Significance
This study offers a comprehensive and scientifically grounded quantitative model predicting the broader economic value of pneumococcal vaccination (PCV) in slowing the development of antimicrobial resistance (AMR) in China. China has high antibiotic use and low PCV coverage, making it an important country to assess in addressing AMR. Based on current published evidence, this model shows that introducing PCV in China’s national immunization program would likely save lives and bring wider societal benefits by reducing antibiotic utilization and treatment failures due to AMR.
Keywords: antibiotic resistance, vaccine, immunization, pneumonia, China
Abstract
Antimicrobial resistance (AMR) poses a serious threat to global public health. However, vaccinations have been largely undervalued as a method to hinder AMR progression. This study examined the AMR impact of increasing pneumococcal conjugate vaccine (PCV) coverage in China. China has one of the world’s highest rates of antibiotic use and low PCV coverage. We developed an agent-based DREAMR (Dynamic Representation of the Economics of AMR) model to examine the health and economic benefits of slowing AMR against commonly used antibiotics. We simulated PCV coverage, pneumococcal infections, antibiotic use, and AMR accumulation. Four antibiotics to treat pneumococcal diseases (penicillin, amoxicillin, third-generation cephalosporins, and meropenem) were modeled with antibiotic utilization, pharmacokinetics, and pharmacodynamics factored into predicting AMR accumulation. Three PCV coverage scenarios were simulated over 5 y: 1) status quo with no change in coverage, 2) scaled coverage increase to 99% in 5 y, and 3) accelerated coverage increase to 85% over 2 y followed by 3 y to reach 99% coverage. Compared to the status quo, we found that AMR against penicillin, amoxicillin, and third-generation cephalosporins was significantly reduced by 6.6%, 10.9%, and 9.8% in the scaled scenario and by 10.5%, 17.0%, and 15.4% in the accelerated scenario. Cumulative costs due to AMR, including direct and indirect costs to patients and caretakers, were reduced by $371 million in the scaled and $586 million in the accelerated scenarios compared to the status quo. AMR-reducing benefits of vaccines are essential to quantify in order to drive appropriate investment.
The rapid rise in antimicrobial resistance (AMR) is a healthcare emergency (1). It threatens the prevention of infectious diseases and the efficacy of treatment options, leading to worse patient outcomes and resulting deaths. A 2014 study estimates that 700,000 deaths per year are attributable to AMR, which is projected to grow to 10 million deaths by 2050, costing up to US$100 trillion globally (2). Vaccination is a solution that can contribute to slowing the pace of AMR. Vaccinations can hinder AMR progression by reducing the incidence of infections (1). Vaccinations can also decrease the need for antibiotic use, reducing the selection and pressure for resistance to accumulate (3). Resistance to vaccines is unlikely compared to drugs because vaccines are inherently used prophylactically to combat pathogens through multiple pathways (4). Despite a growing recognition that vaccines can deliver AMR-reducing benefits, vaccines’ AMR-related value is not incorporated in most studies, leading to undervaluation and underinvestment (5).
The pneumococcal conjugate vaccine (PCV), covering up to 13 different serotypes of Streptococcus pneumoniae (SP), has demonstrated that AMR-reducing benefits are possible. Previous studies in the United States, Europe, and Africa have shown that PCV introduction has led to reduction in antibiotic use and decreased resistant invasive disease episodes (6). A 2016 study estimated that universal coverage with PCV could avert 11.4 million days per year of antibiotic use in children under 5 y of age (7). However, these AMR-reducing benefits of PCV have not been shown at the country level related to specific vaccination policies in order to have policymakers incorporate PCV’s AMR-related value in decision making.
China has one of the highest burdens of childhood pneumonia cases caused by SP at around 454,000 new episodes per year (8). SP is a major cause of morbidity and mortality in China, especially among children under 5 (9). The World Health Organization estimates that China accounts for 12% of SP infections and 3.6% of SP deaths worldwide in children under 5 (10). China’s Expanded Program on Immunization (EPI) currently does not include PCV among its mandatory vaccines freely available to registered children (11). The most recent data on PCV coverage among children in China was only at 4.7% in 2014 (12).
Antibiotic use and misuse are also widespread in China, both within healthcare and animal agriculture systems (13, 14). In healthcare, Chinese patients often view antibiotics as a panacea, asking for antibiotics from providers even when unwarranted (15). For example, a 2014 Chinese national survey showed that 52.9% of outpatient visits resulted in antibiotic prescriptions, while only 39.4% of patients required them based on their clinical conditions (16). China’s high burden of pneumococcal disease, low PCV coverage, and high antibiotic utilization make it an important country to demonstrate the AMR-related value of pneumococcal vaccination.
This study examined the health and economic impact of increasing PCV coverage in China, focusing on the value of PCV in slowing AMR progression. This research responds to calls for a global coordinated effort to conduct research on AMR-sensitive evaluations and integrated AMR strategies (1, 5, 7). We developed and utilize an agent-based model to simulate PCV coverage, pneumococcal infections, antibiotic use, AMR accumulation, and resulting health and economic outcomes. This work demonstrates the broader economic value of pneumococcal vaccination in slowing the development of AMR in China.
Methods
Here, we describe our model, which follows the Overview, Design concepts, and Detail (ODD) protocol for agent-based models (17). Below is the concise methods, with further detail in SI Appendix, Part B. We developed an agent-based microsimulation model known as Dynamic Representation of the Economics of Anti-Microbial Resistance (DREAMR) (18) to examine the health and economic impact of increasing PCV coverage in China, focusing on the value of PCV in slowing AMR progression (19). An agent-based model was selected as it allows for adding specific characteristics to comprehensively simulate the dynamics of increased vaccination to compare the outcomes generated from current status quo vaccination rates versus increased vaccinations. A similar model was utilized to examine the impact of AMR on the treatment of pneumococcal diseases in Ethiopia (19). This model was developed in NetLogo 6.0.2, free and accessible software with a multiagent programmable modeling environment (20). Model inputs are presented in Tables 1 and 2, with pharmacokinetics and pharmacodynamics (PKPD) parameters in SI Appendix, Part B. The model simulations are described below.
Table 1.
Population and disease characteristics: Input parameters of the DREAMR Model
| Parameter | Unit | Value | SD or uncertainty range | Source |
| Demographics | ||||
| Total population (2015) | Thousands | 1,397,028.6 | — | United Nations (21) |
| Population, age 0–4 (2015) | Thousands | 85,884.7 | — | United Nations (21) |
| Rural population (2017) | % | 42.0 | — | United Nations (31) |
| Life expectancy at birth (2017) | Years | 76.4 | — | United Nations (63) |
| Inflation rate (2017–2018, 2018–2019) | % | 2.1, 2.9 | — | World Bank (65) |
| GDP per capita (2019) | USD | 10,261.7 | — | World Bank (60) |
| Epidemiology | ||||
| SP colonization rate | % | 24.5 | — | Wang et al. (51) |
| Pneumococcal pneumonia | ||||
| Incidence | Cases/100,000 | 619 | 534–736 | Wahl et al. (30) |
| Case fatality rate | % | 4.1 | 4.03–4.18 | Chen et al. (27) |
| Pneumococcal meningitis | ||||
| Meningitis incidence | Cases/100,000 | 14 | 8.9–19.2 | Chen et al. (27) |
| SP isolation rate | % | 9.5 | — | Chen et al. (27) |
| Case fatality rate | % | 8.3 | 6.57–10.28 | Chen et al. (27) |
| Hospitalization rate | % | 100.0 | — | Assumption |
| Incidence of sequelae | ||||
| Hearing loss | % | 5.7 | 4.26–7.54 | Li et al. (43) |
| Seizure | % | 6.7 | 5.10–8.61 | Li et al. (43) |
| DALY weights for sequelae | ||||
| Hearing loss | 0.158 | — | Salomon et al. (62) | |
| Seizure | 0.263 | — | Salomon et al. (62) | |
| Pneumococcal AOM | ||||
| AOM incidence (age 0–2) | Cases/1,000 | 71.7 | — | Wang et al. (28) |
| AOM incidence (age 3–5) | Cases/1,000 | 158.8 | — | Wang et al. (28) |
| SP isolation rate | % | 39.2 | — | Wen et al. (29) |
| Hospitalization rate | % | 0.0 | — | Assumption |
| Clinical resolution rate: amoxicillin | % | 92.8 | — | Le Saux et al. (37) |
| Clinical resolution rate: placebo | % | 84.2 | — | Le Saux et al. (37) |
| Hearing loss (0–11 mo) | Cases/10,000 | 22.8 | — | Monasta et al. (56) |
| Hearing loss (1–4 y) | Cases/10,000 | 9.3 | — | Monasta et al. (56) |
| DALY weight for hearing loss | 0.158 | — | Salomon et al. (62) | |
| Care seeking | ||||
| Urban | % | 75.4 | 70.02–80.32 | Zhang et al. (32) |
| Rural | % | 76.54 | 73.84–79.02 | Gao et al. (33) |
| Self-medicate: urban | % | 14.1 | 13.83–14.37 | NHC (35) |
| Self-medicate: rural | % | 14.4 | 13.99–14.81 | NHC (35) |
| Mortality rate odds ratio (nonseeking vs. seeking care) | OR | 7.56 | 3.77–15.1 | Reyes et al. (36) |
| Health facility utilization | ||||
| Urban (inpatient & outpatient) | ||||
| Municipal hospital | % | 89.5 | — | Li et al. (34) |
| County hospital | % | 7.0 | — | Li et al. (34) |
| Not reported | % | 3.5 | — | Li et al. (34) |
| Rural (inpatient & outpatient) | ||||
| Municipal hospital | % | 1.77 | — | Li et al. (34) |
| County hospital | % | 12.39 | — | Li et al. (34) |
| Township or village hospital | % | 30.09 | — | Li et al. (34) |
| Private clinic | % | 30.91 | — | Li et al. (34) |
| Not reported | % | 24.75 | — | Li et al. (34) |
| Proportion of inpatient care | ||||
| Municipal hospital | % | 1.5 | — | Calculated (34) |
| County hospital | % | 2.0 | — | Calculated (34) |
| Township or village hospital | % | 1.8 | — | Calculated (34) |
| Private clinic | % | 0.0 | — | Calculated (34) |
| Not reported | % | 1.4 | — | Calculated (34) |
AOM, acute otitis media; DALY, disability-adjusted life year; GDP, gross domestic product; OR, odds ratio; NHC, National Health Commission, China; SD, standard deviation; SP, Streptococcus pneumoniae; USD, US dollar.
Table 2.
Vaccine, antibiotic treatments, and costs: Input parameters of the DREAMR Model
| Parameter | Unit | Value | SD or uncertainty range | Source |
| Vaccine characteristics, PCV | ||||
| Direct vaccine effect* | ||||
| Pneumococcal bacteremia | % | 94 | — | Shen et al. (22) |
| Pneumococcal meningitis | % | 94 | — | Shen et al. (22) |
| Pneumonia: inpatient | % | 29 | — | Shen et al. (22) |
| Pneumonia: outpatient | % | 7 | — | Shen et al. (22) |
| AOM mild | % | 8 | — | Shen et al. (22) |
| AOM moderate/severe | % | 17 | — | Shen et al. (22) |
| Indirect vaccine effect* | ||||
| Pneumococcal bacteremia | % | 64 | — | Shen et al. (22) |
| Pneumococcal meningitis | % | 64 | — | Shen et al. (22) |
| Pneumococcal: inpatient 0–1 | % | 22 | — | Shen et al. (22) |
| Pneumococcal: inpatient 2–4 | % | 17 | — | Shen et al. (22) |
| PCV coverage rate 2014 | % | 4.7 | — | Yuan et al. (12) |
| Antibiotic utilization | ||||
| Pneumococcal pneumonia | ||||
| Penicillin G | % | 31.6 | 25.04–38.70 | Li (45) |
| Amoxicillin | % | 39.5 | 32.47–46.81 | Li (45) |
| Third-generation cephalosporins | % | 28.9 | 22.61–35.95 | Li (45) |
| Pneumococcal meningitis | ||||
| Meropenem | % | 100 | — | Adjusted Zhu et al. (46) |
| Pneumococcal AOM | ||||
| Penicillin G | % | 31.6 | 25.04–38.70 | Li (45) |
| Amoxicillin | % | 39.5 | 32.47–46.81 | Li (45) |
| Third-generation cephalosporins | % | 28.9 | 22.61–35.95 | Li (45) |
| Antibiotic treatment duration | ||||
| Treatment follow-up | Days | 3 | — | Bradley et al. (38) |
| Pneumococcal pneumonia: inpatient | Days | 7.1 | — | Zhang et al. (44) |
| Pneumococcal pneumonia: outpatient | Days | 7 | — | Assumption |
| Pneumococcal meningitis | Days | 21 | — | Li et al. (43) |
| Pneumococcal AOM | Days | 7 | — | COEC (42) |
| Antibiotic resistance | ||||
| Penicillin G | % | 53.8 | — | Li et al. (43) |
| Amoxicillin | % | 8.1 | — | Lyu et al. (47) |
| Third-generation cephalosporins | % | 20.8 | — | Lyu et al. (47) |
| Meropenem | % | 36.0 | — | Liu et al. (48) |
| Distribution of costs | ||||
| Direct medical cost: outpatient | % | 62.8 | — | Li et al. (34) |
| Direct medical cost: inpatient | % | 88.3 | — | Li et al. (34) |
| Mean medical expenditures by health facility | ||||
| Outpatient | ||||
| Municipal hospital/private clinic | USD | 173.46 | 43.37† | Li et al. (34); Ma et al. (59) |
| County hospital | USD | 125.98 | 31.50† | Li et al. (34); Ma et al. (59) |
| Township or village hospital | USD | 74.33 | 18.58† | Li et al. (34); Ma et al. (59) |
| Inpatient | ||||
| Municipal hospital/private clinic | USD | 820.31 | 205.07† | Ma et al. (59) |
| County hospital | USD | 595.75 | 148.94† | Ma et al. (59) |
| Township or village hospital | USD | 351.52 | 84.88† | Ma et al. (59) |
AOM, acute otitis media; COEC, China Otorhinolaryngology Editor Committee; PCV, pneumococcal conjugate vaccine; SD, standard deviation; USD, US dollar.
*Vaccine overall effect calculated as [%vaccinated × direct effect + (1 − %vaccinated × direct effect) × indirect effect].
The SD was assumed to be 25% of the mean.
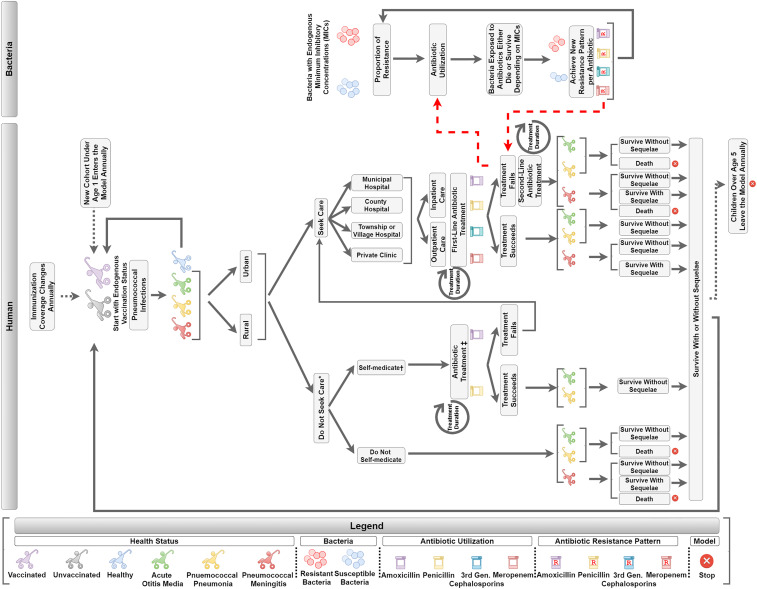
The model has two interactive parts, the human and bacteria components. The child and bacteria agents move sequentially through the steps depicted in Fig. 1. The human component simulates the progression of healthcare seeking and treatment if a child were to become infected by SP. This includes probabilities for whether or not a child seeks care and at what type of health facility, whether a child receives outpatient or inpatient care, and what antibiotics are used for treatment.
Fig. 1.
DREAMR model structure. Legend: Gen, generation. *Care seeking focuses on formal healthcare systems. †Pneumococcal meningitis patients are assumed not to self-medicate. ‡Self-medication involves use of oral antibiotics.
The bacteria component focused on four antibiotics commonly used to treat pneumococcal diseases in China: penicillin, amoxicillin, third-generation cephalosporins, and meropenem. The entities in the bacteria component are the bacteria agents, where each bacterium was classified as either resistant or susceptible. When antibiotics are utilized, the human component interacts with the bacteria component, where bacteria may die, or survive and replicate, depending on the antibiotic’s pharmacokinetics and pharmacodynamics properties and the degree of exposure. The change in ratio between resistant and susceptible bacteria, known as the AMR ratio, subsequently influences pneumococcal disease treatment outcomes in the human component. The model simulations begin in 2021 and run for a duration of 5 y to 2026.
Human Component: Vaccination, Care Seeking, and Treatment.
The DREAMR human component simulated 5,000 agents, where each agent represented 10 children, comprising a population of 50,000 children between 0 and 5 y of age. Results were scaled to the pediatric population size in China based on the most current demographic data (21). Each agent in the model was assigned a PCV vaccination status based on coverage data available from the National Immunization Program Information System in China (12). Published literature were utilized to simulate PCV vaccine efficacy (22). The model incorporated effects of herd immunity, where immunized individuals provided protection among the unvaccinated population (23, 24). This study assumed herd immunity occurred at 85% PCV vaccination coverage, where decreased disease incidence rates were applied to simulate the indirect effect of immunization (25, 26).
Modeled child agents faced an incidence of contracting pneumococcal infections, resulting in pneumococcal pneumonia, pneumococcal meningitis, or acute otitis media (AOM). Disease incidence rates and the percentage of cases caused specifically by SP were derived from published literature (27–30). Care seeking and treatment of pneumococcal disease were modeled separately for rural and urban environments (31). Child agents sought or did not seek care from healthcare facilities based on care-seeking rates from the literature (32, 33). Child agents who sought healthcare received treatment from one of the following levels of health facilities: 1) municipal level, 2) county level, 3) township/village level, or 4) private clinics (34). Child agents who did not seek care either self-medicated or remained untreated based on care-seeking rates from the Analysis Report of National Health Services Survey in China (35). Untreated agents faced a greater propensity to develop adverse health outcomes (i.e., disability and death) compared to those who received treatment (36). All children with AOM were treated as outpatients, while all pneumococcal meningitis cases were treated as inpatients (37). Health facility-level hospitalization rates from the literature were applied to pneumococcal pneumonia cases who sought care (34). Antibiotic regimens and treatment durations for each pneumococcal disease were extracted from the literature (38–44). The likelihood of a certain antibiotic to be utilized was based on historical sources (45, 46). The proportion of child agents treated with antibiotics for pneumococcal disease in the human component affected the degree of antibiotic exposure in the bacteria component.
Bacteria Component.
The DREAMR bacteria component focused on four antibiotics commonly used to treat pneumococcal diseases in China: penicillin, amoxicillin, third-generation cephalosporins, and meropenem. We simulated 80,000 bacteria agents, 20,000 per antibiotic, where each bacterium was classified as either resistant or susceptible. The bacterial agents were compartmentalized to and only interacted with their assigned antibiotic. The initial AMR ratios for antibiotics were set based on the resistance patterns in China (43, 47, 48). Bacteria agents were also assigned a minimum inhibitory concentration (MIC) from a gamma distribution based on the antibiotic and resistance status. The method of moments approach was used to obtain parameters needed for the gamma distribution to characterize these distributions (49). Gamma distributions were derived from the mean and SD of the parameter. The susceptibility breakpoints were set to be equal to that of the Clinical Laboratory Standards Institute, at the 90th percentile of the MIC gamma distribution (50).
PKPD were used to determine which bacteria agents would die under antibiotic exposure and the inputs can be found in SI Appendix, Part B. PK characteristics, including the volume of distribution, total body clearance, and elimination rate constants were retrieved from literature and the product information for each antibiotic. The probability that bacteria encountered antibiotic exposure depended both on defined daily doses (DDDs) and the proportion of children colonized with SP (51). DDDs per 1,000 children per day were obtained from the human component, which estimated the proportion of child agents using antibiotics for pneumococcal diseases. DDDs per 1,000 children per day were then divided by the percentage of children colonized to estimate the probability that bacterial agents would be exposed to antibiotics. Large DDDs per 1,000 children per day led to a higher probability for bacteria antibiotic exposure. Since all four antibiotics are time dependent (i.e., efficacy of the antibiotic is dependent on duration of time spent above MIC), we then estimated the percentage of time that exposed antibiotic concentrations were above MIC for each bacterium, which is based on the dose, dosing interval, MIC value, and PK of the antibiotic. A bacterial agent would die once the percentage of time it was exposed to antibiotics above its MIC value became greater than the threshold of 50% (52–54). Susceptible strains of bacteria faced a higher probability of being killed compared to resistant strains due to a lower MIC distribution. Killed bacteria agents were replaced through replication of susceptible and resistant bacteria agents maintaining the latest AMR ratio.
Human Component: Health and Economic Outcomes.
The AMR ratio from the bacteria component subsequently set the proportion of treatment failures in the human component (55). Treatment failures were due to antibiotic agents unable to overcome higher MIC values from increasing antimicrobial resistance. Child agents with treatment failures from first-line antibiotics switched to second-line therapy, and overall treatment durations were prolonged (37–43). Child agents who self-medicated and experienced treatment failure sought care from formal health facilities. Patients either died or recovered from the disease episode based on case-fatality rates from the literature (27). Agents who survived from pneumococcal meningitis and AOM also faced a probability of developing long-term sequelae (40, 56). We simulated the number of cases, deaths, disabilities, average DDDs, AMR ratios, cumulative treatments, and treatment failures over time. Difference in treatment failures with and without vaccination is equivalent to the number of averted resistant infections.
Economic outcomes were also assessed by using the cost-of-illness method, estimating related direct medical costs, direct-nonmedical costs, productivity losses for caregivers, and productivity losses due to death/disability (34, 57–59). Direct medical and nonmedical costs to treat pneumococcal disease at different health facility levels in China were abstracted from the literature (34, 59). Productivity losses were estimated based on China’s GDP per capita per day and the duration of lost productivity (60, 61). Caregivers lost income over the duration of illness, and children who died or became disabled lost productivity from age 15 until the average age of retirement at 60. Productivity loss for children who lived with a disability due to pneumococcal meningitis and AOM was reduced by the relevant disability-adjusted life year (DALY) weights (62–64). All costs are presented in US dollars (2019). For model inputs in local currency, all cost values were inflated using the local inflation rate to 2019 values, and then converted from yuan to US dollars using the 2019 exchange rate of 0.145 USD/yuan (65, 66). A 3% compounded discount rate was used to estimate the present value of future productivity losses. Study results were presented from a societal perspective.
Scenario and Sensitivity Analyses.
Three scenarios of PCV vaccination coverage were modeled, all over 5 y (2021 to 2026), to simulate changes in AMR. The first scenario simulated the status quo, where PCV coverage remained at current levels at 4.74% for 5 y. The second “scaled” scenario linearly increased PCV coverage from status quo to 99% over 5 y. We set the maximum PCV coverage to 99% based on China’s nationally reported immunization coverage for other childhood vaccines (67). In the third “accelerated” scenario, PCV coverage increased from status quo to 85% in the first 2 y, and to 99% over the next 3 y. This was designed to simulate the adoption of PCV into China’s EPI, to quickly reach 85% coverage. Our results compare the scaled and accelerated scenarios to status quo.
Uncertainty arising from various processes in the DREAMR agent-based model was minimized by averaging base-case results across 5,000 simulations. Sensitivity analyses were conducted to evaluate how uncertainty of input parameters impacted key outcomes. A tornado diagram was made to identify inputs that were most influential of cumulative costs.
Results
The impact of PCV in reducing pneumococcal disease burden is shown in Table 3. Based on the current PCV coverage, we predicted that China will have 2.6 million cases of pneumococcal pneumonia, around 5,400 cases of pneumococcal meningitis, and 24.0 million cases of pneumococcal AOM over 5 y. Compared to the status quo, the scaled scenario reduced the disease burden of pneumococcal pneumonia to 2.4 million cases (9.4% decrease), pneumococcal meningitis to 3,100 cases (42.5% decrease), and pneumococcal AOM to 22.0 million cases (8.3% decrease). The accelerated scenario further reduced the disease burden of pneumococcal pneumonia to 2.2 million cases (14.7% decrease), pneumococcal meningitis to 1,900 cases (65.3% decrease), and pneumococcal AOM to 20.9 million cases (13.0% decrease).
Table 3.
Health and economic outcomes of PCV13
| Outcomes | Status quo (SQ) | Scaled (difference from SQ) | % Change (scaled vs. SQ) | P value | Accelerated (difference from SQ) | % Change (accel. vs. SQ) | P value |
| Cumulative disease cases | |||||||
| Pneumococcal pneumonia, n | 2,608,071 | −245,505 | −9.41% | <0.01* | −382,513 | −14.67% | <0.01* |
| Pneumococcal meningitis, n | 5,377 | −2,283 | −42.46% | <0.01* | −3,510 | −65.28% | <0.01* |
| Pneumococcal AOM, n | 23,991,850 | −1,995,811 | −8.32% | <0.01* | −3,118,694 | −13.00% | <0.01* |
| Cumulative adverse health outcomes | |||||||
| Overall deaths, n | 51,245 | −4,650 | −9.07% | <0.01* | −7,508 | −14.65% | <0.01* |
| Disabilities, n | 3,920 | −338 | −8.62% | <0.01* | −545 | −13.91% | <0.01* |
| Treatment behavior | |||||||
| Cumulative first-line treatments, n | 14,298,861 | −1,183,174 | −8.27% | <0.01* | −1,847,888 | −12.92% | <0.01* |
| Cumulative second-line treatments, n | 7,019,911 | −618,753 | −8.81% | <0.01* | −966,773 | −13.77% | <0.01* |
| Average defined daily dose, per 1,000 patient days | 1.04 | −0.09 | −8.64% | <0.01* | −0.14 | −13.55% | <0.01* |
AOM, acute otitis media; PCV13, 13-valent pneumococcal conjugate vaccine; SQ, status quo. Point estimates were derived from taking averages across 5,000 base case simulations.
P < 0.01.
While the status quo scenario predicted around 51,200 deaths from pneumococcal diseases over 5 y, the scaled and accelerated scenarios reduced the number of overall deaths to 46,600 (9.1% decrease) and 43,700 (14.7% decrease) deaths, respectively. Increased PCV coverage and decreased disease incidence resulted in reductions in cumulative first-line treatments for pneumococcal diseases by 8.3% in the scaled scenario and 12.9% in the accelerated scenario. There was also a similar reduction in cumulative second-line treatments.
The AMR-related value of PCV vaccines is presented in Table 4. Based on China’s current PCV coverage, we simulated that resistance for penicillin, amoxicillin, and third-generation cephalosporins would increase by 6.5%, 0.9%, and 1.3%, respectively, over 5 y. Compared to the status quo, the scaled scenario significantly reduced resistance for penicillin, amoxicillin, and third-generation cephalosporins by 6.6%, 10.9%, and 9.8%, respectively. In the accelerated scenario, resistance for penicillin, amoxicillin, and third-generation cephalosporins reduced further by 10.5%, 17.0%, and 15.4%, respectively. In all three scenarios, no significant change in AMR ratio was observed for meropenem, likely due to low incidence of pneumococcal meningitis resulting in low utilization of the antibiotic within the model.
Table 4.
AMR-reducing benefits from increasing pneumococcal vaccination in China
| Outcomes | Status quo (SQ) | Scaled (difference from SQ) | % Change (scaled vs. SQ) | P value | Accelerated (difference from SQ) | % Change (accel. vs. SQ) | P value |
| Incremental change in resistance | |||||||
| Penicillin, % | 6.50 | −0.43 | −6.58 | <0.01* | −0.68 | −10.52 | <0.01* |
| Amoxicillin, % | 0.89 | −0.10 | −10.93 | <0.01* | −0.15 | −16.99 | <0.01* |
| Third-generation cephalosporins, % | 1.31 | −0.13 | −9.76 | <0.01* | −0.20 | −15.43 | <0.01* |
| Meropenem, % | 0.00 | 0.00 | −25.68 | 0.82 | 0.00 | −36.69 | 0.72 |
| Treatment failures | |||||||
| Cumulative treatment failures, n | 7,019,917 | −618,728 | −8.81 | <0.01* | −966,756 | −13.77 | <0.01* |
| Treatment failures (pneumonia), n | 688,387 | −67,223 | −9.77 | <0.01* | −104,728 | −15.21 | <0.01* |
| Treatment failures (meningitis), n | 1,769 | −735 | −41.55 | <0.01* | −1,162 | −65.70 | <0.01* |
| Treatment failures (AOM), n | 6,330,659 | −550,899 | −8.70 | <0.01* | −861,150 | −13.60 | <0.01* |
| Proportion of treatments resulting in failures, % | 32.93 | −0.13 | −0.40 | <0.01* | −0.22 | −0.66 | <0.01* |
| Average annual costs per child in first-line treatment due to resistance | |||||||
| Overall costs, USD | $1.30 | -$0.13 | −9.98 | <0.01* | -$0.21 | −15.87 | <0.01* |
| Direct medical costs, USD | $0.40 | -$0.04 | −10.53 | <0.01* | -$0.07 | −16.89 | <0.01* |
| Direct nonmedical costs, USD | $0.23 | -$0.02 | −9.48 | <0.01* | -$0.03 | −14.94 | <0.01* |
| Productivity losses for caretaker, USD | $0.67 | -$0.07 | −9.83 | <0.01* | -$0.10 | −15.58 | <0.01* |
| Average annual costs per child in second-line treatment | |||||||
| Overall costs, USD | $3.96 | -$0.38 | −9.63 | <0.01* | -$0.61 | −15.32 | <0.01* |
| Direct medical costs, USD | $1.23 | -$0.13 | −10.17 | <0.01* | -$0.20 | −16.34 | <0.01* |
| Direct nonmedical costs, USD | $0.69 | -$0.06 | −9.12 | <0.01* | -$0.10 | −14.37 | <0.01* |
| Productivity losses for caretaker, USD | 5.86 | −0.32 | −5.53 | <0.01* | -$0.31 | −15.02 | <0.01* |
| Direct costs due to resistance in first-line treatment, USD | 555,553,463 | −50,709,315 | −8.80 | <0.01* | −79,279,354 | −13.92 | <0.01* |
| Year 1 | 101,223,059 | −1,175,063 | −1.16 | <0.01* | −2,886,681 | −2.85 | <0.01* |
| Year 2 | 107,242,100 | −5,104,673 | −4.76 | <0.01* | −11,161,258 | −10.41 | <0.01* |
| Year 3 | 111,473,671 | −9,547,907 | −8.57 | <0.01* | −20,216,120 | −18.14 | <0.01* |
| Year 4 | 115,806,071 | −14,376,376 | −12.41 | <0.01* | −21,883,454 | −18.90 | <0.01* |
| Year 5 | 119,808,562 | −20,505,297 | −17.12 | <0.01* | −23,131,842 | −19.31 | <0.01* |
| Productivity losses for caretaker due to resistance in first-line treatment, USD | 838,365,043 | −73,360,011 | −8.75 | <0.01* | −114,707,201 | −13.68 | <0.01* |
| Direct costs due to resistance in second-line treatment, USD | 1,701,472,159 | −163,787,441 | −9.34 | <0.01* | −260,645,092 | −14.99 | <0.01* |
| Year 1 | 314,812,910 | −3,622,235 | −1.15 | <0.01* | −8,943,720 | −2.84 | <0.01* |
| Year 2 | 330,591,265 | −16,159,263 | −4.89 | <0.01* | −35,500,946 | −10.74 | <0.01* |
| Year 3 | 342,349,973 | −31,072,801 | −9.08 | <0.01* | −65,185,799 | −19.04 | <0.01* |
| Year 4 | 353,040,336 | −46,847,363 | −13.27 | <0.01* | −73,099,863 | −20.71 | <0.01* |
| Year 5 | 360,677,674 | −66,085,780 | −18.32 | <0.01* | −77,914,765 | −21.60 | <0.01* |
| Productivity losses for caretaker due to resistance in second-line treatment, USD | 877,157,654 | −83,044,609 | −9.47 | <0.01* | −131,791,660 | −15.02 | <0.01* |
| Cumulative costs due to resistance, USD† | 3,972,548,320 | −370,901,377 | −9.34 | <0.01* | −586,423,306 | −14.76 | <0.01* |
| Projected productivity losses from death/disability due to resistance, USD | 456,401,958 | −36,685,990 | −8.04 | <0.01* | −66,593,295 | −14.59 | <0.01* |
AMR, antimicrobial resistance; AOM, acute otitis media; SQ, status quo; USD, US dollar. Point estimates were derived from taking averages across 5,000 base case simulations. Productivity losses are calculated from 15 y of age until retirement at 60 for all affected individuals (DALY weights of 1 for death, 0.158 for hearing loss, and 0.263 for seizure were applied for disability).
P < 0.01.
Includes direct costs due to resistance in first- and second-line treatments and resulting productivity losses for caretakers.
Increased PCV vaccinations resulted in fewer antibiotic treatment failures for pneumococcal pneumonia, pneumococcal meningitis, and AOM. At status quo vaccination coverage, we estimated that there would be 7.0 million treatment failures for pediatric pneumococcal diseases over 5 y. The scaled and accelerated scenarios decreased treatment failures by 618,000 (8.8% decrease) and 967,000 (13.8% decrease) over 5 y, respectively.
Under status quo PCV coverage, cumulative direct costs of resistance in first-line treatments are predicted to be $555 million, growing from $101 million in year 1 to $120 million in year 5. These costs were reduced by 9.1% ($51 million) in scaled and 14.3% ($79 million) in accelerated scenarios over 5 y. Productivity losses for the child’s caretaker due to AMR is also projected to decrease from the status quo $838 million by 8.8% ($73 million) and 13.7% ($115 million) for the scaled and accelerated scenarios, respectively. There is a similar reduction in cumulative direct costs of resistance in second-line treatments.
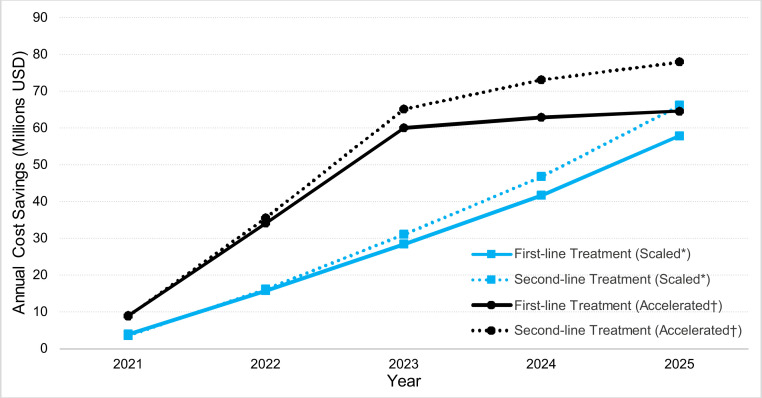
Overall AMR-related costs for pediatric pneumococcal treatment in China was predicted to be $4.0 billion over 5 y under current PCV coverage. Increasing PCV coverage would reduce AMR-related costs by $371 million (9.3% decrease) and $586 million (14.8% decrease) over 5 y in scaled and accelerated coverage scenarios, respectively. Moreover, this did not include AMR-related productivity losses due to death and disability, which were predicted to be $456 million based on current PCV coverage, and reduced by $37 million (8.0% decrease) and $67 million (14.6% decrease) in scaled and accelerated coverage scenarios, respectively. A year-by-year change in cumulative AMR-related costs for the scaled and accelerated scenarios can be seen in Fig. 2.
Fig. 2.
AMR-related cost savings from increased PCV coverage in China. Legend: AMR, antimicrobial resistance; PCV, pneumococcal conjugate vaccine; USD, US dollars. *Scaled scenario linearly increased PCV coverage from 4.7 to 99% over 5 y. †Accelerated scenario increased PCV coverage linearly from 4.7 to 85% in the first 2 y, and to 99% over the next 3 y.
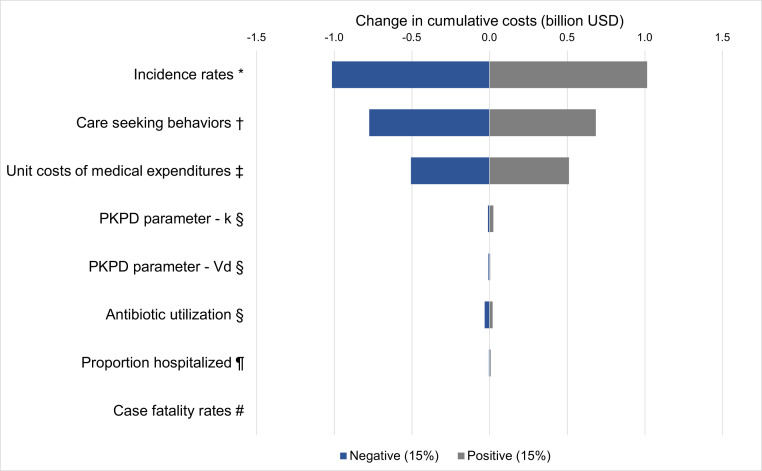
A tornado diagram was constructed in Fig. 3, demonstrating the comparative influence of model inputs on our cost outcomes. We ranged each key parameter by ±15%. The model showed most sensitivity to incidence rates (−$1.0 billion, $1.0 billion), care seeking behaviors (−$775 million, $685 million), and unit costs of medical expenditures (−$506 million, $512 million). The model outcomes were less sensitive to changes in key PKPD parameters, antibiotic utilization, proportion of hospitalized patients, and case fatality rates.
Fig. 3.
Sensitivity analysis: tornado diagram of select parameters. Legend: k, elimination rate constant; PKPD, pharmacokinetic–pharmacodynamic; Vd, volume of distribution. Note: Sensitivity analyses were conducted by adjusting each group of related input parameters together by ±15% to examine the impact on change in cumulative costs. *Incidence of pneumococcal pneumonia, pneumococcal meningitis, and AOM were concurrently ranged. †Care seeking at formal healthcare facilities was varied for all modeled diseases. ‡All average unit costs of medical expenditures were ranged across health facilities. §PKPD parameters, k, Vd, and antibiotic utilization (%), were varied across all four modeled antibiotics. ¶The proportion of individuals hospitalized was ranged for pneumococcal pneumonia. #Case fatality rates were ranged for pneumococcal pneumonia and pneumococcal meningitis.
Discussion
This study demonstrates the broader economic value of pneumococcal vaccination in slowing the development of AMR in China. We show that increased PCV coverage not only reduces the disease burden, but also provides AMR-reducing benefits including lower AMR ratios, reduced treatment failures, averted second-line therapies, and cost savings from ineffective or unnecessary treatments. AMR-related costs that could be averted by increased PCV coverage in China ranged in the billions of US dollars over 5 y.
The AMR-reducing benefits of vaccines are essential to incorporate in valuation of vaccines to drive appropriate levels of investment. Economic evaluations of vaccinations have not traditionally incorporated the AMR-reducing benefits of vaccines, having focused on a narrower set of benefits (1, 5). Our results suggest that PCV has been undervalued by not taking this benefit into account, which could affect decision makers’ willingness to invest in this vaccine.
Our findings demonstrate that introducing PCV to China’s EPI program would not only save lives but also bring wider societal benefits by reducing antibiotic utilization, averting treatment failures, and saving costs due to AMR. Since pneumococcal vaccination is currently not mandated by the Chinese government, it is highly expensive and coverage is very low (4.7%) (12). Interested parents must pay out-of-pocket for their children to receive PCV at select hospitals with prices around $130 per dose (68). As the vaccine is recommended to be given in a series of four doses for children at 2, 4, 6, and 12–15 mo of age, this comes to a costly total of $520, deterring parents from vaccinating their children and/or receiving the full course of the vaccine. Including PCV in the EPI schedule would make the vaccine affordable and accessible to the population, allowing China to quickly scale up PCV coverage similar to our accelerated scenario. High PCV coverage can trigger herd immunity, providing protection from pneumococcal diseases even among those who are not vaccinated. Moreover, the large forecasted demand could provide China with the bargaining power to negotiate down the vaccine price or manufacture its own PCV vaccine to curb vaccine costs. Some Chinese companies are currently conducting research to develop a pneumococcal vaccine, which may contribute to these efforts in the long run (69).
Vaccination is not the only solution to combatting the threat of AMR. The Chinese government has made gradual improvements in antimicrobial stewardship, including recent revisions to antibiotic use guidelines and enforcement of stricter regulations (70). In 2012, the Chinese government passed the law “Administrative Measures for the Clinical Use of Antibacterial Drugs,” which strengthened the management and regulation of clinical uses of antibacterial drugs. A recent study conducted in primary care institutions in Hubei Province showed that antimicrobial stewardship interventions can reduce the costs and volumes of antibiotic procurements (71).
Although there are not many studies with which we can compare our results, our findings are consistent with previous studies on AMR and PCV in China. A 2016 study predicted that introduction of PCV in China would decrease cases of AOM by 10.0% and cases of pneumococcal pneumonia by 15.3%, which is similar to our results in the accelerated scenario (13.0% and 14.7%, respectively) (72). We also compared published historical growth rates of SP resistance across antibiotics to our model outputs. In a study that tracked SP resistance from 2011 to 2016 in cities across China, the investigators found an 8% increase in resistance for penicillin, which is similar to our findings of 6.5% (73). For amoxicillin, the authors found a 5% increase in resistance compared to our model results of 0.9%, which is more conservative as we focused on pediatric cases (73). For meropenem and third-generation cephalosporins, comparable estimates were not found in mainland China.
One of the other benefits of this study is the development of the DREAMR agent-based model to be able to dynamically simulate AMR accumulation. While these results are specific to antibiotic utilization for pediatric pneumococcal diseases in China, the DREAMR model could be used to examine the impact of increased PCV coverage on AMR accumulation across various countries utilizing different antibiotics.
This study has a number of limitations to note. First, model results are limited by the quality and availability of data. Systematic national surveillance data for SP was not available for China, and information on rare cases of pneumococcal meningitis were especially difficult to find. Data were also limited on inpatient distribution of patients at different health facilities. We obtained model inputs from Chinese studies to the greatest extent possible, including literature written in both English and Chinese languages. Second, the model was not able to account for within-country heterogeneity, including large disparities across provinces within China. Using available data, we gathered inputs for both urban and rural locations to account for as much variation as possible. Third, this study focused on antibiotic use for pediatric pneumococcal diseases, which is a limited fraction of overall antibiotic utilization. AMR ratios may be different when also considering utilization of antibiotics across other conditions and age groups. Fourth, the current model does not incorporate treatment adherence, serotype replacement, or other rarer diseases associated with SP. Greater data availability in these areas would facilitate model upgrades to examine additional impact. Finally, this study focused on human use of antibiotics, without incorporating animal agriculture use. Further research should be conducted taking a One Health approach to examine antibiotic utilization across human and animal sectors.
As AMR poses a serious threat to healthcare, the benefit of vaccination in hindering AMR progression needs to be reevaluated. This study illustrates the AMR-reducing benefits of pneumococcal vaccination in China, including reduction in treatment failures and large cost savings. Given the sizable pneumococcal disease burden, low PCV coverage, and high antibiotic use, our research reveals the significant AMR-related value of introducing PCV in China’s national immunization program. These results are useful for governments, partners such as the Bill and Melinda Gates Foundation, Gavi (the Vaccine Alliance), UNICEF, and the World Health Organization, as well as the vaccine and AMR communities. By contributing to slowing the pace of AMR, PCV not only saves lives but also adds to global efforts to combat AMR. It contributes to global vaccination and AMR goals in addition to supporting the United Nations Sustainable Development Goals and the Global Health Security Agenda (74). The AMR-reducing benefit of vaccines should be further examined to ensure adequate commitments and investments are made for vaccines to not only save lives but also protect the effectiveness of existing medicines.
Supplementary Material
Acknowledgments
We thank Jim Herrington, Gauri Rao, Brian Conlon, and Ashley Marx for their valuable insights. We also thank the Research and Scholarship in Pharmacy (RASP) Program Directors and the Global Health Economics for Pharmacy (GHEP) team (Colleen Higgins, Ashley Y. Lee, and Tatenda Yemeke) at the University of North Carolina Eshelman School of Pharmacy for their feedback and tremendous support. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004933118/-/DCSupplemental.
Data Availability
Source code for the DREAMR model was custom written in NetLogo. The main structural codes have been shared on GitHub (https://github.com/GHEP-UNC/DREAMR/blob/main/structural%20code).
References
- 1.Bloom D. E., Black S., Salisbury D., Rappuoli R., Antimicrobial resistance and the role of vaccines. Proc. Natl. Acad. Sci. U.S.A. 115, 12868–12871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neil J., Tackling drug-resistant infections globally: Final report and recommendations (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. Accessed 1 March 2021.
- 3.The Boston Consulting Group , Vaccines to tackle drug resistant infections: An evaluation of R&D opportunities (2018). https://vaccinesforamr.org/wp-content/uploads/2018/09/Vaccines_for_AMR.pdf. Accessed 1 March 2021.
- 4.Kennedy D. A., Read A. F., Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc. Natl. Acad. Sci. U.S.A. 115, 12878–12886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevilla J. P., Bloom D. E., Cadarette D., Jit M., Lipsitch M., Toward economic evaluation of the value of vaccines and other health technologies in addressing AMR. Proc. Natl. Acad. Sci. U.S.A. 115, 12911–12919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klugman K. P., Black S., Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proc. Natl. Acad. Sci. U.S.A. 115, 12896–12901 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laxminarayan R., et al., Access to effective antimicrobials: A worldwide challenge. Lancet 387, 168–175 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Rudan I.et al.; Child Health Epidemiology Reference Group (CHERG) , Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health 3, 010401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudan I.et al.; WHO/UNICEF’s Child Health Epidemiology Reference Group (CHERG) , Causes of deaths in children younger than 5 years in China in 2008. Lancet 375, 1083–1089 (2010). [DOI] [PubMed] [Google Scholar]
- 10.O’Brien K. L.et al.; Hib and Pneumococcal Global Burden of Disease Study Team , Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 374, 893–902 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y., et al., The landscape of vaccines in China: History, classification, supply, and price. BMC Infect. Dis. 18, 502 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan P., Jin Y., Zheng J., Cao L., Surveillance of category II vaccines in China, 2014 [in Chinese]. Chin. J. Vaccines Immun. 22, 143–158 (2016). [Google Scholar]
- 13.Zhang W., et al., Outpatient antibiotic use and assessment of antibiotic guidelines in Chinese children’s hospitals. Eur. J. Clin. Pharmacol. 64, 821–828 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Collignon P., Voss A., China, what antibiotics and what volumes are used in food production animals? Antimicrob. Resist. Infect. Control 4, 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie J., Lin W., Zhang W., Patient knowledge and antibiotic abuse: Evidence from an audit study in China. J. Health Econ. 30, 933–949 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Wang P., Wang X., Zheng Y., Xiao Y., Use and prescription of antibiotics in primary health care settings in China. JAMA Intern. Med. 174, 1914–1920 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Grimm V., et al., A standard protocol for describing individual-based and agent-based models. Ecol. Modell. 198, 115–126 (2006). [Google Scholar]
- 18.Chen H. H., Ozawa S., Structural code, DREAMR (Dynamic Representation of the Economics of Antimicrobial Resistance). https://github.com/GHEP-UNC/DREAMR/blob/main/structural%20code. Accessed 25 February 2021. [Google Scholar]
- 19.Chen H. H., Stringer A., Eguale T., Rao G. G., Ozawa S., Impact of antibiotic resistance on treatment of pneumococcal disease in Ethiopia: An agent-based modeling simulation. Am. J. Trop. Med. Hyg. 101, 1042–1053 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilensky U., NetLogo, Version 6.0.2 (Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL, 1999).
- 21.United Nations, Department of Economic and Social Affairs, Population Division , World population prospects: The 2017 revision (2017). https://population.un.org/wpp/. Accessed 1 March 2021.
- 22.Shen K., et al., Estimating the cost-effectiveness of an infant 13-valent pneumococcal conjugate vaccine national immunization program in China. PLoS One 13, e0201245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine P. E., Herd immunity: History, theory, practice. Epidemiol. Rev. 15, 265–302 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Greenwood B., The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank P. R., Szucs T. D., Cost-effectiveness of 13-valent pneumococcal conjugate vaccine in Switzerland. Vaccine 30, 4267–4275 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Cao L., Wang H., Zheng J., Yuan P., National immunization coverage survey in China after integrated more vaccines into EPI since 2008 [in Chinese]. Chin. J. Vaccines Immun. 18, 419–424 (2012). [Google Scholar]
- 27.Chen Y., et al., Burden of pneumonia and meningitis caused by Streptococcus pneumoniae in China among children under 5 years of age: A systematic literature review. PLoS One 6, e27333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P. C., Chang Y. H., Chuang L. J., Su H. F., Li C. Y., Incidence and recurrence of acute otitis media in Taiwan’s pediatric population. Clinics (São Paulo) 66, 395–399 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen R., et al., [Pathogenic bacteria distribution and drug susceptibility in children with acute otitis media in Pearl River Delta.] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 25, 884–887 (2011). [PubMed] [Google Scholar]
- 30.Wahl B., et al., Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 6, e744–e757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United Nations, Department of Economic and Social Affairs, Population Division , World urbanization prospects: The 2018 revision (2018). https://population.un.org/wpp/. Accessed 1 March 2021.
- 32.Zhang Q., et al., A population-based study on healthcare-seeking behaviour of persons with symptoms of respiratory and gastrointestinal-related infections in Hong Kong. BMC Public Health 20, 402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao W., Dang S., Yan H., Wang D., Care-seeking pattern for diarrhea among children under 36 months old in rural western China. PLoS One 7, e43103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., et al., Disease burden of community acquired pneumonia among children under 5 y old in China: A population based survey. Hum. Vaccin. Immunother. 13, 1681–1687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Center for Health Statistics and Information , “The fifth analysis report of National Health Services survey in China, 2013” [in Chinese] (Report 5, Center for Health Statistics and Information, National Health Commission, Beijing, China, 2013).
- 36.Reyes H., et al., Infant mortality due to acute respiratory infections: The influence of primary care processes. Health Policy Plan. 12, 214–223 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Le Saux N., et al., A randomized, double-blind, placebo-controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age. CMAJ 172, 335–341 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley J. S.et al.; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America , The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimberlin D., Brady M., Jackson M., Long S., Red Book: 2015 Report of the Committee on Infectious Diseases (American Academy of Pediatrics, Elk Grove Village, IL, ed. 30, 2015). [Google Scholar]
- 40.Arditi M., et al., Three-year multicenter surveillance of pneumococcal meningitis in children: Clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics 102, 1087–1097 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Baxter Healthcare Corporation , Penicillin G potassium [package insert] (Baxter Healthcare Corporation, San Diego, CA, 2016).
- 42.China Otorhinolaryngology Editor Committee , Pediatric acute otitis medica diagnosis and treatment guidelines [in Chinese]. Chin. J. Otorhinolaryngol. Head Neck Surg. 43, 884–885 (2008). [Google Scholar]
- 43.Li C., et al., Clinical characteristics and etiology of bacterial meningitis in Chinese children >28 days of age, January 2014–December 2016: A multicenter retrospective study. Int. J. Infect. Dis. 74, 47–53 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Zhang X., et al., Pneumonia and influenza hospitalizations among children under 5 years of age in Suzhou, China, 2005–2011. Influenza Other Respir. Viruses 11, 15–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li R., Antibiotic choices for respiratory system infections. China and Foreign Med. Res. 12, 152–153 (2014). [Google Scholar]
- 46.Zhu M., Zhu J., Li H., Liu P., Lin Z., Clinical analysis of 13 neonates with group B streptococcal meningitis [in Chinese]. Chin. J. Pediatr. 52, 133–136 (2014). [PubMed] [Google Scholar]
- 47.Lyu S., et al., Vaccine serotypes of Streptococcus pneumoniae with high-level antibiotic resistance isolated more frequently seven years after the licensure of PCV7 in Beijing. Pediatr. Infect. Dis. J. 35, 316–321 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Liu C., et al., Serotypes and patterns of antibiotic resistance in strains causing invasive pneumococcal disease in children less than 5 years of age. PLoS One 8, e54254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briggs A., Claxton K., Sculpher M., Decision analytic modelling in the economic evaluation of health technologies. PharmacoEconomics 17, 443–444 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Clinical Laboratory Standards Institue , Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fifth Informational Supplement (Committee for Clinical Laboratory Standards, Wayne, PA, 2015). [Google Scholar]
- 51.Wang L., Fu J., Liang Z., Chen J., Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae in China: A meta-analysis. BMC Infect. Dis. 17, 765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuti J. L., Optimizing antimicrobial phamacodynamics: A guide for your stewardship program. Rev. Med. Clin. Las Condes 27, 615–624 (2016). [Google Scholar]
- 53.Craig W. A., Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22, 89–96 (1995). [DOI] [PubMed] [Google Scholar]
- 54.Levison M. E., Levison J. H., Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. North Am. 23, 791–815, vii (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris M.et al.; British Thoracic Society Standards of Care Committee , British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 66 (suppl. 2), ii1–ii23 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Monasta L., et al., Burden of disease caused by otitis media: Systematic review and global estimates. PLoS One 7, e36226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummond M., Cost-of-illness studies: A major headache? Pharmacoeconomics 2, 1–4 (1992). [DOI] [PubMed] [Google Scholar]
- 58.Hodgson T. A., Meiners M. R., Cost-of-illness methodology: A guide to current practices and procedures. Milbank Mem. Fund Q. Health Soc. 60, 429–462 (1982). [PubMed] [Google Scholar]
- 59.Ma X., Yu X., Hou Y., He J., China Health and Family Planning Statistics Yearbook 2017 [in Chinese] (Peking Union Medical College Publishing House, Beijing, 2017).
- 60.World Bank; OECD , World Bank National accounts data and OECD national accounts data files, GDP per capita (current US$) (2019). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD Accessed 1 March 2021.
- 61.Drummond M., Sculpher M., Claxton K., Stoddart G., Torrance G., Methods for the Economic Evaluation of Health Care Programmes (Oxford University Press, New York, 2015). [Google Scholar]
- 62.Salomon J. A., et al., Disability weights for the global burden of disease 2013 study. Lancet Glob. Health 3, e712–e723 (2015). [DOI] [PubMed] [Google Scholar]
- 63.United Nations Development Programme , The next frontier: Human development and the anthropocene: China (2020). hdr.undp.org/sites/all/themes/hdr_theme/country-notes/CHN.pdf. Accessed 1 March 2021.
- 64.Lucas M. J., Brouwer M. C., van de Beek D., Neurological sequelae of bacterial meningitis. J. Infect. 73, 18–27 (2016). [DOI] [PubMed] [Google Scholar]
- 65.World Bank , Inflation, consumer prices (annual %)—China. (2019). https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG?locations=CN. Accessed 13 November 2020.
- 66.World Bank , Official exchange rate (LCU per US$, period average)—China (2019). https://data.worldbank.org/indicator/PA.NUS.FCRF?locations=CN. Accessed 13 November 2020.
- 67.WHO; UNICEF , China: WHO and UNICEF estimates of immunization coverage: 2019 revision (2019). https://www.who.int/immunization/monitoring_surveillance/data/chn.pdf. Accessed 1 March 2021.
- 68.Maurer K. A., et al., Cost-effectiveness analysis of pneumococcal vaccination for infants in China. Vaccine 34, 6343–6349 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Mason B., ImmunoBiology limited announces intention to build partnerships for PnuBioVax, its universal pneumococcal vaccine. https://www.einnews.com/pr_news/497272811/immunobiology-limited-announces-intention-to-build-partnerships-for-pnubiovax-its-universal-pneumococcal-vaccine Accessed 26 September 2019.
- 70.China National Health Office , Notice on clinical management of antimicrobial drugs (2018). www.nhc.gov.cn/ewebeditor/uploadfile/2018/05/20180515171055352.pdf. Accessed 1 March 2021.
- 71.Tang Y., Liu C., Zhang Z., Zhang X., Effects of prescription restrictive interventions on antibiotic procurement in primary care settings: A controlled interrupted time series study in China. Cost Eff. Resour. Alloc. 16, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mo X., Gai Tobe R., Liu X., Mori R., Cost-effectiveness and health benefits of pediatric 23-valent pneumococcal polysaccharide vaccine, 7-valent pneumococcal conjugate vaccine and forecasting 13-valent pneumococcal conjugate vaccine in China. Pediatr. Infect. Dis. J. 35, e353–e361 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Zhao C., et al., Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolates from 17 Chinese cities from 2011 to 2016. BMC Infect. Dis. 17, 804 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Global Health Security Agenda , Global Health Security Agenda (2018). https://www.ghsagenda.org/. Accessed 1 March 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source code for the DREAMR model was custom written in NetLogo. The main structural codes have been shared on GitHub (https://github.com/GHEP-UNC/DREAMR/blob/main/structural%20code).