Abstract
Background and purpose:
Cancer stem cells (CSCs), as the subpopulation of cancer cells, are associated with carcinogenesis, chemoresistance, and metastasis in malignancies. Also, CSCs are considered as the major reason for treatment failure in prostate cancer (PCa). Alantolactone (ALT), exerts anticancer activity in different types of cancers. In the present study, the relationship between ALT and CSCs in PCa metastasis and the molecular mechanisms involved in the progression of PCa were investigated.
Experimental approach:
In this study, to evaluate cell viability, MTT assay was performed. Then, PC3 cells were treated with nontoxic concentrations of ALT and after this step wound-healing assay, colony-formation assay and chemosensitization assay were applied to determine cell migration, the ability of colony formation, and chemoresistance, respectively. Also, real-time polymerase chain reaction and western blotting were used for the determination of genes and protein expression, respectively.
Findings/Results:
Our finding showed that ALT at nontoxic concentrations (0.01 and 0.1 μM) for 72 h suppressed the STAT3 phosphorylation and signaling pathway. Also, ALT was able to modulate the stemness of PCa cells through downregulation of expression of SOX2, Oct-4, Nanog, CD133, CD44, and upregulation of p53 expression. On the other hand, we further found that ALT in nontoxic concentrations sensitized PCa cells to cisplatin
Conclusion and implications:
ALT combated the stemness of cancer cells and metastasis by antagonizing of STAT3 signaling pathway. In addition, ALT exhibited anti-metastatic properties and may have potential as a new chemotherapy agent for the reduction of PCa metastasis.
Keywords: Alantolactone, Cancer stem cell, Prostate cancer, Sesquiterpene lactone
INTRODUCTION
Prostate cancer (PCa), as one of the most common cancers in men, is a major cause of cancer-associated death worldwide (1). Its metastasis has a heavy financial burden on countries' health systems (2). The molecular mechanism of PCa metastasis is still unknown, so one of the hot topics of scientists' research is the clarification of the PCa metastasis (3). According to previous studies, like most human tumors, PCa has a heterogeneous cell population. The main small subpopulation of these cells is cancer stem cells which have a key role in tumor initiation, unlimited growth, proliferation, invasion, and heterogeneity of the tumor mass and is one of the main challenges in the treatment of PCa (4). Cancer stem cells (CSCs) play a crucial role in PCa development, metastasis, and chemo-/radiotherapy resistance (5). CSCs are characterized by the ability of self-renewal, high metastatic potential, and chemotherapy resistance. These cells express pluripotent stem cell markers on the cell surface as the hallmark of stemness (6).
A complex network of regulatory pathways is contributed to the functional regulation of CSCs including nuclear factor-κB (NF-κB), Wnt/β-catenin, Hedgehog, Notch, epidermal growth factor/vascular-endothelial growth factor, and signal transducer and activator of transcription 3 (STAT3) (7).
STAT3 is one of the main oncogenes with upregulated expression levels in prostate tumors and further hematological and solid tumors (8). Line of evidence confirms that sustained activity of STAT3 is a substantial factor in the maintenance of pluripotency and self-renewal in embryonic stem cells and CSCs. Importantly, the aberrant activity of STAT3 affects CSCs modulation of master genes that control stemness (9). For better illustration of master genes function in stemness, several investigations demonstrated that octamer-binding transcription factor 4 (Oct-4), sex-determining region Y (SOX2), Nanog, Kruppel-like factor 4, and c-Myc are some of the transcription factors that preserve the stemness property of embryonic stem cells. Moreover, several studies showed that these transcription factors play a key role in tumorigenesis, metastasis of tumor cells, and recurrence of cancer. Furthermore, increased expression of these transcription factors led to increased invasion and metastasis of cancer cells (10,11). Therefore, STAT3 is a possible candidate for therapeutic targeting of CSCs function for the treatment of advanced tumors (8).
It is confirmed that STAT3 contributes to the regulation of stemness properties in cancer cells by modulating p53 expression level. STAT3 activity decreases the expression of p53 (12,13). Therefore, the inhibitory effect of p53 on stemness factors is suppressed and supports the stemness proprieties in cancer cells (14). PCa treatment faces main challenges including metastasis, drug resistance, and tumor recurrence which are due to the presence and function of CSCs in tumor mass, therefore exploring novel therapeutic agents is an urgent demand.
Natural compounds are diverse resources for the identification of new therapeutic agents for targeting CSCs (15). Sesquiterpene lactones are a group of natural compounds that have been extracted from Asteraceae family plants, and their anti-inflammatory and anticancer activities have been confirmed previously (16).
Alantolactone (ALT) is one of the most active sesquiterpene lactones existing in Inula helenium with antibacterial, antifungal, and anticancer activities (17). Extensive investigations on ALT have suggested that this compound has an inhibitory effect on STAT3 (16,17,18). Nevertheless, the molecular mechanism of ALT in the metastasis of cancer cells is still unclear. This study was aimed to investigate the molecular mechanism of ALT in the stemness of PCa cells by targeting STAT3 in the PCa-PC3 cell line.
MATERIAL AND METHODS
Reagents
ALT (Cat. No: SML0415), cisplatin, interleukin (IL)-6, and Dulbecco's phosphate- buffered saline (DPBS) were purchased from Sigma-Aldrich (St Louis, MO; USA). The primary antibodies against p-STAT3 (Tyr705), STAT3, SOX2, and Nanog, and secondary antibody (mouse IgGκ light chain binding protein conjugated to horseradish peroxidase) were obtained from Santa Cruz Biotechnology (Santa Cruz Biotechnology, CA, USA). RPMI 1640, fatal bovine serum (FBS), penicillin, and streptomycin were purchased from Biowest (Nuaille, France). GeneAll Hybrid-R TM total RNA purification kit (GeneAll, Korea) was used for total RNA extraction. Additionally, cDNA synthesis kit and SYBR green qPCR master mix were purchased from Yekta Tajhiz Azma (Iran).
Cell culture
Prostate cancer cell line, PC3, was purchased from the National Cell Bank of Iran (NCBI), Tehran, Iran. The cells were cultured in RPMI1640 medium with 10% FBS, streptomycin (100 μg/mL) and penicillin (100 U/mL). These cells were cultured at 37 °C provided in an incubator with 5% CO2 and 90% humidified atmosphere.
MTT assay for cell viability
Cell viability was assessed by MTT assay. The cells were cultured in a 96-well plate with 5 × 103 cells per well. Then PC3 cells were treated with various concentrations of ALT (0.01-150 μM) for 24, 48, and 72 h. PC3 cells in the control group were treated with dimethyl sulfoxide (DMSO). A stock solution was prepared in PBS at 5 mg/mL, filtered, and kept at -20 °C under dark condition. After the treatment period, the cells were washed with PBS and then, 20 μL of MTT solution (MTT reagent) was added to each well (final concentration of MTT was 0.5 mg/mL). Then, they were incubated at 37 °C with 5% CO2 and humidified atmosphere for 3 h. For analysis of the formation of formazan crystals, an inverted microscope was used. The media of the wells were removed and for solvation of formazan crystals, 100 μL of DMSO was added to each well. After 5 min of mild shaking, the optical absorption at the 570 nm and a reference filter of 630 nm was assessed by BioTek microplate reader (USA). IC50 values were assessed by nonlinear fitting based on the log-transformed dose-response curve.
RNA extraction
In order to extract total RNA, PC3 cells seeded in a culture dish at a density of 1 × 106 and incubated at 37 °C for 24 h. Then, these cells were treated with nontoxic concentrations of ALT (0.01 and 0.1 μM) for 72 h. Control cells were also treated with DMSO. Total RNA was extracted from the treated and untreated cells according to the manufacturer's protocol. At first, the media was removed from the wells, and then, they were washed by PBS buffer. In the following, 1 mL of cell lysis solution (RiboEx™) was added to each well. Then, the content of the wells was transferred into the microtubes and was kept at room temperature for 5 min. Afterward, 200 μL chloroform was added and were incubated at room temperature for 2 min. In the next step, they were centrifuged at 12000 g for 15 min at 4 °C. Then, the upper phase was transferred to another microtube and the same volume of resuspension Buffer 1 was added to the solution. After that, 700 μL of the solution was transferred into a mini spin column and centrifuged at room temperature and 10000 g for 30 sec. Then, 500 μL of SW1 and RNW buffers were added to the mini spin column and were centrifuged at 10000 g for 30 s at room temperature. In the end, 50 μL of RNase-free water was added to the middle part of the mini spin column and was centrifuged at room temperature and 10000 g for 1 min. The extracted RNA was restored at - 70 °C. The quantity and quality of the extracted RNA were checked by a 260/280 absorption ratio and agarose gel electrophoresis, respectively.
Stemness-related mRNA profiling
More than 1 μg of RNA was used for complementary DNA (cDNA) synthesis. cDNA synthesis was done by random hexamer. The tubes were first kept at room temperature for 5 min. Then, they were kept at 50 °C for 30 min. After that, to deactivate RNase, the tubes were placed at 95 °C for 5 min. All the primer sets were designed by oligo 7 software and the specificity of primers were checked by NCBI Primer-BLAST. The primers sequence is presented in Table 1. Gene expression was assessed by Syber green-based quantitative PCR by biomolecular systems thermal- magnetic induction cycler (Mic, Bio Molecular Systems, Australia). After normalization of the results using a reference gene (GAPDH), the relative expression of the target genes was assessed via 2-ΔΔct formula (Livak and Schmittgen method).
Table 1.
List of primer sequences used for the real-time polymerase chain reaction
| Gene name | Primer sequence ( 5′-3′) | Product size(bp) |
|---|---|---|
| Nanog | Forward: AGTCCCAAAGGCAAACAACCCACTTC Reverse: TGCTGGAGGCTGAGGTATTTCTGTCTC |
161 |
| SOX-2 | Forward: CACATGTCCCAGCACTACCAG Reverse: CCCCTCCCATTTCCCTCGTT |
141 |
| OCT-4 | Forward: CCATGCATTCAAACTGAGGT Reverse: CCTTTGTGTTCCCAATTCCT |
146 |
| CD133 | Forward: GCAAATCACCAGGTAAGAACCC Reverse: CAAGAATTCCGCCTCCTAGCA |
193 |
| CD44 | Forward: GCAACTCCTAGTAGTACAACGGAA Reverse: AGCTGTCCCTGTTGTCGAA |
123 |
| p53 | Forward: TCAGTCTACCTCCCGCCATA Reverse: TTACATCTCCCAAACATCCCT |
322 |
| GAPDH | Forward: GGTCTCCTCTGACTTCAACA Reverse: AGCCAAATTCGTTGTCATAC |
116 |
Western blot analysis
PC3 cells were seeded with 106 cells/dish density in a cell culture dish and then, treated with a nontoxic concentration of ALT (0.01 and 0.1 μM) for 72 h. Control cells were also treated with DMSO. After this, to extract total protein, all of the cells in our study were incubated in lysis buffer containing protease inhibitor for 20 min. Subsequently, the lysate was centrifuged at 14000 RPM for 10 min at 4 °C and the quantity of protein was measured by Bradford assay. In the following step, protein electrophoresis was carried out using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then, the proteins were transferred to polyvinylidene difluoride (PVDF). Then, the blotted membrane was blocked by 5% skim milk solution and after that, an adequate concentration of the primary antibodies (p-STAT3 (Tyr705), STAT3, Nanog, SOX2) were added and incubated at 4 °C overnight (GAPDH antibody was used as the internal control). Then, the membrane incubated with horseradish peroxidase- conjugated secondary antibodies for 3 h. In the following, the signals were visualized by enhanced chemiluminescence (ECL) method (GE Healthcare, Boston, MA, USA) and exposed to X-ray film. Finally, the protein bands quantified using ImageJ software.
Wound-healing assay
For analysis of cell migration ability, in vitro wound-healing assay was performed. PC3 cells seeded into 6-well plate (5 × 105 cells per well) and allowed to reach 80-90% confluency. When cells reached confluence, a wound was made with a 10 μL blunt-end pipette tip. Consequently, cell debris was removed with PBS and cells treated with ALT (0.01 and 0.1 μM) for 72 h. Control cells were also treated with DMSO. In the following, the images of the same wound area were captured at 0, 24, 72 h after scratching. Finally, the size of the wound area was analyzed by Image J software.
Colony-formation assay
Colony-formation assay was applied for investigating the effect of ALT on PC-3 cells' viability. PC-3 cells were seeded at 500 cells per well into a 6-well plate. On the next day, the cells were treated by ALT at 0.01 and 0.1 μM for 72 h. Control cells were also treated with DMSO. After 72 h, the cells incubated for 14 days until the formation of colonies (about 50 cells in every colony). Then, the colonies were fixed by methanol/acetic acid (3:1) solution for 20 min, and the fixed colonies were stained by crystal violet (0.5%) for 10 min. Colonies with more than 50 cells counted under a microscope.
Chemosensitization assay
The toxicity of different concentrations of cisplatin (ranging from 1 to 100 μM) in untreated cells and pretreated cells with ALT was assessed by MTT assay already described. The IC50 of cisplatin in cells were determined in untreated cells and ALT-pretreated cells (0.01 and 0.1 μM) after 72 h of pretreatment cells. The effect of ALT on the toxicity of cisplatin was presented as an IC50 shift and percentage of cell viability.
Statistical analyses
All of the statistical analyses were carried out using GraphPad 8 (GraphPad Software, USA) and were presented as mean ± standard deviation (SD). For multiple comparisons between groups, one-way analysis of variance (ANOVA) with post-hoc Tukey's test was utilized. P < 0.05 was considered statistically as a significant difference.
RESULTS
Effect of ALT on the viability of PC3 cells
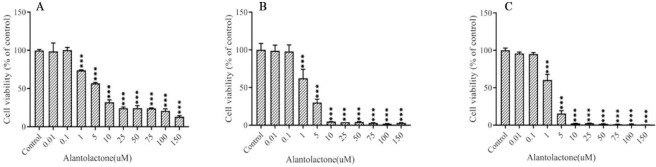
In order to investigate the cytotoxic effects of ALT on prostate cancer cells (PC-3), MTT assay was used in various concentrations of ALT (0, 0.01, 0.1, 1, 5, 10, 25, 50, 75, 100, and 150 μM) in different periods 24, 48, and 72 h (Fig. 1). Our results demonstrated that ALT decreased cell viability in a concentration- and time-dependent manner. IC50 values were obtained as 3.063, 1.666, and 1.557 μM for 24, 48, and 72 h, respectively. Since the cell viability at 0.01 and 0.1 μM were not significantly different from the control group in all time periods, therefore the concentration of 0.01 and 0.1 μM, for 72 h were used for the following experiments.
Fig. 1.
The effect of ALT on PC3 cell viability was evaluated using MTT assay. PC3 cells were treated with various concentrations of ALT in (A) 24 h, (B) 48 h, and (C) 72 h. The control group cells were treated with DMSO. The results were presented as mean ± SD, n = 3. ***P < 0.001 Indicates significant differences compared to the control group. ALT, Alantolactone.
ALT inhibits IL-6-induced STAT3 tyrosine 705 phosphorylation
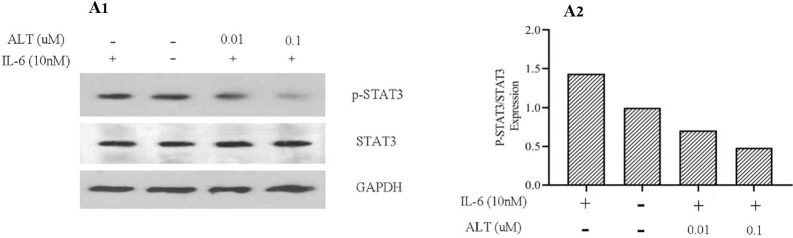
Studies on PCa suggested that STAT3 and its upstream activator i.e. IL-6 play a major role in metastasis of PCa and can be considered as a therapeutic target in PCa treatment (8). Besides, immunohistological studies reported that levels of phosphorylated STAT3 (p-STAT3 (Tyr705)) and its mRNA were significantly elevated in metastatic PCa (8,19). In order to clarify the inhibitory molecular mechanism of ALT on STAT3 phosphorylation, we first pretreated PC3 cells with a nontoxic concentration of ALT (0.01 and 0. 1 μM) for 72 h. In the following step, these cells were treated with IL-6 (10 ng/mL) for 30 min. Our results demonstrated that STAT3 phosphorylation was increased in the IL-6-treated cells (Fig. 2). Furthermore, in the ALT-pretreatment groups (0.01 and 0.1 μM), ALT inhibited IL-6 stimulated STAT3 activity in a dose-dependent manner. We also evaluated the effect of ALT on STAT3 expression. The results showed that ALT was not significantly affected STAT3 expression in a dose-dependent manner.
Fig. 2.
ALT inhibits inducible STAT3 activity and STAT3 tyrosine 705 phosphorylation. PC3 cells were pretreated with a nontoxic concentration of ALT (0.01 and 0.1 μM) for 72 h. Subsequently, these cells were stimulated by IL-6 (10 ng/mL) for 30 min and analyzed with western blotting (in one repeat). GAPDH was used to normalize the expression value. ALT, Alantolactone.
ALT decreased mesenchymal stem cell surface markers
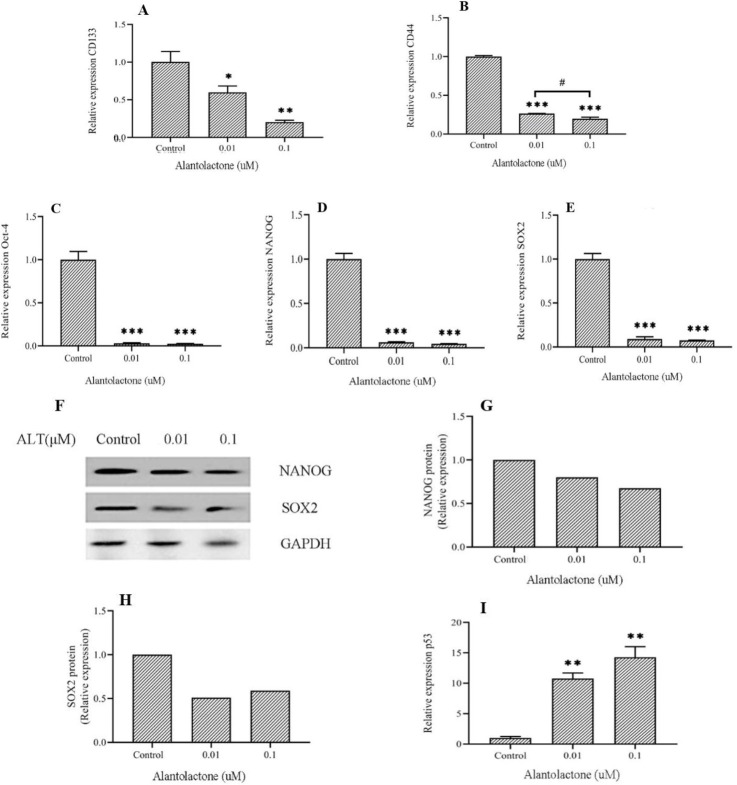
In order to investigate the effect of ALT on cell surface markers of CSCs, the expression levels of CD133 and CD44 were assessed by real-time polymerase chain reaction (RT-PCR) (Fig. 3A and B). The results showed that ALT at 0.01 and 0.1 μM significantly decreased the expression of CD44 and CD133 in comparison with the control group, in a concentrations- dependent manner. Based on our findings, ALT decreased the subpopulation of cancer cells with CSCs properties in PC3 cells in a dose- dependent manner.
Fig. 3.
ALT decreased the expression level of stemness's factors in PC3 cells. Protein and mRNA levels of stemness's factors in PC3 cells, which were treated by nontoxic concentrations of ALT for 72 h, were evaluated with western blotting and RT-PCR. (A-E, and I) mRNA expression levels of CD133, CD44, Oct-4, Nanog, SOX2, and p53. (F-H) Protein expression levels of SOX2 and Nanog (in one repeat), GAPDH was used as a reference gene. All data represent the mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 Indicate significant differences compared to the control group. #P < 0.05 refers to the significant differences between indicated groups. ALT, Alantolactone; RT-PCR, real-time polymerase chain reaction.
ALT decreased expressions of genes involved in stemness and self-renewal of PC3 cells
Several studies depicted that uncontrolled self-renewal of cells causes the initiation and progression of various cancers. A growing body of evidence showed that tumors may have a subpopulation of cells that possess stem cell features including self-renewal and pluripotency (20). Stemness is defined the potential of cancer cells for self-renewal and differentiation to other cells that are associated with cancer progression and metastasis. RT- PCR and western blotting were used to investigate the effect of ALT on certain chief genes involved in the stemness of PC3 cells through STAT3 signaling pathway. Based on the results obtained from the gene expression levels, ALT was significantly decreased the expression of Oct-4, Nanog, and SOX2 in a dose-dependent manner (Fig. 3C-E). Furthermore, analyzing of protein expression levels, showed that this compound reduced the expression of SOX2 and Nanog proteins compared to the control group (Fig. 3F-H).
ALT increased the expression of p53 in PC3 cells
Loss of p53 is one of the main causes of many cancers development. In the lack of this guardian of the genome, cells are not protected enough from mutation. As a tumor suppressor, p53 plays a key role in many cellular events such as DNA replication and repair, cell cycle arrest, proliferation, apoptosis, angiogenesis inhibition, and cellular stress responses. According to recent studies, p53 plays a key role in asymmetric and symmetric divisions in stem cells and CSCs (21). Recent studies suggest that p53 is one of the gene targets of STAT3. Activation of STAT3 was led to inhibition of p53 expression in cancer cells (12). To evaluate the effect of ALT on p53 expression through STAT3 signaling pathway, p53 expression was assessed via RT-PCR. Our findings suggested that ALT significantly increased p53 expression in a dose-dependent manner (Fig. 3I).
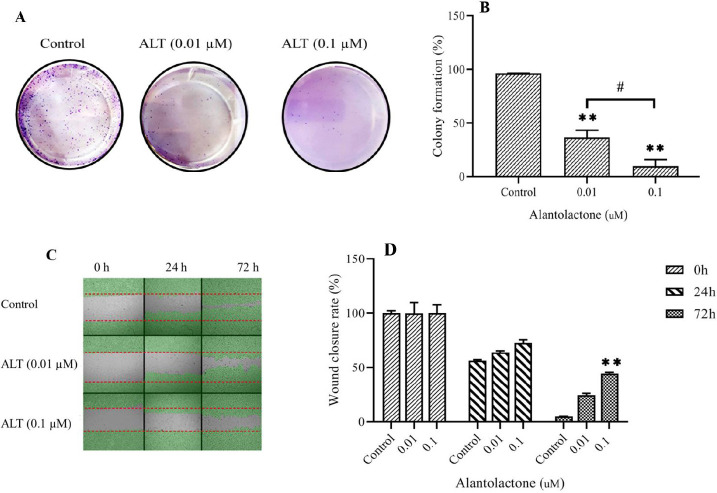
ALT decreased cell migration and colony formation ability in PC3 cells
Invasion and cloning ability of PC3 cells were assessed by wound healing assay and colony formation, respectively. The results of the colony formation assay showed that the cell cloning ability was inhibited in a dose-dependent manner (Fig. 4A and B). In cells treated with ALT at 0.01 and 0.1 μM, the colony formation ability was significantly decreased compared to the control group cells. Moreover, cell invasion was significantly decreased in a dose-dependent manner (Fig. 4C and D). An inverted microscope was used to evaluate the morphologic changes and the effect of ALT on pseudopodia formation in PC3 cells. Pseudopodia are specialized membrane protrusions that facilitate the dissemination of metastatic cancer cells. Our results indicated that ALT decreased pseudopodia formation in a dose-dependent manner (Fig. 5).
Fig. 4.
ALT decreased cell migration and colony formation ability in PC3 cells. The wound-healing assay and colony formation assay was carried out in PC3 cells undergoing the nontoxic concentration of ALT. (A and B) The representative images of the colony and the quantitative analysis number of colonies, respectively. (C) Represents the image of the wound area at 0, 24, 72 h. (D) The quantitative analysis of the migration rate is presented in the column charts. All data represent the mean ± SD. n = 3. **P < 0.01 indicates significant differences compared to the control group; #P < 0.05 refers to the significant differences between indicated groups. ALT, Alantolactone.
Fig. 5.
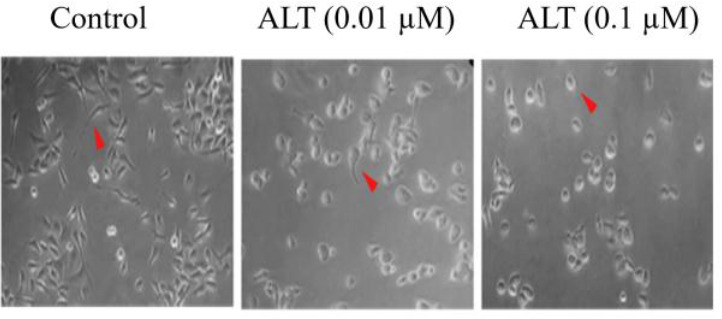
ALT decreased pseudopodia formation in PC3 cells. Change in the morphology of PC3 cells evaluated with inverted phase-contrast microscopy. PC3 cells treated with the nontoxic concentration of ALT (0.01 and 0.1 μM); the magnification was 40×. ALT, alantolactone.
ALT enhanced chemosensitization of PC3 cells
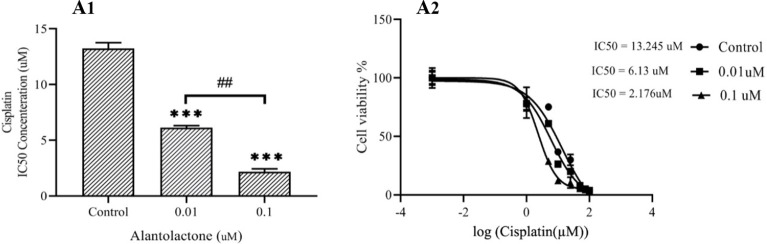
In order to evaluate the effect of ALT on drug resistance in PC3 cells, we evaluated the sensitivity of PC3 cells to cisplatin. Our results indicated that nontoxic concentrations of ALT made the cells sensitive to cisplatin. Also, pretreatment of cells with the nontoxic concentrations of ALT, strongly enhanced the anti-proliferative effects of cisplatin in PC3 cells in a dose-dependent manner. As shown in Fig. 6, pretreatment of PC3 cells by ALT (0.01 and 0.1 μM) for 72 h significantly shifted the cisplatin inhibitory concentration (IC50) from 13.245 μM to 6.13 and 2.176 μM, respectively.
Fig. 6.
ALT enhanced the chemosensitization of PC3 cells. PC3 cells were pretreated with ALT at 0.01 and 0.1 μM for 72 h. Then, these cells were treated with various concentrations of cisplatin (1 to 100 μM) for 24 h. All data were represented as mean ± SD, n = 3. ***P < 0.001 indicates significant differences compared to the control group. ##P < 0.01 refers to the significant differences between indicated groups. ALT, Alantolactone.
DISCUSSION
PCa is one of the most common malignancies and major causes of cancer- related deaths among men in developed countries (22). One of the main challenges in the treating PCa is the metastasis of this type of cancer to distant organs. Scientists have clearly revealed that, like other human tumors, CSCs play a pivotal role in PCa metastasis (4,5). In the present study, the effect of ALT on metastasis and CSC properties of PCa cells was investigated. To help the optimal treatment of PCa due to the lack of sufficient therapeutic strategy against metastasis of this cancer, our study investigated the targeted treatment of this disease by discovering cellular and molecular pathways of metastasis. To the best of our knowledge, there is no previous study to elucidate the molecular mechanisms of ALT on metastasis of PCa through reduced stemness properties of cancer cells. The results showed that ALT inhibited the CSC features of PCa cells as well as metastasis and chemoresistance in these cells by inhibiting the STAT3 signaling pathway.
Clinical studies on PCa patients have shown that the main clinical complication in PCa is bone metastasis which brings about in death of 80% of patients with bone metastasis (23). In addition to bone, lymph nodes, brain, and visceral organs such as the liver and lungs are involved by PCa (24). Therefore, metastasis was considered a substantial cause of PCa related deaths. In spite of various treatments used for combating PCa including surgery, radiation therapy, and chemotherapy, tumor recurrence occurs in 20-30% of the patients after 5 years. Of note, PCa usually occurs in old men and these people are sensitive to chemotherapy medicines. Therefore, one of the therapeutic strategies for the reduction of the side effects of chemotherapy and the recurrence of PCa is using natural compounds (25).
The potential capacity of natural compounds as anticancer agents has recently attracted a lot of attention (26,27). Recent reports suggested that the compounds extracted from plants are strongly effective in cancer treatment (28). Sesquiterpene lactones are a subclass of sesquiterpenes and a secondary compound existing in Asteraceae family plants. These compounds have different anti-malarial, wound healing, anti-inflammatory, and anti-tumor effects (29). ALT was shown to exert various biological activity including antimicrobial, anti-inflammatory, and anticancer (30). Previous studies have reported that ALT induces apoptosis in many types of cancer cells. For example, Maryam et al. reported that ALT inhibited STAT3, induced apoptosis, and increased doxorubicin toxicity in lung cancer cells by inducing oxidative stress (17). Also, Khan et al. reported that ALT inhibits STAT3 phosphorylation and oxidative stress in liver cancer cells by reducing GSH levels, thereby inducing apoptosis in these cells (16). Lu et al. showed that ALT induced apoptosis in breast cancer cells by reducing the expression of nuclear expression levels of p65 and nuclear factor erythroid 2 -related factor 2 (Nrf2), increasing p38 phosphorylation and inhibiting the NFκB signaling pathway (30).
Despite various studies on ALT, the molecular mechanisms affecting cancer cell metastasis are still poorly understood. One of the molecular candidates which recently evaluated as a therapeutic target in the invasiveness of PCa is STAT3. It is the most important oncogenes of PCa that becomes constitutively activated by phosphorylation at Tyr705 and finally translocated into the nucleus to affects the target genes in the nucleus and causes anti-apoptosis, anti-proliferation, and anti-metastasis effects (8). STAT3 signaling pathway has been reported to have an essential role in the expression of the genes involved in stem cell properties which ultimately result in invasion and metastasis of cancer cells (10,31).
CSCs are also known as tumor-initiating cells which play a fundamental role in self- renewal and formation of new tumors. Also, evidence indicated that CSCs are involved in metastasis, invasion, and chemoresistance of tumors. Nowadays, one of the researcher's goals is to try to target CSCs based on surface markers and intrinsic signaling pathways of CSCs (10,14,32). Therefore, inhibitors of STAT3 signaling pathway have the potential for cancer treatment. In the present study, we showed that ALT acts as a STAT3 inhibitor. The results of our study suggested that this compound inhibited phosphorylation of STAT3 at Tyr705 and hence, it prevented the translocation of STAT3 into the nucleus and inhibited the expression of target genes. Our results were supported by Chun et al. which indicated that ALT directly interacted with SH2 domain of STAT3 and significantly reduced phosphorylation and dimerization in MDA- MB231 breast cancer cells. It has been reported that this compound inhibits metastasis and cell invasion, colony formation, and suppresses tumor growth in breast cancer cells by reducing the expression levels of cyclin D1, c-myc, cyclooxygenase-2 (COX-2), matrix metalloproteinase-9 (MMP-9) that are STAT3 target genes (18). Khan et al. also reported that ALT suppressed the activation of STAT3 in HepG2 cells by inhibition of Tyr705 phosphorylation in SH2 domains (16). Moreover, Amara et al. conducted that ALT inhibited STAT3 activation and blocked nuclear translocation. This study also showed that ALT is involved in inducing apoptosis in these lung cancer cells by inhibiting the expression of anti-apoptotic genes and increasing the expression of pro-apoptotic genes. ALT also inhibits lung cancer by inhibiting the expression of inducible nitric oxide synthase (iNOS), COX-2, and MMP-9 as markers of invasion and metastasis (17).
STAT3 was capable to regulate the biological characteristics of CSCs by affecting Nanog, Oct-4, and SOX2 (10). According to our findings, ALT decreased gene expression of Oct-4 and gene and protein expression of SOX2 and Nanog. In addition to the abovementioned transcription factors, several cell surface markers are widely used for identifying CSCs. Recent findings demonstrated that, in different cancer cells especially in PCa and breast cancer, cells with CD133+/CD44+ properties have a higher invasion and metastasis (6,33). Our findings also suggested that ALT decreased the expression of CD133 and CD44 in a dose- dependent manner. This finding is consistent with the results of our cell invasion study which showed that ALT inhibited PC3 cell migration in a dose-dependent manner. Our results confirmed by Liu et al. study, reported that ALT suppressed cell migration and inhibited colony formation ability of MCF-7 cells (30).
Therefore, these results suggested that ALT through inhibition of gene expression involved in self-renewal and CSC surface markers reduced the stemness of PC3 cells, and finally, it inhibited cancer cell migration and invasion.
Recent studies demonstrated that STAT3 activation can be considered as a potential suppressive target for chemosensitizing strategies; because constitutive activation of STAT3 signaling pathway plays an important role in the chemoresistance of many cancers (34). Our findings confirmed that STAT3 plays a major role in PC3 cell's resistance to cisplatin. Also, we found that ALT decreased cisplatin IC50 for PC3 cells in a dose-dependent manner. According to these findings, by inhibiting STAT3 activity, ALT overcame the resistance of PC3 cells to cisplatin.
Studies on cancer cells found that p53 is one of the gene targets of STAT3. Increased expression of STAT3 by binding to p53 promoter inhibits the expression of this protein (13). As a guardian of the genome, p53 has a substantial role in the regulation of senescence, apoptosis, and cell-cycle arrest. Moreover, recent studies have indicated that in addition to the role of p53 in genome stability, this protein plays a key role in the differentiation of pluripotent stem cells and in reprogramming for the generation of pluripotency in cells. Remarkably, activation of p53 signaling pathways by inhibition of stem cell-related mechanisms can lead to inhibition of cancer metastasis (35,36). We found that ALT enhanced the expression of p53 in a dose- dependent manner. According to our results and given the association between STAT3 and p53 in cancer cells, it is possible that ALT also affects the expression of genes involving in stemness through the STAT3-p53 signaling pathway. However, further investigations are needed in the future.
CONCLUSION
ALT noticeably can mitigate the migration of PCa cells and attenuate stemness properties as well as colony formation mainly through suppressing the STAT3 signaling pathway, suggesting that ALT may have enormous anticancer potential, especially the treatment of metastatic PCa.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest in this study.
AUTHORS' CONTRIBUTION
MH. Khadem-Ansari devised the main conceptual ideas and participated in the design of the work. MH. Khadem-Ansari, Sh. Gholizadeh-Ghaleh Aziz, and M. RajabiBazl provided biological materials and reagents. G. babaei performed the experiments and wrote the initial draft of the manuscript. MH. Khadem-Ansari, Sh. Gholizadeh-Ghaleh Aziz and M. Rajabi Bazl participated in the analysis of the work and reviewed and edited the manuscript. MH Khadem-Ansari supervised the study.
ACKNOWLEDGMENTS
This study was financially supported by Urmia University of Medical Sciences, Urmia, I.R. Iran under Grant No.2527. We gratefully acknowledge them for their contribution to this research.
REFERENCES
- 1.Azemikhah M, Ashtiani HA, Aghaei M, Rastegar H. Evaluation of discoidin domain receptor-2 (DDR2) expression level in normal, benign, and malignant human prostate tissues. Res Pharm Sci. 2015;10(4):356–363. [PMC free article] [PubMed] [Google Scholar]
- 2.Rycaj K, Tang DG. Molecular determinants of prostate cancer metastasis. Oncotarget. 2017;8(50):88211–88231. doi: 10.18632/oncotarget.21085. DOI: 10.18632/oncotarget.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorai T, Diouri J, O'Shea O, Doty SB. Curcumin inhibits prostate cancer bone metastasis by up- regulating bone morphogenic protein-7 in vivo. J Cancer Ther. 2014;5(4):369–386. doi: 10.4236/jct.2014.54044. DOI: 10.4236/jct.2014.54044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moltzahn F, Thalmann GN. Cancer stem cells in prostate cancer. Transl Androl Urol. 2013;2(3):242–253. doi: 10.3978/j.issn.2223-4683.2013.09.06. DOI: 10.3978/j.issn.2223-4683.2013.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris KS, Kerr BA. Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. Stem Cells Int. 2017;2017:8629234,1–9. doi: 10.1155/2017/8629234. DOI: 10.1155/2017/8629234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellón EA, Valenzuela R, Lillo J, Castillo V, Contreras HR, Gallegos I, et al. Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different Gleason grades and metastasis. Biol Res. 2012;45(3):297–305. doi: 10.4067/S0716-97602012000300011. DOI: 10.4067/S0716-97602012000300011. [DOI] [PubMed] [Google Scholar]
- 7.Radpour R. Tracing and targeting cancer stem cells: new venture for personalized molecular cancer therapy. World J Stem Cells. 2017;9(10):169–178. doi: 10.4252/wjsc.v9.i10.169. DOI: 10.4252/wjsc.v9.i10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Don-Doncow N, Marginean F, Coleman I, Nelson PS, Ehrnström R, Krzyzanowska A, et al. Expression of STAT3 in prostate cancer metastases. Eur Urol. 2017;71(3):313–316. doi: 10.1016/j.eururo.2016.06.018. DOI: 10.1016/j.eururo.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galoczova M, Coates P, Vojtesek B. STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett. 2018;23(1):12–32. doi: 10.1186/s11658-018-0078-0. DOI: 10.1186/s11658-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Deng J, Ren HY, Jia P, Zhang W, Li MQ, et al. STAT3 influences the characteristics of stem cells in cervical carcinoma. Oncol Lett. 2017;14(2):2131–2136. doi: 10.3892/ol.2017.6454. DOI: 10.3892/ol.2017.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjimichael C, Chanoumidou K, Papadopoulou N, Arampatzi P, Papamatheakis J, Kretsovali A. Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells. 2015;7(9):1150–1184. doi: 10.4252/wjsc.v7.i9.1150. DOI: 10.4252/wjsc.v7.i9.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y, Kim JK, Yoo JY. NFκB and STAT3 synergistically activate the expression of FAT10, a gene counteracting the tumor suppressor p53. Mol Oncol. 2014;8(3):642–655. doi: 10.1016/j.molonc.2014.01.007. DOI: 10.1016/j.molonc.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25(17):7432–7440. doi: 10.1128/MCB.25.17.7432-7440.2005. DOI: 10.1128/MCB.25.17.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spike BT, Wahl GM. p53, Stem cells, and reprogramming: tumor suppression beyond guarding the genome. Genes Cancer. 2011;2(4):404–419. doi: 10.1177/1947601911410224. DOI: 10.1177/1947601911410224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor WF, Jabbarzadeh E. The use of natural products to target cancer stem cells. Am J Cancer Res. 2017;7(7):1588–1605. [PMC free article] [PubMed] [Google Scholar]
- 16.Khan M, Li T, Khan MKA, Rasul A, Nawaz F, Sun M, et al. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. Biomed Res Int. 2013;2013:719858,1–11. doi: 10.1155/2013/719858. DOI: 10.1155/2013/719858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maryam A, Mehmood T, Zhang H, Li Y, Khan M, Ma T. Alantolactone induces apoptosis, promotes STAT3 glutathionylation and enhances chemosensitivity of A549 lung adenocarcinoma cells to doxorubicin via oxidative stress. Sci Rep. 2017;7(1):6242,1–18. doi: 10.1038/s41598-017-06535-y. DOI: 10.1038/s41598-017-06535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun J, Li RJ, Cheng MS, Kim YS. Alantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cells. Cancer Lett. 2015;357(1):393–403. doi: 10.1016/j.canlet.2014.11.049. DOI: 10.1016/j.canlet.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 19.Culig Z, Puhr M. Interleukin-6 and prostate cancer: current developments and unsolved questions. Mol Cell Endocrinol. 2018;462(Pt A):25–30. doi: 10.1016/j.mce.2017.03.012. DOI: 10.1016/j.mce.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Amini S, Fathi F, Mobalegi J, Sofimajidpour H, Ghadimi T. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat Cell Biol. 2014;47(1):1–11. doi: 10.5115/acb.2014.47.1.1. DOI: 10.5115/acb.2014.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetzer Y, Solomon H, Koifman G, Molchadsky A, Horesh S, Rotter V. The paradigm of mutant p53- expressing cancer stem cells and drug resistance. Carcinogenesis. 2014;35(6):1196–1208. doi: 10.1093/carcin/bgu073. DOI: 10.1093/carcin/bgu073. [DOI] [PubMed] [Google Scholar]
- 22.Mei W, Lin X, Kapoor A, Gu Y, Zhao K, Tang D. The contributions of prostate cancer stem cells in prostate cancer initiation and metastasis. Cancers. 2019;11(4):434–456. doi: 10.3390/cancers11040434. DOI:10.3390/cancers11040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziaee S, Chu GCY, Huang JM, Sieh S, Chung LWK. Prostate cancer metastasis: roles of recruitment and reprogramming, cell signal network and three- dimensional growth characteristics. Transl Androl Urol. 2015;4(4):438–454. doi: 10.3978/j.issn.2223-4683.2015.04.10. DOI: 10.3978/j.issn.2223-4683.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan D, Senbanjo L, Majumdar S, Franklin RB, Chellaiah MA. Androgen receptor expression reduces stemness characteristics of prostate cancer cells (PC3) by repression of CD44 and SOX2. J Cell Biochem. 2018;120(2):2413–2428. doi: 10.1002/jcb.27573. DOI: 10.1002/jcb.27573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N, et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35(30):8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. DOI: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabrizi FHA, Irian S, Amanzadeh A, Heidarnejad F, Gudarzi H, Salimi M. Anti-proliferative activity of Fumaria vaillantii extracts on different cancer cell lines. Res Pharm Sci. 2016;11(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- 27.Homayoun M, Ghasemnezhad Targhi R, Soleimani M. Anti-proliferative and anti-apoptotic effects of grape seed extract on chemo-resistant OVCAR-3 ovarian cancer cells. Res Pharm Sci 2020. 2020;15(4):390–400. doi: 10.4103/1735-5362.293517. DOI: 10.4103/1735-5362.293517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmadi F, Mojarrab M, Ghazi-Khansari M, Hosseinzadeh L. A semipolar fraction of petroleum ether extract of Artemisia aucheri induces apoptosis and enhances the apoptotic response to doxorubicin in human neuroblastoma. SKNMC cell line Res Pharm Sci. 2015;10(4):335–344. [PMC free article] [PubMed] [Google Scholar]
- 29.Shoaib M, Shah I, Ali N, Adhikari A, Tahir MN, Shah SWA, et al. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement Altern Med. 2017;17(1):27–38. doi: 10.1186/s12906-016-1517-y. DOI: 10.1186/s12906-016-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Liu M, Wang S, He Y, Huo Y, Yang Z, et al. Alantolactone induces apoptosis and suppresses migration in MCF-7 human breast cancer cells via the p38 MAPK, NF-κB and Nrf2 signaling pathways. Int J Mol Med. 2018;42(4):1847–1856. doi: 10.3892/ijmm.2018.3751. DOI: 10.3892/ijmm.2018.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial- mesenchymal transition. Cells. 2020;9(1):217–240. doi: 10.3390/cells9010217. DOI: 10.3390/cells9010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Gao X, Wang S, Yuan X, Pang W, Chen J, et al. Cancer stem cells are regulated by STAT3 signalling in wilms tumour. J Cancer. 2018;9(8):1486–1499. doi: 10.7150/jca.23277. DOI: 10.7150/jca.23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantari E, Asgari M, Nikpanah S, Salarieh N, Asadi Lari MH, Madjd Z. Co-expression of putative cancer stem cell markers CD44 and CD133 in prostate carcinomas. Pathol Oncol Res. 2017;23(4):793–802. doi: 10.1007/s12253-016-0169-z. DOI: 10.1007/s12253-016-0169-z. [DOI] [PubMed] [Google Scholar]
- 34.Qin JJ, Yan L, Zhang J, Zhang WD. STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J Exp Clin Cancer Res. 2019;38(1):195–211. doi: 10.1186/s13046-019-1206-z. DOI: 10.1186/s13046-019-1206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin T, Lin Y. p53 Switches off pluripotency on differentiation. Stem Cell Res Ther. 2017;8(1):44–51. doi: 10.1186/s13287-017-0498-1. DOI: 10.1186/s13287-017-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, He Y, Dubois W, Wu X, Shi J, Huang J. Distinct regulatory mechanisms and functions for p53-activated and p53-repressed DNA damage response genes in embryonic stem cells. Mol Cell. 2012;46(1):30–42. doi: 10.1016/j.molcel.2012.01.020. DOI: 10.1016/j.molcel.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]