Abstract
Background and purpose:
Mercuric chloride (Merc) can cause kidney toxicity. Harmine (Harm), an herbal alkaloid has various pharmacological and medicinal effects mainly because of its antioxidant activity. In this study, therefore, Harm's protective mechanisms on Merc-induced nephrotoxicity in BALB/c male mice were investigated.
Experimental approach:
Forty-eight male mice were randomly divided into six groups (n = 8). Groups were received saline, Merc (0.5 mL/day of 0.5 ppm aqueous), Harm (5, 10, 15 mg/kg/day), Merc + Harm (5, 10, 15 mg/kg/day) for 14 consecutive days. Saline and Harm were administrated intraperitoneally and Merc dissolved in drinking water. Urea and creatinine serum levels, body weight, kidney weight, quantitative and qualitative histological alterations, apoptosis rate, total antioxidant capacity (TAC), superoxide dismutase (SOD), and nitric oxide (NO) levels were evaluated.
Findings/Results:
There was a significant reduction in total body and kidney weights, renal histological criteria, TAC, SOD levels in the Merc group compared to the control group (P < 0.05), whereas these parameters in the Merc + Harm groups, were significantly increased compared to the Merc group (P < 0.05). Urea and creatinine serum levels, levels of NO, and apoptosis were significantly higher in the Merc group than the control, while these parameters were decreased in the Merc + Harms groups in comparison with the Merc group (P < 0.05).
Conclusion and implications:
Harm protected Merc-induced renal damage in mice. This protection was observed in both histological and biochemical respects. The beneficial effect of Harm was related to its antioxidant properties that diminish NO production and apoptosis induction in the kidney.
Keywords: Antioxidants, Apoptosis, Harmine, Mercuric chloride, Kidney
INTRODUCTION
Mercuric chloride (Merc, HgCl2) existing in many sources such as soil, dental amalgam restorations, seafood can tread to the body by inhalation, ingestion, and skin absorbance. Its accumulation in the body exerts toxicity into the central nervous system, liver, and mainly the kidneys. Heavily exposed to Merc develops certain symptoms like hand tremors, insomnia, emotional instability, and Merc salts deposition in the kidneys can cause severe damage in this organ (1,2,3). Furthermore, Merc is considered a risk factor for the induction of cell death in different tissues. Oxidative stress following Merc exposure can reform to hazardous Merc ions and affects tissues by catastrophic and metabolism disruption (4). Also, Merc toxicity increases the production of pro-inflammatory and nitric oxide (NO) molecules in mice (5). However, no effective method exists to avoid the entrance of toxins into the body, thus only medications and herbal medicine can help suffered persons.
Plant-derived antioxidant constituents by inhibiting the production of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-a) can reduce inflammation.
Moreover, the antioxidant supplements and antioxidant-rich foods can reduce oxidative damage by scavenging free radicals in the human body. Harmine (Harm; 7-methoxy-1- methyl-9H-pyrido[3, 4-b] indole) is an active form of the plant Peganum harmala (6). It is an herbal alkaloid related to the beta-carboline family with various pharmacological and medicinal effects mainly because of its antioxidant activity (7). Surprisingly, Harm induces apoptosis in cancerous cells both by internal and external pathways and regulates the transcription factors and pro-inflammatory cytokines (8). While Harm inhibits TNF-α activity and production of NO in lipopolysaccharide in RAW264 mouse cells and human THP-1 healthy cells (9).
So far, no academic study has evaluated the beneficial effects of Harm on Merc-induced renal injury. Therefore, the present attempt has been carried out to investigate the biochemical and renal histological alterations following Harm administration on Merc-induced nephrotoxicity in BALB/c male mice.
MATERIALS AND METHODS
Animal treatment and sampling
In this study, forty-eight BALB/c mice weighing 30 ± 2 g were used. Animals were kept in special cages at 22 ± 2 °C and 12/12-h light/dark cycle and had free access to water and food. The investigation was approved by the Animal Ethics Committee of Kermanshah University of Medical Sciences (Ethics Code: IR. KUMS. REC. 1398.087). The animals were randomly divided into 8 study groups, 6 each, as shown in Table 1. The animals received saline, Merc, and Harm (Sigma, USA) once a day for 14 consecutive days (10,11).
Table 1.
Characteristics of the studied groups.
| Groups | Treatments |
|---|---|
| Control | Saline (0.09%) |
| Merc | HgCl2 (0.5 mL/day of 0.5 ppm aqueous) |
| Harm1 | Harmine (5 mg/kg) |
| Harm2 | Harmine (10 mg/kg) |
| Harm3 | Harmine (15 mg/kg) |
| Merc + Harm1 | Hgcl2 (0.5 mL/day) + Harmine (5 mg/kg) |
| Merc + Harm2 | Hgcl2 (0.5 mL/day) + Harmine (10 mg/kg) |
| Merc + Harm3 | Hgcl2 (0.5 mL/day) + Harmine (15 mg/kg) |
Merc, Mercuric chloride, Harm, harmine
The weight of all animals was measured and recorded at the beginning and the end of the experiment. A day after the last treatment, the mice were anesthetized by intraperitoneal (I.P) injection of ketamine HCl (100 mg/kg) and xylazine (10 mg/kg) (12). The kidneys were excised, weighed, and reserved for biochemical and molecular applications. The blood samples were aspirated from the heart, centrifuged at 4000 rpm for 15 min, and then the isolated serum was transferred to new micro-tubes and stored in a -20 °C refrigerator.
Histological evaluation of the kidney
For histological evaluation, the kidney tissue was suspended in 10% formalin saline for 72 h to exact tissue fixation and prevention of tissue autolysis. Subsequently, dehydration of the fixed samples was carried out by increasing concentrations of ethanol followed by a clarification in xylene. Next, the kidney tissue was transferred to a paraffin bath to remove xylene and infiltrate the paraffin. Using a microtome, 5 μm thickness sections were made from paraffin-embedded blocks. Then, 5 microscopic sections of each sample were randomly selected and followed by hematoxylin-eosin (H&E) staining. The slices were studied by a histologist and pathologist using light microscopy. The images were captured by the use of a conventional camera. Control samples were considered as normal, and the histological alterations caused by the agents were compared to the control group (13).
For estimating the number of the corpuscles, 20 randomly selected fields of 100× magnification captured pictures from each section were monitored. Then to determine the number of the corpuscles this number was divided into 20. To achieve the mean diameters of renal corpuscles, at least 150 of the round or almost rounded renal corpuscles were randomly captured (400×) with a zigzag method by a blind observer and followed by statistical analysis using a specialized software package (AE-3; Motic S.L.U., Spain).
Briefly, the diameter of each renal corpuscle was estimated as the mean length of two drawing lines, vertical to each other that connected the distance between opposed basement membranes of the outer cell layer and the mean of these lines were considered as the diameter of renal corpuscle (14). The qualitative changes, including vascular congestion, dilated distal and proximal tubules, intra-tubular proteinaceous casts, tubular cell detachment, and intra-cellular vacuolization, were also evaluated in the 50 randomly-selected fields of each kidney sample (13).
Measurement of urea and creatinine
Three mL of the blood sample was collected from the heart and placed into Vacutainer blood collecting tubes (BD Biosciences; USA). Then, the serums were retrieved following 30 min of centrifugation of the blood samples at 1000 g (4 °C). All samples were centrifuged within 35- to 40 min after collection. The serum samples were frozen at -80 °C for future biochemical measurements of urea (BUN) and creatinine using an appropriate kit (PARS-AZMON; Iran) and accessed by an autoAnalyzer (RA 1000; Technicon Instruments, USA).
Evaluation of NO level of renal tissue
The NO level of lysed kidneys was measured based on Griess colorimetric assay. Briefly, 100 μL of the zinc sulfate-deproteinized sample was transferred into the wells. Then, 100 μL of chloride vanadium, 50 μL of sulfonamide, and 50 μL of N-(1-naphthyl) ethylenediamine dihydrochloride (NEED) solutions were added to the samples. The samples' optical density (OD) was measured by an ELISA reader at a wavelength of 540 nm.
Determination of superoxide dismutase level of renal tissue
To measure the amount of superoxide dismutase (SOD), the Zell Bio kit (GmbH, Germany) and spectrophotometric apparatus were used. The reaction of 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) with anion superoxide, produced from pyrogallol, was inhibited by the SOD enzyme. Then, the other steps were conducted following the manufactures' instructions, and the light absorption of the compound was read by ELISA at a wavelength of 570 nm. In order to obtain the percent inhibition induced by SOD enzyme, the corresponding formula was used based on the kit manufacturer.
Measurement of total antioxidant capacity levels of the renal tissue
Total antioxidant capacity (TAC) was assessed using the Trolox equivalent antioxidant capacity (TEAC) method and Randox kit (UK), and finally, TAC was determined according to the Randox kit protocol. Briefly, a stock solution was prepared from 2,2′-azino-bis (3-ethylbenzothiazoline-6- sulphonic acid) (ABTS, 8 mM), and potassium persulfate K2S2O8 (3 mM) formed ABTS+, which was developed 24 h before the analysis operation. Trolox was used as the standard, 25 μL of extract, and 275 μL of ABTS mix was added to the well. A Multiskan plate reader (Thermo Electron Corporation, Beverly, MA, USA) was used to read the absorbance at 734 nm.
Cell death detection
The induction of apoptosis in the renal tissues was calculated using a cell death detection ELISA-kit (Cat.No.11544675001; Roche, Germany) with a spectrophotometer method according to the manufacturer's instruction. One mg of selected renal tissues (without the appearance of vasculature) was thrown in a microtube containing 100 μL of lysis buffer and vortexed, then centrifuged in 1000 g for 10 min, and finally washed by phosphate-buffered saline (PBS) in triplicate. The cells were suspended in 20 μL of PBS and transferred to a 96-well plate. 80 μL of immunoreagent was added to each well, which was covered by a foil for 2 h at 20 °C under gently shaking (300 rpm). Following rinsing with the solutions 1 and 2, the absorbance rate of the samples was read at 490 ± 10 nm of wavelength.
Statistical analysis
Data were analyzed using SPSS (Version16) software. Quantitative parameters were statistically compared using one-way ANOVA followed by the post hoc test of Tukey, while the qualitative ones by λ2. The data are presented as mean ± standard error of the mean (SEM), and P < 0.05 was considered as significant.
RESULTS
Harm and Merc effects on weights of mice and kidney
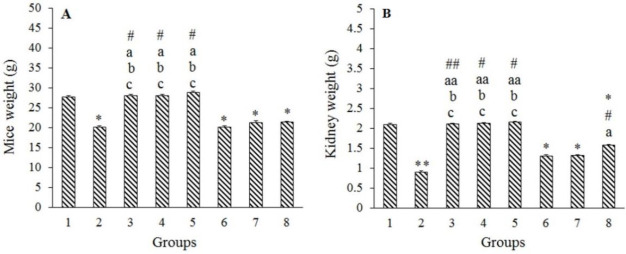
Significant reductions in total body and kidney weights were detected in the Merc group compared to the control group (P < 0.01), whereas there were no significant changes in these two variables between the Harm groups and the control group (P > 0.05). Although in Harm groups, the values of these two parameters were boosted in a dose-dependent manner, this increase was not statistically significant (P > 0.05). In addition, either total body weight or kidney weights showed a significant decrease in Merc + Harm groups in comparison with the control group (P < 0.05). Co-administration of Harm at 15 mg/kg with Merc significantly increased the kidney weight compared to Merc group (P < 0.05). Comparing Merc + Harm groups, Merc + Harm groups, a growing trend in Harm dosage was observed, which was only significant in terms of kidney weight in Merc + Harm3 compared to the other two groups (Fig. 1A and B).
Fig. 1.
Effects of Harm (5, 10, 15 mg/kg) and Merc (0.5 mL/day of 0.5 ppm aqueous) on mice and kidney weights. Values are presented as mean ± SEM; n = 6. Groups 1 (Control) received normal saline 0.09%; group 2 received Merc at 0.5 mL/day; groups 3-5 (Harm1-3) received Harm at 5, 10, 15 mg/kg, respectively; groups 6-8 received Merc at 0.5 mL/day and Harm in doses of 5, 10, 15 mg/kg, respectively. *P < 0.05 and **P < 0.01 indicate significant differences compared to the control groups; #P < 0.05 and ##P < 0.01 versus Merc group; aP < 0.05 and aaP < 0.01 in comparison with Merc + Harm1 group; bP < 0.05 in contrast to Merc + Harm2 group; cP < 0.05 against Merc + Harm3 group. Merc, Mercuric chloride, Harm, harmine.
Histological characteristics of kidney
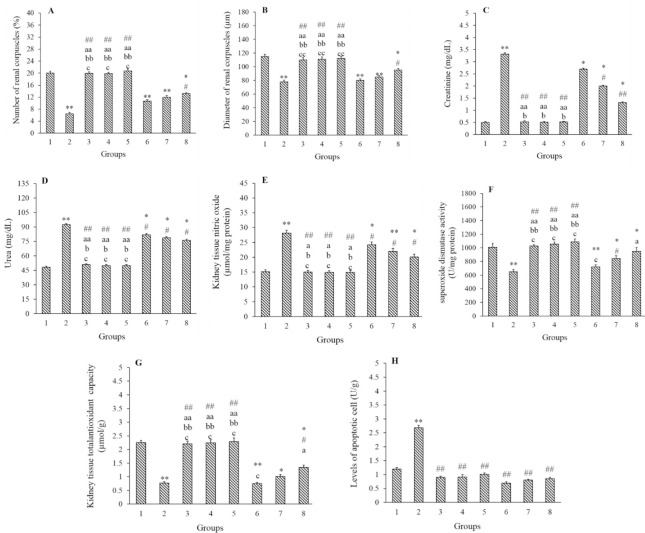
As shown in Fig. 2 and Table 2, there was an increase in qualitative histology criteria in the Merc, Merc + Harm1, and Merc + Harm2 groups (P < 0.01), while a slight histopathological alteration was detected in Merc + Harm3 group (P < 0.05). Generally, the histopathologic indices in the Merc + Harm group compared to the Merc group were improved significantly in Merc + Harm 1and 2 (P < 0.05), and Merc + Harm3 (P < 0.01). Further, the quantitative parameters on renal tissues were evaluated. The number and the diameter of renal corpuscles in the Merc group were significantly decreased compared to the control group (P < 0.01). These two parameters in the Harm groups showed no significant differences in comparison with the control group (P > 0.05). Despite an increase in the number and the diameter of renal corpuscles in Harms intra-group, this difference was not statistically significant (P > 0.05). A significant decrease in the number and diameter of renal corpuscles was observed in Merc + Harm groups compared to the control group (P < 0.05). In addition, there was a significant increase in the number and diameter of renal corpuscles in the Harms and Merc + Harm3 groups compared to the Merc group (P < 0.05). Although these two parameters were elevated dose dependently in Merc + Harms group, these changes were not statistically significant (Fig. 3A-B).
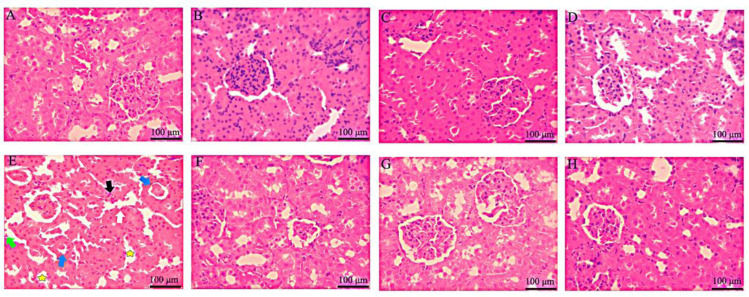
Fig. 2.
Hematoxylin and eosin stained sections of renal tissue in control and experimental groups following administration of Merc and different doses of Harm (H&E. ×400). Scale bar = 100 μm. (A) Received normal saline 0.09%, control group; (B-D) received Harm in doses of 5, 10, 15 mg/kg, respectively; (E) received Merc in a dose of 0.5 mL/day; (F-H) received Merc in a dose of 0.5 mL/day and Harm in doses of 5, 10, 15 mg/kg, respectively. White, black, blue, and green arrows referred to the intra-cellular vacuolization, vascular congestion, intra-tubular proteinaceous casts, and tubular cell detachment, respectively; yellow star pointed to the tubular dilatation. Merc, Mercuric chloride, Harm, harmine.
Table 2.
Qualitative histology alteration in renal tissue of mice following administration of Merc (0.5 mL/day), Harm1- 3 (5, 10, 15 mg/kg, respectively) or their combination.
| Histopathology indices | Control | Merc | Harm1 | Harm2 | Harm3 | Merc + Harm1 | Merc + Harm2 | Merc + Harm3 |
|---|---|---|---|---|---|---|---|---|
| Intra-cellular vacuolization | 0 | III | 0 | 0 | 0 | IV | III | I |
| Tubular dilatation | 0 | VII | 0 | 0 | 0 | IV | II | I |
| Vascular congestion | 0 | X | 0 | 0 | 0 | IV | II | 0 |
| Intra-tubular proteinaceous casts | 0 | IX | 0 | 0 | 0 | IV | II | 0 |
| Tubular cell detachment | 0 | V | 0 | 0 | 0 | II | III | 0 |
| Total | 0 | XXIV** | 0 aa | 0 aa | 0 aa | IIX**, a | XII**, a | II*, aa |
Merc, Mercuric chloride, Harm, harmine.*P < 0.05 and **P < 0.01 indicate significant differences compared with the control group; aP < 0.05 and aaP < 0.01 against Merc + Harm1 group.
Fig. 3.
Effects of Harm (5, 10, 15 mg/kg) and Merc (0.5 mL/day) on the parameters related to the function and the histology of kidney, nitric oxide, superoxide dismutase, total anti-oxidant capacity, and apoptosis. Values are resented as mean ± SEM; n = 6. Groups 1 (control) received normal saline 0.09%; group 2 received Merc at 0.5 mL/day; groups 3-5 (Harm1-3) received Harm at 5, 10, 15 mg/kg, respectively; groups 6-8 received Merc at 0.5 mL/day and Harm in doses of 5, 10, 15 mg/kg, respectively. *P < 0.05 and **P < 0.01 indicate significant differences compared to the control groups; #P < 0.05 and ##P < 0.01 versus Merc group; aP < 0.05 and aaP < 0.01 in comparison with Merc + Harm1 group; bP < 0.05 and bbP < 0.01 in contrast to Merc + Harm2 group; cP < 0.05 and ccP < 0.01 against Merc + Harm3 group. Merc, Mercuric chloride, Harm, harmine.
Influences of Harm and Merc on serum creatinine and BUN levels
Figure 3C and D indicate that the serum levels of creatinine and BUN were significantly increased in the Merc group compared to the control group (P < 0.01). The results offered no significant difference in these two variables between Harm and the control groups (P > 0.05). There were no significant differences in Harms intra-group comparison. Moreover, serum levels of BUN and creatinine were significantly higher in the Merc + Harm groups than the control group (P < 0.05). Administration of Harm significantly decreased both parameters, in Harm + Merc groups, compared to Merc group (P < 0.05). Although the serum creatinine and BUN levels were reduced with increasing dose in the Merc + Harm groups, the decrease was not statistically significant.
Effects of Harm and Merc on NO in renal tissue of mice
Measurement of NO levels of renal tissue in Merc groups showed a significant increase compared to the control group (P < 0.05). There were no significant differences in this parameter between the Harm groups and the control group (P > 0.05). Although in Harm groups, the NO level was reduced in a dose-dependent manner, this alteration was not statistically significant (P > 0.05). In Merc + Harm groups, there was a significant increase in this parameter compared to the control group (P < 0.05). NO levels were significantly declined in Harm receiving groups compared to Merc groups (P < 0.05), while there were no significant differences between Merc + Harms groups (P > 0.05, Fig. 3E).
Impacts of Harm and Merc on SOD and TAC levels in renal tissue of mice
There was a significant decrease in SOD and TAC levels in the Merc group compared to the control group (P < 0.01). In comparison with the control group, these parameters did not show any significant differences with Harm groups (P > 0.05). In comparison with the control group, a significant decrease in SOD levels in groups of Merc + Harm1 and 2 were observed (P < 0.05). Also, the TAC level was significantly lower in Merc + Harm groups in comparison with the control group (P < 0.05). In groups of Harms and Merc + Harm2 and 3, a significant increase in SOD levels was detected compared to the Merc group. Furthermore, there was a significant increase in TAC levels in Harms and Merc + Harm3 groups compared to the Merc group (P < 0.05). A significant dose-dependent increase was observed for these two parameters in the Merc + Harms groups (P < 0.05, Fig. 3F-G).
Cell death detection following Harm and Merc in renal tissue
According to Fig. 3H, the number of apoptotic cells showed a significant increase in Merc group than the control group (P < 0.01), at the same time, no statistical alterations were detected between control and other experimental groups (P > 0.05). Apoptotic cells were significantly decreased in Harms and Merc + Harm groups compare to the Merc group (P < 0.01), indicating the restoration characteristic of Harm in apoptosis inhibition.
DISCUSSION
Chronic exposure to Merc can lead to the induction of pathological tissue transformation (15,16). Merc toxicity is the second most common heavy metals contamination. Renal toxicity is one of the crucial side effects of acute or chronic Merc exposures. Herbal compounds can inhibit toxicity of exogenous agents. Thus, the antioxidant features available in medicinal plants are considered as an accepted option for protection against heavy metal toxicity (17). Several studies have reported the antioxidant properties of Harm β-carboline (widely distributed in the plants) (7). No researches have been conducted to investigate the antioxidant effect of Harm (one of the major active components of Peganum harmala plant) following Merc-toxicity induction in the kidney. In this study, for the first time, we have investigated the effects of Harm on renal injury induced by Merc administration in male mice.
The results showed that the Merc reduced the body and kidney weights, number and diameter of renal components, TAC and SOD levels, increased serum levels of renal enzymes (creatinine and BUN), NO, and apoptotic cells in male mice, whilst Harm administration improved these parameters.
The decrease in body weight after Merc administration probably indicated the food intake reduction or nephrotoxicity, which is reported with earlier research (16). Of course, the duration of treatment and the prescribed doses are two critical factors in weight loss due to exposure to Merc. Also, the reduction of kidney weight in the group treated with Merc, demonstrate the fat and protein degeneration occurred in experimental mice. Harm was effective in restoring weight loss as well as kidney weight in Merc receiving mice. In line with our results, the body and kidney weights loss following Merc and cadmium administration has been reported in male Wistar rats (16). Further, the results of a study showed that the oral administration of Peganum harmala hydroalcoholic extract improved the lost weight in thiourea-treated mice (17). Also, oral administration of Harm within 7 days improved nutrient uptake compared to the saline-receiving mice.
In this study, the Harm administration in Merc receiving groups increased the number and diameter of renal corpuscles. Also, Gao et al. reported that rhubarb anthraquinones could relieve histopathological alterations induced by Merc administration in rats (18). According to the renal histological studies, the kidney tissue in Harm-receiving groups was detected as normal with no trace of renal necrosis. While Merc permeation to the kidney tissue exerts swelling, lumen enlargement in distal and proximal tubules, and variation in the shape of the epithelium of renal tubules, all are related to the oxidative stress induction in the vast area of kidney tissues. Moreover, Harm administration in the Merc receiving groups decreased tubular necrosis and glomerular damage. Numerous researches have suggested the qualitative renal tissue damage due to Merc exposure in laboratory animals (18,19). Previous investigations showed the positive effects of Harm on renal injury induced by lipopolysaccharide and nicotine in mice (20,21,22).
Serum urea and creatinine are two critical indicators of renal function. The promotion of them following Merc treatment indicates the harmful effects of Merc on the function of the kidney. In addition to impaired renal function by an increased level of urea and creatinine, the glomerular damage due to decreased renal excretion of this substance is speculated. It has been reported that the Merc treatment induces impaired glomerular function (19). On the other hand, the increased levels of urea can induce oxidative stress by increasing free radicals' level in the body (23). These radicals alter the enzymatic activity by attacking biological molecules such as DNA, proteins, and lipids, which eventually causes cell damage and cell death. Further, the creatinine levels demonstrate the filtration performance through the visceral layer of Bowman's capsule (24). Previous studies reported increased serum creatinine and urea after Merc induction (5,16). Based on our results, the Harm in a dose- dependent manner caused a significant reduction of urea and creatinine serum levels. Thus, this substance alleviated the Merc deleterious effect on renal function. The results of the study of El Baky et al. revealed that the Peganum harmala extract significantly decreased the serum urea and creatinine levels in streptozotocin-induced diabetes mice (25). Also, Salahshoor et al. reported the positive effects of Harm in the reduction of these two parameters caused by nicotine (21).
In the present study, the Merc administration caused an increase in NO level of renal tissue, which is in agreement with other studies (26). NO increasing results in a cascade reaction and then free radicals' production. Further, the increased level of NO is associated with the increased rate of cyclooxygenase 2 and nuclear factor kappa B expression, leading to inflammation induction, apoptosis, and tissue damage (27,28). NO can enhance the excessive entry of calcium to cellular cytosol and induce a poisonous effect on cells. Excessive NO production and increased iNOS and nNOS expression may induce nephrotoxicity and nephritic diseases. Based on the results of this study, the Harm prescription in Merc receiving mice could significantly decrease the NO serum levels. Thus, the harm, as an antioxidant, can alleviate the effects on renal tissue damage caused by Merc exposure. Antioxidants can diminish the NO production by rapid degeneration and perturbation in NO production system. Harm treatment could moderate lipid peroxidation and increase the antioxidant capacity of renal tissue, which consequently decreases the intracellular oxidative stress levels. The same effect of Harm also was seen in the mice treated with rotenone (29) and nicotine (21).
Moreover, this study demonstrated that the Merc reduced the concentration of renal antioxidant, including SOD and TAC, which was consistent with the results of Gao et al. (18). One study illustrated that the rise in urea and creatinine serum levels could lead to a direct reduction of antioxidant levels in the body (23). The Harm administration in the Merc receiving mice significantly increased the levels of SOD and TAC. Thus, we can conclude that the Harm can reduce the levels of free radicals leading to decreased levels of NO and increased level of antioxidant activity. The previous study revealed that the Peganum harmala extract significantly reduced the SOD level in diabetic mice (25). Berrougui et al. reported that the harmaline and harm alkaloids compound exist in Peganum harmala extract with significant free radical-scavenging capacity and the ability to inhibit the low- density lipoprotein peroxidation (30). The current study displayed that Harm administration was able to moderate the lipid peroxidation and increase the TAC of renal tissue, and consequently decrease oxidative stress levels.
Apoptosis (programmed cell death) is a cellular process operated by a variety of chemicals or environmental motivations like heavy metals, ultraviolet radiation, oxidative stress, calcium ionophores, xenobiotic agents, and anoxia (31). In the present study, the level of apoptosis in the kidney was found to be increased following Merc treatment as a consequential report earlier (32,33). The production of elevated levels of free radicals and lipid peroxidation following Merc administration could damage intracellular proteins and DNA of the kidney which finally the apoptosis occurs. This phenomenon could be characterized as the universal process of hazardous agents (34). This study emphasized the antioxidant activity of Harm along with other plants with beneficial therapeutic effects by suppressing apoptosis in hazarded tissues.
CONCLUSION
The findings of the present study confirmed that the Harm administration could reduce the toxic effects of Merc on renal tissue in a dose- dependent manner. Also, the Harm reduced the oxidative stress and free radicals' production in the kidney caused by Merc through its antioxidant capacity. Furthermore, the antioxidant effect of Harm marked by a decrease in NO level and increased TAC and SOD levels of renal tissue could reduce apoptosis and renal damage in mice.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest in this study.
AUTHORS' CONTRIBUTION
All authors contributed equally to this work. C, Jalili and A. Ghanari take the responsibility for the integrity of the work as a whole from inception to publication of the article.
ACKNOWLEDGEMENTS
The authors express their gratitude to the Research Deputy of Kermanshah University of Medical Sciences for the financial support of this research project with Grant No. 980112.
REFERENCES
- 1.Flora SJS, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7(7):2745–2788. doi: 10.3390/ijerph7072745. DOI: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andayesh S, Hadiani M, Mousavi S, Shoeibi S. Determination of heavy metals; mercury, cadmium, lead and arsenic in different types of canned tuna fish in Tehran. Res Pharm Sci. 2012;7(5):S134. [Google Scholar]
- 3.Haghgoo S, Kalami M, Hadiani M. Method development for determination of Arsenic, Cadmium and Mercury in marine origin dietary supplements by atomic absorption spectrometry (AAS) Res Pharm Sci. 2012;7(5):S663. [Google Scholar]
- 4.Cargnelutti D, Tabaldi LA, Spanevello RM, de Oliveira Jucoski G, Battisti V, Redin M, et al. Mercry toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere. 2006;65(6):999–1006. doi: 10.1016/j.chemosphere.2006.03.037. DOI: 10.1016/j.chemosphere.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Piacenza F, Malavolta M, Cipriano C, Costarelli L, Giacconi R, Muti E, et al. L-Arginine normalizes NOS activity and zinc-MT homeostasis in the kidney of mice chronically exposed to inorganic mercury. Toxicol Lett. 2009;189(3):200–205. doi: 10.1016/j.toxlet.2009.05.021. DOI: 10.1016/j.toxlet.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Babaei Pour A, Moghadar N. Larval effect of extract of harmine and harmalin from Peganum harmala on juvenile of Protostrongylus rufescens. Res Pharm Sci. 2012;7(5):S63. [Google Scholar]
- 7.Moura DJ, Richter MF, Boeira JM, Pêgas Henriques JAP, Saffi J. Antioxidant properties of β-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis. 2007;22(4):293–302. doi: 10.1093/mutage/gem016. DOI: 10.1093/mutage/gem016. [DOI] [PubMed] [Google Scholar]
- 8.Hamsa TP, Kuttan G. Harmine activates intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma. Chin Med. 2011;6(1):11–18. doi: 10.1186/1749-8546-6-11. DOI: 10.1186/1749-8546-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki Y, Kawano Y. Inhibitory effects of herbal alkaloids on the tumor necrosis factor-α and nitric oxide production in lipopolysaccharide-stimulated RAW264 macrophages. Chem Pharm Bull. 2011;59(3):388–391. doi: 10.1248/cpb.59.388. DOI: 10.1248/cpb.59.388. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari V, Bhattacharya L. Adverse effects of mercuric chloride on thyroid of mice, musculus albinus and pattern of recovery of the damaged activity. J Environ Biol. 2004;25(1):109–111. [PubMed] [Google Scholar]
- 11.Réus GZ, Stringari RB, de Souza B, Petronilho F, Dal-Pizzol F, Hallak JE, et al. Harmine and imipramine promote antioxidant activities in prefrontal cortex and hippocampus. Oxid Med Cell Longev. 2010;3(5):325–331. doi: 10.4161/oxim.3.5.13109. DOI: 10.4161/oxim.3.5.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiravi A, Jalili C, Vaezi G, Ghanbari A, Alvani A. Acacetin attenuates renal damage-induced by ischemia-reperfusion with declining apoptosis and oxidative stress in mice. Int J Prev Med. 2020;11(1):22. doi: 10.4103/ijpvm.IJPVM_512_18. DOI: 10.4103/ijpvm.IJPVM_512_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shokri V, Jalili C, Raissi F, Akhshi N, Ghanbari A. Evaluating the effects of acacetin versus a low dose of cisplatin drug on male reproductive system and kidney in mice: with emphasis on inflammation process. Andrologia. 2020;52(1):e13444,1–9. doi: 10.1111/and.13444. DOI: 10.1111/and.13444. [DOI] [PubMed] [Google Scholar]
- 14.Chehrei S, Moradi M, Ghiabi HR, Falahi M, Kaviani S, Ghanbari A. Pentoxifylline besides naltrexone recovers morphine-induced inflammation in male reproductive system of rats by regulating Toll-like receptor pathway. Andrologia. 2017;49(9):e12749,1–8. doi: 10.1111/and.12749. DOI: 10.1111/and.12749. [DOI] [PubMed] [Google Scholar]
- 15.Clifton JC. Mercury exposure and public health. Pediatr Clin North Am. 2007;54(2):237–269. doi: 10.1016/j.pcl.2007.02.005. DOI: 10.1016/j.pcl.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Haouem S, NaJJAR MF, El Hani A. Stimultaneous effects of cadmium and mercury on some biochemical parameters of kidney function in male rats. J Curr Chem Pharm Sc. 2015;5(1):26–30. [Google Scholar]
- 17.Hamden K, Masmoudi H, Ellouz F, ElFeki A, Carreau S. Protective effects of Peganum harmala extracts on thiourea-induced diseases in adult male rat. J Environ Biol. 2008;29(1):73–77. [PubMed] [Google Scholar]
- 18.Gao D, Zeng LN, Zhang P, Ma ZJ, Li RS, Zhao YL, et al. Rhubarb anthraquinones protect rats against mercuric Chloride (HgCl(2))-induced acute renal failure. Molecules. 2016;21(3):298–109. doi: 10.3390/molecules21030298. DOI: 10.3390/molecules21030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabi S. Toxic effects of mercury. 1st ed. India: Springer; 2014. pp. 117–134. DOI: 101007/978-81-322-1922-4. [Google Scholar]
- 20.Niu X, Yao Q, Li W, Zang L, Li W, Zhao J, et al. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice. Eur J Pharmacol. 2019;849:160–169. doi: 10.1016/j.ejphar.2019.01.062. DOI: 10.1016/j.ejphar.2019.01.062. [DOI] [PubMed] [Google Scholar]
- 21.Salahshoor MR, Roshankhah S, Motavalian V, Jalili C. Effect of harmine on nicotine-induced kidney dysfunction in male mice. Int J Prev Med. 2019;10:97. doi: 10.4103/ijpvm.IJPVM_85_18. DOI: 10.4103/ijpvm.IJPVM_85_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalili F, Jalili C. Protective effect of Harmine on kidney disorders induced by nicotine in male mice. Int Pharmacy Acta. 2018;1(1):132–133. DOI: 10.22037/ipa.v1i1.20025. [Google Scholar]
- 23.Naghibi B, Ghafghazi T, Hajhashemi V, Talebi A, Taheri D. The effect of vitamin E in prevention of vancomycin-induced nephrotoxicity in rats. Res Pharm Sci. 2006;2:104–111. [Google Scholar]
- 24.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. DOI: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 25.El Baky HH, AAA ER, Mekawia EM, Ibrahema EA, Shalapy NM. The anti-diabetic and anti-lipidemic effects of Peganum harmala seeds in diabetic rats. Der Pharm Lett. 2016;8(10):1–10. [Google Scholar]
- 26.Omanwar S, Saidullah B, Ravi K, Fahim M. Modulation of vasodilator response via the nitric oxide pathway after acute methyl mercury chloride exposure in rats. Biomed Res Int. 2013;2013:530603,1–8. doi: 10.1155/2013/530603. DOI: 10.1155/2013/530603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honma S, Takahashi N, Shinohara M, Nakamura K, Mitazaki S, Abe S, et al. Amelioration of cisplatin- induced mouse renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Eur J Pharmacol. 2013;715(1-3):181–188. doi: 10.1016/j.ejphar.2013.05.023. DOI: 10.1016/j.ejphar.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35(3):553–571. doi: 10.1016/j.gtc.2006.07.002. DOI: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 29.EL Madani M, Attia A, Abd el salam RM, ElShenawy M, Arbid MS. Neuropharmacological effects of naringenin, harminee and adenosine on parkinsonism induced in rats. Der Pharm Lett. 2016;8(5):45–57. [Google Scholar]
- 30.Berrougui H, Isabelle M, Cloutier M, Hmamouchi M, Khalil A. Protective effects of Peganum harmala L extract, harmine and harmaline against human low- density lipoprotein oxidation. J Pharm Pharmacol. 2006;58(7):967–974. doi: 10.1211/jpp.58.7.0012. DOI: 101211/jpp5870012. [DOI] [PubMed] [Google Scholar]
- 31.Turk E, Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Kuzu M. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol Trace Elem Res. 2019;189(1):95–108. doi: 10.1007/s12011-018-1443-6. DOI: 101007/s12011-018-1443-6. [DOI] [PubMed] [Google Scholar]
- 32.Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q, et al. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: Involvement of AKT/Nrf2 and NF-κB pathways. Food Chem Toxicol. 2018;113:296–302. doi: 10.1016/j.fct.2018.02.003. DOI: 10.1016/j.fct.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Caglayan C, Kandemir FM, Yildirim S, Kucukler S, Eser G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J Trace Elem Med Biol. 2019;54:69–78. doi: 10.1016/j.jtemb.2019.04.007. DOI: 10.1016/j.jtemb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Hamzeh M, Hosseinimehr SJ, Khalatbary AR, Mohammadi HR, Dashti A, Amiri FT. Atorvastatin mitigates cyclophosphamide-induced hepatotoxicity via suppression of oxidative stress and apoptosis in rat model. Res Pharm Sci. 2018;13(5):440–449. doi: 10.4103/1735-5362.236837. DOI: 10.4103/1735-5362.236837. [DOI] [PMC free article] [PubMed] [Google Scholar]