Summary
Vaccine elicitation of broadly neutralizing antibodies (bnAbs) is a key HIV-research goal. The VRC01-class of bnAbs targets the CD4-binding site on the HIV-envelope trimer and requires extensive somatic hypermutation to neutralize effectively. Despite substantial progress, vaccine-induced VRC01-class antibodies starting from unmutated precursors have exhibited limited neutralization breadth, particularly against viruses bearing glycan276, present on most circulating strains. Here, using sequential immunization of immunoglobulin-humanized mice expressing diverse unmutated VRC01-class antibody precursors, we elicited serum responses capable of neutralizing viruses bearing glycan276 and isolated multiple lineages of VRC01-class bnAbs, including two with >50% breadth on a 208-strain panel. Crystal structures of representative bnAbs revealed the same mode of recognition as known VRC01-class bnAbs. Structure-function studies further pinpointed key mutations and correlated their induction with specific immunizations. VRC01-class bnAbs can thus be matured by sequential immunization from unmutated ancestors to >50% breadth, and we delineate immunogens and regimens inducing key somatic hypermutation.
Keywords: antibody VRC01, broadly neutralizing antibody, glyan276, HIV-1 vaccine, somatic hypermutation
Introduction
Among various HIV-1 broadly neutralizing antibodies (bnAbs), VRC01-class antibodies target the conserved CD4-binding site (CD4bs) and are among the broadest neutralizing antibodies thus far identified (Huang et al., 2016; Scheid et al., 2011; Wu et al., 2010; Zhou et al., 2010; Zhou et al., 2015; Zhou et al., 2013). Passive transfer of VRC01-class bnAbs, either alone or in combination with other bnAbs, can prevent SHIV infection in animal models (Gautam et al., 2016; Hessell et al., 2016; Pegu et al., 2014) and demonstrate potent antiviral activity in humans (Mendoza et al., 2018; Scheid et al., 2016). Therefore, elicitation of VRC01-class bnAbs has been a heavily pursued goal in the HIV-1 vaccine field.
VRC01-class antibodies share three common characteristics: a VH1-2-derived heavy chain, a 5-amino acid (aa) complementarity-determining region (CDR) L3, and a short CDR L1 (West et al., 2012; Zhou et al., 2010; Zhou et al., 2013), and their germline precursors are uncommon in the human naïve B cell population (Jardine et al., 2016a; Zhou et al., 2013). Substantial progress has been made in recent years to develop germline targeting immunogens, including an engineered envelope (Env) outer domain eOD-GT8 and a modified 426c Env (Jardine et al., 2013; McGuire et al., 2013), and nanoparticles displaying them (Jardine et al., 2015; McGuire et al., 2016) that allow the activation and amplification of VRC01-class bnAb precursors in transgenic mouse models (Dosenovic et al., 2015; McGuire et al., 2016; Sok et al., 2016; Tian et al., 2016) and the isolation of VRC01-class precursors from naïve human peripheral blood mononuclear cells (PBMCs) (Jardine et al., 2016a). Investigators have also created transgenic mouse models to express much of the human immunoglobulin repertoire including VH1-2 or to have VRC01-class precursor gene sequences engineered into the mouse Ig locus (Dosenovic et al., 2015; Jardine et al., 2015; McGuire et al., 2016; Tian et al., 2016). Some models predominantly express the rearranged VRC01-class precursors consisting of a germline reverted heavy chain with a fixed mature CDR H3 (Dosenovic et al., 2015; Jardine et al., 2015; McGuire et al., 2016), while an alternate model incorporates a human VH1-2 gene segment that recombines with native mouse D and JH gene segments to create a diverse repertoire of heavy chain precursors (Tian et al., 2016); addition of a rearranged unmutated human VK3-20 light chain with a 5aa CDR L3 from mature VRC01 generates the VH1-2/LC model and further increases the frequency of VRC01-class precursor B cells, thus allowing the assessment of immunization strategies for driving affinity maturation (Tian et al., 2016).
Despite the above progress, vaccine induction of VRC01-class bnAbs has been stymied by two hurdles: (i) requirement for substantial somatic hypermutation (SHM) and (ii) overcoming the steric barriers posed by glycan on loop D residue N276 (glycan276), which is directly proximal to the CD4bs and present in >90% of circulating HIV-1 strains (Kong et al., 2016; Travers, 2012). Natural infection-elicited bnAbs can possess very high levels of SHM and requiring years to develop (Falkowska et al., 2012; Huang et al., 2016; Umotoy et al., 2019; Wu et al., 2010; Wu et al., 2015; Wu et al., 2011; Zhou et al., 2013). Nearly all VRC01-class bnAbs isolated from HIV patients evolve to have deletions or glycine mutations in CDR L1, to avoid a steric clash with glycan276 when bound to gp120 (Zhou et al., 2015; Zhou et al., 2013). The long and complex course of affinity maturation required for VRC01-class bnAbs casts doubt on whether a vaccine regimen can replicate such a feat. Immunizations of transgenic mice with unmutated VRC01-class precursors have so far elicited VRC01-class neutralizing antibodies (nAbs) with less than 10% nucleotide SHM that cannot effectively overcome the steric hinderance of glycan276 (Escolano et al., 2016; Jardine et al., 2016b; Tian et al., 2016).
In this study, we tested a strategy of sequential immunization using the previously described VH1-2/LC mice (Tian et al., 2016) because this model generates a diverse repertoire of VH1-2 heavy chains that can be paired with germline reverted precursor-light chains with 5aa CDR L3. We compared a “multi-strain, heterologous boost” strategy, which presents the conserved CD4bs in different gp120 cores or trimers for immune focusing on the CD4bs, to a previously tested strategy based on germline-targeting 426c cores with different levels of glycan shielding of the CD4bs (Tian et al., 2016) or repeated eOD-GT8 60mer priming followed by diverse Env boosts. With both the new strategy and the 426c-core strategy, we elicited modest levels of cross-reactive serum neutralization titers. We further identified multiple lineages of VRC01-class bnAbs, including two neutralizing >50% of a 208-strain panel, and carried out structure-function analyses, revealing key sites of SHM and mechanisms for surmounting glycan276. This study provides proof-of-concept for the induction of VRC01-class antibodies of greater than 50% neutralization breadth that are capable of recognizing glycan276-bearing strains, and uses longitudinal analysis to pinpoint the development of key affinity maturation mutations in response to specific immunogens.
Results
Sequential immunization elicits broad serum neutralizing activities in mice expressing diverse VRC01-class precursors
To test whether we could elicit neutralizing VRC01-class antibodies in our transgenic mouse model, we designed a sequential immunization strategy utilizing multi-strain heterologous boosts including early boosts with native-like and glycan276-bearing Env trimers (Figure 1A, G2). This contrasts to our previous strategy in which we boosted with 426c Env proteins with increasing glycan coverage around the CD4bs (Tian et al., 2016). We also used eOD-GT8 60mer, rather than eOD-GT6 60mer, due to its higher affinity to diverse VRC01-class germline revertants (Jardine et al., 2015). The 2nd boost, C13.G4.2-Ferritin, is a chimeric gp120 core nanoparticle that has an extra V5 glycan at N462 than eOD-GT8 (Figure 1A) and moderate affinity to VRC01-class germline-revertant antibodies but presents the CD4bs in a more native-like context than the engineered gp120 outer domain (Figure S1). For the 3rd boost, we used a stabilized trimeric Env, 426c-degly3.DS.SOSIP, with three glycans near the CD4bs removed. Lastly, we boosted with diverse wild type (no glycan deletion) SOSIP trimers and, in some cases, with a resurfaced SIV gp120 core RSC3 (Wu et al., 2010). Our aim was to focus the immune response to the CD4bs with heterologous boosts to promote antibodies that could recognize the CD4bs in diverse native contexts.
Figure 1. Sequential immunization induces cross-reactive serum neutralizing titers against HIV strains bearing glycan276.
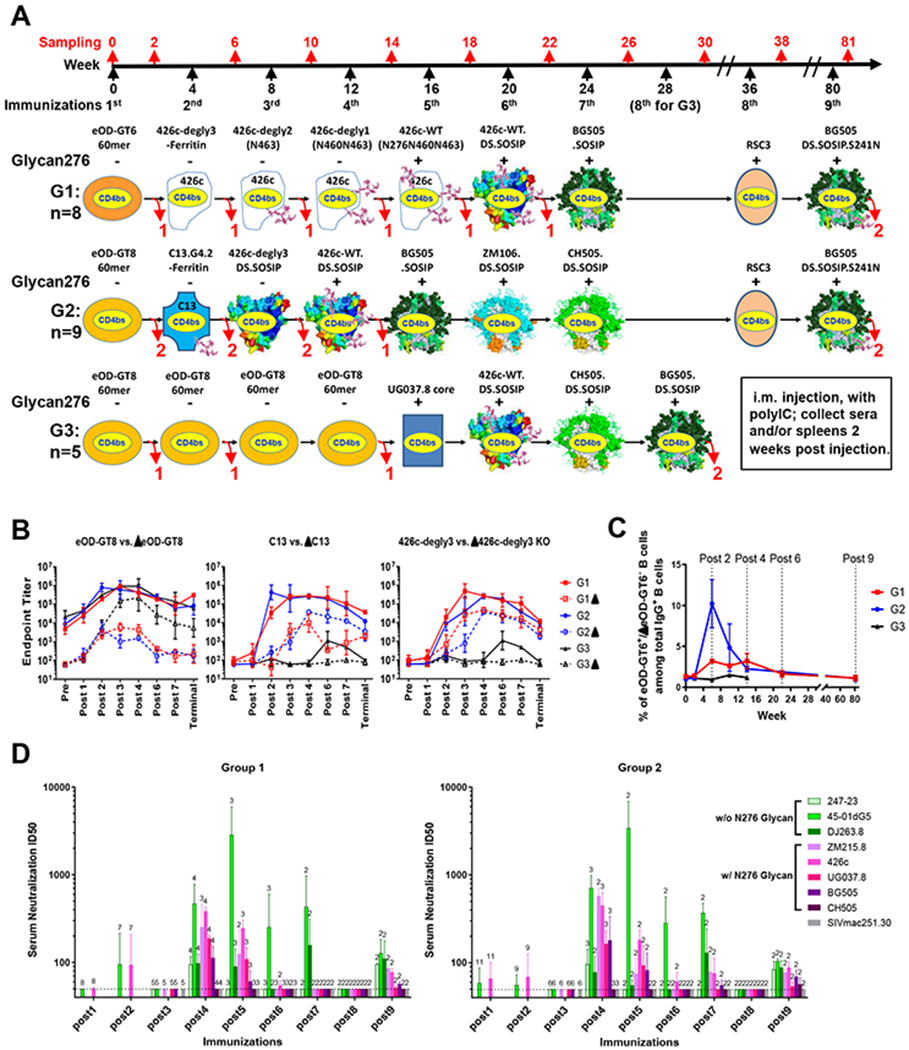
(A) Immunization schema of three groups of VH1-2/LC mice: two sequential immunization groups (G1 and G2) and an eOD-repeated priming control group (G3). The presence (+) or absence (−) of glycan276 in each immunogen is indicated. Important glycans either proximal to the CD4bs or used to fill the BG505 N241 glycan hole are also depicted on relevant immunogens. The number of mice sacrificed at various timepoints is delineated with red numbers below curved arrows.
(B) ELISA endpoint titers of post immune sera to eOD-GT8, C13 gp120 core, and 426c-degly3 gp120 core and their respective CD4bs-disrupting (Δ) mutants.
(C) CD4bs-specific B cell percentage among total IgG+ B cells of sacrificed mice at different time points, measured by FACS with eOD-GT6 or eOD-GT8 and their respective ΔCD4bs probes.
(D) Serum neutralization titers against a panel of 8 tier II viruses, including 5 with glycan276 (pink-purplish bars), and SIV control. Number of available samples for each test is noted above each bar. The dashed lines mark the detection limit of 50 of ID50.
For comparison, we repeated our previous immunization approach with 426c gp120 (Tian et al., 2016) in group 1 (G1) and also tested a third group (G3) that applied four eOD-GT8 60mer immunizations and a gp120 core (UG037.8) boost before diverse trimer boosts (Figure 1A) to investigate whether repeated immunization with a strong germline-targeting immunogen could drive affinity maturation and elicit VRC01-class bnAbs. We previously demonstrated that repeated immunization using only native-like Env BG505.SOSIP does not elicit nAbs in these mice, likely because this Env does not engage the germline precursors.
We used binding titers to eOD-GT8 and its CD4bs-disrupting (Δ) mutant to assess the overall CD4bs-specific serum response (Figure 1B). Both sequential immunization groups G1 and G2 maintained high differential binding between eOD-GT8 and ΔeOD-GT8 during immunizations, indicating that high levels and proportions of CD4bs-specific antibodies were elicited by sequential immunizations. Moreover, after the 2nd immunization, the G1 and G2 mice maintained strong differential binding to two germline-targeting gp120 cores, C13 and 426c-degly3, compared to their corresponding ΔCD4bs mutants, ΔC13 and Δ426c-degly3 (Tian et al., 2016). In contrast, the G3 mice had much smaller differential binding between eOD-GT8 and ΔeOD-GT8 after three immunizations, mainly due to increased titers to ΔeOD-GT8, suggesting that higher proportions of off-target (non-CD4bs) responses were elicited. The four eOD-GT8 60mer immunizations elicited no serum response to either C13 or 426c-degly3 gp120 cores. The G3 mice displayed low gp120-core reactivity after the UG037.8 core and trimer immunizations, but much lower than the G1 and G2 mice at corresponding time points (post 6-terminal). These results demonstrated that eOD-GT8 60mer immunization alone was not sufficient to elicit heterologous gp120 response; and a gp120 core boost, as in G1 and G2, was likely required to guide VRC01-class precursors to develop into Env-recognizing antibodies.
We sacrificed mice at various time points to obtain spleens for B cell specificity analysis and to obtain CD4bs-specific BCR sequences (Figure 1A and 1C). Because this mouse model has a background (8-10%) of eOD-GT8-binding B cells expressing predominantly unmutated VRC01-class precursors (Figure S2), we used the relatively more native eOD-GT6 and its ΔCD4bs mutant, ΔeOD-GT6, as probes to determine the frequency of CD4bs-specific (eOD-GT6+ and Δ−) B cells among total IgG+ B cells by fluorescence-activated cell sorting (FACS) (Figures 1C and S2A, see also STAR Methods). The CD4bs-specific B cell frequency was about 1% in preimmune mice, increased to 3% and 10% at post 2 in G1 and G2 mice respectively, then gradually deceased to 2-3% at post 3 and 4, and further decreased to the baseline level by the end of immunizations in G1 and G2 mice (Figure 1C). In contrast, the CD4bs-specific B cell frequency in G3 mice remained at the baseline level (1-1.5%) throughout the immunizations (Figure 1C). These results indicated that both sequential immunization strategies, but not repetitive eOD-GT8 60mer immunizations, boosted the CD4bs-specific B cell frequency, especially at post 2.
We next checked the serum neutralizing activities of all mice at different time points against a panel of eight VRC01-sensitive Env-pseudoviruses including five with glycan276 (Figure 1D and Table S1). Both G1 and G2 mice displayed cross-reactive serum neutralizing activity by week 14 (post 4) with mean ID50 titers ranging from 78 to 708 (Table S1). None of the sera neutralized the negative control SIVmac250.30, or virus CH505.TF. For both G1 and G2 mice, the serum neutralizing titers peaked after the 4th and 5th immunizations, and decreased with further boosts to an undetectable level at post 8, but recovered after the last injection of BG505.DS.SOSIP.S241N. The terminal sera of G1 and G2 animals neutralized 7 or 8 viruses, and one of the G2 mice also neutralized CH505.TF (Figure 1D and Table S1). In contrast, the G3 mice showed little or no neutralizing activity against five tested viral strains, including the sensitive glycan276-deficient viruses 45-01dG5 and 426c-degly3 (Table S1). Therefore, two different sequential immunization regimens, with as few as four immunizations, could elicit cross-reactive serum neutralizing activity against glycan276-bearing viruses.
Sequential immunization elicits antibodies capable of neutralizing glycan276-bearing viruses
To isolate VRC01-class nAbs, we performed two-color antigen-specific B cell sorting with eOD-GT6 and ΔeOD-GT6 probes on the splenocytes of mice sequentially sacrificed between week 0 and week 22 (post 6) (Figures S2A and S3). However, as in our previous study (Tian et al., 2016), from 1476 sorted B cells, we only isolated antibodies capable of neutralizing either glycan276-deficient viruses or autologous 426c virus, but were unable to neutralize heterologous glycan276-bearing viruses (Figure S3).
To select more cross-reactive B cells, we added a third probe BG505.SOSIP, in addition to eOD-GT6/ΔeOD-GT6, to sort for BG505.SOSIP+/eOD-GT6+/ΔeOD-GT6− IgG+ B cells (Figures 2 and S2A). With this sorting strategy, we isolated VRC01-class antibodies with significantly higher VH1-2 nucleotide mutation frequencies in post 4 and post 6 G1 or G2 mice than those isolated by prior two-color sorting from the same mice (Figure 2A). Although the highest VH1-2 mutation frequency at these time points was still below 10%, we identified four week 14 (post 4) antibodies — G1:2404-E12, G2:2410A-A4, G2:2410A-A5 and G2:2410B-G6 — that bound two glycan276-bearing Env antigens, 426cWT.DS.SOSIP and BG505.DS.SOSIP trimers (Figure 2B). We then tested the neutralization activity of these four antibodies against a panel of ten viral strains, nine of which bore glycan276 (Figure 2C). Also included was a week 22 (post 6) antibody G1:2405p1-72 that was identified by both two- and three-probe sorts and had been shown to neutralize glycan276-bearing virus 426c (Figures 2C and S3C). All five antibodies neutralized at least one heterologous glycan276-bearing virus. Although the four post 4 antibodies did not neutralize viral strains BG505 or 426c, G1:IgG 2404-E12, with no mutation in the heavy chain (Figure 2D), neutralized a heterologous glycan276-bearing virus UG021.16, while the other three, each identified in multiple sorted B cells, neutralized 4-5 heterologous viruses: 45-01dG5, JRFL.JB, 001428-2.42, UG021.16, and/or RHPA.7, with the latter four viruses bearing glycan276. The post 6 IgG G1:2405p1-72, represented by 6 sorted B cells, also neutralized a heterologous glycan276-bearing virus JRFL.JB in addition to 45-01dG5 and 426c (Figure 2C). Therefore, IgG cloning from three-color sorted B cells confirmed that both G1 and G2 sequential immunization strategies could elicit VRC01-class nAbs against heterologous glycan276-bearing viruses with as few as four immunizations.
Figure 2. VRC01-class nAbs elicited after only four immunizations neutralize multiple heterologous glycan276-bearing viruses.

(A) Three-color sort identified VRC01-class antibodies with higher VH1-2 nucleotide mutation frequencies than identified by two-color sort. The scatter graph shows the VH1-2 nucleotide mutation frequencies of VRC01-class antibody heavy chains identified from the B cells of three mice: G1 post 4 (wk14) mouse 2404, G2 post 4 (wk14) mouse 2410 and G1 post 6 (wk22) mouse 2405, obtained by either a: two-color sort (solid symbols: sort for eOD-GT6+/ΔeOD-GT6− cells) or b: three-color sort (open symbols: sort for BG505.SOSIP+/eOD-GT6+/ΔeOD-GT6− cells). n, number of analyzed antibody heavy chains identified from each mouse with specified B cell-sorting method. The median and 95% confidence interval for VH1-2 mutation frequencies of each set of heavy chains are shown. Mann-Whitney test was performed for statistical analysis. ns, non-significant; ***, p<0.001; ****, p<0.0001.
(B) Heat map showing the Area Under the Curve (AUC) for ELISA-binding curves of selected VRC01-class antibodies (columns) isolated by three-color B cell sorting from post 4 mice to various Env antigens (rows). The name of each antibody, such as G1:2404-E12, is displayed under the corresponding column.
(C) Neutralization titers (IC50) of selected VRC01-class antibodies isolated from G1 or G2 mice post 4 (wk14) or 6 (wk22) immunizations against a panel of 10 Tier II viruses of clade A-D. The presence (G) or absence (−) of N-linked glycans at residues 276, 460 and 462(3) for each virus is shown. The number in the parentheses following each antibody name indicates the number of sorted B cells expressing the same antibody or its clonal relative(s).
(D) The heavy chain sequences of the antibodies tested in (B). Dots indicate residues identical to the germline IGHV1-2*02.
See also Figures S2–S3.
Isolation of multiple lineages of VRC01-class bnAbs with up to 54% breadth on a 208-virus panel
While serum neutralization titers of the G1 and G2 mice did not increase with further boosts after the first 4-5 immunizations, terminal sera maintained detectable neutralization of some viral strains in our sentinel panel (Figure 1D). Flow cytometry analysis of post 4, post 6 and post 9 mouse splenocytes revealed that while both eOD-GT6+ B cell and BG505.SOSIP+ B cell frequencies decreased from post 4 (2-4% of total IgG+ B cells) to post 9 (0.7-1.6%), a small fraction (0.01-0.17%) of BG505.SOSIP and eOD-GT6 cross-reactive CD4bs-specific B cells remained mostly unchanged (Table S2), suggesting that the heterologous boosts between post 4 and post 9 preserved cross-reactive CD4bs-specific B cells. From the three-probe sorted cross-reactive B cells in the four remaining terminal (post 9) G1 and G2 mice, we isolated 75 VRC01-class antibodies with VH1-2 nucleotide mutation frequencies reaching 21% (Figures 3A–3C). From each terminal mouse, at least one VRC01-class multi-member clonal lineage (with the same CDR H3 and similar V-region mutation pattern) was identified (Figure 3B and Table S3). The VH1-2 heavy chains of the 13 lineages had 12-29 aa mutations in the V-region, including many also observed in known VRC01-class bnAbs (Table S3 and Figure S5A). All the heavy chains contained the key amino acid W100b in the CDR H3, which has been associated with neutralizing VRC01-class antibodies (Tian et al., 2016) and shown to be important for Env binding (West et al., 2012; Yacoob et al., 2016). Nine of the lineages had CDR L1 deletion and/or Gly/Ser mutation, thought to be important for accommodating glycan276 (Zhou et al., 2013). The CDR L1s of four other lineages, although not shortened or mutated to smaller residues, did show extensive mutation (Table S3).
Figure 3. Sequential immunizations elicit multiple lineages of VRC01-class bnAbs.

(A) FACS plots showing the BG505.SOSIP and eOD-GT6 double positive B cells of wk81 animals, from which VRC01-class bnAbs were cloned.
(B) Summary of VRC01-class antibody lineages cloned from the wk81 mice.
(C) Nucleotide mutation frequencies of the VH1-2 heavy chains amplified from different time points of the immunization course. * B cells were sorted with BG505.SOSIP probe in addition to eOD-GT6 and ΔeOD-GT6. # Sorted with BG505.SOSIP, eOD-GT8 and ΔeOD-GT8. Mean and SD are shown.
(D) Neutralization titers (IC50) of representative members of each VRC01-class bnAb lineage against a panel of 11 tier II viruses and SIV control.
(E) Summary of the 208-virus panel neutralization results for six selected VRC01-class bnAbs. The number of viruses neutralized at specified IC50 range and its percentage among the 208 viruses (in parentheses) are listed for each bnAb. Median and geometric mean titers are calculated only for samples with IC50 <50 µg/ml.
(F) Dendrograms showing the neutralization breadth and potency of six selected VRC01-class bnAbs against a 208-virus panel. Neighbor-joining trees display the protein distance of gp160 sequences from 208 HIV-1 isolates. A scale bar (upper-left corner) denotes 10% (0.1) distance in amino acid sequence. Neutralization potency is indicated by the color of the branch for each virus and the color coding is shown on the upper-right corner.
See also Figures S2, S4–S5 and Tables S2–S4.
We expressed 17 representative IgGs, including at least one member of each lineage, in a mouse IgG2a backbone and tested their neutralization against a panel of 11 viruses from different clades, including seven with glycan276 (Figure 3D). Except 2406a, 2408d and 2413d, which neutralized only glycan276-deficient viruses, members of the other 10 lineages neutralized at least one glycan276-bearing virus in addition to glycan276-deficient viruses, with antibodies 2408b, 2408c.3, 2411a, 2413a, 2413b and 2413c neutralizing 7-10 of the 11 viruses (Figure 3D). We tested these 6 IgGs against a 208-virus panel for neutralization breadth and potency. The six antibodies in the order above neutralized 18%, 23%, 51%, 34%, 54% and 39% of the 208 viruses with the geometric mean IC50 ranging from 1.65 to 5.78 µg/ml (Figure 3E and Table S4). Most of the neutralized viruses had glycan276. Neutralization curves against representative glycan276-bearing viruses by the six nAbs had typical sigmoidal shapes indicating complete neutralization (Figure S4A). 2411a and 2413b were the broadest, neutralizing 51% and 54% of viruses, respectively. Neutralization fingerprinting analysis revealed these six nAbs to cluster with known VRC01-class antibodies, with 2413b clustered with VRC18.02 and the other five forming a separate sub-cluster (Figure S4B). Therefore, both sequential immunization strategies elicited multiple lineages of VRC01-class nAbs that could neutralize glycan276-bearing viruses and exhibited up to 54% breadth on a 208-virus panel.
Furthermore, next generation sequencing (NGS) of the VH1-2 and VK3-20 specific amplicons from total IgG+ splenic B cells of each terminal mouse detected both heavy chains and light chains of most identified VRC01-class lineages (Table S5). In the four terminal mice G1:2406, G1:2408, G2:2411 and G2:2413, the heavy chain frequencies of the identified VRC01-class nAb lineages among total VH1-2 reads reached 2.32%, 0.066%, 0.007% and 3.123%, respectively (Table S5), matching or surpassing the heavy chain read prevalence (up to ~1.4%) of VRC01-class bnAb lineages in the human donor of VRC01 (Wu et al., 2015). Given that the VH1-2 usage rate in this mouse model is about 40% (Tian et al., 2016), the VRC01-class nAb frequency in the whole IgG repertoire of each mouse would be about 0.93% (2.32% x 40%), 0.026%, 0.003% and 1.249%, respectively. Therefore, the sequential immunizations can elicit abundant levels of VRC01-class nAbs.
Crystal structures of antibodies 2411a and 2413a in complex with gp120 reveal how antibodies overcome glycan276 to achieve broad neutralization
To define the structural mode of gp120 recognition of elicited VRC01-class bnAbs, we chose two bnAbs, 2411a and 2413a, representing lineages from two different mice for crystal structure-based analyses. 2411a was the most potent and 2nd broadest (51%) among these mouse bnAbs but, unlike human VRC01-class bnAbs, had a full-length CDR L1 without any mutation to glycine; 2413a also had a glycine residue introduced by SHM (Table S3). We crystallized antigen-binding fragments (Fab) of 2411a and 2413a each in complex with a modified d45-01dG5 gp120 core (Wu et al., 2012; Wu set al., 2015), in which residue 278 was mutated to T to enable glycosylation at N276. The 2411a-gp120 crystals were obtained by incubating d45-01dG5-K278T expressed from GNTI−/− cells with 2411a Fab followed by a limited Endo H digestion to remove glycans on gp120 that were not protected by the antibody. Crystals of the 2413a-d45-01dG5-K278T complex, however, could only be obtained with gp120 deglycosylated by Endo H. Complex crystals diffracted to 3.45 Å and 2.75 Å, respectively (Table S6). Comparison with the VRC01-gp120 structure (Zhou et al., 2010) indicated antibodies 2411a and 2413a to have the same mode of recognition as VRC01, with antibody footprints on gp120 that overlapped closely with that of VRC01 (Figures 4A and 4B).
Figure 4. Crystal structures of two elicited antibodies confirm the same mode of gp120 recognition as VRC01-class bnAbs and revealed key mutated residues binding glycan276.

(A) Crystal structure of 2411a in complex with gp120 core (left) and its footprint (green) on the core (right). Epitope of VRC01 is highlighted with red line. HIV-1 gp120 was shown in gray cartoon with loop D, the CD4-binding loop and V5 highlighted in yellow, orange and brown, respectively. Heavy chain is shown in light green with its CDRs in different shades of green. Light chain is shown in slate with its CDRs in different shades of blue.
(B) Crystal structure of 2413a in complex with gp120 core (left) and its footprint (cyan) on the core (right). Heavy chain is shown in light cyan with its CDRs in different shades of cyan. Light chain is shown in light blue with its CDRs in different shades of blue.
(C) Conservation of key VRC01-class antibody interactions with HIV-1 gp120 by antibodies 2411a and 2413a. Top panels depict the antibody mimicry of the gp120-CD4 interactions: SHM-derived Y54 and F54 (magenta) mimicry of the CD4 F43 to bind the gp120 “F43 pocket”; salt bridges between gp120 D368 to antibody R71. gp120 and antibody are shown in transparent surface and cartoon, respectively, with interacting residues shown in sticks. Lower panels show the conserved interactions between CDR H3 W100b (or W100c) and loop D, as well as interactions between CDR L3 E96 and V5.
(D) Interaction between 2411a light chain residues with glycan276 moieties. CDR L1 and L3 residues interacting with glycans were labelled with contact areas shown. Somatic mutations in CDR L1 were highlighted in magenta. Potential hydrogen bonds between glycan276 and 2411a light chain atoms were marked with dash lines.
Structural analysis indicated both 2411a and 2413a to have developed key interactions conserved in VRC01-class antibodies. For example, residue 54 (in the CDR H2) in both antibodies was mutated from the germline VH1-2-encoded G to Y or F, respectively, to mimic the interaction of HIV-1 gp120 with CD4 residue F43 (Kwong et al., 1998) (Figure 4C and Table S7). Similar mutations have been observed in other VRC01-class antibodies, including W54 in VRC03 and VRC-PG20 , Y54 in N6 , and F54 in 12A21 (Huang et al., 2016; Scheid et al., 2011; Wu et al., 2011). Other highly favorable conserved interactions, such as the VH1-2 germline residue R71 interaction with gp120 CD4bs residue D368, VRC01 CDR H3 W100b interaction with Loop D D279, and CDR L3 E96 interaction with Loop V5 G459, were also observed in both antibody-gp120 structures (Figure 4C). It has been observed that the germline VH1-2-encoded Y33 always matures to a residue with a less bulky side chain, such as T33 in VRC01, I33 in N6 and 12A12, and F33 in VRC-PG20 and 3BNC117; Y33V and Y33F mutations were also observed in 2411a and 2413a, respectively. Modelling with the 2411a and 2413a structures indicated that a bulky Y33 clashed with loop D of gp120. Maturation to residues with smaller side chains potentially led to increased breadth and potency against HIV-1.
Previous studies of VRC01 class antibodies indicate that these antibodies evolve to have shorter or more flexible CDR L1 via deletion or mutation to glycine, allowing the CDR L1 to avoid clashing with the conserved glycan276, and thus improve access to native trimer and neutralization (Zhou et al., 2013). Neutralization assays indicated that 2411a and 2413a were able to neutralize HIV-1 strains with glycan276. However, the CDR L1 of 2411a did not contain deletions nor mutation to glycine, but instead had 5 mutations including Q27Y, S27aF, V28I, S30R and S31D in its CDR L1. Although we failed to crystalize 2411a Fab with fully glycosylated gp120, limited Endo H digestion of the 2411a Fab - d45-01dG5-K278T complex yielded crystals and the structure revealed N-linked NAG-NAG-BMA moieties attached to N276 on gp120 (Figure 4A). Glycan276 lodged between 2411a CDR L1 and the gp120, extending from loop D to V5 (Figures 4A and 4D). The NAG1, NAG2 and BMA3 moieties had 144 Å2, 143 Å2 and 89 Å2 binding surface for 2411a, respectively, providing 25% of the 2411a epitope on gp120, and 63% of the light chain contact (Table S7). NAG1 formed extensive hydrogen bonds with residues I28, S29, D31 and Y32 in CDR L1 and E90 and Y91 in CDR L3. Facing the middle of CDR L1, NAG2 interacted with residues Y27, F27a, I28 and S29 in CDR L1 and E90 and Y91 in CDR L3. BMA3 formed stacking interactions with Y27 and hydrogen bond with F27a of CDR L1 (Figure 4D). Overall, the light chain of 2411a provided 256 Å2 binding surface for glycans on N276, of which 64% of the surface areas were from mutated residues, such as the Y27, F27a, I28, and D31 in CDR L1 (Table S7). These data demonstrated an alternative strategy for VRC01-class antibodies to accommodate glycan276: by direct binding rather than passive accommodation.
Mutagenesis analyses identified key mutations required for breadth and potency of elicited transgenic mouse VRC01-class bnAbs
The most potent and 2nd broadest of all the elicited VRC01-class nAbs, 2411a, had 21 V-region mutated residues in the heavy chain and 11 in the light chain (Figures 3E and 5A). We reverted each of these 32 residues, or two at a time (when adjacent), to respective germline residues (Figure 5A). These partially reverted antibodies were tested for binding to 18 Env antigens and for neutralization on a representative 10-virus panel comprising HIV-1 strains from different clades with 9 bearing glycan276 (Figure 5B). We determined the mean fold changes in ELISA binding titer (OD450) to each antigen and the mean fold changes in neutralization titer to each tested virus of every revertant antibody versus the parental antibody (Figure 5B and STAR Methods). Compared to the parental 2411a, most revertants did not cause significant diminution in either antigen binding or neutralization; however, reverting the following residues reduced both binding and neutralization, with the fold-change in binding and neutralization titers shown in parentheses for each antibody: Y33V/M34L (3.9, 29) in the CDR H1; N52K (5.4, 21), N53R/S54Y (6.5, 29), G56A/T57V (5.5, 25) and Q61H (1.4, 5.1) in the CDR H2; as well as Q27Y (1.8, 15) and S27aF (1.6, 5.2) in the CDR L1 (Figure 5B). Heavy chain V37M may also be important since its revertant caused 1.9-fold decrease in neutralization (Figure 5B).
Figure 5. Mutagenesis studies pinpoint key affinity-matured residues responsible for broad binding and neutralization activity of bnAbs 2411a and 2413a.

(A) List of revertant antibodies. Each mutated residue of 2411a and selected residues in 2413a were individually reverted to its germline residue, and mutant antibodies that contain only the specified reverted SHM point mutations were made.
(B) The impact of individual residues reverted to germline on 2411a binding and neutralization titers. Left, heat map showing the ELISA binding (OD450) of parental and revertant 2411a IgGs to various HIV-Env antigens. Right, the neutralization titers (IC50) of parental 2411a and 2413a as well as their revertants against a panel of 10 tier II viruses. Middle, the mean fold changes in ELISA binding titers to each antigen and neutralization titers to each tested virus of every SHM revertant antibody compared to its parental antibody. Revertant antibodies with ELISA fold change >1.5 and/or neutralization fold change >2.0 were highlighted in red, both in A and B. The 2411a-H-V37M revertant with a neutralization fold change of 1.91 was labeled in dark red.
(C) Mutability analysis identifies the key mutated residues as rare mutations. The positional mutation frequency of IGHV1-2 and IGKV3-20 genes was estimated from 6883 and 21915 antibody clones from 293 non-HIV human antibody repertoires, respectively (see STAR Methods for detail). The logo graphs show the positional mutation frequency for all mutations on each position of the VH1-2 (top) and VK3-20 (bottom) genes. The 2411a and 2413a mutated residues with lower than 0.5% frequency are deemed rare and labeled in red. The germline residues of the identified key mutations as in (B) are labeled in teal for comparison.
To differentiate the impact of each single residue in the heavy chain Y33V/M34L, N53R/S54Y and G56A/T57V dual residue mutants, we created single residue revertants Y33V, S54Y and T54V respectively, and assessed their neutralization. The Y33V revertant matched the effect of its corresponding dual mutant Y33V/M34L on the neutralization of the 10 viruses, with single and double mutants each showing 30-fold mean increase in IC50 compared to parental 2411a. In contrast, the heavy chain S54Y and T57V revertants, which decreased the neutralization activity by 24- and 14-fold respectively, did not match the 29- and 25-fold impact caused by their corresponding dual mutants N53R/S54Y and G56A/T57V, respectively. These results suggested that heavy chain mutation M34L probably did not contribute to the functionality of the antibody, but heavy chain Y33V, N53R, S54Y, G56A and T57V each improved antibody neutralization potency and breadth. Furthermore, in another VRC01-class nAb 2413a, reverting mutations at the same heavy chain positions, Y33F, S54F and T57V, also significantly increased neutralization IC50s by 2.1, 19, and 12-fold, respectively (Figures 5A and 5B). In addition, 2413a light chain had a S29G mutation in the CDR L1. Reverting this G29 to the bigger germline residue Ser compromised the neutralization index by 4.2-fold.
Consistent with these mutagenesis results, gene-specific substitution profiles of VH1-2 heavy chains (n=6883) and VK3-20 light chains (n=21915) from 293 non-HIV human antibody repertoires (Guo et al., 2019) identified the same residues in 2411a and/or 2413a, namely heavy chain Y33V2411a, S54Y2411a , S54F2413a, T57V2411a/2413a, kappa chain Q27Y2411a and S27aF2411a, as rare mutations that had <0.5% of chance to occur naturally by activation-induced deaminase (AID) activity without any selection (Figure 5C). Notably, nearly all the above identified key amino acid mutations were also gp120-contact residues in 2411a and 2413a co-crystal structures (Figure 4 and Table S7) as well as the VRC01-gp120 complex (Zhou et al., 2010), supporting their importance in antibody function. Given their low frequency and functional significance, it is likely that these rare mutations were actively selected by the immunogens.
Longitudinal antibody-sequence analyses reveal developmental SHM pathways induced by sequential immunization
Because mice were sacrificed after each step of sequential immunizations, we could not connect VRC01-class bnAb lineages in terminal mice with earlier time points. However, each immunogen did seem to induce similar patterns of SHMs in the V-regions of diverse VH1-2/VK3-20 antibodies (Tian et al., 2016). To understand how key SHM developed along the immunization course and what immunogens elicited them, we analyzed hundreds of VH1-2 and VK3-20 antibody sequences amplified from antigen-sorted CD4bs-specific single B cells following each step of sequential immunizations, aligned them to their respective germline V-genes and displayed the mutated residues in logo graphs (Figure S5). We also calculated frequencies and numbers of key mutated residues identified by structural analysis (Figure 4) and mutagenesis study (Figure 5) in these antibody sequences (Figure 6A). The results showed sequential immunization to gradually increase both the frequency and the number of key and total mutations in both G1 and G2 mice (Figures 6 and S5). In most cases, new mutations were introduced on top of previous mutations at each step of the sequential immunizations. At post 9, the aa-affinity maturation level reached the peak in the antibodies isolated from three-probe sorted B cells (Figures 6 and S5). Notably, many of these mutations are frequently observed in known VRC01-class bnAbs (Figure S6) and are located at Env contact sites (Figures 6C and S5, highlighted residues). These important residues include K19R, Y33F/V/L/I, V37M/I/L, N52K/R, N53K/R/V/T/L/Y, S54G/R/N/Y/F/W/T/H, G56A, T57V/I/P/R, Q61R/H/K/W and V89I/L/M/T in the VH1-2 heavy chain and Q27Y/F/H, S27aF, deletions and glycine mutations in the CDR L1 of the VK3-20 light chain.
Figure 6. Key mutations elicited by tested immunogens during sequential immunizations.

(A)Summary of occurrence frequency of key mutations among amplified VRC01-class IgG sequences at different time points along sequential immunization with different immunogens. Refer to (B) and Figure S5 for number of VH1-2 and VK-320 sequences analyzed.
(B)Scatter plot showing the number of key mutations in each VH1-2 heavy chain amplified at different time points along sequential immunizations. The number of VH1-2 sequences at each time point is labeled above each corresponding plot.
(C)Mapping of the development of maturation residues. The 2411a-gp120 complex is shown in cartoon representation with paratope residues shown in red. Critical residues for the mature 2411a antibody are shown in spheres and colored according to their percentages of occurrence from blue to magenta. Mutations at heavy chain residues 54 and 61 critical for interacting with the gp120 “F43 pocket” and loop V5 are the first ones to be observed. In contrast, mutations on CDR L1 required for effective accommodation of glycan276 occurred only after the 9th immunization.
See also Figures S5–S7.
We next focused on how affinity maturation developed during sequential immunizations. In naïve mice, VRC01-class BCRs had few alterations in either VH1-2 heavy chain or VK3-20 light chain. Priming with eOD-GT6/8 60mer induced heavy chain (H-) Q61R/H (Figures 6A and S5A), a key change that inserts into the gp120 cavity formed by β24 and V5 loop and forms a critical contact site (Table S7) (Zhou et al., 2010). eOD-GT8 60mer also elicited the key H-S54G/R/N/F/T mutation, which is frequently seen in VRC01-class bnAbs (Figure S6) and interacts with gp120 CD4bs residue D368 (Zhou et al., 2010). For the 2nd immunization, 426c-degly3-Ferritin in group 1 elicited heavy chain N53K and S54R/N/T (Figures 6 and S5), which rendered an intermediate antibody the neutralizing activity against glycan276-deficient viruses (Figure S7); while C13-Ferritin in group 2, in addition to strengthening the previous heavy chain mutations Q61H and S54G, elicited two key mutations H-Y33F and H-N52K that interacted with gp120 loop D (Figures 6 and S5, Table S7). Both immunogens also started to elicit changes in the CDR L1 region of light chain (Figure 6 and S5). The acquisition of CDR H2 S54 mutations in some post 1 G2 mice and post 2 mice of both groups correlated with their serum neutralization activity against glycan276-deficient 45-01dG5 and 426c mutant viruses (Figure 1D and Table S1), demarking the first milestone of affinity maturation for VRC01-class antibodies neutralization of a glycan276-deficient virus.
The 3rd immunization of G1 mice with 426c-degly2 core, which had a V5 glycan (at N463) like the C13 core, induced the same heavy chain K19R and Y33F mutations as C13-Ferritin and additional light chain alterations such as deletions (represented by letter “O” in Figure S5B) and/or glycine mutations in CDR L1. These additional mutations may help to accommodate the added N463 glycan in the immunogen either by directly contact or by creating additional contacts at other parts of gp120 to compensate for the steric hindrance caused by this glycan. For example, H-R19 has been shown to interact with the N197 glycan (Jardine et al., 2016b); H-F33 likely interacted with loop D (Table S7d). The smaller CDR L1 may give the antibody more room to wiggle around the V5 glycan. The 4th immunogen, 426c-degly1 core, had one more V5 glycan at N460 added back, and elicited the key CDR H2 mutations G56A and T57V that interacted with loop D of gp120 (Table S7d). So did the 4th immunogen of group 2, the fully glycosylated 426c.DS.SOSIP trimer. Concurrent with the elicitation of the key CDR H2 mutations G56A and/or T57V, we observed cross-strain neutralization against glycan276-bearing viruses by the post 4 sera (Figure 1D). We highlight the elicitation of CDR H2 mutations G56A and T54V and the correlated antibody neutralization of some glycan276-bearing viruses as the second milestone of affinity maturation.
The 5th-6th immunizations in G1 mice appeared to have decreased the frequencies of key VH1-2 mutations enriched at post 4 appeared to have decreased (Figure 6A and 6B). This coincided with the decrease of mean VH1-2 nucleotide mutation frequency (Figure 2A) and serum neutralization potency and breadth (Figure 1D) from post 4 to post 6. Due to the lack of mice sacrificed at post 6-8, we had no direct information on how the later boosts affected the SHM patterns of elicited VRC01-class antibodies; however, both G1 and G2 mice had significantly decreased serum neutralization activity since post 4 (Figure 1D), suggesting lack of effective boosting by these immunogens. We speculated that dominant off-target responses caused by Env trimers and insufficient conservation at the CD4bs between different boosting immunogens were the cause and thus the limiting factors for further effective boosting. However, the final boost with N241 glycan-filled BG505.DS.SOSIP, which lacked a dominant off-target epitope, the N241 glycan hole, recovered the broader serum neutralization activity lost at post 8 (Figure 1D) and the VRC01-class antibodies isolated by the three-color B cell sorting from this time point (post 9) exhibited the highest SHM levels (Figure 3C). Moreover, these antibodies possessed nearly all the key mutated residues (Figure 6) and showed broad neutralizing activities against a 208-virus panel (Figure 3).
Combining data obtained from two different sequential immunization strategies, we summarized our findings on the relationships between immunogens, the key mutations induced by the immunogens, and the neutralization activity of corresponding sera or elicited VRC01-class nAbs, and propose a 4-step immunization strategy for eliciting VRC01-class bnAbs (Figure 7). First, wild type or glycan masked eOD-GT8 60mer (Duan et al., 2018) is used to increase the levels of VRC01-class precursors and introduce the initial key mutations CDR H2 Q61R and S54G/R that increase the affinity of the VH1-2 heavy chain to the CD4bs of Env outer domain. These VRC01-class antibodies have little binding activity to wild type gp120 or neutralizing activity against even the most sensitive glycan276-deficient viruses. A second boost with a gp120 core-based germline-targeting nanoparticle immunogen, such as C13.G4.2-Ferritin, can further expand VRC01-class precursors and select for gp120-binding antibodies by introducing key mutations at the antibody-gp120 contact sites, including heavy chain K19R, Y33F and N52K. The elicited antibodies have increased neutralizing activity against glycan276-deficient viruses such as 45-01dG5. The C13 gp120 core contains an N462 glycan that partially shields the CD4bs and is conserved in many HIV-1 viruses. The CD4bs-surrounding glycans may exert selection to enrich mutations that overcome the blocking effect of the glycans. A heterologous third immunization with 426c-degly1 core containing more glycans surrounding the CD4bs (N460 and N463) can induce additional key CDR H2 mutations, G56A and T57V, that appeared to correlate with arising neutralizing activity against glycan276-bearing viruses. Finally, a polishing boost with N241 glycan hole filled BG505.DS.SOSIP trimer further affinity matured the VRC01-class lineages by adding more VRC01-class mutations not only in the heavy chain but also in the CDR L1 for better accommodation or binding of glycan276. More heterologous boosting may further expand the breadth and potency of VRC01-class lineages, but measures must be taken to minimize off-target epitopes in the boost immunogens, which should have a sufficiently conserved CD4bs with previous immunogens to maintain immune focus on the CD4bs.
Figure 7. Relationships between immunogens, development of antibody mutations, and neutralization activity of corresponding serum or elicited antibodies in a proposed sequential immunization scheme.

Immunogens are shown in gray surfaces with potential antibody contact areas colored green for heavy chain and slate for light chain. Glycans on the HIV-1 Env are shown in sticks and colored teal for their C atoms. Glycans N276, N460 and N463 on the 2nd and 3rd boost immunogens are shown in spheres. The V region of elicited antibody is shown in cartoon representation with heavy chain colored green and light chain colored slate. Prevalent somatic hypermutations induced by each immunization is highlighted as magenta spheres.
Discussion
In prior studies using transgenic mice with unmutated VRC01 class precursors, , vaccine-elicited antibodies have been either unable to neutralize HIV-1 (Dosenovic et al., 2015; McGuire et al., 2016) or have display limited neutralization breadth due to their inability to accommodate glycan276 (Jardine et al., 2016b; Tian et al., 2016). Recently, PGT121-like bnAbs with high levels of somatic hypermutation have been elicited by sequential immunization from a mouse model that exclusively expresses the inferred germline PGT121 precursors with fixed CDR H3 and CDR L3 containing the predetermined human D and J gene sequences (Escolano et al., 2016). This study suggests that high SHM and cross-reactive neutralizing antibodies can be elicited by immunization given the presence of sufficient bnAb precursors. Our study extended this proof of concept to VRC01-class bnAbs, using a mouse model in which the VRC01 germline VH gene recombines with diverse mouse D and JH gene segments and pairs with an unmutated rearranged light chain to create a diverse repertoire of VRC01-class precursors that are expressed at lower levels (25% of total IgG+ B cells) than the PGT121 precursor mouse model (close to 100%). By sequential immunization, we successfully amplified the B cells expressing VRC01-class precursors, drove their SHM levels to over 20%, and elicited cross-reactive serum neutralizing activity. Thirteen VRC01-class nAb lineages were elicited, the best of which displayed 54% neutralization breadth.
Among the 13 elicited VRC01-class nAb lineages, the heavy chains had 12-29 V-region mutations and the light chains had 7-18. Some of these, such as heavy chain G31D and M34I, are at intrinsic AID hot spots and frequently identified in non-HIV VH1-2 or VK3-20 antibodies, whereas others are uncommon in non-HIV antibodies and appear to be positively selected in VRC01-class antibodies. The systemic mutagenesis studies of bnAbs 2411a and 2413a confirmed that a small number of key mutations were required for neutralization breadth; these included Y33F/V in the CDR H1, N52K, N53K/R, H-S54G/R/F/Y/W, G56A and T57V in the CDR H2, Q61R/H in the heavy chain frame work 3, as well as mutations in the CDR L1 that either directly bind to or help to accommodate glycan276. Crystal structures of 2411a and 2413a with gp120 cores demonstrated that these key mutations provided important binding interactions. These key mutations are consistent with motifs identified in other studies that either determines the minimally required mutations for VRC01 function (Jardine et al., 2016b) or delineates the important motifs needed for the rapid development of a VRC01-class bnAb lineage in an HIV patient (Umotoy et al., 2019). Elicitation and accumulation of these key mutations correlated with increased gp120 binding titers and neutralization activities of the antibodies: from no gp120-binding to gp120-binding but no neutralization, to neutralization of glycan276-deficient viruses, and finally to neutralization of glycan276-bearing viruses.
Loop D glycan276 resides at the edge of the CD4bs and shields the CD4bs from easy access by antibodies (Stewart-Jones et al., 2016), and therefore is a hurdle that must be dealt with by any CD4bs-directed bnAb. Nearly all known VRC01-class bnAbs avoid clashing with this glycan by shortening the length or increasing flexibility of their CDR L1s. We found CDR L1 deletions or glycine mutations to occur at early stages of immunization, but without the key VH1-2 heavy chain mutations, antibodies with these mutations did not neutralize. In this study, 9 of 13 elicited VRC01-class nAb lineages have CDR L1 deletions and/or mutations from larger residues to the smaller glycine or serine. However, the four remaining lineages, as represented by the bnAb 2411a with 51% neutralizing breadth, do not have a deletion or glycine in their CDR L1s, but instead have bulky residues like tyrosine and phenylalanine through somatic hypermutation. Crystal structure of 2411a in complex with a glycan276-bearing gp120 core revealed that these bulky residues, i.e. Y27 and F27a, directly interacted with glycan276 and thus enhanced the binding of the antibody to the gp120 core. These findings demonstrate an alternative mechanism of accommodating glycan276. The effective maturation of VRC01 class antibody lineages during natural infection may also require early engagement of glycan276 (Umotoy et al., 2019).
Ideally, an HIV vaccine that induces nAbs would be comprised of the least number of immunizations in the shortest time frame. In this study, although we isolated VRC01-class bnAbs after 9 immunizations, the peak serum neutralization breadth and potency was achieved as early as post 4. Moreover, VRC01-class nAbs capable of neutralizing heterologous glycan276-bearing viruses were isolated after four immunizations, confirming the post 4 serum neutralizing activity. Therefore, these immunizations were sufficient to elicit cross-reactive serum neutralization against at least some glycan276-bearing viruses. Despite the lower frequency of CD4bs-specific B cells and lower serum neutralization activity at post 9 than at post 4, the cross-reactive VRC01-class nAbs isolated after nine immunizations had higher SHM levels. A possible explanation for this observation is that after nine immunizations there were fewer but more affinity matured VRC01-class bnAbs in the serum and the overall serum neutralization titers were lower than post 4 due to the low concentrations of these bnAbs. Since the later (6-8th) boosts negatively impacted the serum neutralization titers, it is not clear whether the higher SHM levels of post 9 nAbs were caused by the additional heterologous boosts or simply time, which need be tested in future studies.
Two different sequential immunizations, both utilizing gp120 core nanoparticles as 2nd boost, succeeded in eliciting VRC01-class bnAbs, while the repeated eOD-GT8 60mer priming with later gp120 and Env-trimer boosts did not, demonstrating that the early boost with germline-targeting gp120 cores is an important intermediate immunization step. Longitudinal analysis of antibody sequences isolated after each immunization helped to reveal which immunogens elicited the key mutations required for achieving the neutralization potency and breadth, and thus depicted a vaccination pathway to eliciting VRC01-bnAbs.
Limitations of Study
This work provide evidence that it is possible to elicit and affinity mature VRC01 class antibodies to the point of effective cross-reactive neutralization. An important limitation is the use of a mouse model with a much higher frequency of VRC01 class precursor B cells than found in humans: approximately 25% of mouse IgG+ splenic B cells compared a frequency of 1 in 250,000 in humans. Thus, this mouse model does not necessarily predict immune responses that will be seen in humans and is more likely suited to identify immunogens and immunization strategies that have potential for success in human vaccine studies. We can screen immunogens in this model, to identify those best for priming VRC01-class responses, as well as those best for intermediate and final boosting. The eOD-GT8 nanoparticle immunogen is currently being tested in a phase 1-clinical study to assess its ability to stimulate VRC01-class lineages, but it is not expected to generate sufficient SHM to yield bnAbs. This mouse model and the immunization strategies described herein may thus help to guide the selection of intermediate and final boost immunogens for future human studies.
STAR*METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Peter D. Kwong (pdkwong@nih.gov) and John R. Mascola (jmascola@nih.gov).
Materials Availability
All materials generated in this study are available upon request with a completed Materials Transfer Agreement.
Data and Code Availability
NGS data for the terminal mice VH1-2 and VK3-20 sequences can be downloaded from the NCBI Short Read Archive (SRA) under accession number PRJNA623583.
The sequences of the cloned antibodies from immunized VH1-2/LC mice (described as antibodies prepended by 2403, 2404, 2405, 2406, 2408, 2410, 2411, 2413 in the Key Resources Table) have been deposited in GenBank: MT551660 - MT551703 and MT551704 - MT551747. The structures of the 2411a-gp120 and 2413a-gp120 complexes are available in protein data bank under accession codes of 7JKS and 7JKT, respectively.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| 2406a | This paper | MT551685, MT551729 |
| 2408a | This paper | MT551686, MT551730 |
| 2408b | This paper | MT551687, MT551731 |
| 2408c | This paper | MT551688, MT551732 |
| 2408c.2 | This paper | MT551689, MT551733 |
| 2408c.3 | This paper | MT551690, MT551734 |
| 2408c.4 | This paper | MT551692, MT551735 |
| 2408d | This paper | MT551691, MT551736 |
| 2408e | This paper | MT551693, MT551737 |
| 2408e.2 | This paper | MT551694, MT551738 |
| 2408f | This paper | MT551695, MT551739 |
| 2411a | This paper | MT551696, MT551740 |
| 2411a.2 | This paper | MT551697, MT551741 |
| 2411b | This paper | MT551698, MT551742 |
| 2411b.2 | This paper | MT551699, MT551743 |
| 2413a | This paper | MT551700, MT551744 |
| 2413b | This paper | MT551701, MT551745 |
| 2413c | This paper | MT551702, MT551746 |
| 2413d | This paper | MT551703, MT551747 |
| KIIM | This paper | MT551684, MT551728 |
| 1538-79 | Tian et al., 2016 | KX462817, KX462842 |
| 2405p1-12 | This paper | MT551667, MT551711 |
| 2405p1-72 | This paper | MT551668, MT551712 |
| 2405p1-80 | This paper | MT551669, MT551713 |
| 2404-36 | This paper | MT551665, MT551709 |
| 2404-18 | This paper | MT551663, MT551707 |
| 2404-20 | This paper | MT551664, MT551708 |
| 2403-93 | This paper | MT551662, MT551706 |
| 2403-59 | This paper | MT551661, MT551705 |
| 2403-16 | This paper | MT551660, MT551704 |
| 2410-05 | This paper | MT551670, MT551714 |
| 2410-07 | This paper | MT551672, MT551716 |
| 2410-78 | This paper | MT551676, MT551720 |
| 2410-19 | This paper | MT551673, MT551717 |
| 2410-06 | This paper | MT551671, MT551715 |
| 2410-68 | This paper | MT551675, MT551719 |
| 2410-42 | This paper | MT551674, MT551718 |
| 2410-93 | This paper | MT551677, MT551721 |
| 2414-27 | This paper | MT551681, MT551725 |
| 2414-44 | This paper | MT551682, MT551726 |
| 2414-61 | This paper | MT551683, MT551727 |
| 2404-E12 | This paper | MT551666, MT551710 |
| 2410A-A4 | This paper | MT551678, MT551722 |
| 2410A-A5 | This paper | MT551679, MT551723 |
| 2410B-G6 | This paper | MT551680, MT551724 |
| VRC01 | Wu et al., 2010 | RRID: AB_2491019 |
| anti-mouse CD3 Cy5.5PerCP | BD Pharmingen | Cat# 551163; RRID: AB_394082 |
| anti-mouse CD4 Cy5.5PerCP | BioLegend | Cat# 100540; RRID: AB_893326 |
| anti-mouse CD8 Cy5.5PerCP | BD Pharmingen | Cat# 551162; RRID: AB_394081 |
| anti-mouse F4/80 Cy5.5PerCP | BioLegend | Cat# 123128; RRID: AB_893484 |
| anti-mouse B220 TrPE | BD Pharmingen | Cat# 551489; RRID: AB_394219 |
| anti-mouse IgD BV711 | BioLegend | Cat# 405731; RRID: AB_2563342 |
| anti-mouse IgM Cy7PE | eBioscience | Cat# 25-5790-82; RRID: AB_469655 |
| anti-mouse IgG1 FITC | BD Pharmingen | Cat# 553443; RRID: AB_394862 |
| anti-mouse IgG2a FITC | BD Pharmingen | Cat# 553390; RRID: AB_394828 |
| anti-mouse IgG2b FITC | BD Pharmingen | Cat# 553395; RRID: AB_394833 |
| anti-mouse IgG3 FITC | BD Pharmingen | Cat# 553403; RRID: AB_394840 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| C13.G3-AviHis | Tian et al., 2016 | KX462845 |
| ΔC13-AviHis | Tian et al., 2016 | N/A |
| C13.G4-Ferritin | This paper | N/A |
| 426c-degly3 core-Ferritin | Tian et al., 2016 | KX462844 |
| 426c-degly3 core-AviHis | Tian et al., 2016 | KX518319 |
| Δ426c-degly3 core-AviHis | Tian et al., 2016 | KX518320 |
| 426c-degly2 core monomer | Tian et al., 2016 | KX518321 |
| 426c-degly1 core monomer | Tian et al., 2016 | KX518322 |
| 426c-WT.core monomer | Tian et al., 2016 | KX518323 |
| 426c-WT.DS.SOSIP | Tian et al., 2016 | KX462847 |
| eOD-GT8 60mer | Tian et al., 2016 | KX527857 |
| eOD-GT8-AviHis | Tian et al., 2016 | KX527855 |
| ΔeOD-GT8-AviHis | Tian et al., 2016 | KX527856 |
| eOD-GT6 60mer | Tian et al., 2016 | KX527854 |
| eOD-GT6-AviHis | Tian et al., 2016 | KX527852 |
| ΔeOD-GT6-AviHis | Tian et al., 2016 | KX527853 |
| BG505.SOSIP.T332N | Tian et al., 2016 | N/A |
| BG505.DS.SOSIP | (Kwon et al., 2015) | N/A |
| BG505.DS.SOSIP.S241N | This paper | N/A |
| RSC3 | NIH AIDS Reagent Program; Wu et al., 2010 | Cat# 12042 |
| ZM106.DS.SOSIP | Joyce et al, 2017 | N/A |
| CH505.DS.SOSIP | (Cheng et al., 2019) | N/A |
| UG037.8 core | This paper | N/A |
| Poly I:C | Invivogen | Cat# tlrl-pic-5 |
| d45-01dG5 gp120 core | Wu et al., 2015 | PDB: 4XVS_G |
| SuperScript® II Reverse Transcriptase | ThermoFisher Scientific Inc. | Cat# 18064014 |
| SuperScript® III Reverse Transcriptase | ThermoFisher Scientific Inc. | Cat# 18080044 |
| HotStarTaq Plus DNA Polymerase | Qiagen | Cat# 203607 |
| Galanthus nivalis (GNA)-lectin gel | EY Laboratories, Inc. | Cat# A-7401-2 |
| Ni Sepharose™ excel | GE Healthcare | Cat# 17-3712-03 |
| Protein G Sepharose™ 4 Fast Flow | GE Healthcare | Cat# 17-0618-05 |
| SureBlue™ TMB Microwell Peroxidase Substrate | Kirkegaard & Perry Laboratories, Inc. | Cat# 52-00-00 |
| Extravidin-PE | Sigma | Cat# E4011 |
| Streptavidin-APC | ThermoFisher Scientific Inc. | Cat# SA1005 |
| ViViD (LIVE/DEAD® fixable violet dead cell stain) | ThermoFisher Scientific Inc. | Cat# L34964 |
| Critical Commercial Assays | ||
| Bio-Layer Interferometry Antigenicity Analysis | ForteBio | Octet® HTX System |
| MiSeq Reagent Nano kit, ver2 | Illumina, Inc. | Cat# MS-102-2003 |
| Ovation Ultralow System V2 1-96 kit | NuGen Technologies, Inc. | Cat# 0344NB-08 |
| Deposited Data | ||
| Structure of 2411a in complex with glycosylated HIV-1 gp120 core | This paper | PDB: 7JKS |
| Structure of 2413a in complex with deglycosylated HIV-1 gp120 core | This paper | PDB: 7JKT |
| Deep sequencing of B cell repertoire | This paper | PRJNA623583 |
| Experimental Models: Cell Lines | ||
| Mouse: ES clone for VH1-2/LC mouse model | Tian et al., 2016 | N/A |
| Human: Expi293F™ cells | ThermoFisher Scientific Inc. | Cat# A14528; RRID: CVCL_D615 |
| Human: FreeStyle™ 293F cells | ThermoFisher Scientific Inc. | Cat# R79007 |
| Experimental Models: Organisms/Strains | ||
| Mus musculus: VH1-2/LC mouse model; mixed 129/Sv and C57BL/6 strains | Tian et al., 2016 | N/A |
| Recombinant DNA | ||
| VRC2742: mouse IgG2a heavy chain expression vector | This paper | N/A |
| VRC3353: mouse Kappa chain expression vector | This paper | N/A |
| Sequence-Based Reagents | ||
| Primers for single cell RT-PCR in the VH1-2/LC mouse models | Tian et al., 2016 | Table S6 |
| VH1-2 NGS 5’ primer: xj-VH1-1st ACAGGAGCCCACTCCCAGGTGCAG |
Tian et al., 2016 | Table S6 |
| VH1-2 NGS 3’ primer: 3’musCg-2nd CCAGGGGCCAGTGGATAGACHGATGG |
Tian et al., 2016 | Table S6 |
| VK3-20 NGS 5’ primer: 5’ hVK3-20.6 CGCAGCTTCTCTTCCTCCTG |
Tian et al., 2016 | Table S6 |
| VK3-20 NGS 3’ primer: 3’musCk-1st ACTGGATGGTGGGAAGATGGA |
Tian et al., 2016 | Table S6 |
| Software and Algorithms | ||
| GraphPad Prism 8.01 Software | GraphPad Prism Software, Inc. | RRID: SCR_002798 |
| IMGT/V-Quest | (Brochet et al., 2008; Giudicelli et al., 2011) | http://www.imgt.org/IMGT_vquest/vquest; RRID: SCR_010749 |
| WebLogo | (Crooks et al., 2004) | http://weblogo.berkeley.edu/logo.cgi; RRID: SCR_010236 |
| mGSSP | (Sheng et al., 2017) | https://github.com/scharch/SONAR |
| SONAR | (Schramm et al., 2016) | https://github.com/scharch/SONAR |
| CLUSTALO | (Sievers and Higgins, 2018) | http://clustal.org/omega/; RRID: SCR_001591 |
| HKL2000 | HKL Research, Inc. | https://hkl-xray.com/; |
| The PyMol Molecular Graphics System, v1.8.6 | Schrödinger, LLC | https://pymol.org/2/; RRID: SCR_000305 |
| Phenix | (Adams et al., 2004) | https://sbgrid.org/software/; RRID: SCR_014224 |
| FlowJo v.9.9.1 and v.10.6.2 | FlowJo | https://www.flowjo.com; RRID: SCR_008520 |
| R v3.5.2 | The Comprehensive R Archive Network | https://cran.r-project.org/ |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
The VH1-2/LC mouse has been described (Tian et al., 2016). The usage percentage of human VH1-2 and unmutated VRC01 light chain (VK3-20 with 5aa CDRL3) in the splenic B cells of this transgenic mouse model was determined to be ~40% and 94%, respectively (Tian et al., 2016), and thus ~37% of splenic B cells are expected to be VRC01-class precursors that express both the VH1-2 and unmutated VRC01 LC. The VRC01-class precursor frequency in IgG+ splenic B cells appears to be lower, ~25% (24 out of 96 IgG+ B cells) as determined by single B cell RT-PCR. The model was generated in Boston Children’s Hospital, but the mice for immunization studies were bred, housed and cared for in accordance with local, state, federal, and institute policies in an American Association for Accreditation of Laboratory Animal Care-accredited facility at the NIH. All animal experiments were reviewed and approved by the Animal Care and Use Committee of the Vaccine Research Center, NIAID, NIH. The animal work was covered under protocol VRC 14-467 for breeding, and VRC 14-480 for immunization.
METHOD DETAILS
Immunizations
For each immunization, 100 µl of immunogen mix, containing 30 µg (for boost) or 60 µg (for prime) of specified, filter-sterilized protein immunogen and 60 µg of poly I:C in PBS, was injected to the inner thigh of the two rear legs of each mouse. The VH1-2/LC mice were immunized monthly for 7 times, and additional immunizations were administered at wk28 for group 3 and at week 36 and 80 for groups 1 and 2. Two weeks after each injection, blood from all animals was collected for serological analyses, and 1-2 animals from each group may be sacrificed for spleen collection. The blood and spleen from a naïve mouse were also collected as reference.
Immunogens
Most immunogens have been described before (Joyce et al., 2017; Tian et al., 2016; Wu et al., 2010). See also Key Resources table for details. Previously undescribed immunogens include:
C13.G4.2-Ferritin, also named C3.2.6WW-Ferritin, is a ferritin nanoparticle of VRC-designed germline-targeting gp120 core C13 (Tian et al., 2016) with minor modifications. C13 gp120 core is an engineered UG037.8-based chimeric gp120 core with a few residues in the D and V5 loops grafted from the 45_01dG5 strain, and it combined both the epitope residues from UG037.8 for recognition of VRC01 germline-reverted heavy chain and the residues from 45_01dG5 for binding of VRC01 germline-reverted light chain (Tian et al., 2016 and Figure S1). A third generation monomeric C13 core, C13.G3, binds VRC01-germline revertant at 173 nM (Figure S1C). The 4th generation C13.G4.2 included a few more deletions and mutations that further reduced the V1V2 and V3 loop stems, made the core more flexible, or enhanced its binding to VRC01 germline-reverted antibody (Figure S1B). The ferritin nanoparticle form of C13.G4.2 displayed strong binding to various VRC01-class bnAb germline revertants, better than 426c-degly3-Ferritin but less than eOD-GT8 60mer (Figure S1, D and E).
426c-degly3.DS.SOSIP: a mutant of 426c-WT.DS.SOSIP (Tian et al., 2016) that lacks the N276, N460 and N463 glycans with S278A, T462A and T465A point mutations.
BG505.DS.SOSIP.S241N: BG505.DS.SOSIP (Pancera et al., 2014) with the “glycan hole” at residue 241 filled by the introduction of S241N mutation.
Protein production
All proteins were produced in transiently transfected Expi293 or 293F cells as previously described (Pancera et al., 2014; Tian et al., 2016). The untagged Env immunogens, including eODGT6(8) 60mers, gp120 cores, and gp120 core ferritin nanoparticles, were purified with Galanthus nivalis (GNA)-lectin gel (EY Laboratories, Inc.) and followed with a size exclusion chromatography (SEC). The various gp140 SOSIP trimer were purified with the 2G12/SEC method as previously described (Pancera et al., 2014). For ELISA and sorting probes, avi-his-tagged eOD-GT6, eOD-GT8, 426-degly3 core, C13 gp120 core and their corresponding CD4bs-disrupting mutants, ΔeOD-GT6 (D279K/D368V), ΔeOD-GT8 (D279K/D368R), Δ426-degly3 core (D279K/D368R) and ΔC13 (D279K), were purified with Ni-NTA beads (GE healthcare) and followed by a gel filtration chromatography for monomeric proteins. For antibody production, heavy and light chain plasmids were co-transfected (1:1 ratio) in Expi293 cells using Expi293fectin (Invitrogen) according to the manufacturer’s protocol. The supernatants were harvested six days later, and the IgG was purified using protein G or A Sepharose (GE Healthcare) and buffer-exchanged into PBS.
To make the antigen binding fragment (Fab) of antibodies for crystallization, a variant plasmid of the heavy chain (IgG-3C) was made in which an HRV3C cleavage site was inserted in the heavy-chain hinge region (Kong et al., 2019). Full-length IgG-3C proteins were produced in Expi293 cells and cleaved by HRV3C overnight at 4oC. The cleavage mixture was passed through a protein A column to remove the uncleaved IgG and constant fragment (Fc). The Fabs were further purified using a Superdex 200 column (GE Healthcare) in a buffer containing 5 mM HEPES pH7.5 and 150 mM NaCl.
HIV-1 gp120 core d45-01dG5-K278T was constructed by introducing K278T into the d45-01dG5 gp120 plasmid (Wu et al., 2015) by site-directed mutagenesis (GeneImmune, New York) to restore the glycosylation site on N276. The d45-01dG5-K278T gp120 core was expressed by transient transfection of GNTI−/− cells and purified with Ni2+-NTA column as described previously (Wu et al., 2015).
ELISA
ELISA was performed as previously described (Tian et al., 2016). Corning Costar half-area assay plates were coated with antigens at 100 ng/well at 4C overnight, blocked with 1:10 diluted blocking solution (Immune Technology Corp.) for purified mAbs at room temperature for 1 hr, and incubated with serial diluted sera or antibodies at room temperature for 1 hr. The plates were then washed with PBS with 0.05% Tween (Sigma) 5 times, and incubated with 1:5,000 diluted HRP-conjugated goat anti-human IgG or goat-anti-mouse IgG (Bio-Rad) at room temperature for 1 hr. The plates were washed again and the SureBlue TMB Microwell Peroxidase Substrate (Kirkegaard & Perry Laboratories, Inc.) was added to each well. The color reaction was terminated with 1N sulfuric acid and the absorbance at 450 nm (OD450) was recorded. Background absorbance was determined based on wells blotted with a non-related primary antibody and endpoint titer was determined as the largest serum dilution or the lowest primary antibody concentration at which the OD450 is three times over the background level.
TZM-bl neutralization assay
Pseudoviruses for use in TZM-bl neutralization assays were produced in 293T cells by cotransfection of a pSG3∆Env backbone plasmid and a full HIV-1 Env gp160-encoding plasmid (Li et al., 2005). Briefly, 2X106 cells in 20ml cDMEM were seeded in T75 flasks the day prior to cotransfection. For transfection, 40μl of FuGene 6 reagent (Promega) was diluted into 800μl of room-temperature Opti-MEM I reduced serum medium (Thermo Fisher), followed by addition of 10μg of pSG3∆Env backbone plasmid. 3.3μg of HIV Env plasmid was then added to the mixture, mixed, and incubated for 30 minutes at room temperature. Transfection mixture was then added to media of previously seeded 293T cells in the T75 flask and then distributed evenly on cells. The following day, media was replaced with 20ml fresh cDMEM. Virus was harvested the following day by filtering cell supernatants with 0.45μm Steriflip units (EMD Millipore) and aliquotted.
To measure neutralization of purified antibodies, 10μl of five-fold serially diluted mAbs in cDMEM was incubated with 40ul of diluted HIV-1 Env-pseudotyped virus and incubated for 30 minutes at 37oC in a 96-well CulturPlate (Perkin Elmer). 20 μl of TZM-bl cells (10,000 cells/well) with or without 70μg/ml DEAE-Dextran was then added and incubated overnight at 37oC. Each experiment plate also had a column of cells only (no Ab or virus) and a column of virus only (no Ab) as controls for background TZM-bl luciferase activity and maximal viral entry, respectively. Serial dilutions were performed with a change of tips at each dilution step to prevent carryover. The following day, all wells received 100μl of fresh cDMEM and were incubated overnight at 37oC. The following day, 50μl of Steadylite Plus Reporter Gene Assay System (PerkinElmer) was added to all wells, and plates were shaken at 600RPM for 15 minutes. Luminometry was then performed on a SpectraMax L (Molecular Devices) luminometer. Percent neutralization is determined by calculating the difference in average RLU between virus only wells (cells + virus column) and test wells (cells + plasma/Ab sample + virus), dividing this result by the average RLU of virus only wells (cell + virus column) and multiplying by 100. Background is subtracted from all test wells using the average RLU from the uninfected control wells (cells only column) before calculating the percent neutralization. Neutralizing plasma antibody titers are expressed as the antibody concentration required to achieve 50% or 80% neutralization (ID50 or ID80) and calculated using a dose-response curve fit with a 5-parameter nonlinear function.
Selected monoclonal antibodies were assessed on a panel of 208 geographically and genetically diverse Env pseudoviruses representing the major subtypes and circulating recombinant forms (Kong et al., 2016). Assays were performed by microneutralization in an optimized and qualified automated 384-well format using robotic liquid handling (Sarzotti-Kelsoe et al., 2014).
Flow cytometry and B cell sorting
Mouse spleen samples were processed for single B cell sorting based on previously described methods (Tiller et al., 2009). In brief, single cell suspension of splenocytes was stained sequentially with ViVid and a staining mix containing anti-CD3 Cy5.5PerCP, anti-CD4 Cy5.5PerCP, anti-CD8 Cy5.5PerCP, anti-F4/80 Cy5.5PerCP, anti-B220 TrPE, anti-IgD BV711, anti-IgM Cy7PE, anti-IgG FITC, eOD-GT6(8)-PE and ΔeOD-GT6(8)-APC. Memory B cells were selected for the phenotype B220+, CD3−, CD4−, CD8−, F4/80−, IgM−, IgD−, and IgG+. To make antigen-specific probes, 50 nM of biotinylated Avi-tagged eOD-GT6(8) monomer and its CD4bs-disrupting variant ΔeOD-GT6(8) (D279K, D368V/R) were coupled to Streptavidin-PE and Streptavidin-APC (Life Technologies), respectively, in equal molar ratios. Similarly, C-terminally Avi-tagged BG505.SOSIP were conjugated with equal molar of Streptavidin-AF700. Memory B cells with either eOD-GT6(8)-PE+ and ΔeOD-GT6(8)-APC- only (two-color sort) or plus BG505.SOSIP-AF700+ (three-color sort) were selected and single-cell sorted into 96 well plates containing lysis buffer on a BD FACSAria III sorter and immediately stored at −80˚C. Spleen samples from naive littermates were used as controls and to set up the gates for sorting.
In the VH1-2/LC mice, sorting with eOD-GT8 and ΔeOD-GT8 for VRC01-class BCRs generated much high background than using the eOD-GT6 and ΔeOD-GT6 probes, 7.6-9.5% vs. 1.0-1.4% of total IgG+ B cell population in naïve mice (Figure S2B and 1C). The percentage of eOD-GT8+/ΔeOD-GT8− B cells increased to 13-18% in G1 and G2 mice after two or three boosts and then decreased to just above the background level (~10%) by the end of all nine immunizations (Figure S2B). The BCRs derived from the eOD-GT8-sorted B cells, even after a further selection for BG505.SOSIP+ population in the post 9 (wk81) samples, were still mostly unmutated germline-like VRC01-class antibodies (0.3-0.7% VH1-2 nt mutation frequency). In contrast, the BCR sequences from the eOD-GT6-sorted BG505.SOSIP+ B cells of the same samples had much higher SHM levels, 13.5-14.2% (Figure S2). These results suggested that eOD-GT8 was much more inclusive than eOD-GT6 for VRC01-class precursors and preferentially fished out the germline VRC01-class BCRs, whereas eOD-GT6 only selected for a small fraction of the relatively more affinity mutated VRC01-class BCRs. Given that eOD-GT6 is more similar to gp120 than eOD-GT8 and the eOD-GT6-reactive VRC01-class precursors might be more likely to develop into gp120-recognizing Abs, we had decided to use eOD-GT6, rather than eOD-GT8, as the primary sorting probe for this study.
Single B cell RT-PCR, gene amplification, and cloning
Reverse transcription and subsequent PCR amplification of heavy and light chain variable genes were performed using SuperScript III (Thermofisher Scientific Inc.) as previously described (Tian et al., 2016). All PCR reactions were performed in 25 ml volume with 1-2.5 ml of cDNA transcript using HotStar Taq DNA polymerase master mix (Qiagen). Specific VH1-2 and VK3-20 5’ primers in combination with designed 3’ primers aligning to the 5’ regions of the mouse gamma (1, 2a, 2b and 3) or kappa constant segments (Tian et al., 2016 and Key Resources Table) were used for nested PCR to amplify VH1-2 and VK3-20 heavy and light chains. PCR products were then sequenced using Sanger sequencing and corrected for PCR errors before further analysis by IMGT/V-quest (Brochet et al., 2008; Giudicelli et al., 2011) and expression. Except for Table S2 where IMGT antibody numbering was used to mark the antibody sequence alignments, in all other figures, tables and the main text, the Kabat antibody numbering scheme was used for the identified antibodies. The confirmed heavy or light chain sequences containing the whole V(D) J or VJ segments were synthesized by GenScript and cloned into a mouse IgG2a (VRC2742) or mouse Kappa (VRC3353) mammalian expression vector for antibody expression.
Next generation sequencing (NGS) of B cell receptor repertoire
NGS analysis of VRC01-class BCR repertoire were performed as described previously (Kong et al., 2019). Mouse memory B cells (ViVid− CD3− CD4− CD8− F4/80− B220+ IgD− IgM− IgG+) from the spleens of post 9 mice 2406, 2408, 2411 and 2413 were bulk sorted into tubes containing 10% FBS/RPMI-1640 medium. Normally, 100,000 to 400, 000 memory B cells were collected from each sample. mRNAs were extracted from sorted cells using Oligotex Direct mRNA Mini Kit (Qiagen). cDNAs were synthesized from mRNA transcripts using Smart-Seq2 method as described previously (Picelli et al., 2014). PCR was performed with VH1-2 or VK3-20 specific primers (for VH1-2, xj-VH1-1st: ACAGGAGCCCACTCCCAGGTGCAG and 3’musCg-2nd: CCAGGGGCCAGTGGATAGACHGATGG; for VK3-20, 5’ hVK3-20.6: CGCAGCTTCTCTTCCTCCTG and 3’musCk-1st: ACTGGATGGTGGGAAGATGGA) using 2x KAPA HIFI HotStart ReadyMix (Kapa Biosystems), and the amplified VH1-2 and VK3-20 rearranged V(D)J fragments were further gel purified with Gel Extraction Kit (Qiagen).
Sequencing were performed by NISC Comparative Sequencing Program. Briefly, Illumina adapters were added to 50 ng of amplicon DNA using the Ovation Ultralow System V2 1-96 kit (NuGen Technologies, Inc.). Amplicons were pooled in equal volume ratio. An aliquot of the pool was run on a MiSeq (Illmina, Inc.) using a MiSeq Reagent Nano kit, ver2. This QC run consisted of 25 cycles followed by an index read. The pool was then rebalanced based on the percentage of reads seen for each amplicon’s index. The final pool was then sequenced on the Miseq in Rapid mode using version 2 chemistry to generate a minimum of 11 M paired-end 300 base reads per amplicon. Post-run processing of data was performed using RTA 1.18.64 and CASAVA 1.8.2.
Preparation of germline revertants of elicited VRC01-class nAbs
DNA fragments encoding the VH1-2 or VK3-20 regions of the elicited VRC01-class nAbs, 2411a or 2413a, containing one or two (adjacent) mutated residues reverted to the corresponding germline residues synthesized by Genscript and cloned into wild type 2411a or 2413a heavy chain or light chain expression vector at the AgeI/SalI (for heavy chain) or AgeI/BsiWI (for light chain) site, respectively. The resulting heavy or chain with the specific residue(s) reverted to germline was then paired with the corresponding mature light or heavy chain for antibody production in transiently transfected Expi293 cells to obtain the specific germline-reverted 2411a or 2413a antibody.
Neutralization fingerprinting analysis
The neutralization fingerprint of a monoclonal antibody is defined as the potency pattern with which the antibody neutralizes a set of diverse viral strains. The neutralization fingerprints of VRC01-class antibodies identified in this paper and a set of published monoclonal antibodies were compared and clustered according to fingerprint similarity, as described previously (Georgiev et al., 2013). Neutralization data of 208 HIV-1 viral strains were used in the fingerprint analysis. Antibodies with less than 10% breadth were excluded from this analysis.
Bio-layer interferometry antigenicity analysis
Kinetics and affinities of antibody-antigen interactions were measured on an Octet (Forte Bio) using Anti-human IgG Fc and Anti- mouse IgG Fc capture biosensors following the instructions. Briefly, purified VRC01 and VRC01gl were loaded (50 µg/ml) on anti-human or anti-mouse IgG Fc biosensors (AHC, AMC, ForteBio). Following a 1% BSA/PBS buffer wash step, C13.G3 core monomer at 3 different concentrations was associated and then allowed to dissociate into 1% BSA/PBS buffer. A 1:1 ratio of antibody to antigen was assumed for the complex to calculate molar concentration and an irrelevant anti-Influenza antibody was used as a negative control in all experiments. Reference wells containing anti-influenza antibody were subtracted from samples wells. All experiments were performed at 30˚C. Octet Analysis Software was used for data analysis, curve fitting and determination of Kd, Kon and Koff. We deemed that no fit was observed if fewer than 3 curves could be fit with an R2 R 0.95.
Crystallization and structure determination of the antibody Fab in complex with HIV-1 gp120
Protein complex of Fab 2411a and HIV-1 d45-01dG5-K278T core gp120 were formed by mixing gp120 with the antibody Fab in a 1:1.2 molar ratio. After 10 minutes incubation, limited Endoglycosidase H deglycosylation of the complex was carried out at room temperature for 2 hrs with 10 units of enzyme per µg gp120 at pH 6.1. The complex was then purified by size exclusion chromatography on a Superdex 200 column (GE Healthcare) with buffer containing 5 mM HEPES, pH 7.5 and 150 mM NaCl. Fractions with gp120-antibody complexes were concentrated to ~10 mg/ml and used for crystallization experiments. Protein complex of Fab 2413a and HIV-1 gp120 core was made by co-incubation with fully deglycosylated HIV-1 d45-01dG5-K278T core gp120 as described previously (Zhou et al., 2010).
Initial crystallization screening was performed with 576 conditions using a Mosquito crystallization robot by the vapor diffusion method in sitting drops containing 0.1 μl of protein and 0.1 μl of reservoir solution at 20 °C. Crystal conditions observed were manually reproduced in hanging drops by mixing 0.50 μl protein with 0.5 μl reservoir solution. Crystals of 2411a Fab and d45-01dG5-K278T were obtained in a condition containing 12 % PEG3350, 100 mM MgCl2, 2 M NaCl and 100 mM Imidazole, pH 6.5. Crystals were flash frozen in liquid nitrogen in the presence of 15 % 2R,3R-butanediol before data collection. Crystals of 2413a Fab and deglycosylated d45-01dG5-K278T were obtained in a condition containing 5.3 % PEG 8000, 130 mM (NH4)2SO4, 100 mM HEPES, pH 7.5, and 2.2 % isopropanol. 30 % Ethylene glycol was added as cryoprotectant to freeze crystals for data collection.
Diffraction data for were collected at the SER-CAT beamline ID-22 (Advanced Photon Source, Argonne National Laboratory) with 1.0000Å x-ray and processed with the HKL2000 suite. Molecular replacement procedures were performed to solve the structures by using PHASER, and iterative model building and refinement were carried out in COOT and PHENIX, respectively. A cross validation (Rfree) test set consisting of 5 % of the data was used throughout the refinement processes.
Gene-specific mutation profile construction
The positional mutation frequency of IGHV1-2 and IGKV3-20 genes was estimated from 6883 and 21915 antibody clones from 293 non-HIV human antibody repertoires (Guo et al., 2019), respectively. Briefly, sequences originated from each V gene were assigned to clones using SONAR (Schramm et al., 2016). From each clone, one sequence was randomly selected and was aligned to the assigned germline gene using ClustalO (Sievers and Higgins, 2018). mGSSP was used to generate gene-specific substitution profile for each gene (Sheng et al., 2017). A “rare mutation” is defined as a mutation with a lower than 0.5% positional mutation frequency among all sequences of the same V-gene (VH1-2 or VK3-20) from the 293 non-HIV human antibody repertoires. The “rare mutations” are rare in non-HIV donors but might be enriched in HIV patients or vaccinated subjects due to selection force exerted by the pathogens or immunogens to benefit the host.
QUANTIFICATION AND STATISTICAL ANALYSIS
For statistical comparison of two individual groups, unpaired nonparametric t-Test (Mann-Whitney test) was performed with two tailed distributions and equal variance. For comparison of the mutation frequencies at multiple time points, ANOVA Kruskal-Wallis test was performed using GraphPad Prism 8.01 Software (GraphPad Prism Software, Inc.).
SUPPLEMENTAL INFORMATION
Table S7. Interaction between Antibody 2411a and HIV-1 gp120, Related to Figure 4.
Supplementary Material
ACKNOWLEDGEMENTS
We thank D. Ambrozak and R. Nguyen for assistance in single-cell sorting, C. Chiedi, M. Dillon, and S. Gloria for animal care and maintenance, NISC Comparative Sequencing Program for NGS of B cell transcripts, members of the Structural Biology Section of the Vaccine Research Center for helpful discussions, and B. Hartman for help in manuscript submission. We thank J. Baalwa, D. Ellenberger, F. Gao, B. Hahn, K. Hong, J. Kim, F. McCutchan, D. Montefiori, L. Morris, E. Sanders-Buell, G. Shaw, R. Swanstrom, M. Thomson, S. Tovanabutra, C. Williamson, and L. Zhang for contributing the HIV-Env plasmids used in our neutralization panel. We thank C. Moore, C. Whittaker, J. Rathmann, G. Padilla, and A.B. McDermott for assistance with neutralization assessments on the 208-strain panel. Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH, by NIH grant P01-AI104722 (to L.S.), by NIAID, Division of AIDS, Center for HIV/AIDS Vaccine Immunology- Immunogen Discovery (CHAVI-ID) 5UM1 AI100645 (to F.W.A.), and by Bill and Melinda Gates Foundation (OPP1162123 to T.Z.). Use of sector 22 (Southeast Region Collaborative Access team) at the Advanced Photon Source was supported by the US Department of Energy, Basic Energy Sciences, Office of Science, under contract number W-31-109-Eng-38. F.W. Alt is an investigator of the Howard Hughes Medical Institute.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Adams PD, Gopal K, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Pai RK, Read RJ, Romo TD, et al. (2004). Recent developments in the PHENIX software for automated crystallographic structure determination. J Synchrotron Radiat 11, 53–55. [DOI] [PubMed] [Google Scholar]
- Brochet X, Lefranc MP, and Giudicelli V (2008). IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 36, W503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Xu K, Kong R, Chuang GY, Corrigan AR, Geng H, Hill KR, Jafari AJ, O’Dell S, Ou L, et al. (2019). Consistent elicitation of cross-clade HIV-neutralizing responses achieved in guinea pigs after fusion peptide priming by repetitive envelope trimer boosting. PLoS One 14, e0215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, and Brenner SE (2004). WebLogo: a sequence logo generator. Genome Res 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, et al. (2015). Immunization for HIV-1 Broadly Neutralizing Antibodies in Human Ig Knockin Mice. Cell 161, 1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Chen X, Boyington JC, Cheng C, Zhang Y, Jafari AJ, Stephens T, Tsybovsky Y, Kalyuzhniy O, Zhao P, et al. (2018). Glycan Masking Focuses Immune Responses to the HIV-1 CD4-Binding Site and Enhances Elicitation of VRC01-Class Precursor Antibodies. Immunity 49, 301–311 e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolano A, Steichen JM, Dosenovic P, Kulp DW, Golijanin J, Sok D, Freund NT, Gitlin AD, Oliveira T, Araki T, et al. (2016). Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell 166, 1445–1458 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowska E, Ramos A, Feng Y, Zhou T, Moquin S, Walker LM, Wu X, Seaman MS, Wrin T, Kwong PD, et al. (2012). PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. J Virol 86, 4394–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, et al. (2016). A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev IS, Doria-Rose NA, Zhou T, Kwon YD, Staupe RP, Moquin S, Chuang GY, Louder MK, Schmidt SD, Altae-Tran HR, et al. (2013). Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science 340, 751–756. [DOI] [PubMed] [Google Scholar]
- Giudicelli V, Brochet X, and Lefranc MP (2011). IMGT/V-QUEST: IMGT standardized analysis of the immunoglobulin (IG) and T cell receptor (TR) nucleotide sequences. Cold Spring Harb Protoc 2011, 695–715. [DOI] [PubMed] [Google Scholar]
- Guo Y, Chen K, Kwong PD, Shapiro L, and Sheng Z (2019). cAb-Rep: A Database of Curated Antibody Repertoires for Exploring Antibody Diversity and Predicting Antibody Prevalence. Front Immunol 10, 2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, et al. (2016). Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med 22, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Kang BH, Ishida E, Zhou T, Griesman T, Sheng Z, Wu F, Doria-Rose NA, Zhang B, McKee K, et al. (2016). Identification of a CD4-Binding-Site Antibody to HIV that Evolved Near-Pan Neutralization Breadth. Immunity 45, 1108–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, et al. (2013). Rational HIV immunogen design to target specific germline B cell receptors. Science 340, 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine JG, Kulp DW, Havenar-Daughton C, Sarkar A, Briney B, Sok D, Sesterhenn F, Ereno-Orbea J, Kalyuzhniy O, Deresa I, et al. (2016. a). HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351, 1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Skog PD, Thinnes TC, Bhullar D, Briney B, et al. (2015). HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 349, 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine JG, Sok D, Julien JP, Briney B, Sarkar A, Liang CH, Scherer EA, Henry Dunand CJ, Adachi Y, Diwanji D, et al. (2016. b). Minimally Mutated HIV-1 Broadly Neutralizing Antibodies to Guide Reductionist Vaccine Design. PLoS Pathog 12, e1005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MG, Georgiev IS, Yang Y, Druz A, Geng H, Chuang GY, Kwon YD, Pancera M, Rawi R, Sastry M, et al. (2017). Soluble Prefusion Closed DS-SOSIP.664-Env Trimers of Diverse HIV-1 Strains. Cell Rep 21, 2992–3002. [DOI] [PubMed] [Google Scholar]
- Kong L, Ju B, Chen Y, He L, Ren L, Liu J, Hong K, Su B, Wang Z, Ozorowski G, et al. (2016). Key gp120 Glycans Pose Roadblocks to the Rapid Development of VRC01-Class Antibodies in an HIV-1-Infected Chinese Donor. Immunity 44, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R, Duan H, Sheng Z, Xu K, Acharya P, Chen X, Cheng C, Dingens AS, Gorman J, Sastry M, et al. (2019). Antibody Lineages with Vaccine-Induced Antigen-Binding Hotspots Develop Broad HIV Neutralization. Cell 178, 567–584 e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YD, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, et al. (2015). Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol 22, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, and Hendrickson WA (1998). Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Gray MD, Dosenovic P, Gitlin AD, Freund NT, Petersen J, Correnti C, Johnsen W, Kegel R, Stuart AB, et al. (2016). Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nat Commun 7, 10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Hoot S, Dreyer AM, Lippy A, Stuart A, Cohen KW, Jardine J, Menis S, Scheid JF, West AP, et al. (2013). Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med 210, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, et al. (2018). Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, et al. (2014). Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, et al. (2014). Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 6, 243ra288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, and Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 9, 171–181. [DOI] [PubMed] [Google Scholar]
- Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, et al. (2016). HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. (2011). Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science (New York, NY) 333, 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm CA, Sheng Z, Zhang Z, Mascola JR, Kwong PD, and Shapiro L (2016). SONAR: A High-Throughput Pipeline for Inferring Antibody Ontogenies from Longitudinal Sequencing of B Cell Transcripts. Front Immunol 7, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Schramm CA, Kong R, Program NCS, Mullikin JC, Mascola JR, Kwong PD, and Shapiro L (2017). Gene-Specific Substitution Profiles Describe the Types and Frequencies of Amino Acid Changes during Antibody Somatic Hypermutation. Front Immunol 8, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, and Higgins DG (2018). Clustal Omega for making accurate alignments of many protein sequences. Protein Sci 27, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D, Pauthner M, Briney B, Lee JH, Saye-Francisco KL, Hsueh J, Ramos A, Le KM, Jones M, Jardine JG, et al. (2016). A Prominent Site of Antibody Vulnerability on HIV Envelope Incorporates a Motif Associated with CCR5 Binding and Its Camouflaging Glycans. Immunity 45, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, Kong R, Thomas PV, Wagh K, Zhou T, Behrens AJ, et al. (2016). Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 165, 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Cheng C, Chen X, Duan H, Cheng HL, Dao M, Sheng Z, Kimble M, Wang L, Lin S, et al. (2016). Induction of HIV Neutralizing Antibody Lineages in Mice with Diverse Precursor Repertoires. Cell 166, 1471–1484 e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Busse CE, and Wardemann H (2009). Cloning and expression of murine Ig genes from single B cells. J Immunol Methods 350, 183–193. [DOI] [PubMed] [Google Scholar]
- Travers SA (2012). Conservation, Compensation, and Evolution of N-Linked Glycans in the HIV-1 Group M Subtypes and Circulating Recombinant Forms. ISRN AIDS 2012, 823605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umotoy J, Bagaya BS, Joyce C, Schiffner T, Menis S, Saye-Francisco KL, Biddle T, Mohan S, Vollbrecht T, Kalyuzhniy O, et al. (2019). Rapid and Focused Maturation of a VRC01-Class HIV Broadly Neutralizing Antibody Lineage Involves Both Binding and Accommodation of the N276-Glycan. Immunity 51, 141–154 e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP Jr., Diskin R, Nussenzweig MC, and Bjorkman PJ (2012). Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A 109, E2083–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wang C, O’Dell S, Li Y, Keele BF, Yang Z, Imamichi H, Doria-Rose N, Hoxie JA, Connors M, et al. (2012). Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J Virol 86, 5844–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. (2010). Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang Z, Schramm CA, Joyce MG, Kwon YD, Zhou T, Sheng Z, Zhang B, O’Dell S, McKee K, et al. (2015). Maturation and Diversity of the VRC01-Antibody Lineage over 15 Years of Chronic HIV-1 Infection. Cell 161, 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. (2011). Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333, 1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, et al. (2010). Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lynch RM, Chen L, Acharya P, Wu X, Doria-Rose NA, Joyce MG, Lingwood D, Soto C, Bailer RT, et al. (2015). Structural Repertoire of HIV-1-Neutralizing Antibodies Targeting the CD4 Supersite in 14 Donors. Cell 161, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, et al. (2013). Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity 39, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NGS data for the terminal mice VH1-2 and VK3-20 sequences can be downloaded from the NCBI Short Read Archive (SRA) under accession number PRJNA623583.
The sequences of the cloned antibodies from immunized VH1-2/LC mice (described as antibodies prepended by 2403, 2404, 2405, 2406, 2408, 2410, 2411, 2413 in the Key Resources Table) have been deposited in GenBank: MT551660 - MT551703 and MT551704 - MT551747. The structures of the 2411a-gp120 and 2413a-gp120 complexes are available in protein data bank under accession codes of 7JKS and 7JKT, respectively.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| 2406a | This paper | MT551685, MT551729 |
| 2408a | This paper | MT551686, MT551730 |
| 2408b | This paper | MT551687, MT551731 |
| 2408c | This paper | MT551688, MT551732 |
| 2408c.2 | This paper | MT551689, MT551733 |
| 2408c.3 | This paper | MT551690, MT551734 |
| 2408c.4 | This paper | MT551692, MT551735 |
| 2408d | This paper | MT551691, MT551736 |
| 2408e | This paper | MT551693, MT551737 |
| 2408e.2 | This paper | MT551694, MT551738 |
| 2408f | This paper | MT551695, MT551739 |
| 2411a | This paper | MT551696, MT551740 |
| 2411a.2 | This paper | MT551697, MT551741 |
| 2411b | This paper | MT551698, MT551742 |
| 2411b.2 | This paper | MT551699, MT551743 |
| 2413a | This paper | MT551700, MT551744 |
| 2413b | This paper | MT551701, MT551745 |
| 2413c | This paper | MT551702, MT551746 |
| 2413d | This paper | MT551703, MT551747 |
| KIIM | This paper | MT551684, MT551728 |
| 1538-79 | Tian et al., 2016 | KX462817, KX462842 |
| 2405p1-12 | This paper | MT551667, MT551711 |
| 2405p1-72 | This paper | MT551668, MT551712 |
| 2405p1-80 | This paper | MT551669, MT551713 |
| 2404-36 | This paper | MT551665, MT551709 |
| 2404-18 | This paper | MT551663, MT551707 |
| 2404-20 | This paper | MT551664, MT551708 |
| 2403-93 | This paper | MT551662, MT551706 |
| 2403-59 | This paper | MT551661, MT551705 |
| 2403-16 | This paper | MT551660, MT551704 |
| 2410-05 | This paper | MT551670, MT551714 |
| 2410-07 | This paper | MT551672, MT551716 |
| 2410-78 | This paper | MT551676, MT551720 |
| 2410-19 | This paper | MT551673, MT551717 |
| 2410-06 | This paper | MT551671, MT551715 |
| 2410-68 | This paper | MT551675, MT551719 |
| 2410-42 | This paper | MT551674, MT551718 |
| 2410-93 | This paper | MT551677, MT551721 |
| 2414-27 | This paper | MT551681, MT551725 |
| 2414-44 | This paper | MT551682, MT551726 |
| 2414-61 | This paper | MT551683, MT551727 |
| 2404-E12 | This paper | MT551666, MT551710 |
| 2410A-A4 | This paper | MT551678, MT551722 |
| 2410A-A5 | This paper | MT551679, MT551723 |
| 2410B-G6 | This paper | MT551680, MT551724 |
| VRC01 | Wu et al., 2010 | RRID: AB_2491019 |
| anti-mouse CD3 Cy5.5PerCP | BD Pharmingen | Cat# 551163; RRID: AB_394082 |
| anti-mouse CD4 Cy5.5PerCP | BioLegend | Cat# 100540; RRID: AB_893326 |
| anti-mouse CD8 Cy5.5PerCP | BD Pharmingen | Cat# 551162; RRID: AB_394081 |
| anti-mouse F4/80 Cy5.5PerCP | BioLegend | Cat# 123128; RRID: AB_893484 |
| anti-mouse B220 TrPE | BD Pharmingen | Cat# 551489; RRID: AB_394219 |
| anti-mouse IgD BV711 | BioLegend | Cat# 405731; RRID: AB_2563342 |
| anti-mouse IgM Cy7PE | eBioscience | Cat# 25-5790-82; RRID: AB_469655 |
| anti-mouse IgG1 FITC | BD Pharmingen | Cat# 553443; RRID: AB_394862 |
| anti-mouse IgG2a FITC | BD Pharmingen | Cat# 553390; RRID: AB_394828 |
| anti-mouse IgG2b FITC | BD Pharmingen | Cat# 553395; RRID: AB_394833 |
| anti-mouse IgG3 FITC | BD Pharmingen | Cat# 553403; RRID: AB_394840 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| C13.G3-AviHis | Tian et al., 2016 | KX462845 |
| ΔC13-AviHis | Tian et al., 2016 | N/A |
| C13.G4-Ferritin | This paper | N/A |
| 426c-degly3 core-Ferritin | Tian et al., 2016 | KX462844 |
| 426c-degly3 core-AviHis | Tian et al., 2016 | KX518319 |
| Δ426c-degly3 core-AviHis | Tian et al., 2016 | KX518320 |
| 426c-degly2 core monomer | Tian et al., 2016 | KX518321 |
| 426c-degly1 core monomer | Tian et al., 2016 | KX518322 |
| 426c-WT.core monomer | Tian et al., 2016 | KX518323 |
| 426c-WT.DS.SOSIP | Tian et al., 2016 | KX462847 |
| eOD-GT8 60mer | Tian et al., 2016 | KX527857 |
| eOD-GT8-AviHis | Tian et al., 2016 | KX527855 |
| ΔeOD-GT8-AviHis | Tian et al., 2016 | KX527856 |
| eOD-GT6 60mer | Tian et al., 2016 | KX527854 |
| eOD-GT6-AviHis | Tian et al., 2016 | KX527852 |
| ΔeOD-GT6-AviHis | Tian et al., 2016 | KX527853 |
| BG505.SOSIP.T332N | Tian et al., 2016 | N/A |
| BG505.DS.SOSIP | (Kwon et al., 2015) | N/A |
| BG505.DS.SOSIP.S241N | This paper | N/A |
| RSC3 | NIH AIDS Reagent Program; Wu et al., 2010 | Cat# 12042 |
| ZM106.DS.SOSIP | Joyce et al, 2017 | N/A |
| CH505.DS.SOSIP | (Cheng et al., 2019) | N/A |
| UG037.8 core | This paper | N/A |
| Poly I:C | Invivogen | Cat# tlrl-pic-5 |
| d45-01dG5 gp120 core | Wu et al., 2015 | PDB: 4XVS_G |
| SuperScript® II Reverse Transcriptase | ThermoFisher Scientific Inc. | Cat# 18064014 |
| SuperScript® III Reverse Transcriptase | ThermoFisher Scientific Inc. | Cat# 18080044 |
| HotStarTaq Plus DNA Polymerase | Qiagen | Cat# 203607 |
| Galanthus nivalis (GNA)-lectin gel | EY Laboratories, Inc. | Cat# A-7401-2 |
| Ni Sepharose™ excel | GE Healthcare | Cat# 17-3712-03 |
| Protein G Sepharose™ 4 Fast Flow | GE Healthcare | Cat# 17-0618-05 |
| SureBlue™ TMB Microwell Peroxidase Substrate | Kirkegaard & Perry Laboratories, Inc. | Cat# 52-00-00 |
| Extravidin-PE | Sigma | Cat# E4011 |
| Streptavidin-APC | ThermoFisher Scientific Inc. | Cat# SA1005 |
| ViViD (LIVE/DEAD® fixable violet dead cell stain) | ThermoFisher Scientific Inc. | Cat# L34964 |
| Critical Commercial Assays | ||
| Bio-Layer Interferometry Antigenicity Analysis | ForteBio | Octet® HTX System |
| MiSeq Reagent Nano kit, ver2 | Illumina, Inc. | Cat# MS-102-2003 |
| Ovation Ultralow System V2 1-96 kit | NuGen Technologies, Inc. | Cat# 0344NB-08 |
| Deposited Data | ||
| Structure of 2411a in complex with glycosylated HIV-1 gp120 core | This paper | PDB: 7JKS |
| Structure of 2413a in complex with deglycosylated HIV-1 gp120 core | This paper | PDB: 7JKT |
| Deep sequencing of B cell repertoire | This paper | PRJNA623583 |
| Experimental Models: Cell Lines | ||
| Mouse: ES clone for VH1-2/LC mouse model | Tian et al., 2016 | N/A |
| Human: Expi293F™ cells | ThermoFisher Scientific Inc. | Cat# A14528; RRID: CVCL_D615 |
| Human: FreeStyle™ 293F cells | ThermoFisher Scientific Inc. | Cat# R79007 |
| Experimental Models: Organisms/Strains | ||
| Mus musculus: VH1-2/LC mouse model; mixed 129/Sv and C57BL/6 strains | Tian et al., 2016 | N/A |
| Recombinant DNA | ||
| VRC2742: mouse IgG2a heavy chain expression vector | This paper | N/A |
| VRC3353: mouse Kappa chain expression vector | This paper | N/A |
| Sequence-Based Reagents | ||
| Primers for single cell RT-PCR in the VH1-2/LC mouse models | Tian et al., 2016 | Table S6 |
| VH1-2 NGS 5’ primer: xj-VH1-1st ACAGGAGCCCACTCCCAGGTGCAG |
Tian et al., 2016 | Table S6 |
| VH1-2 NGS 3’ primer: 3’musCg-2nd CCAGGGGCCAGTGGATAGACHGATGG |
Tian et al., 2016 | Table S6 |
| VK3-20 NGS 5’ primer: 5’ hVK3-20.6 CGCAGCTTCTCTTCCTCCTG |
Tian et al., 2016 | Table S6 |
| VK3-20 NGS 3’ primer: 3’musCk-1st ACTGGATGGTGGGAAGATGGA |
Tian et al., 2016 | Table S6 |
| Software and Algorithms | ||
| GraphPad Prism 8.01 Software | GraphPad Prism Software, Inc. | RRID: SCR_002798 |
| IMGT/V-Quest | (Brochet et al., 2008; Giudicelli et al., 2011) | http://www.imgt.org/IMGT_vquest/vquest; RRID: SCR_010749 |
| WebLogo | (Crooks et al., 2004) | http://weblogo.berkeley.edu/logo.cgi; RRID: SCR_010236 |
| mGSSP | (Sheng et al., 2017) | https://github.com/scharch/SONAR |
| SONAR | (Schramm et al., 2016) | https://github.com/scharch/SONAR |
| CLUSTALO | (Sievers and Higgins, 2018) | http://clustal.org/omega/; RRID: SCR_001591 |
| HKL2000 | HKL Research, Inc. | https://hkl-xray.com/; |
| The PyMol Molecular Graphics System, v1.8.6 | Schrödinger, LLC | https://pymol.org/2/; RRID: SCR_000305 |
| Phenix | (Adams et al., 2004) | https://sbgrid.org/software/; RRID: SCR_014224 |
| FlowJo v.9.9.1 and v.10.6.2 | FlowJo | https://www.flowjo.com; RRID: SCR_008520 |
| R v3.5.2 | The Comprehensive R Archive Network | https://cran.r-project.org/ |


