. ACS Nano. 2013;7(11):9814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunn J, Smithrud D, Guo P, Fang H, Haque F. Real-time sensing and discrimination of single chemicals using the channel of Phi29 DNA packaging nanomotor. ACS Nano. 2012;6(4):3251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derrington IM, Butler TZ, Collins MD, Manrao E, Pavlenok M, Niederweis M, Gundlach JH. Nanopore DNA sequencing with MspA. Proc Natl Acad Sci U S A. 2010;107(37):16060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahfoud M, Sukumaran S, Hülsmann P, Grieger K, Niederweis M. Topology of the porin MspA in the outer membrane of Mycobacterium smegmatis. J Biol Chem. 2006;281(9):5908–15. [DOI] [PubMed] [Google Scholar]
- 32.Howard P, Lee JS, Stefureac R, Kraatz H-B, Long Y. Transport of α-helical peptides through α-hemolysin and aerolysin pores. Biochemistry. 2006;45(30):9172–9. [DOI] [PubMed] [Google Scholar]
- 33.Podobnik M, Kisovec M, Anderluh G. Molecular mechanism of pore formation by aerolysin-like proteins. Philos Trans R Soc Lond B Biol Sci. 2017;372(1726):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao C, Ying YL, Hu ZL, Liao DF, Tian H, Long YT. Discrimination of oligonucleotides of different lengths with a wild-type aerolysin nanopore. Nat Nanotechnol [Internet]. 2016;ll(8):713–8. Available from: 10.1038/nnano.2016.66. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Gu LQ, Tian K. The aerolysin nanopore: From peptidomic to genomic applications. Nanoscale. 2018;10(29):13857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao C, Long YT. Biological nanopores: Confined spaces for electrochemical single-molecule analysis. Acc Chem Res. 2018;51(2):331–41. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Wang YQ, Li MY, Ying YL, Long YT. Direct sensing of single native RNA with a single-biomolecule interface of aerolysin nanopore. Langmuir. 2018;34(49):14940–5. [DOI] [PubMed] [Google Scholar]
- 38.Soskine M, Moeyaert B, Cheley S, Bayley H, Biesemans A, Maglia G. An engineered ClyA nanopore detects folded target proteins by selective external association and pore entry. Nano Lett. 2012;12(9):4895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nomidis SK, Hooyberghs J, Maglia G, Carlon E. DNA capture into the ClyA nanopore: Diffusion-limited versus reaction-limited processes. J Phys Condens Matter. 2018;30(30):1–6. [DOI] [PubMed] [Google Scholar]
- 40.Soskine M, Biesemans A, De Maeyer M, Maglia G. Tuning the size and properties of ClyA nanopores assisted by directed evolution. J Am Chem Soc. 2013;135(36):13456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niedzwiecki DJ, Mohammad MM, Movileanu L. Inspection of the engineered FhuA Δc/Δ4L protein nanopore by polymer exclusion. Biophys J. 2012;103(10):2115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammad MM, Borer PN, McPike MP, Iyer R, Howard KR, Movileanu L. Engineering a rigid protein tunnel for biomolecular detection. J Am Chem Soc. 2012;134(22):9521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HY, Li Y, Qin LX, Heyman A, Shoseyov O, Willner I, Long YT, Tian H. Single-molecule DNA detection using a novel SP1 protein nanopore. Chem Commun. 2013;49(17):1741–3. [DOI] [PubMed] [Google Scholar]
- 44.Qin LX, Li Y, Li DW, Jing C, Chen BQ, Ma W, Heyman A, Shoseyov O, Willner I, Tian H, Long YT. Electrode-position of single-metal nanoparticles on stable protein 1 membranes: Application of plasmonic sensing by single nanoparticles. Angew Chemie - Int Ed. 2012;51(1):140–4. [DOI] [PubMed] [Google Scholar]
- 45.Huang G, Voet A, Maglia G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat Commun. 2019;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wloka C, Mutter NL, Soskine M, Maglia G. Alpha-helical Fragaceatoxin C nanopore engineered for double-stranded and single-stranded nucleic acid analysis. Angew Chemie Int Ed Engl. 2016;55(40):12494–8. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Khalid S, Sansom MSP, Bayley H. Outer membrane protein G: Engineering a quiet pore for biosensing. Proc Natl Acad Sci U S A. 2008;105(17):6272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanganna Gari RR, Seelheim P, Liang B, Tamm LK. Quiet outer membrane protein G (OmpG) nanopore for biosensing. ACS Sensors. 2019;4:1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fahie MA, Chen M. Electrostatic interactions between OmpG nanopore and analyte protein surface can distinguish between glycosylated isoforms. J Phys Chem B. 2015;119(32):10198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe AJ, Hsueh YC, Blanden AR, Mohammad MM, Pham B, Thakur AK, Loh SN, Chen M, Movileanu L. Interrogating detergent desolvation of nanopore-forming proteins by fluorescence polarization spectroscopy. Anal Chem. 2017;89(15):8013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yildiz Ö, Vinothkumar KR, Goswami P, Kühlbrandt W. Structure of the monomeric outer-membrane porin OmpG in the open and closed conformation. EMBO J. 2006;25(15):3702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bokori-Brown M, Martin TG, Naylor CE, Basak AK, Titball RW, Savva CG. Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein. Nat Commun. 2016;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Colibus L, Sonnen AFP, Morris KJ, Siebert CA, Abrusci P, Plitzko J, Hodnik V, Leippe M, Volpi E, Anderluh G, Gilbert RJC. Structures of lysenin reveal a shared evolutionary origin for pore-forming proteins and its mode of sphingomyelin recognition. Structure. 2012;20(9):1498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrestha N, Bryant SL, Thomas C, Richtsmeier D, Pu X, Tinker J, Fologea D. Stochastic sensing of Angiotensin II with lysenin channels. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munguira ILB, Takahashi H, Casuso I, Scheuring S. Lysenin toxin membrane insertion is pH-dependent but independent of neighboring lysenins. Biophys J. 2017;113(9):2029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goyal P, Krasteva PV, Van Gerven N, Gubellini F, Van Den Broeck I, Troupiotis-Tsaïlaki A, Jockheere W, Pehau-Arnaudet G, Pinkner JS, Chapman MR, Hultgren SJ, Howorka S, Fronzes R, Remaut H. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature. 2014;516(7530):250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu H, Giordano F, Ning Z. Oxford nanopore MinION sequencing and genome assembly. Genom Proteom Bioinf. 2016;14(5):265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Assad ON, Gilboa T, Spitzberg J, Juhasz M, Weinhold E, Meller A. Light-enhancing plasmonic-nanopore biosensor for superior single-molecule detection. Adv Mater. 2017;29(9):1–9. [DOI] [PubMed] [Google Scholar]
- 59.Budzik JM, Hosseini M, Mackinnon AC, Taxy JB. Disseminated Nocardia farcinica: Literature review and fatal outcome in an immunocompetent patient. Surg Infect (Larchmt). 2012;13(3):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt J Membrane platforms for biological nanopore sensing and sequencing. Curr Opin Biotechnol. 2016;39:17–27. [DOI] [PubMed] [Google Scholar]
- 61.Singh PR, Bárcena-Uribarri I, Modi N, Kleinekathöfer U, Benz R, Winterhalter M, Mahendran K. Pulling peptides across nanochannels: Resolving peptide binding and translocation through the hetero-oligomeric channel from nocardia farcinica. ACS Nano. 2012;6(12):10699–707. [DOI] [PubMed] [Google Scholar]
- 62.Collier RJ, Young JAT. Anthrax toxins. Rev Physiol Biochem Pharmacol. 2004;152:135–64. [DOI] [PubMed] [Google Scholar]
- 63.Krantz BA, Melnyk RA, Zhang S, Juris SJ, Lacy DB, Wu Z, Finkelstein A, Collier RJ. A phenylalanine clamp catalyzes protein A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2007;777(2005):777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krantz BA, Finkelstein A, Collier RJ. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J Mol Biol. 2006;355(5):968–79. [DOI] [PubMed] [Google Scholar]
- 65.Capone R, Blake S, Restrepo MR, Yang J, Mayer M. Designing nanosensors based on charged derivatives of gramicidin A. J Am Chem Soc. 2007;129(31):9737–45. [DOI] [PubMed] [Google Scholar]
- 66.Abou Chaaya A, Le Poitevin M, Cabello-Aguilar S, Balme S, Bechelany M, Kraszewski S, Picaud F, Cambedouzou J, Balanzat E, Janot JM, Thami T, Miele P, Dejardin P. Enhanced ionic transport mechanism by gramicidin A confined inside nanopores tuned by atomic layer deposition. J Phys Chem C. 2013;117(29):15306–15. [Google Scholar]
- 67.Del Rio Martinez JM, Zaitseva E, Petersen S, Baaken G, Behrends JC. Automated formation of lipid membrane microarrays for ionic single-molecule sensing with protein nanopores. Small. 2015;11(1):119–25. [DOI] [PubMed] [Google Scholar]
- 68.Kelkar DA, Chattopadhyay A. The gramicidin ion channel: A model membrane protein. Biochim Biophys Acta-Biomembr. 2007;1768(9):2011–25. [DOI] [PubMed] [Google Scholar]
- 69.Lepoitevin M, Ma T, Bechelany M, Janot JM, Balme S. Functionalization of single solid state nanopores to mimic biological ion channels: A review. Adv Colloid Interface Sci [Internet]. 2017;250:195–213. Available from: 10.1016/j.cis.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Mannella CA, Forte M, Colombini M. Toward the molecular structure of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1992;24(1):1988–9. [DOI] [PubMed] [Google Scholar]
- 71.Krammer EM, Homblé F, Prévost M. Concentration dependent ion selectivity in VDAC: A molecular dynamics simulation study. PLoS One. 2011;6(12):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zalk R, Israelson A, Garty ES, Azoulay-zohar H, Shoshan-barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. J Biochem Sci. 2005;83:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watanabe H, Gubbiotti A, Chinappi M, Takai N, Tsumoto K, Kawano R. Analysis of pore formation and protein translocation using large biological nanopores. Anal Chem. 2017;89:11269–77. [DOI] [PubMed] [Google Scholar]
- 74.Leung C, Hodel AW, Brennan AJ, Lukoyanova N, Tran S, House CM, Kondos SC, Whisstock JC, Dunstone MA, Trapani JA, Voskoboinik I, Saibil HR, Hoogenboom BW. Real-time visualization of perforin nanopore assembly. Nat Nanotechnol. 2017;12:467–75. [DOI] [PubMed] [Google Scholar]
- 75.Spicer BA, Conroy PJ, Law RHP, Voskoboinik I, Whisstock JC. Perforin—A key (shaped) weapon in the immunological arsenal. Semin Cell Dev Biol [Internet]. 2017;72:117–23. Available from: 10.1016/j.semcdb.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 76.Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, Bhakdi S. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci U S A. 2001;98(6):3185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yasuga H, Kawano R, Takinoue M, Tsuji Y, Osaki T, Kamiya K, Miki N, Takeuchi S. Logic gate operation by DNA translocation through biological nanopores. PLoS One. 2016;11(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagamune H, Ohkura K, Sukeno A, Cowan G, Mitchell TJ, Ito W, Ohnishi O, Hattori K, Yamato M, Hirota K, Miyake Y, Maeda T, Kourai H. The human-specific action of intermedilysin, a homolog of Streptolysin O, is dictated by domain 4 of the protein. Microbiol Immunol. 2004;48(9):677–92. [DOI] [PubMed] [Google Scholar]
- 79.Palmer M, Harris R, Freytag C, Kehoe M, Tranum-Jensen J. Assembly mechanism of the oligomeric streptolysin O pore: The early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 1998;17(6):1598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lawrence SL, Gorman MA, Feil SC, Tweten RK, Morton CJ, Parker MW, Mulhern TD, Kuiper MJ, Ratner AJ, Tweten RK. Structural basis for receptor recognition by the article structural basis for receptor recognition by the human CD59-responsive. Structure [Internet]. 2016;24(9):1488–98. Available from: 10.1016/j.str.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutierrez MG, Saka HA, Chinen I, Zoppino FCM, Yoshimori T, Bocco JL, Colombo MI. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc Natl Acad Sci U S A. 2007;104(6):1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olson R, Gouaux E. Crystal structure of the Vibrio cholerae cytolysin (VCC) pro-toxin and its assembly into a heptameric transmembrane pore. J Mol Biol. 2005;9:997–1016. [DOI] [PubMed] [Google Scholar]
- 83.De S, Olson R. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc Natl Acad Sci U S A. 2011;108(18):7385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gribov NN, Theeuwen SJCH, Caro J, Radelaar S. A new fabrication process for metallic point contacts. Microelectron Eng. 1997;35:317–20. [Google Scholar]
- 85.Li J, McMullan C, Branton D, Aziz MJ, Stein D, Golovchenko JA. Ion-beam sculpting at nanometre length scales. Nature [Internet]. 2002;412(6843):166–9. Available from: http://www.nature.com/doifinder/10.1038/35084037. [DOI] [PubMed] [Google Scholar]
- 86.Storm AJ, Chen JH, Zandbergen HW, Dekker C. Translocation of double-strand DNA through a silicon oxide nanopore. Phys Rev E. 2005;71(5):1–10. [DOI] [PubMed] [Google Scholar]
- 87.Choi W, Jeon ES, Chun KY, Kim YR, Park KB, Kim KB, Han CS. A low-noise silicon nitride nanopore device on a polymer substrate. PLoS One. 2018;13(7):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwok H, Briggs K, Tabard-Cossa V. Nanopore fabrication by controlled dielectric breakdown. PLoS One. 2014;9(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park SR, Peng H, Ling XS. Fabrication of nanopores in silicon chips using feedback chemical etching. Small. 2007;3(1):116–9. [DOI] [PubMed] [Google Scholar]
- 90.Storm AJ, Chen JH, Ling XS, Zandbergen HW, Dekker C. Fabrication of solid-state nanopores with single-nanometre precision. Nat Mater. 2003;2(8):537–40. [DOI] [PubMed] [Google Scholar]
- 91.Siwy ZS, Davenport M. Graphene opens up to DNA. Nat Nanotechnol. 2010;5(10):697–8. [DOI] [PubMed] [Google Scholar]
- 92.Merchant CA, Healy K, Wanunu M, Ray V, Peterman N, Bartel J, Fischbein MD, Venta K, Luo Z, Johnson ATC, Drndic M. DNA translocation through graphene nanopores. Nano Lett. 2010;10(8):2915–21. [DOI] [PubMed] [Google Scholar]
- 93.Dresselhaus MS, Jorio A, Hofmann M, Dresselhaus G, Saito R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010;10(3):751–8. [DOI] [PubMed] [Google Scholar]
- 94.Wang J Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis. 2005;17(1):7–14. [Google Scholar]
- 95.Tsuchiya H, Macak JM, Sieber I, Schmuki P. Self-organized high-aspect-ratio nanoporous zirconium oxides prepared by electrochemical anodization. Small. 2005;1(7):722–5. [DOI] [PubMed] [Google Scholar]
- 96.Ingham CJ, ter Maat J, de Vos WM. Where bio meets nano: The many uses for nanoporous aluminum oxide in biotechnology. Biotechnol Adv. 2012;30(5):1089–99. [DOI] [PubMed] [Google Scholar]
- 97.Santos A, Kumeria T, Losic D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal Chem. 2013;44:25–38. [Google Scholar]
- 98.Shi Y, Hamsen C, Jia X, Kim KK, Reina A, Hofmann M, Hsu AL, Zhang K, Li H, Juang ZY, Dresselhaus MS, Li LJ, Kong J. Synthesis of few-layer hexagonal boron nitride thin film by chemical vapor deposition. Nano Lett. 2010;10(10):4134–9. [DOI] [PubMed] [Google Scholar]
- 99.Liu S, Lu B, Zhao Q, Li J, Gao T, Chen Y, Zhang Y, Liu Z, Fan Z, Yang F, Liping Y, Yu D. Boron nitride nanopores: Highly sensitive DNA single-molecule detectors. Adv Mater. 2013;25(33):4549–54. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Shi Q, Liu Y, Wang Y, Meng Z, Xiao C, Deng K, Rao D, Lu R. Hexagonal boron nitride with designed nanopores as a high-efficiency membrane for separating gaseous hydrogen from methane. J Phys Chem C. 2015;119(34):19826–31. [Google Scholar]
- 101.Song L, Ci L, Lu H, Sorokin PB, Jin C, Ni J, Kvashnin AG, Kvashnin DG, Lou J, Yakobson BI, Ajayan PM. Large scale growth and characterization of atomic hexagonal boron nitride layers. Nano Lett. 2010;10(8):3209–15. [DOI] [PubMed] [Google Scholar]
- 102.Zhan Y, Liu Z, Najmaei S, Ajayan PM, Lou J. Large-area vapor-phase growth and characterization of MoS 2 atomic layers on a SiO 2 substrate. Small. 2012;8(7):966–71. [DOI] [PubMed] [Google Scholar]
- 103.Liu K, Feng J, Kis A, Radenovic A. Atomically thin molybdenum disulfide nanopores with high sensitivity for dna translocation. ACS Nano. 2014;8(3):2504–11. [DOI] [PubMed] [Google Scholar]
- 104.Wu MY, Krapf D, Zandbergen M, Zandbergen H, Batson PE. Formation of nanopores in a SiN/SiO 2 membrane with an electron beam. Appl Phys Lett [Internet]. 2005;87(11):4–7. Available from: 10.1063/1.2043247. [DOI] [Google Scholar]
- 105.Shim J, Rivera J, Bashir R. In endometrial hyperplasias, the molecular-genetics and morphometry-based EIN classification more accurately predicts cancer-progression than the WHO94. Cancer. 2005;103(11):2304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steinbock LJ, Otto O, Chimerel C, Gonall J, Keyser UF. Detecting DNA folding with nanocapillaries. Nano Lett. 2010;10(7):2493–7. [DOI] [PubMed] [Google Scholar]
- 107.Gao C, Ding S, Tan Q, Gu L-Q. Method of creating a nanopore-terminated probe for single-molecule enantiomer discrimination. Anal Chem. 2009;81(1):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.An R, Uram JD, Yusko EC, Ke K, Mayer M, Hunt AJ. Ultrafast laser fabrication of submicrometer pores in borosilicate glass. Opt Lett [Internet]. 2008;33(10):1153–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18483543%0A; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2722875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steinbock LJ, Bulushev RD, Krishnan S, Raillon C, Radenovic A. DNA translocation through low-noise glass nanopores. ACS Nano. 2013;7(12):11255–62. [DOI] [PubMed] [Google Scholar]
- 110.Yusko EC, Johnson JM, Majd S, Prangkio P, Rollings RC, Li J, Yang J, Mayer M. Controlling protein translocation through nanopores with bio-inspired fluid walls. Nat Nanotechnol. 2011;6(4):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernández-Ainsa S, Muus C, Bell NAW, Steinbock LJ, Thacker VV, Keyser UF. Lipid-coated nanocapillaries for DNA sensing. Analyst. 2013;138(1):104–6. [DOI] [PubMed] [Google Scholar]
- 112.Venkatesan BM, Polans J, Comer J, Sridhar S, Wendell D, Aksimentiev A, Bashir R. Lipid bilayer coated Al2O3 nanopore sensors: Towards a hybrid biological solid-state nanopore. Biomed Microdevices. 2011;13(4):671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Farimani AB, Dibaeinia P, Aluru NR. DNA origami-graphene hybrid nanopore for DNA detection. ACS Appl Mater Interfaces. 2017;9(1):92–100. [DOI] [PubMed] [Google Scholar]
- 114.Goodwin S, Gurtowski J, Ethe-Sayers S, Deshpande P, Schatz M, McCombie WR. Oxford Nanopore Sequencing, hybrid error correction, and de novo assembly of a eukaryotic genome. Genome Res. 2015;25:1750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heron M Deaths: Leading causes for 2013. Natl Vital Stat Reports. 2016;65(2):1–95. [PubMed] [Google Scholar]
- 116.Shaw LM, Korecka M, Clark CM, Lee VMY, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6(4):295–303. [DOI] [PubMed] [Google Scholar]
- 117.Mawuenyega KG, Kasten T, Sigurdson W, Bateman RJ. Amyloid-beta isoform metabolism quantitation by stable isotope labeled kinetics. Anal Biochem. 2013;440(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Racke MM, Boone LI, Hepburn DL, Parsadainiana M, Bryan MAT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid. J Neurosci. 2005;25(3):629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Song C, Deng P, Que L. Rapid multiplexed detection of beta-amyloid and total-tau as biomarkers for Alzheimer’s disease in cerebrospinal fluid. Nanomedicine [Internet]. 2018;14(6):1845–52. Available from: 10.1016/j.nano.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 120.Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. Molecular pathogenesis of Alzheimer’s disease: an update. Ann Neurosci [Internet]. 2017;24(1):46–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28588356%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5448443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharma N, Singh AN. Exploring biomarkers for Alzheimer’s disease. J Clin Diagn Res. 2016;10(7):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fayyad M, Salim S, Majbour N, Erskine D, Stoops E, Mollenhauer B, El-Agnaf OMA. Parkinson’s disease biomarkers based on alpha-synuclein. J Neurochem [Internet]. 2019; 1–20. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jnc.14809. [DOI] [PubMed] [Google Scholar]
- 123.Grasso M, Piscopo P, Confaloni A, Denti MA. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules. 2014;19(5):6891–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin W, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: A potential biomarker of disease status. Neurology. 2019;70:1411–8. [DOI] [PubMed] [Google Scholar]
- 125.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: Loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(6):1314–22. [DOI] [PubMed] [Google Scholar]
- 126.Li X, Tong X, Lu W, Yu D, Diao J, Zhao Q. Label-free detection of early oligomerization of α-synuclein and its mutants A30P/E46K through solid-state nanopores. Nanoscale [Internet]. 2019;11(13):6480–8. Available from: 10.1039/c9nr00023b. [DOI] [PubMed] [Google Scholar]
- 127.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montinbe TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and α-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010;133(3):713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crippa JAS, Hallak JEC, Zuardi AW, Guimarães FS, Tumas V, dos Santos RG. Is cannabidiol the ideal drug to treat non-motor Parkinson’s disease symptoms? Eur Arch Psychiatry Clin Neurosci [Internet]. 2019;269(1):121–33. Available from: 10.1007/s00406-019-00982-6. [DOI] [PubMed] [Google Scholar]
- 129.Tavassoly O, Kakish J, Nokhrin S, Dmitriev O, Lee JS. The use of nanopore analysis for discovering drugs which bind to a -synuclein for treatment of Parkinson’s disease. Eur J Med Chem [Internet]. 2014;88:42–54. Available from: 10.1016/j.ejmech.2014.07.090. [DOI] [PubMed] [Google Scholar]
- 130.Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: Insights from Huntington’s disease. Trends Neurosci [Internet]. 2010;33(11):513–23. Available from: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 131.Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5(10):958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Victorson D, Carlozzi NE, Frank S, Beaumont JL, Cheng W, Gorin B, Duh MS, Samuelson D, Tulsky D, Gutierrez S, Nowinski CJ, Mueller A, Shen V, Sung V. Identifying motor, emotional-behavioral, and cognitive deficits that comprise the triad of HD symptoms from patient, caregiver, and provider perspectives. Tremor Other Hyperkinet Mov. 2014;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: Time-dependent alterations in synaptic and receptor function. Neuroscience [Internet]. 2011;198:252–73. Available from: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conradi S, Grimby L, Lundemo G. Pathophysiology of fasciculations in ALS as studied by electromyography of single motor units. Muscle Nerve. 1982;5(3):202–8. [DOI] [PubMed] [Google Scholar]
- 135.Orrell RW, Habgood JJ, Malaspina A, Mitchell J, Greenwood J, Lane RJM, deBelleroche JS. Clinical characteristics of SOD1 gene mutations in UK families with ALS. J Neurol Sci. 1999;169:56–60. [DOI] [PubMed] [Google Scholar]
- 136.Juergens SM, Kurland LT, Okazaki H, Mulder DW. ALS in Rochester, Minnesota, 1925-1977. Neurology. 1980;30(5):463–463. [DOI] [PubMed] [Google Scholar]
- 137.Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science [Internet]. 2006;312:1389–92. Available from: http://www.sciencemag.org/content/312/5778/1389.abstract. [DOI] [PubMed] [Google Scholar]
- 138.Held XA, Major P, Sahin XA, Reenan RA, Lipscombe XD, Wharton XKA. Circuit dysfunction in SOD1 - ALS model first detected in sensory feedback prior to motor neuron degeneration is alleviated by BMP signaling. J Neurosci. 2019;39(12):2347–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci [Internet]. 2004;27(1):723–49. Available from: http://www.annualreviews.org/doi/10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 140.Kolb SJ, Kissel JT. Spinal muscular atrophy: A timely review. Arch Neurol. 2011;68(8):979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodillo E, Marini ML, Heckmatt JZ, Dubowitz V. Scoliosis in spinal muscular atrophy: Review of 63 cases. J Child Neurol. 1989;4(2):118–23. [DOI] [PubMed] [Google Scholar]
- 142.Müller B, Melki J, Burlet P, Clerget-Darpoux F. Proximal spinal muscular atrophy (SMA) types II and III in the same sibship are not caused by different alleles at the SMA locus on 5q. Am J Hum Genet. 1992;50(5):892–5. [PMC free article] [PubMed] [Google Scholar]
- 143.Burghes AHM, Beattie CE. Spinal muscular atrophy: Why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci [Internet]. 2009;10(8):597–609. Available from: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cartegni L, Hastings ML, Calarco JA, de Stanchina E, Krainer AR. Determinants of Exon 7 splicing in the spinal muscular atrophy genes, SMN1 and SMN2. Am J Hum Genet. 2005;78(1):63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glascock JJ, Osman EY, Wetz MJ, Krogman MM, Shababi M, Lorson CL. Decreasing disease severity in symptomatic, Smn −/−; SMN2 +/+, spinal muscular atrophy mice following scAAV9-SMN delivery. Hum Gene Ther 2012;23(3):330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa EG, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes parkinson and Lewy body dementia. Ann Neurol [Internet]. 2004;55(2):164–73. Available from: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 147.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufner D, Kenny RA, Korczyn A, Kosaka K, Lee VMY, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinsk EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology. 2005;65(12):1863–72. [DOI] [PubMed] [Google Scholar]
- 148.Gibb RG, Esiri MM, Lees AJ. Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia). Brain. 1985;110:1131–53. [DOI] [PubMed] [Google Scholar]
- 149.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A [Internet]. 1998;95(11):6469–73. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe DM, Mira SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Loveston S, Collerton D, Jansen ENH, Ballard C, de Vos RAI, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24. [DOI] [PubMed] [Google Scholar]
- 151.Tavassoly O, Lee JS. Methamphetamine binds to α-synuclein and causes a conformational change which can be detected by nanopore analysis. FEBS Lett [Internet]. 2012;586(19):3222–8. Available from: 10.1016/j.febslet.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 152.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D. Early onset multiple sclerosis: A longitudinal study. Neurol Sci. 2000;21:1006–10. [DOI] [PubMed] [Google Scholar]
- 153.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–73. [DOI] [PubMed] [Google Scholar]
- 154.Wingerchuk DM, Lucchinetti CF, Noseworthy JH. Multiple sclerosis: Current pathophysiological concepts. Lab Investig. 2001;81(3):263–81 [DOI] [PubMed] [Google Scholar]
- 155.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev [Internet]. 2014;13:518–24. Available from: 10.1016/j.autrev.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 156.Nortvedt MW, Riise T, Frugard J, Mohn J, Bakke A, Skar AB, Nyland H, Glad SB, Myhr KM. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler. 2007;13:106–12. [DOI] [PubMed] [Google Scholar]
- 157.Korn T Pathophysiology of multiple sclerosis. J Neurol. 2008;255:2–6. [DOI] [PubMed] [Google Scholar]
- 158.Munger KL, Ebers GC, Sadovnick AD, Veith R, O’Reilly E, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2012;63:60–5. [DOI] [PubMed] [Google Scholar]
- 159.Unser S, Bruzas I, He J, Sagle L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors. 2015;15(7):15684–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baran C, Smith GST, Bamm VV, Harauz G, Lee JS. Divalent cations induce a compaction of intrinsically disordered myelin basic protein. Biochem Biophys Res Commun [Internet]. 2010;391(1):224–9. Available from: 10.1016/j.bbrc.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 161.Koeppen AH. Friedreich’s ataxia: Pathology, pathogenesis, and molecular genetics. J Neurol Sci [Internet]. 2011;303:1–12. Available from: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vorgerd M, Schols L, Hardt C, Ristow M, Epplen JT, Zange J. Mitochondrial impairment of human muscle in Friedreich ataxia in vivo. Neuromuscul Disord. 2000;10(6):430–5. [DOI] [PubMed] [Google Scholar]
- 163.Patel PI, Isaya G. Friedreich Ataxia: From GAA triplet–repeat expansion to frataxin deficiency. Am J Hum Genet. 2001;69:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McGinty RJ, Rubinstein RG, Neil AJ, Dominska M, Kiktev D, Petes TD, Mirkin SM. Nanopore sequencing of complex genomic rearrangements in yeast reveals mechanisms of repeat-mediated double-strand break repair. Genome Res. 2017;27(12):2072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Menestrina G Ionic channels formed by Staphylococcus aureus alpha-toxin: Voltage-dependent inhibition by divalent and trivalent cations. J Membrain Biol. 1986;90:177–90. [DOI] [PubMed] [Google Scholar]
- 166.Miles G, Bayley H, Cheley S. Properties of Bacillus cereus hemolysin II: A heptameric transmembrane pore. Protein Sci. 2002;11:1813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thormann A, Teuscher N, Pfannmöller M, Rothe U, Heilmann A. Nanoporous aluminum oxide membranes for filtration and biofunctionalization, Small, 2007;3(6):1032–40. [DOI] [PubMed] [Google Scholar]