Unicellular organisms and epithelial cells from animals and plants must adapt to abrupt changes in their environment. Similarly, cells bathed in the protective milieu intérieur of multicellular organisms must monitor their intracellular nutrient and energy levels to control and adapt key cellular processes such as growth, proliferation, autophagy, and transcriptional programs. To perform these adaptations, cells have evolved a capacity to sense nutrients and induce signaling cascades from the nutrient sensors. Some sensors exist in soluble form within the cytosol while others sit in the plasma membrane and intracellular membranes (1). Early studies in yeast identified several nutrient sensors among membrane transporters or transporter-like proteins rather than bona fide receptors (2, 3), leading to the hybrid concept of “transceptor”: a transporter that has evolved to interact with signaling proteins in a substrate-dependent manner (4). In PNAS, Talaia et al. (5) characterize such transceptor interaction between a lysosomal amino acid transporter and a signaling complex implicated in two neurodegenerative diseases, amyotrophic lateral sclerosis and frontotemporal dementia. Their study unveils a plug-and-socket mechanism where a flexible protruding loop of the complex plugs into the central cavity of the transporter, suggesting a mechanism for the interplay between amino acid transport and amino acid signaling. Interestingly, this mechanism bears some resemblance with the interaction between another lysosomal amino acid transporter and a signaling complex intervening in the mechanistic target of rapamycin complex 1 (mTORC1) pathway elucidated in very recent studies (6, 7).
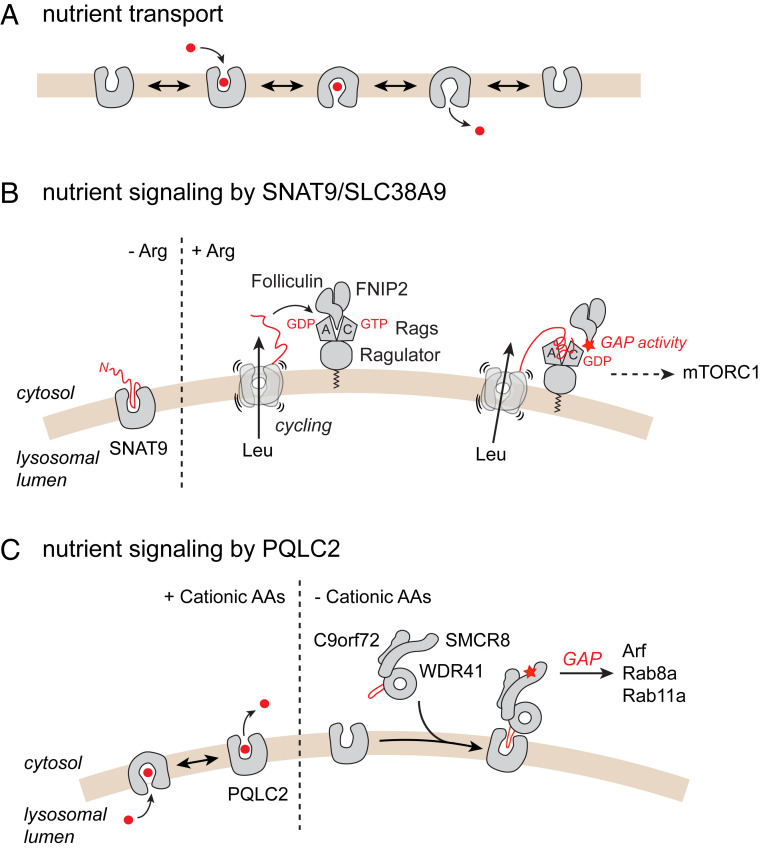
The transceptor concept is based on a key property of membrane transporters: To translocate small molecules or ions across biological membranes, these proteins must undergo structural changes that alternately expose their substrate-binding site to one or the other side of the membrane (Fig. 1A). This alternating-access mechanism allows translocating nutrients and waste products “uphill” against their transmembrane concentration gradient by coupling this translocation to the cotransport or exchange of an ion pumped by an ion-translocating ATPase. If a specific conformation of the transporter interacts with a signaling protein and activates a signaling cascade, the transporter becomes a transceptor. The protein might eventually lose its transport activity during evolution while conserving its signaling function if it cannot visit all conformations of the alternating-access cycle.
Fig. 1.
Nutrient sensing by lysosomal amino acid transceptors. (A) Alternating-access conformational cycle of a canonical transporter. (B) Arginine-sensing and signaling by the lysosomal amino acid transporter SNAT9/SLC38A9. (C) Cationic amino acid sensing by the lysosomal transporter PQLC2. In both mechanisms (B and C), a short peptide from the transporter itself (SNAT9) or from its cognate signaling protein (WDR41) plugs into the central cavity of the transporter, thus linking the transport and signaling activities. See main text for mechanistic details.
The transceptor concept attracted attention since it was put forward (4), and several nutrient transceptors have been established or suggested in microorganisms, plants, and animals. As the translocated nutrient or its driving ion(s) may have a downstream impact on signaling cascades (see ref. 8 for amino acid-sensing by the yeast transporter Gap1), it is essential to demonstrate a physical interaction between the transporter and a component of the signaling cascade, and the dependence of this interaction on the conformational state of the transporter to establish a genuine transceptor mechanism.
The study of the lysosomal amino acid transporter PQ-loop repeat containing protein 2 (PQLC2) by Talaia et al. (5) and recent studies involving another lysosomal transporter (6, 7) unveiled such transceptor mechanisms in the membrane of an intracellular organelle, the lysosome.
Nutrient Sensing by mTORC1 at the Lysosomal Surface
Lysosomes are membrane-bound hydrolytic organelles responsible for the degradation and recycling of cell and tissue components. They also constitute signaling hubs that integrate environmental and intracellular cues, including intracellular amino acids, to coordinate cell growth and metabolism. Amino acids trigger the recruitment of mTORC1 to the lysosomal surface, where it is activated by the small GTPase Ras homolog enriched in brain (Rheb) in response to energy status and growth factors (1, 9). Several amino acid sensors specific for leucine, arginine, or S-adenosylmethionine are involved in this response (10). Among them, the amino acid transporter SNAT9, also known by its gene name SLC38A9, stands out by its localization in the lysosomal membrane and its ability to sense intralysosomal arginine. SNAT9 has a dual function in this membrane: It exports branched-chain and aromatic amino acids generated by lysosomal proteolysis to the cytosol (11), and it activates the heterodimeric Rag GTPase tethered to the lysosomal membrane by the Ragulator complex (12, 13). In its active state (RagA/BGTP–RagC/DGDP), the Rag GTPase in turn recruits mTORC1.
Arginine stimulates both transport and signaling activities of SNAT9 (11–13). The large, cytosolic N-terminal tail of SNAT9 had been implicated in the interaction with the Ragulator–Rag complex (12, 13); however, the arginine-sensing mechanism remained unclear. In a recent study (7), part of the N terminus was shown to form a β hairpin, which plugs into the central cavity of SNAT9 in cytosol-open conformation and interacts with the arginine-binding pocket at the bottom of the cavity. Arginine competes with the β hairpin, thus releasing it from the central cavity with two outcomes: The amino acid transport activity of SNAT9 is up-regulated because alternating-access movements are not hindered anymore, and the N-terminal tail of SNAT9 can engage in other interactions (Fig. 1B). In another study (6), the N-terminal tail was shown to associate with the inactive state (RagA/BGDP–RagC/DGTP) of the Ragulator–Rag complex by plugging into the cleft between the Rag nucleotide-binding domains. This displaces the folliculin–FNIP2 complex from the inactive Ragulator–Rag complex and activates its GTPase activating protein (GAP) activity toward RagC, thus promoting progression of the Rag heterodimer toward its active state to recruit mTORC1 (Fig. 1B).
Nutrient Signaling by the Lysosomal Transporter PQLC2 to the C9orf72–SMCR8–WDR41 Complex
Another complex formed by the C9orf72, SMCR8, and WDR41 (CSW) proteins responds to amino acid starvation at the lysosomal surface (14). This complex has received growing attention because a hexanucleotide repeat expansion in the C9orf72 gene is a frequent genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia. The C9orf72-mediated diseases are transmitted in an autosomal dominant manner, and both gain-of-function (toxicity of RNA foci or dipeptide repeat proteins) and loss-of-function (C9orf72 haploinsufficiency) mechanisms have been implicated in pathogenesis (15). The C9orf72 and SMCR8 subunits share some homology with folliculin and FNIP2, while WDR41 is a β-propeller protein that associates with the C9orf72–SMCR8 heterodimer (14, 16, 17). Like folliculin, SMCR8 has a GAP activity directed, however, toward Arf and some Rab GTPases instead of Rags (16, 17).
Under amino acid starvation, the CSW complex is recruited to lysosomes through an interaction between its WDR41 subunit and the lysosomal transporter PQLC2 (18). As PQLC2 transports cationic amino acids (19, 20) and the CSW complex selectively responds to cationic amino acid repletion (18), PQLC2 was a candidate transceptor of these amino acids. However, in contrast with established yeast transceptors and SNAT9, PQLC2 lacks prominent domains exposed to the cytosol.
How does PQLC2 transduce cationic amino acid binding to the CSW complex? To address this issue, Talaia et al. (5) built three-dimensional homology models of PQLC2 and WDR41 and performed protein–protein docking simulations to predict how PQLC2 and WDR41 interact. Interestingly, they identified a protruding loop of WDR41 that plugs into the central cavity of the PQLC2 model in cytosol-open conformation. This loop was not resolved in recent cryo-electron microscopy structures of the CSW complex (16, 17), suggesting it is flexible. Talaia et al. (5) then mutated selective residues of the WDR41 loop and the PQLC2 cavity and analyzed the interaction between mutant proteins in vitro and in live cells to test, and validate, their docking model. The WDR41 “plug” could be narrowed down to a 10-amino acid peptide necessary and sufficient for the interaction with the lysosomal transporter.
The CSW complex thus senses cationic amino acid levels by inserting a 10-amino acid plug into a specific conformation of PQLC2 (Fig. 1C), linking the alternating-access cycle of the transporter to the dissociation of the complex.
A plug-and-socket mechanism where a short hairpin, either from the transporter itself (SNAT9) or from a signaling partner (WDR41), inserts into the central cavity of the transporter thus emerges as an efficient way to couple the transport and signaling activities of two lysosomal transceptors. The biological role of the CSW complex remains unclear, and the significance of its regulation by amino acids is still unknown. The mechanism unveiled by Talaia et al. (5) provides useful tools to interrogate these roles and explore their potential relevance for the understanding and treatment of amyotrophic lateral sclerosis and frontotemporal dementia.
Acknowledgments
My research is supported by Agence Nationale de la Recherche Grant ANR-18-CE11-0009-01 and by Centre National de la Recherche Scientifique.
Footnotes
The author declares no competing interest.
See companion article, “Receptor-like role for PQLC2 amino acid transporter in the lysosomal sensing of cationic amino acids,” 10.1073/pnas.2014941118.
References
- 1.Chantranupong L., Wolfson R. L., Sabatini D. M., Nutrient-sensing mechanisms across evolution. Cell 161, 67–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Özcan S., Dover J., Johnston M., Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 17, 2566–2573 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iraqui I., et al., Amino acid signaling in Saccharomyces cerevisiae: A permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol. Cell. Biol. 19, 989–1001 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holsbeeks I., Lagatie O., Van Nuland A., Van de Velde S., Thevelein J. M., The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem. Sci. 29, 556–564 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Talaia G., Amick J., Ferguson S. M., Receptor-like role for PQLC2 amino acid transporter in the lysosomal sensing of cationic amino acids. Proc. Natl. Acad. Sci. U.S.A. 118, e2014941118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fromm S. A., Lawrence R. E., Hurley J. H., Structural mechanism for amino acid-dependent Rag GTPase nucleotide state switching by SLC38A9. Nat. Struct. Mol. Biol. 27, 1017–1023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei H. T., Mu X., Hattne J., Gonen T., A conformational change in the N terminus of SLC38A9 signals mTORC1 activation. Structure, 10.1016/j.str.2020.11.014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saliba E., et al., The yeast H+-ATPase Pma1 promotes Rag/Gtr-dependent TORC1 activation in response to H+-coupled nutrient uptake. eLife 7, e31981. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence R. E., Zoncu R., The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133–142 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Wolfson R. L., Sabatini D. M., The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 26, 301–309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyant G. A., et al., mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 171, 642–654.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S., et al., Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebsamen M., et al., SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amick J., Ferguson S. M., C9orf72: At the intersection of lysosome cell biology and neurodegenerative disease. Traffic 18, 267–276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balendra R., Isaacs A. M., C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat. Rev. Neurol. 14, 544–558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang D., et al., Cryo-EM structure of C9ORF72-SMCR8-WDR41 reveals the role as a GAP for Rab8a and Rab11a. Proc. Natl. Acad. Sci. U.S.A. 117, 9876–9883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su M. Y., Fromm S. A., Zoncu R., Hurley J. H., Structure of the C9orf72 ARF GAP complex that is haploinsufficient in ALS and FTD. Nature 585, 251–255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amick J., Tharkeshwar A. K., Talaia G., Ferguson S. M., PQLC2 recruits the C9orf72 complex to lysosomes in response to cationic amino acid starvation. J. Cell Biol. 219, e201906076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jézégou A., et al., Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Natl. Acad. Sci. U.S.A. 109, E3434–E3443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B., Du H., Rutkowski R., Gartner A., Wang X., LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science 337, 351–354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]