Abstract
Background and purpose:
Crocetin is a natural antioxidant that is found in the crocus flower and Gardenia jasminoides (fruit). Previous studies have reported its anticancer activity both in vivo and in vitro. In addition, crocetin suppresses the growth and migration of human colorectal cancer cells, however, its mechanism of action remains to be elucidated. Therefore, the present study investigated the molecular mechanism of crocetin effect on colorectal cancer cells (HCT-116) in vitro.
Experimental approach:
HCT-116 cells were treated with different concentrations (0, 200, 400, 600, and 800 μM) of crocetin for 24 h. The cell survival rate was measured by MTT assay. Cell migration capacity was evaluated using the wound healing assay. The expression levels of vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP-9) was monitored by RT-PCR. Phosphorylation of focal adhesion kinase (FAK) and p38 mitogen-activated protein kinase (MAPK) was determined using western blot.
Findings/Results:
The proliferation of HCT-116 was inhibited by crocetin at 800 μM (P < 0.001). Crocetin prevented migration of HCT-116 cells (P < 0.05) and suppressed VEGF and MMP-9 mRNA expression (P < 0.001) and increased phosphorylation of p38 (MAPK; P < 0.001). However, no significant change in the phosphorylation of FAK was observed.
Conclusion and implication:
These data suggested that crocetin-induced growth- and migration- suppressing effects on HCT-116 cells may partially depend on the regulation of the p38 (MAPK) signaling pathway.
Keywords: Crocetin, HCT-116 cells, Matrix metalloproteinase 9, p38-mitogen activated protein kinase, Vascular endothelial growth factor
INTRODUCTION
Colorectal cancer (CRC) is the third most common cause of cancer death in the world and its incidence and mortality rates are relatively high among Iranians, and threatens them at younger ages with an increasing prevalence similar to the Western counterparts (1,2). It is well documented that CRC remained as a potentially fatal cancer with a poor prognosis, mainly due to metastasis in the majority of patients (3). Important steps in the distribution of CRC cells to the other organs are the ability of these cells to migrate in the direction of the basement membrane and then invade the surrounding stroma (4).
For the control of metastatic cancer, the development of anticancer therapies has been strongly targeted the cancer migration- and invasion-related molecules (5). Due to the various side effects of the current treatment, much attention has been given to the medicinal value of natural products (3,6).
In modern pharmacological studies, saffron or its active constituents have anti-inflammatory (6) antitumor activities (7), radical scavenger effects (8,9,10)as well as learning and memory improving properties (11). Crocetin as an important natural antioxidant is derived from saffron. Previous studies have reported different aspects of crocetin as a potential drug that inhibits the proliferation of various tumor cells but not normal cells (12,13). It has been reported that crocetin exerts cytotoxic activity and displays anticancer effects through multiple mechanisms including potential interaction with DNA, capable of inducing apoptosis and inhibitory effects on cell proliferation, motility, and migration (14,15,16,17).
Angiogenesis is known as one of the key processes that can lead to invasion and metastasis of cancer cells to distant organs, resulting in more than 90% mortality from cancer. Moreover, anti-angiogenic therapy is recognized as one of the most reliable alternative remedies to control cancer (18). The role of vascular endothelial growth factor (VEGF) on the other hand, is extremely important in the regulation of angiogenesis. It corresponds to tumor growth and development by the proliferation, migration, and invasion of endothelial cells. It has also been documented that VEGF has an influential effect on cancer cell invasion by altering the expression of matrix metalloproteinases (MMPs) (19,20).
Many signaling pathways are participating in cellular events of cancer cells. High levels of phosphor focal adhesion kinase (p-FAK), which initiates downstream signaling pathways, has already been found in different invasive cancer cells including human colon cancer cells (21). The p38 mitogen-activated protein kinase (MAPK) signaling pathway is a key component of the MAPK superfamily, which is triggered by distinct extracellular stimuli and has an important role in cell apoptosis. Malfunctions in this pathway are associated with tumorigenesis and the development of other proliferative diseases (22,23). Exploring the role of the p38 MAPK signaling pathway in CRC development using chemical intervention may offer a reliable theoretical basis for further clarification of CRC pathogenesis. However, the role of p38 MAPK in cancer development is controversial (24). Previous reports indicated that p38 activation resulted in a remarkable reduction in tumor growth in chick embryos (25). In this study, we examined the effects of crocetin on p38 MAPK and FAK signaling pathways in HCT-116 human colorectal cancer cells.
MATERIALS AND METHODS
Materials
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl- tetrazolium bromide (MTT) and dimethyl sulfoxid (DMSO) were purchased from Sigma (USA) and Merck (Germany), respectively. Crocetin was obtained from MP Biomedicals (CAT No. 02193543; USA). Roswell Park Memorial Institute media 1640 (RPMI-1640), fetal bovine serum (FBS), penicillin- streptomycin and trypsin-ethylenediamine tetra acetic acid (EDTA) solution were purchased from Biowest (France). Primary antibodies against p38 MAPK, FAK, p-p38 MAPK, and p-FAK were purchased from Santa Cruz Biotechnology (USA).
Cell culture
HCT-116 human colorectal cancer cells were obtained from the cell bank of Pasteur Institute (Tehran, I.R. Iran), and cultured in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated FBS, 100 unit/mL penicillin, and 100 μg/mL streptomycin, and incubation was carried out at 37 °C in a humidified incubator (95%) in a 5% CO2 atmosphere. Subculture was performed when cells reached 80-90% confluence.
Cell viability assay
The effect of crocetin on cell viability was determined using the MTT assay. Briefly, 5 × 103HCT-116 cells were seeded into each well of a 96-well culture plate for 24 h and followed by treatment with crocetin at 200, 400, 600 and 800 μM. After 24 h of treatment, cells were washed with phosphate-buffered saline (PBS 0.15 M, pH 7.4) twice and incubated with MTT solution (5 mg/mL) for 4 h at 37 °C. After removing the MTT solution, DMSO was added (200 μL/well) and the absorbance was recorded at 570 nm (630 nm as a reference) using an ELISA reader (BioTek, USA). Cell viability was expressed as the percentage of non-treated controls as follows:

Wound healing assay
A wound-healing assay was performed to investigate a possible effect of crocetin on cell migration behavior. In short, HCT-116 cells were trypsinized and seeded in a 12-well culture plate (3 × 105/well). When the cells reached 80-90% confluence, the monolayer cells were scratched with a 200 μL pipette tip to create a wound, and cells were washed with PBS to discard floating cells and then replaced with RPMI-1640 supplemented with two concentrations of crocetin (400 and 800 μM). Untreated and treated cells were photographed both immediately after wounding (0 h) and 24 h after wounding under phase-contrast microscopy (Micros, Austria), and the scratch area was measured using Image J Software (US National Institutes of Health).
Migration rate was expressed via the percentage of scratch closure change according to the following equation (26):

where, At0 is the scratch area at time 0, and Atc is the corresponding scratch area after 24 h.
RNA extraction and cDNA synthesis
The HCT-116 cells were treated with crocetin at 200, 400, 600, and 800 μM for 24 h. The cells were harvested and total RNA extraction was performed with a hybrid RNA extraction kit (GeneAll, Seoul, South Korea). The purity and concentration of extracted RNA were evaluated with NanoDrop TM 2000 Spectrophotometer (Thermo Fisher Scientific, USA). One μg total RNA of each sample was reverse-transcribed into cDNA with random hexamer primers using Hyperscript TM first-strand synthesis kit (GeneAll, Seoul, South Korea).
Reverse transcriptase-polymerase chain reaction analysis
Primer pairs were designed according to melting temperature and primer dimer formation in ncbi/primer- blast (http://blast.ncbi.nlm.nih.gov/blast.cgi). Primers were then checked for primer-dimer and hairpin formation again with gene runner software (http://www.generunner.net). Nucleotide sequences of primers (VEGF, MMP-9, and β-actin) are presented in Table 1. Cycling conditions were as follows: denaturation at 94 °C for 30 s, annealing for VEGF and MMP-9 at 58 °C and for β-actin at 60 °C for 30 s, and extension at 72 °C for 30 s. All polymerase reactions (PCRs) were linear up to 35 cycles. Expression levels of VEGF and MMP-9 were normalized in accordance with the expression levels of β-actin as the housekeeping gene. The products of reverse transcriptase-polymerase chain reaction (RT-PCR) were separated on 1.5% agarose gel containing safe stain and visualized using Gel Doc 2000 System (Bio-Rad). Densitometry analyses of PCR products were performed using Image J Software (US National Institutes of Health).
Table 1.
Nucleotide sequences of primers used for RT-PCR.
| Genes | Forward primers (5'-3') | Reverse primers (5'-3') |
|---|---|---|
| VEGF | AGGAGGAGGGCAGAATCATC | GGCACACAGGATGGCTTGAA |
| MMP-9 | GATGCGTGGAGAGTCGAAA | TAGGTGATGTTGTGGTGGTG |
| β-actin | CTGGAACGGTGAAGGTGACA | TGGGGTGGCTTTTAGGATGG |
Protein extraction and western blotting
The HCT-116 cells were treated with crocetin at 200, 400, 600, and 800 μM for 24 h. The collected cells were washed twice with PBS and lysed in lysis buffer (50 mM tris-HCl at pH 7.5, 150 mM NaCl, 0.5% sodium orthovanadate (Na3VO4 0.5% sodium deoxycholate, 50 mM NaF, 50 mM EDTA, 0.1% SDS, 1% Triton™ X-100) mixed with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 0.5 mM aprotinin, 0.5 mM leupeptin). The protein concentration in the samples was measured based on the Bradford method (27). After electrophoresis, proteins were electrotransferred to polyvinylidene fluoride (PVDF, Millipore®) membranes, and blocked with 3% bovine serum albumin (BSA) in tris- buffered saline, 0.1% Tween® 20 (TBST) buffer (20 mM tris-HCl pH 7.5, 150 mM NaCl and 0.1% Tween® 20) for 2 h at room temperature. The membranes were then probed with diluted primary antibodies (p38 MAPK (Cat No. sc-7972), FAK (Cat No. sc-271126), p-p38 MAPK (Cat No. sc-166182), and p- FAK (Cat No. sc-81493)) in 1% BSA/TBST (overnight, 4 °C), washed three times with TBS-T buffer for 10 min, incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000, Cat No. 1105A211) for 2 h at room temperature, and washed extensively three times with TBS-T buffer for 10 min before detection by chemiluminescence with the ECL kit (Cat No. CMGECL). Ultimately, proteins were visualized by exposing the blots to film and western blotting data were quantified by densitometry using Image J Software.
Statistical analysis
The data were expressed as means ± SD. For statistical analysis of the data, we performed one-way ANOVA and Tukey post hoc tests. P values ≤ 0.05 were considered statistically significant. GraphPad Prism 7.0 (GraphPad Software, San Diego, USA) was used for statistical analysis.
RESULTS
Crocetin inhibits the proliferation of HCT-116 cells
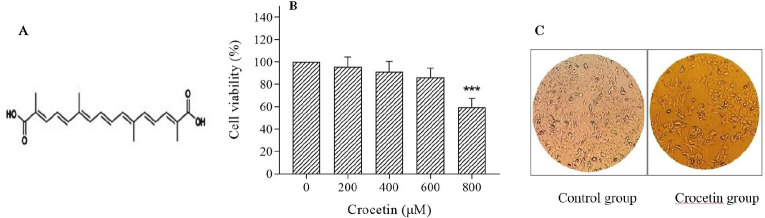
The first trial that was performed in this study was to highlight any potential effect of crocetin on colorectal cancer cell viability/proliferation by treating the human colorectal cancer HCT-116 cells with various concentrations of crocetin (200, 400, 600, and 800 μM) for 24 h. The MTT assay, (Fig. 1) showed that crocetin reduced HCT-116 cell proliferation in a dose-dependent manner, but significant inhibition on cell proliferation only was found at 800 μM concentration of crocetin as compared to the untreated control cells (P < 0.001). The half-maximal inhibitory concentration (IC50) of crocetin was 800 μM at 24 h.
Fig. 1.
(A) The chemical structure of crocetin; (B) cytotoxic effects of crocetin on HCT-116 cells; HCT-116 cells were exposed to crocetin at 200, 400, 600, and 800 μM for 24 h. Cell viability was determined by MTT assay. Cell viability was expressed as % of control; and (C) the morphology of HCT-116 cells under the light microscope was examined (100 × ). Data are reported as the mean ± SD, n = 3. ***P < 0.001 indicates a significant difference compared with the control group.
Crocetin reduced the migration of HCT-116 cells
The effect of crocetin on the migration of HCT-116 colorectal cancer cells was examined. The wound-healing assay or scratch test was conducted to investigate the cell migration. To perform the scratch test, we selected the effective concentrations. As shown in Fig. 2, crocetin was able to inhibit the migration of HCT-116 cells at 400 μM. Following 24 h exposure to crocetin, the rate of scratch closure change in different crocetin groups (0, 400, and 800 μM) was 38.33, 30.69, and 0%, respectively. Compared with the control group, crocetin significantly decreased cell migration in a dose-dependent manner.
Fig. 2.
Effect of crocetin on the migration of HCT-116 cells; cells were exposed to 400 and 800 μM of crocetin for 24 h. (A) The wound-healing assay was carried out to evaluate the inhibitory effects of crocetin on HCT-116 cell migration; (B) quantitative analyses of scratch closure changes. Data are reported as mean ± SD, n = 3). *P < 0.05 and ***P < 0.001 indicate significant differences compared with the control group.
Crocetin down-regulated the expression of VEGF and MMP-9 mRNA in HCT-116 cells
Angiogenesis and tumor cell metastasis mainly associated with an up-regulation of VEGF and MMP-9 expression in cancer cells. The expression of VEGF and MMP-9 genes at the mRNA level was analyzed. The RT-PCR analyses showed a concentration-dependent down-regulation of either gene of VEGF and MMP-9 (Fig. 3). All data at the mRNA level were normalized based on β-actin expression in the corresponding groups.
Fig. 3.
Effect of crocetin on the mRNA expression level of (A) VEGF and (B) MMP-9 in HCT-116 cells. Densitometric analyses of the (C) VEGF and (D) MMP-9 mRNA, which has been normalized to β-actin mRNA expression. Data are reported as mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 indicate significant differences compared with the control group. VEGF, Vascular endothelial growth factor; MMP-9, matrix metalloprotease 9.
Crocetin induced the phosphorylation of p38 MAPK in HCT-116 cells
To identify the signaling pathways involved in anti-proliferative and anti-migration effects of crocetin on HCT-116 colorectal cancer cells, western blot analyses were performed. The expression of p38 MAPK and FAK proteins, which are involved in cell proliferation, differentiation, migration, and metastasis, were assessed by western blot analyses. As shown in Fig. 4, crocetin induced the phosphorylation of p38 MAPK but not FAK compared to the non-treated control cells. The results showed that the phosphorylation levels of p-p38 MAPK molecules were significantly increased by crocetin at 400, 600, and 800 μM compared to the control group (P < 0.01), while crocetin had no evident effect on the expression of p-FAK.
Fig. 4.
Effects of crocetin on the phosphorylation of p38 MAPK and FAK in HCT-116 cells. Cells were treated with crocetin for 24 h. (A) The protein levels of p-p38 MAPK, p-FAK, p38 MAPK, and FAK from whole-cell lysates were analyzed by western blotting. (B) Densitometry analyses revealed that crocetin increased the ratio of p-p38 MAPK/p38MAPK but not p-FAK/FAK. Data are presented as mean ± SD, n = 3. **P < 0.01 and ***P < 0.001 indicate significant differences compared with the control group. MAPK, p38 Mitogen-activated protein kinase; FAK, focal adhesion kinase.
DISCUSSION
Although it has been previously demonstrated that crocetin exerts anticancer effects in several human cancer cell lines (28), the detailed mechanisms of such effects in colorectal cancer cells have not been fully understood. The current study investigated the effects of crocetin on proliferation, migration, VEGF and MMP-9 expression, and signaling pathways in HCT-116 colorectal cancer cells. The initial data indicated that crocetin has a cytotoxic effect against HCT-116 cell line in in vitro model. In the evaluation of the cytotoxicity of crocetin, we found that the IC50 values for HCT-116 were 800 μM. This finding is supported by previous studies, where it has been shown that crocetin in variable concentrations inhibited the proliferation of colorectal cancer cells and other cancer cell lines (29,30). The variability of IC50 in different cell lines is likely due to the heterogeneity of tumor cells, the so-called cell-specific response. Certainly, each cancer cell line is completely unique.
During tumor metastasis, MMPs, zinc-dependent proteases, degrade the extracellular matrix and cancer cells migrate from the original primary tumor mass into neighboring tissues (31,32,33). Cell adhesion, migration, and invasion in order to colonize a new tumor in another site involved in the development and progression of tumor metastasis (31,34), the role of tumoral angiogenesis on the other hand is extremely important in the metastatic progression of tumors and tumor proliferation, which supply oxygen and nutrient needs (35). Our results showed that crocetin at effective concentration remarkably inhibited the exposed cells to be closed after 24 h. Previous studies also reported that crocetin inhibited leukocyte adherence to bovine endothelial cells (36). To highlight the possible mechanism of wound healing inhibition by crocetin in HCT-116 cells, we closely looked at the expression of two strongly related genes (VEGF and MMP-9). It is well known that VEGF is the primary factor and the most potent inducer of angiogenesis and plays a pivotal role in regulating many cellular functions in the formation of new blood vessels, including endothelial cell recruitment, proliferation, and migration by altering the expression of MMPs (37). MMPs regulate vascular permeability and stability and are important for tumor angiogenesis, lymphangiogenesis, and vasculogenesis. Moreover, MMP-9 can support tumor angiogenesis by regulating the availability of VEGF in tumors (38). Our results demonstrated that crocetin significantly down- regulated the expression of VEGF and MMP-9 mRNA levels in HCT-116 cells, suggesting a substantial anticancer capacity. An interesting finding of this study was significant and substantial down-regulation of both examined genes even at lower concentrations. Consistent with our results Zhuang and et al showed that crocetin reduced protein levels of VEGF and MMP-9 in colorectal cancer cells (30).
FAK, as a non-receptor tyrosine kinase, can be activated in response to different stimuli and is a major pathway for cell growth, cell adhering migration, and metastasis (39,40). FAK regulates tumor angiogenesis and metastasis by induction of VEGF expression in tumor cells and the MMPs-mediated matrix degradation, respectively (21,40). It has been reported that the down-regulation of FAK pathway reduces the expression of VEGF and in turn decreases the formation of avascular tumor growth in breast carcinoma cells (41). For instance, epigallocatechin-3-gallate has been found to potently inhibit melanoma growth and metastasis through the inhibition of the MMP-9 and FAK activities (42). It is important to be noticed that, previous findings highlighted novel rationales for therapeutic targeting of FAK (43). However, we failed to show any changes in the phosphorylation of FAK under crocetin exposure in HCT-116 cells, proposing alternative signaling pathways of crocetin's effects on cell migration.
p38 MAPK is activated in response to a wide range of cellular stimuli, including stress, oxidative stress, cytokines, inflammation, and death receptors. Recent investigations have highlighted the role of p38 MAPK in cell proliferation, cell differentiation, apoptosis, cell migration, and cell invasion (24,44). Noteworthy, accumulating data support the increased levels of phosphorylated p38 MAPK have been linked to various malignancies, including head and neck squamous cell carcinomas, follicular lymphoma, breast carcinomas, thyroid, lung, and glioma. On the other hand, there are reports indicating that activation of p38 MAPK results in cancer cell apoptosis initiated by cisplatin, polyphenols (e.g. epigallocatechin-3-gallate), retinoids, and other chemotherapeutic agents. There are numerous supporting reports of signaling pathways including in vivo studies using mice with disrupted genes of the p38 kinases indicating an essential role of the p38 MAPK pathway in tumor suppression (24,45,46). At the same time, it has been documented that activation of the p38 MAPK pathway reduces the expression of MMP-2/MMP-9 in human leukemia cells (47). Western blotting analyses in the current study showed that crocetin could increase the p-p38 MAPK but had no effect on phosphorylation of FAK. These results potently suggest that crocetin inhibits angiogenesis by induction of p38 MAPK pathway. Our results are consistent with plenty of previous reports and we suggest that the anticancer effects of crocetin in HCT-116 cells may occur partially through regulating of p38 MAPK pathway and the inhibition of VEGF and MMP-9 expression are involved in the crocetin-inhibited proliferation and migration rate of HCT-116 cells.
CONCLUSION
In this study, we investigated the antitumor effects and the underlying mechanisms of crocetin, a natural carotenoid, in human HCT-116 colorectal cancer cell line. We found that crocetin potently prevented the growth and proliferation of colorectal cancer cells, demonstrating that crocetin could be a candidate as a potent natural substance or adjuvant against HCT-116 human colorectal cancer cells through its activation of the p38 MAPK signaling pathways and down- regulation of VEGF and MMP-9 mRNA expression.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest in this study.
AUTHORS' CONTRIBUTION
E. Khajeh carried out the experimental methods, analyzed the data, and wrote the article. Y. Rasmi conceived of the presented idea and wrote the article. F. Kheradmand helped to presented idea conceived. P. Aramwit, E. Saboory, M. Nasirzadeh revised the final version of the article. H. Malekinejad developed the experimental framework. B. Daeihassani developed the theoretical framework.
ACKNOWLEDGEMENTS
This work is derived from a Master of Science thesis in Clinical Biochemistry and was financially supported by the Urmia University of Medical Sciences, Urmia, Iran under Grant No. UMSU.1395.2024.
REFERENCES
- 1.Amin A, Bajbouj K, Koch A, Gandesiri M, Schneider-Stock R. Defective autophagosome formation in p53-Null colorectal cancer reinforces crocin-induced apoptosis. Int J Mol Sci. 2015;16(1):1544–1561. doi: 10.3390/ijms16011544. DOI: 10.3390/ijms16011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salimzadeh H, Eftekhar H, Majdzadeh R, Montazeri A, Delavari A. Effectiveness of a theory-based intervention to increase colorectal cancer screening among Iranian health club members: a randomized trial. J Behav Med. 2014;37(5):1019–1029. doi: 10.1007/s10865-013-9533-6. DOI: 10.1007/s10865-013-9533-6. [DOI] [PubMed] [Google Scholar]
- 3.Jin Y, Chen W, Yang H, Yan Z, Lai Z, Feng J, et al. Scutellaria barbata D Don inhibits migration and invasion of colorectal cancer cells via suppression of PI3K/AKT and TGF-β/Smad signaling pathways. Exp Ther Med. 2017;14(6):5527–5534. doi: 10.3892/etm.2017.5242. DOI: 103892/etm20175242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen KA, Doan HQ, Zhou BP, Wang Q, Zhou Y, Rychahou PG, et al. PTEN loss induces epithelial- mesenchymal transition in human colon cancer cells. Anticancer Res. 2009;29(11):4439–4449. [PMC free article] [PubMed] [Google Scholar]
- 5.Lin JJ, Su JH, Tsai CC, Chen YJ, Liao MH, Wu YJ. 11-epi-Sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling pathways. Mar Drugs. 2014;12(9):4783–4798. doi: 10.3390/md12094783. DOI: 10.3390/md12094783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L stigma and petal extracts in mice. BMC Pharmacol. 2002;2(1):7–14. doi: 10.1186/1471-2210-2-7. DOI: 101186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolhassani A, Khavari A, Bathaie SZ. Saffron and natural carotenoids: biochemical activities and anti- tumor effects. Biochim Biophys Acta. 2014;1845(1):20–30. doi: 10.1016/j.bbcan.2013.11.001. DOI: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Farahmand S, Samarghandian S. The effects of Safranal, a constituent of Crocus sativus (saffron), on increased biomarkers of oxidative stress in diabetic rats' lung. Res Pharm Sci. 2012;7(5):S3. [Google Scholar]
- 9.Salahshoor MR, Khashiadeh M, Roshankhah S, Kakabaraei S, Jalili C. Protective effect of crocin on liver toxicity induced by morphine. Res Pharm Sci. 2016;11(2):120–129. [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaffari S, Hatami H, Dehghan G. Saffron ethanolic extract attenuates oxidative stress, spatial learning, and memory impairments induced by local injection of ethidium bromide. Res Pharm Sci. 2015;10(3):222–232. [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26(3):381–386. doi: 10.1002/ptr.3566. DOI: 101002/ptr3566. [DOI] [PubMed] [Google Scholar]
- 12.Gutheil WG, Reed G, Ray A, Anant S, Dhar A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012;13(1):173–179. doi: 10.2174/138920112798868566. DOI: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasirzadeh M, Rasmi Y, Rahbarghazi R, Kheradmand F, Karimipour M, Aramwit P, et al. Crocetin promotes angiogenesis in human endothelial cells through PI3K-Akt-eNOS signaling pathway. EXCLI J. 2019;18:936–949. doi: 10.17179/excli2019-1175. DOI: 10.17179/excli2019-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world's most expensive spice: Saffron. Food Res Int. 2010;43(8):1981–1989. DOI: 10.1016/j.foodres.2010.07.033. [Google Scholar]
- 15.Li S, Jiang S, Jiang W, Zhou Y, Shen XY, Luo T, et al. Anticancer effects of crocetin in human esophageal squamous cell carcinoma KYSE-150 cells. Oncol Lett. 2015;9(3):1254–1260. doi: 10.3892/ol.2015.2869. DOI: 10.3892/ol.2015.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chryssanthi DG, Dedes PG, Karamanos NK, Cordopatis P, Lamari FN. Crocetin inhibits invasiveness of MDA-MB-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011;77(2):146–151. doi: 10.1055/s-0030-1250178. DOI: 10.1055/s-0030-1250178. [DOI] [PubMed] [Google Scholar]
- 17.Bathaie SZ, Hoshyar R, Miri H, Sadeghizadeh M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem Cell Biol. 2013;91(6):397–403. doi: 10.1139/bcb-2013-0014. DOI: 10.1139/bcb-2013-0014. [DOI] [PubMed] [Google Scholar]
- 18.Tong Q, Qing Y, Wu Y, Hu X, Jiang L, Wu X. Dioscin inhibits colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol Appl Pharmacol. 2014;281(2):166–173. doi: 10.1016/j.taap.2014.07.026. DOI: 10.1016/j.taap.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Kim GD, Bae SY, Park HJ, Bae K, Lee SK. Honokiol inhibits vascular vessel formation of mouse embryonic stem cell-derived endothelial cells via the suppression of PECAM and MAPK/mTOR signaling pathway. Cell Physiol Biochem. 2012;30(3):758–770. doi: 10.1159/000341455. DOI: 10.1159/000341455. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Kim HJ, Oh SC, Lee DH. Genipin inhibits the invasion and migration of colon cancer cells by the suppression of HIF-1a accumulation and VEGF expression. Food Chem Toxicol. 2018;116(Pt B):70–76. doi: 10.1016/j.fct.2018.04.005. DOI: 10.1016/j.fct.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Bolós V, Gasent JM, López-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. OncoTargets Ther. 2010;3:83–97. doi: 10.2147/ott.s6909. DOI: 10.2147/ott.s6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SI, Huang CC, Huang CJ, Wang BW, Chang PM, Fang YC, et al. Thimerosal-induced apoptosis in human SCM1 gastric cancer cells: activation of p38 MAP kinase and caspase-3 pathways without involvement of [Ca2+] i elevation. Toxicol Sci. 2007;100(1):109–117. doi: 10.1093/toxsci/kfm205. DOI: 10.1093/toxsci/kfm205. [DOI] [PubMed] [Google Scholar]
- 23.Rabi T, Banerjee S. Novel synthetic triterpenoid methyl 25-Hydroxy-3-Oxoolean-12-en-28-Oate induces apoptosis through JNK and p38 MAPK pathways in human breast adenocarcinoma MCF-7 cells. Mol Carcinog. 2008;47(6):415–423. doi: 10.1002/mc.20399. DOI: 10.1002/mc.20399. [DOI] [PubMed] [Google Scholar]
- 24.Low HB, Zhang Y. Regulatory roles of MAPK phosphatases in cancer. Immune Netw. 2016;16(2):85–98. doi: 10.4110/in.2016.16.2.85. DOI: 10.4110/in.2016.16.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more. Front Biosci. 2008;13:3581–3593. doi: 10.2741/2951. DOI: 10.2741/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Li H, Wang X, Zhang X, Liu W, Wang Y, et al. Effect of polysaccharide from Undaria pinnatifida on proliferation, migration and apoptosis of breast cancer cell MCF7. Int J Biol Macromol. 2019;121:734–742. doi: 10.1016/j.ijbiomac.2018.10.086. DOI: 10.1016/j.ijbiomac.2018.10.086. [DOI] [PubMed] [Google Scholar]
- 27.Kruger NJ. The bradford method for protein quantitation. In: Walker JM, editor. Basic protein and peptide protocols Methods in molecular biology™. Vol. 32. Humana Press, Springer; 1994. pp. 15–21. DOI: 101385/0-89603-268-X:9. [Google Scholar]
- 28.Moradzadeh M, Sadeghnia HR, Tabarraei A, Sahebkar A. Anti-tumor effects of crocetin and related molecular targets. J Cell Physiol. 2018;233(3):2170–2182. doi: 10.1002/jcp.25953. DOI: 10.1002/jcp.25953. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Lee JM, Kim SC, Park CB, Lee PC. Proposed cytotoxic mechanisms of the saffron carotenoids crocin and crocetin on cancer cell lines. Biochem Cell Biol. 2014;92(2):105–111. doi: 10.1139/bcb-2013-0091. DOI: 10.1139/bcb-2013-0091. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang X, Dong A, Wang R, Shi A. Crocetin treatment inhibits proliferation of colon cancer cells through down-regulation of genes involved in the inflammation. Saudi J Biol Sci. 2018;25(8):1767–1771. doi: 10.1016/j.sjbs.2017.04.005. DOI: 10.1016/j.sjbs.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsia TC, Yu CC, Hsiao YT, Wu SH, Bau DT, Lu HF, et al. Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res. 2016;36(11):5989–5997. doi: 10.21873/anticanres.11187. DOI: 10.21873/anticanres.11187. [DOI] [PubMed] [Google Scholar]
- 32.Juan TK, Liu KC, Kuo CL, Yang MD, Chu YL, Yang JL, et al. Tetrandrine suppresses adhesion, migration and invasion of human colon cancer SW620 cells via inhibition of nuclear factor-κB, matrix metalloproteinase-2 and matrix metalloproteinase-9 signaling pathways. Oncol Lett. 2018;15(5):7716–7724. doi: 10.3892/ol.2018.8286. DOI: 10.3892/ol.2018.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. DOI: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv T, Zhang W, Han X. Zerumbone suppresses the potential of growth and metastasis in hepatoma HepG2 cells via the MAPK signaling pathway. Oncol Lett. 2018;15(5):7603–7610. doi: 10.3892/ol.2018.8335. DOI: 10.3892/ol.2018.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang L, Ma X, Tian Q, Cheng Y, Yao H, Liu Z, et al. Inhibition of angiogenesis and invasion by DMBT is mediated by downregulation of VEGF and MMP-9 through Akt pathway in MDA-MB-231 breast cancer cells. Food Chem Toxicol. 2013;56:204–213. doi: 10.1016/j.fct.2013.02.032. DOI: 10.1016/j.fct.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107(1):25–31. doi: 10.1016/j.jep.2006.01.022. DOI: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Pourgholami MH, Morris DL. Inhibitors of vascular endothelial growth factor in cancer. Cardiovasc Hematol Agents Med Chem. 2008;6(4):343–347. doi: 10.2174/187152508785909528. DOI: 10.2174/187152508785909528. [DOI] [PubMed] [Google Scholar]
- 38.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. DOI: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei T, Liu L, Zhou X. Cortex Dictamni extracts inhibit over-proliferation and migration of rat airway smooth muscle cells via FAK/p38/Bcl-2 signaling pathway. Biomed Pharmacother. 2018;102:1–8. doi: 10.1016/j.biopha.2018.03.039. DOI: 10.1016/j.biopha.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 40.Chen YY, Liu FC, Chou PY, Chien YC, Chang WSW, Huang GJ, et al. Ethanol extracts of fruiting bodies of Antrodia cinnamomea suppress CL1-5 human lung adenocarcinoma cells migration by inhibiting matrix metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling pathways. Evid Based Complement Alternat Med. 2012;2012:378415,1–11. doi: 10.1155/2012/378415. DOI: 10.1155/2012/378415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golubovskaya VM. Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci (Landmark Ed) 2014;19:687–706. doi: 10.2741/4236. DOI: 10.2741/4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu JD, Chen SH, Lin CL, Tsai SH, Liang YC. Inhibition of melanoma growth and metastasis by combination with (-)-epigallocatechin-3-gallate and combination with (-)-epigallocatechin-3-gallate and dacarbazine in mice. J Cell Biochem. 2001;83(4):631–642. doi: 10.1002/jcb.1261. DOI: 10.1002/jcb.1261. [DOI] [PubMed] [Google Scholar]
- 43.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–149. doi: 10.1016/j.pharmthera.2014.10.001. DOI: 10.1016/j.pharmthera.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4(9-10):342–359. doi: 10.1177/1947601913507951. DOI: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10(3):125–129. doi: 10.1016/j.molmed.2004.01.007. DOI: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Cerezo-Guisado MI, Zur R, Lorenzo MJ, Risco A, Martín-Serrano MA, Alvarez-Barrientos A, et al. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem Toxicol. 2015;84:125–132. doi: 10.1016/j.fct.2015.08.017. DOI: 10.1016/j.fct.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Liu WH, Chang LS. Caffeine induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J Cell Physiol. 2010;224(3):775–785. doi: 10.1002/jcp.22180. DOI: 10.1002/jcp.22180. [DOI] [PubMed] [Google Scholar]