Abstract
Bayesian estimation of pharmacokinetic parameters (PKP), as discussed in this review, provides a powerful approach towards the individualization of dosing regimens. The method was first described by Lewis Sheiner and colleagues and it is well suited in clinical environs where few blood fluid measures of drugs are available in the clinic. This makes it a valuable tool in the effective implementation of therapeutic drug monitoring. The principle behind the method is Bayes theorem, which incorporates elements of variability in a priori-known population estimates and variability in the pharmacokinetic parameters, and known errors intrinsic to the assay method used to estimate the blood fluid drug concentrations. This manuscript reviews the Bayesian method. The literature was scanned using Pubmed to provide background into the Bayesian method. An Add-in for Excel program was used to show the ability of the method to estimate PKP using sparse blood fluid concentration vs time data. Using a computer program, the method was able to find reasonable estimates of individual pharmacokinetic parameters, assessed by comparing the estimated data to the true PKP. Education of students in clinical pharmacokinetics is incomplete without some mention and instruction of the Bayesian forecasting method. For a complete understanding, a computer program is needed to demonstrate its utility.
Keywords: Clinical pharmacology, Dosage regimen design, Pharmacy education, Therapeutic drug monitoring
1. INTRODUCTION
The initial dosing regimens of most drugs are based on empirical dosing regimens that were established, using pharmacokinetic, safety and efficacy data, during phase 3 clinical trials. Owing to the existence of a narrow therapeutic window, for some drugs, individual estimates of pharmacokinetic parameters (PKP) through therapeutic drug monitoring practices are well suited for tailoring dosage needs. Many traditional methods of estimating PKP are based solely on the plasma concentrations obtained from individual patients and applying them directly into pharmacokinetic equations. The timing of collection and the number of available concentrations are an important consideration; generally, the more concentrations one has available, the better refined will be the estimates of integral PKP such as clearance (CL) and volume of distribution (Vd), and hence optimization of dosage based on those values.
For most phase 1 and phase 2 drug development studies, or in post-marketing pharmacokinetic studies, numerous blood fluid concentrations are taken from the same individual (serial sampling) to determine factors such as the area under the concentration (AUC) vs time curve with substantial accuracy in estimation of each subjects PKP.
In phase 3 studies, population pharmacokinetic methods using nonlinear mixed-effects modelling of sparse data sets from many patients is used to determine measures of central tendency, variability in PKP, and to identify the important clinical covariates. Whichever of these approaches is adopted, some knowledge of the mean and standard deviation is known by the time the drug is given to a patient after it is marketed.
In a discreet patient in a health care setting, however, often clinicians are restricted to only a very few measured plasma concentrations after a single dose. If no blood samples are available, all that can be used to estimate a dose based on PKP is the estimates from population means, which in many cases will deviate from individual patient characteristics. Where blood samples are available, such as for drugs possessing a narrow therapeutic margin (e.g. aminoglycoside antibiotics, antiepileptic drugs, immunosuppressive agents) usually very few, maybe even only a single, measure may be available. This creates a challenge in the obtainment of accurate patient-specific estimates of PKP, which are needed for accurate individualization of the dosage regimen for that patient.
The use of Bayesian pharmacokinetic forecasting and its utility in the individualization of dosage is well documented (1,2,3,4,5,6) and provides a cogent approach to dealing with sparse sample availability. Ideally, the Bayesian method should be incorporated in the education of students of pharmacokinetics (7), including that of students in pharmacy and clinical pharmacology. Although the theoretical concepts are readily incorporated into educational formats, Bayesian estimation relies upon iterative computer procedures to solve for best-estimates of PKP. It is difficult to “bring to life” or fully inform students about the approach in the absence of an appropriate computer programfor use in the classroom, particularly at the undergraduate level (such as for pharmacy students). Ease of use is an important feature for such a program.
The teaching of Bayesian estimation approach has been nicely described by Mehvar (8), who designed an easy-to-use web-based Bayesian estimation algorithm for a one-compartment model, linear elimination and bolus intravascular dosing. Other possible models or routes of elimination, require an alternate program.
This article serves as a review of the Bayesian method using a priori known PKP estimates from a population. Illustration of the method's utility to estimate PKP using sparse sampling is achieved by use of an Add-in program for Microsoft Excel (named PKB-est, freely upon request). Selected literature was included using PubMed using search terms Bayesian, pharmacokinetics, dose, individualization, and forecasting.
2. BACKGROUND: WHY USE BAYESIAN PHARMACOKINETICS?
2.1. Estimating PKP in the clinic
Rational individualization of dosing regimens in the clinic is well achieved using a two-stage process (9). Before a drug is given to a patient, one could estimate an initial dosage requirement using average population measures of PKP, with modification as required (Stage 1). Procainamide provides an example of how this could be done. The average Vd based on completed studies is 2 L/kg in a patient with normal renal function (10). If a patient weighed 70 kg, the estimated dose to achieve a C0h of 6 mg/L would be calculated as 2 L/kg × 70 kg × 6 mg/L = 840 mg of procainamide base. If the patient had heart failure which is determined by estimation of a low creatinine clearance, then a Vd of 1.5 L/kg could be used (10).
Once dosing has commenced there is an opportunity to better refine the dose requirements by obtaining some drug blood- fluid (blood, serum, or plasma) concentration measures (Stage 2). This is usually superior to the population mean data as the blood fluid concentrations are reflective of the patient's own pharmacokinetics. Procainamide for example follows a one-compartment open model and if two blood fluid concentrations were minimally available, some estimation of the patient's PKP could be made for use in adjusting the dosage to achieve a desired blood fluid concentration.
Based on a pair of concentration-time points after a discreet IV bolus dose of a drug that follows a one compartment open model such as procainamide, it is possible to estimate the PKP of Vd, CL, and terminal phase half-life (t½). As an illustrative example, after a dose of 100 mg, two samples were taken at 2 and 6 h after the dose (Fig. 1). The true situation would yield an initial concentration of 4 mg/L, t½ of 6 h, Vd of 25 L, and CL of 2.89 L/h. However, these calculations rely on absolutely accurate measures of the concentrations and assume that the drug truly follows the model consistently throughout the post-dose period.
Fig. 1.
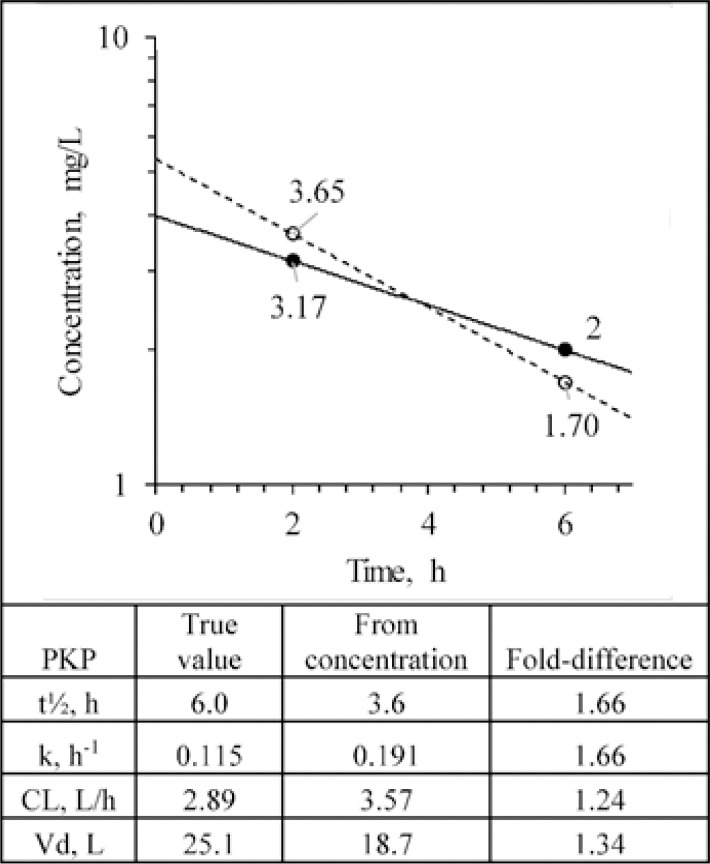
Pharmacokinetic estimation of a drug after intravenous bolus administration based on two drug concentrations in blood fluids. The true values and decline profile of a drug in a patient given 100 mg are shown as solid symbols and solid line. The open symbols and dashed line show what are measured from the assay. The table shows the true PKP values and those estimated from the measured plasma concentrations along with the fold-difference between the estimates. PKP, pharmacokinetic parameters; CL, clearance; Vd, volume of distribution.
No assay, however, is completely and consistently accurate. As part of their development, assays are validated to gain statistical information regarding the specificity and sensitivity of concentration measurements and the associated intrinsic error and variability (11). Over a range of known concentrations, the mean measure of each nominal concentration is determined. The percent coefficient of variation (CV%) is calculated as 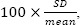 where SD is the standard deviation. This measure reflects the precision of the assay and reflects how replicable a measure might be. For use in bioequivalence studies, for example, an assay should have a CV of less than 15% at most concentrations, and less than 20% at the lowest measurable concentration (12).
where SD is the standard deviation. This measure reflects the precision of the assay and reflects how replicable a measure might be. For use in bioequivalence studies, for example, an assay should have a CV of less than 15% at most concentrations, and less than 20% at the lowest measurable concentration (12).
In addition to the intrinsic degree of error associated with the measure of the plasma concentrations, there may also be some temporal deviation of the decline in concentration over the time period between the two concentrations due to physiological variability, which might differ slightly from another time interval. Either of these occurrences would contribute to an error in the overall mean PKP for this patient, which may lead to plasma concentrations falling outside of the confines of a therapeutic range, even when appropriate pharmacokinetic equations are used.
To illustrate the importance of this consideration, let us assume that for our two blood samples the random analytical error (± 15%) led to concentrations being measured that differed from the true values (Fig. 1). The measured concentrations allow for the estimate PKP by applying the equation below (Fig. 1):
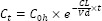
It is apparent that there is quite a significant disparity between the true PKP values from the ones estimated from the plasma concentrations.
There is no way of knowing the exact error in the measures, but error, nevertheless, should be expected. In this case, this random error can, in turn, lead to a miscalculation in the estimation of the patient's PKP, which has important implications in the calculation of the optimal dose regimen. This random error is expected to follow a normal distribution and is additive based on intrinsic variability in a number of analytical steps such as pipetting of samples and solvents, extraction, derivatization yield, and volumes injected into the analytical instrument. Taking a larger number of concentration vs time points is apt to yield a more accurate value, but this is not a reasonable expectation in a clinical setting. Indeed, in many cases, one might only have a single concentration available, which would render impossible even the most rudimentary calculation of PKP based on the estimation of t½ as shown in Fig. 1.
2.2. Combination population-individual data methods: the Bayesian approach
Bayes theorem, which serves as the basis for Bayesian PKP estimation, is grounded in the logic that the likelihood of an observation is dependent on factors known to be involved in the observation, and the variability known to be present in those factors (13). Its use in pharmacokinetics was first described in 1979 by Sheiner et al. (14). It requires a relevant pharmacokinetic model, a measure of random error associated with the concentration measures and the known population estimates and variances for the model-associated PKP (14,15). The inclusion of the population PKP estimates is important because the likelihood is high that a patient's individual PKP will be within a range of values incorporated within its measures of known central tendency and surrounding variance. The combination of these population inputs offers an advantage from using only the measured concentrations with their inherent random error, a factor that becomes more problematic with lower numbers of samples.
In Bayesian pharmacokinetics, the likelihood of the PKP parameter being true is measured by an objective function (OBJBayes), which incorporates elements of the model and known a priori measures of central tendency, variance and errors. The likelihood is optimized by minimization of the OBJBayes using computerization nonlinear algorithms for solving complex relationships. For pharmacokinetics OBJBayes is defined as (14):
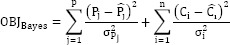
where:
Pj and 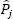 are the estimated and the observed (population) jth PK parameter, respectively.
are the estimated and the observed (population) jth PK parameter, respectively.
Ci and Ĉi are the observed and the patient's estimated (based on the PK model and Pj) concentrations, respectively.
The σ refers to the standard deviations of the population PK parameter (Pj) and the random error for the ith concentration measurement.
The example in Fig. 1 can be used to demonstrate the use of the OBJBayes. The true patient data is reflective of a person with true average values of CL and Vd, with population SD being 25% for each in this case. The observed concentrations are associated with a certain degree of intrinsic error, which is reflective of the validation of the assay. At the concentrations levels measured, the percent coefficient of variation, a measure of precision, is 15%, meaning that the observed concentrations have possible values of 3.65 ± 0.55 and 1.70 ± 0.26 mg/L. Thus, the OBJBayes is calculated as:
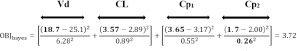
In the equation, the values of 18.7 and 3.57 represent the values estimated based on the slope of decline over time in the log- transformed concentrations using the measured concentrations (Fig. 1). However, there is likely error associated with the concentration measures and hence these estimates of PKP are expectedly prone to some degree of error. In the right-hand part of the equation, if we assume that the patient has the population means of CL and Vd, the concentrations would be 3.17 and 2.00 mg/L as depicted in Fig. 1; these are subtracted from the observed values. The square of each of the differences in the numerator parts of the equation is each divided by the appropriate SD squared term, which is a form of weighting each component by the known variability in the assay measure and in the population PKP.
By iteratively substituting values for the patient's CL and Vd, which requires a computer program, and in turn re-estimating the plasma concentrations based on the pharmacokinetic model, it is possible to minimize OBJBayes. In most cases, this process can be minimized to a finite value. Once this is achieved, the final values of PKP represent the most likely estimates of those values for that patient. Another clinically useful aspect is that as the number of samples increases with continual therapy, any subsequent concentration measures may be added to the existing values and minimization of the OBJBayes rerun through the program, allowing further fine-tuning the PKP estimates of the patient (increasing the number of concentrations tends to focus more of the OBJBayes on the patient-specific concentrations and less on population data).
3. INFLUENCE OF VARYING MAGNITUDE OF VARIANCE IN ASSAY PRECISION AND POPULATION ESTIMATES ON A BAYESIAN ESTIMATION
The Bayesian method incorporates both assay precision and variance in PKP to arrive at a most likely measure of pharmacokinetic parameters. Using the availability of two paired concentrations vs time points (Fig. 2), with varying degrees of assay variability and variance in the PKP of CL and Vd, it is possible to examine the benefits of the Bayesian method under those different conditions.
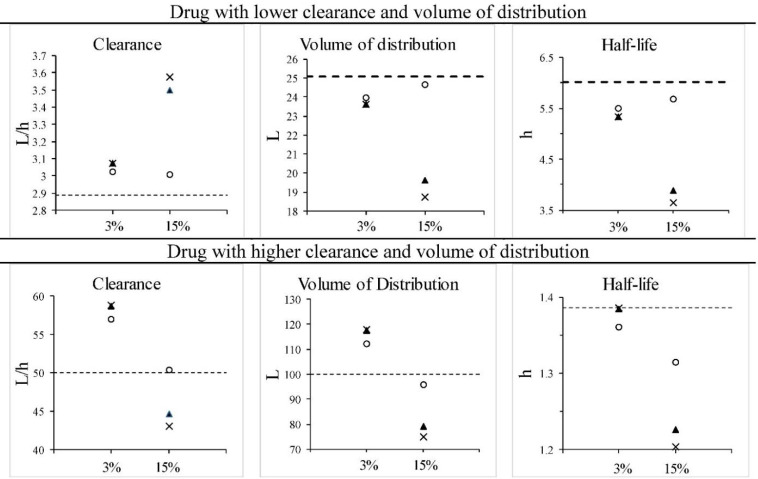
Fig. 2.
Two drugs following a one-compartment model given as an intravenous bolus, with PKP being low (upper panels) or high (lower panels) in value. Error in concentrations is introduced at the limits of the assay coefficient of variation (as depicted in Fig. 1). Dashed lines indicate the true values. The x-axis scale shows the CV% of the assay (3 or 15%). The symbols represent: ×, values from regression analysis (see Fig. 1); ○, Bayesian estimate with 10% CV in population PKP; ▲, Bayesian estimate with 50% CV% in population PKP. PKP, pharmacokinetic parameters; CV, coefficient of variation.
The use of the exponential regression analysis of the two points alone without considering assay variability or variance in the population PKP leads to the largest error in the primary PKP of clearance and the volume of distribution (Fig. 2). When the assay is very precise, the estimates of the PKP become more precise either using the two-point method or the Bayesian method. Likewise increasing the population variance has the effect of increasing the error in PKP estimation. This is true of both low PKP or higher PKP true values. On the other hand, the use of the Bayesian method resulted in estimates of primary PKP that were closer to the true values than by use of the two- point exponential method alone. Even with high variability in the PKP, the Bayesian method brought the estimates closer to the true values.
It would be expected that with a higher level of variability in the assay that there would be a reduced ability of the two-point method to accurately estimate the true value. When this is combined with a lower variability in the population PKP, it is apparent that the Bayesian method can very closely estimate the true variables. Even when combined with a high measure of variability in the PKP (50% CV), the Bayesian method still outperformed the two-point method.
In any event, it would never be possible to determine whether or not the measured concentrations had zero, or the maximum possible degree of error based on the CV%. We would also have to consider that on occasion an individual concentration measure even with a specified CV% will exceed the boundaries of the CV, and this would lead to even more error in the estimate of the PKP if the two-point method were used.
A limitation of the example shown in Fig. 2 is that it illustrates the expectation for the simplest example of a drug, which follows a one-compartment model and bolus intravascular injection. Not only is this a rarely used form of drug administration, but also most drugs follow a multicompartmental model. An even more compelling issue is that the scenario includes two concentrations. What would one be able to do in a realistic clinical situation when only one sample measure is known after a dose? The Bayesian method allows for the ability to provide most likely estimates of PKP regardless of the route of administration, type of compartmental model, number of available samples, or kinetics of elimination, as long as the computer program used has the ability to incorporate these factors.
4. COMPUTER PROGRAMS AVAILABLE FOR BAYESIAN ESTIMATION OF PHARMACOKINETIC PARAMETERS
Several commercially available products are marketed for Bayesian estimations of PKP (16), but these are designed for use in the clinic and the licensing costs are likely prohibitive for many educational institutions (Table 1).
Table 1.
Known programs for performing Bayesian estimations of pharmacokinetic parameters in patients. Each of the sites was accessed on April 2nd, 2020.
| Program | Cost | Advertised models |
|---|---|---|
| Adult and pediatric Kinetics http://www.rxkinetics.com/apk.html | $390 network license | Aminoglycoside antibiotics, one and two- compartment intravenous |
| BestDose http://www.lapk.org/bestdose.php | Free | Selected drugs, one and two compartment, multiple routes |
| DoseMeRx https://doseme-rx.com/ | Not stated* | Vancomycin iv |
| Insight-Rx https://www.insight-rx.com/ | Not stated* | Numerous drugs mostly intravenous |
| Precise PK https://precisepk.com/pricing | $99 to $149 per month | Numerous drugs and routes |
| TCIWorks | ||
| Advertised website links (6,7,17) are inactivated | Unknown | Aminoglycosides and vancomycin |
| PKB-est | Free upon request | General use |
* Currently not stated but apparently higher than Precise PK (16).
Because they focus on therapeutic drug monitoring for a few specific drugs, they also automatically calculate dose regimens for the user. While this is undoubtedly optimal for clinical practice, in the classroom this may allow the program to do too much of the work, and thus remove instructors' desire to have the student perform the necessary tailoring of the dose regimen themselves using the Bayesian PKP estimates. There are some complimentary share-ware no-cost versions of software available as well (17), of which some may require programming expertise.
In the absence of a program, the teaching of this technique to students becomes theoretical only and lacks hand-on or demonstrable pedagogy. Some of the programs are very expensive making them inaccessible for accessibility and for the comprehensive training of students about the method. The limitation in training has been identified as a limitation to the use of the Bayesian method in the clinic (18). There are two free of cost programs available, Bestdose and PKB-est. The latter is an add-in program for Microsoft Excel for Windows (Redmond WA) which is based on the Visual Basic language within Excel. Examples of the utility of this feature to be used within Excel include PKSolver (19) and uSIMPK (20) (for teaching pharmacokinetics through simulations).
5. PREPARING FOR A BAYESIAN ESTIMATION
The following information is required to facilitate a Bayesian estimation of PKP. This includes:
Time after the last dose or for constant infusion
The number of doses represented by that dose since dosing began
Concentration at that time
An appropriate pharmacokinetic model is also a prerequisite for a Bayesian fit. This has to be based on prior pharmacokinetic information in the literature, something that an instructor can expect students to find (a useful example of finding drug information). Sometimes it is known that in certain disease states, such as renal disease, that the mean and SD of pharmacokinetic data differ from those of the general population. In such cases, it would be better to input those values into the dialogue box for mean and SD of population parameters.
After selecting the model and appropriate pharmacokinetic estimations of central tendency and variability, the other integral piece of information needed is the error intrinsic to the assay being used to measure the blood fluid concentrations.
6. DEMONSTRATING THE UTILITY OF THE METHOD USING PKB-EST
The PBB-est Add-in for Excel program was used here to demonstrate the utility of the Bayesian estimation process using a minimum of blood samples (between one and three per patient) and randomly generated patient pharmacokinetic data. For each model, the NORMINV function in Excel was used to create sample sizes of at least 25 simulated subjects given model drugs possessing PKP with set population mean and SD. The assay precision was set at 10% CV. For each regimen the same dose of the drug was given to each of the simulated patients and then, to mimic the clinical situation where only limited blood collection would be available, between 1 and 3 randomly selected concentrations were obtained. The values of the true concentrations were calculated based on the preset randomized patient PKP of each simulated patient. After these samples were drawn and a concentration calculated, random error using the RAND function in Excel was applied to adhere to the 10% CV of the assay.
For the one-compartment model with linear elimination simulated data, the population mean ± SD for the CL/F were preset to either 12 ± 4 or 3.0 ± 1.0 L/h. The Vd/F was preset to either 55 ± 11 or 100 ± 15 L. The oral dose population ka was 2.0 ± 1.0 h-1. For the two-compartment model the population PKP were preset to 0.3 ± 0.06, 3.0 ± 1.5, 0.08 ± 0.02 h-1 and 25 ± 5 L for k21, α, β, and Vc, respectively. The population ka for oral doses was 1.5 ± 1.0 h-1. For drugs following a single nonlinear elimination process, population Vmax, km, Vd, and ka were set to 12 ± 4 mg/h, 4 ± 1 mg/L, 45 ± 5 L, and 2 ± 1 h-1, respectively. For repeat dose regimens, dosing intervals were set to 8 or 12 h.
These population values, regimen, and concentrations were then input into the Bayesian modules and the predicted PKP were compared to the preset “actual” patient values.
Overall assessment of the ability of the Add- in program to find the patient values was accomplished using linear regression to find the correlation coefficient (r2), the slope of the relationships and significance of the relationship. A P-value of less than 0.01 was deemed to be significant.
The relative error and relative squared errors of the Bayesian PKP estimates from the true values, and the population mean from the true value for each patient, were first determined. The difference between the errors and squared errors of the Bayesian PKP and the population values were then calculated, along with the associated 95% confidence intervals (21).
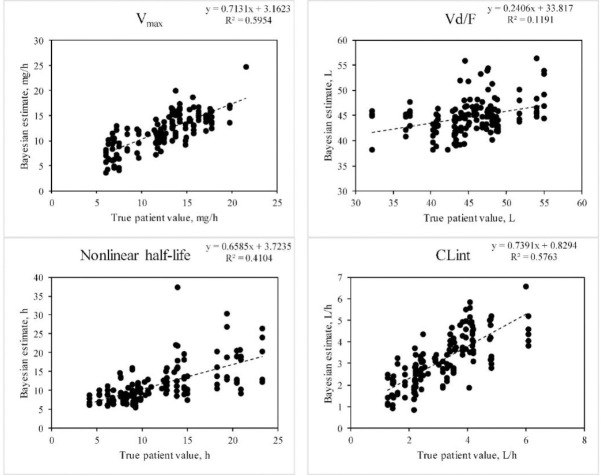
With the simulated drugs and sparse sampling, regardless of the model very good correlations were apparent between measures of elimination with actual values were realized using the Bayesian approach. This included CL, Vmax, and CLint (Table 2, Figs. 3-5). The Bayesian approach tended to maintain the estimates to the population values for ka and km. Consequently, for these two parameters, there were no significant correlations realized. This may be attributed to the fact that these parameters are less likely to be related to overall measures of exposure compared to the values that yielded stronger correlations.
Table 2.
Summary of all regression analysis r values from comparisons of Bayesian estimated pharmacokinetic parameters obtained using PKB-est to the true values (correlations of significance are depicted in Figs. 3-5). Significant relationships (P < 0.01) are denoted as *.
| Parameters | One-compartment linear elimination | Two-compartment linear elimination | One-compartment nonlinear elimination |
|---|---|---|---|
| CL/F | 0.92* | 0.95* | − |
| Vdss/F | 0.44* | 0.68* | 0.35* |
| Vc/F | − | 0.32* | − |
| t½α | − | 0.62* | − |
| t½β | 0.75* | 0.17* | − |
| ka | 0.17 | 0.23 | 0.076 |
| Vmax | − | − | 0.60* |
| km | − | − | 0.17 |
| CLint | − | − | 0.76* |
CL, clearance; Vdss, volume of distribution at steady state; Vc, volume of central compartment; t½α, distribution halflife; t½β, elimination half-life; ka, absorption rate constant; Vmax, maximum velocity; km, Michaelis-Menten constant; CLint, intrinsic clearance.
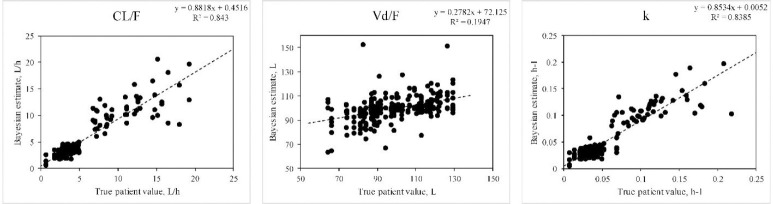
Fig. 3.
Ability of the Bayesian approach to estimate PKP when doses of a drug following a one-compartment model with linear elimination were given as various dosing regimens to simulated patients. Correlation between true values and the Bayesian estimates were significant (P < 0.01) for each parameter except for ka (not shown). Data represent 25 determinations for each of intravenous bolus, intermittent infusion, and oral dosing (single, multiple, and steady-state dosing) and continuous intravenous infusion. Data were estimated on sparse data (between 1, 2, or 3 blood fluid concentrations per subject). PKP, pharmacokinetic parameters; CL, clearance; Vd, volume of distribution.
Fig. 5.
Ability of the Bayesian approach to estimate pharmacokinetic parameters when doses of a drug conforming to a one-compartment model with nonlinear elimination as described in Fig. 3. Significant correlations between the true patient values and the Bayesian estimates are shown. The ka and km were not significant. The half-life shown is the expected value when a dose is given that is well below the km of the drug (calculated as 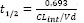 . Data construction and sampling are as described in Fig. 3.
. Data construction and sampling are as described in Fig. 3.
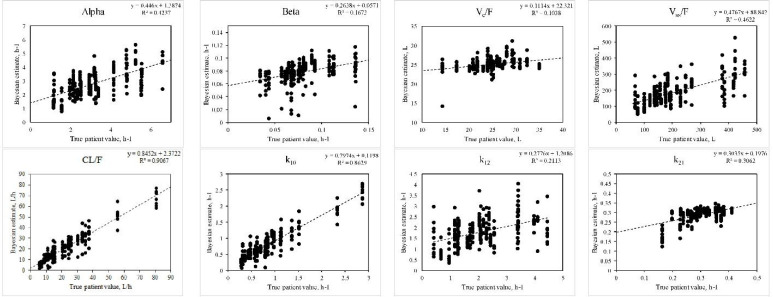
Fig. 4.
Ability of the Bayesian approach to estimate PKP for a drug following a two-compartment model given as described in Fig. 3. Correlations between true values and the Bayesian estimates were significant except for ka. Intercompartmental estimates are k10, k12, and k21, and the Vc and Vss are the Vd of the central compartment, and steady-state, respectively. Data construction and sampling is as described in Fig. 3. PKP, pharmacokinetic parameters; CL, clearance; Vd, volume of distribution.
The Vd estimates were significantly correlated in each case, although not as strongly as the estimates pertaining to drug elimination (Table 2).
For the one-compartment model with linear elimination (Table 3), a significant improvement in precision (mse) and bias (me) achieved by the Bayesian method versus the population estimates alone were apparent for the absorption rate constant for the Bayesian compared to the population approach. Although there was no significant improvement in precision offered by the Bayesian approach, in all cases for the other PKP, the relative mse was negative, indicating a tendency towards improved precision compared to the use of the mean population estimates only. With a two- compartment model (Table 3), significant improvements in precision were apparent for each of the CL/F, both Vd terms, and the t½α.
Table 3.
Relative bias and precision of the ability of the Bayesian method using PKB-est to estimate the true patient parameters compared to that of the population mean data only. Random sparse simulated patient sampling (total of 1 to 3 samples per individual per regimen) was used for the Bayesian estimation for simulated drugs adhering to a one or two-compartment models. Compiled data includes all dose regimens available (intravenous bolus, intravenous infusion, oral dosing as single or repeated dose administration). The error and squared error of the differences in the population means and true values, and the Bayesian estimates and true values, were first calculated. The data shown are the means of the differences between those errors (me, mean error; mse, mean squared error) of the Bayesian minus the population errors, and the associated 95% confidence intervals.
| pharmacokinetic parameters | Difference of Bayesian minus population (95% CI) | |||
|---|---|---|---|---|
| Errors | One-compartment linear elimination | Two-compartment linear elimination | One-compartment nonlinear elimination | |
| CL/F | me | 0.063 (-0.13, 0.26) | 1.3 (-0.48, 3.2) | − |
| mse | -0.92 (-1.87, 0.033) | -250 (-340, -160)* | − | |
| Vdss/F | me | -0.25 (-1.6, 1.1) | 11 (1.0, 21) | -0.28 (-0.84, 0.27) |
| mse | -44 (-93, 5.5) | -6400 (-8500, -4200)* | 0.60 (-3.8, 5.0) | |
| Vc/F | me | − | 0.11 (-0.086, 0.30) | − |
| mse | − | -2.1 (-3.5, -0.65)* | − | |
| t½α | me | − | 0.047 (0.033, 0.062) | − |
| mse | − | -0.0056 (-0.010, -0.0017)* | − | |
| t½β | me | -1.3 (-3.5, 0.86) | 1.45 (0.419, 2.48)* | − |
| mse | -42 (-92, 8.5) | 57 (-16, 130) | − | |
| ka | me | -0.25 (-0.45, -0.050)* | -0.48 (-0.57, -0.40)* | -0.12 (-0.23, -0.020)* |
| mse | -0.56 (-0.77, -0.36)* | 0.11 (-0.17, 0.39) | 0.041 (-0.13, 0.21) | |
| Vmax | me | − | − | 0.034 (-0.54, 0.61) |
| mse | − | − | -8.1 (-11, -5.5)* | |
| km | me | − | − | -0.050 (-0.13, 0.028) |
| mse | − | − | 0.0933 (-0.0307, 0.217) | |
| CLint | me | − | − | 0.0408 (-0.112, 0.193) |
| mse | − | − | -0.568 (-0.854, -0.282)* | |
CL, clearance; Vdss, volume of distribution at steady state; Vc, volume of central compartment; t½α, distribution halflife; t½β, elimination half-life; ka, absorption rate constant; Vmax, maximum velocity; km, Michaelis-Menten constant; CLint, intrinsic clearance.
With nonlinear elimination, the Bayesian approach (Table 3) displayed significant improvement in precision compared to a solely population approach for the Vmax and CLint, and reduced bias (but not precision) for ka. Interestingly, bias and precision for km or Vd were not significantly improved by the use of the Bayesian method.
7. ESTABLISHED CLINICAL UTILITY OF THE BAYESIAN APPROACH
The ability of the Bayesian approach to better estimate PKP, and in turn dosing needs for individual patients, has been established for a number of drugs which have well-documented and defined narrow therapeutic ranges of concentrations. These include vancomycin (22,23), mycophenolic acid (although specifics of the program used was not provided) (24), theophylline (25,26), aminoglycoside antibiotics (27,28,29), antiepileptic drugs (29,30,31), and digoxin (32). In many of these examinations, the use of the Bayesian method using a computer program yielded more precise modifications of doses to achieve target drug concentrations that did a non-Bayesian approach.
8. CONCLUSION
The description of the Bayesian method for therapeutic drug monitoring was first proposed by Sheiner and colleagues (14) and has long been acknowledged (33) to hold promise in therapeutic drug monitoring. The same research group (14) had also earlier described (34) the use of nonlinear mixed-effects modelling, which is used extensively in current drug development to seek population estimates of PKP and covariates that can influence them.
A comprehensive education in the clinical use of pharmacokinetics ideally should include some exposure to the Bayesian forecasting technique, which offers utility in the clinical setting for estimation of PKP (1,2,3,4,5,6,35,36). For the educational environment, there are limited affordable options for the availability of Bayesian estimation programs that can be used to demonstrate how a Bayesian estimation program works (Table 1). Although here we used a free-to-use computer program to demonstrate the utility of Bayesian estimation, the other available programs listed in Table 1 were mostly developed to provide estimations of Bayesian PK and dosing requirements in for specific drugs in the clinic where therapeutic drug monitoring is critical for their safe and effective use. Those programs were compared recently in a study examining their ability to predict vancomycin dosing requirements (16).
PKB-est is available free to instructors of clinical pharmacokinetics or clinical pharmacology by contacting the author (DRB).
Conflicts of interest statement
All authors declared no conflict of interest in this study.
Authors' contribution
D.R. Brocks wrote the bulk of the manuscript and performed some of the data analysis. The computer program used was written by D.R. Brocks. D.A. Hamdy participated in the data analysis using the computer program and contributed to the writing of the manuscript.
9. REFERENCES
- 1.Vozeh S, Hillman R, Wandell M, Ludden T, Sheiner L. Computer-assisted drug assay interpretation based on Bayesian estimation of individual pharmacokinetics: application to lidocaine. Ther Drug Monit. 1985;7(1):66–73. doi: 10.1097/00007691-198503000-00011. DOI: 10.1097/00007691-198503000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Crowley JJ, Koup JR, Cusack BJ, Ludden TM, Vestal RE. Evaluation of a proposed method for phenytoin maintenance dose prediction following an intravenous loading dose. Eur J Clin Pharmacol. 1987;32(2):141–148. doi: 10.1007/BF00542186. DOI: 10.1007/BF00542186. [DOI] [PubMed] [Google Scholar]
- 3.Beach CL, Farringer JA, Peck CC, Crawford MH, Ludden TM, Clementi WA. Clinical assessment of a two-compartment Bayesian forecasting method for lidocaine. Ther Drug Monit. 1988;10(1):74–79. [PubMed] [Google Scholar]
- 4.Privitera MD, Homan RW, Ludden TM, Peck CC, Vasko MR. Clinical utility of a Bayesian dosing program for phenytoin. Ther Drug Monit. 1989;11(3):285–294. doi: 10.1097/00007691-198905000-00011. DOI: 10.1097/00007691-198905000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima T, Ohno T, Koido K, Hashimoto H, Terakado H. Accuracy of predicting the vancomycin concentration in Japanese cancer patients by the Sawchuk-Zaske method or Bayesian method. J Oncol Pharm Pract. 2020;26(3):543–548. doi: 10.1177/1078155219851834. DOI: 10.1177/1078155219851834. [DOI] [PubMed] [Google Scholar]
- 6.Hennig S, Holthouse F, Staatz CE. Comparing dosage adjustment methods for once-daily tobramycin in paediatric and adolescent patients with cystic fibrosis. Clin Pharmacokinet. 2015;54(4):409–421. doi: 10.1007/s40262-014-0211-9. DOI: 10.1007/s40262-014-0211-9. [DOI] [PubMed] [Google Scholar]
- 7.Donagher J, Martin JH, Barras MA. Individualised medicine: why we need Bayesian dosing. Intern Med J. 2017;47(5):593–600. doi: 10.1111/imj.13412. DOI: 10.1111/imj.13412. [DOI] [PubMed] [Google Scholar]
- 8.Mehvar R. Development and application of an on-line module for teaching Bayesian forecasting principles in a clinical pharmacokinetics course. Am J Pharm Educ. 2000;64(2):121–125. DOI: aj640203. [Google Scholar]
- 9.Mehvar R. Estimation of pharmacokinetic parameters based on the patient-adjusted population data. Am J Pharm Educ. 2006;70(5):96,1–8. doi: 10.5688/aj700596. DOI: 10.5688/aj700596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shammas FV, Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15(2):94–113. doi: 10.2165/00003088-198815020-00002. DOI: 102165/00003088-198815020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari G, Tiwari R. Bioanalytical method validation: an updated review. Pharm Methods. 2010;1(1):25–38. doi: 10.4103/2229-4708.72226. DOI: 10.4103/2229-4708.72226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Committee for Medicinal Products for Human Use. Guideline on bioanalytical method validation. London: European Medicines Agency; 2011. cited 2020 April 15. EMEA/CHMP/EWP/192217/2009 Rev 1 Corr 2** Available from: https://wwwemaeuropaeu/en/documents/scientific- guideline/guideline-bioanalytical-method- validation_enpdf . [Google Scholar]
- 13.Bijak J, Bryant J. Bayesian demography 250 years after Bayes. Popul Stud (Camb) 2016;70(1):1–19. doi: 10.1080/00324728.2015.1122826. DOI: 10.1080/00324728.2015.1122826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheiner LB, Beal S, Rosenberg B, Marathe VV. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979;26(3):294–305. doi: 10.1002/cpt1979263294. DOI: 10.1002/cpt1979263294. [DOI] [PubMed] [Google Scholar]
- 15.Sheiner LB, Beal SL. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non-Bayesian methods. J Pharm Sci. 1982;71(12):1344–1348. doi: 10.1002/jps.2600711209. DOI: 10.1002/jps.2600711209. [DOI] [PubMed] [Google Scholar]
- 16.Turner RB, Kojiro K, Shephard EA, Won R, Chang E, Chan D, et al. Review and validation of Bayesian dose-optimizing software and equations for calculation of the vancomycin area under the curve in critically Ill patients. Pharmacotherapy. 2018;38(12):1174–1183. doi: 10.1002/phar.2191. DOI: 10.1002/phar.2191. [DOI] [PubMed] [Google Scholar]
- 17.Bourne D. Pharmacokinetic software. https://www.pharmpk.com/soft.html. 2020 .
- 18.Alsultan A, Abouelkheir M, Alqahtani S, Aljabri A, Somily AM, Alsubaie S, et al. Optimizing vancomycin monitoring in pediatric patients. Pediatr Infect Dis J. 2018;37(9):880–885. doi: 10.1097/INF.0000000000001943. DOI: 10.1097/INF.0000000000001943. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add- in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. doi: 10.1016/j.cmpb.2010.01.007. DOI: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Brocks DR. uSIMPK An Excel for Windows-based simulation program for instruction of basic pharmacokinetics principles to pharmacy students. Comput Methods Programs Biomed. 2015;120(3):154–163. doi: 10.1016/j.cmpb.2015.04.006. DOI: 101016/jcmpb201504006. [DOI] [PubMed] [Google Scholar]
- 21.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–512. doi: 10.1007/BF01060893. DOI: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 22.Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042. doi: 10.1128/AAC.02042-17. DOI: 10.1128/AAC.02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–57. doi: 10.1016/j.addr.2014.05.016. DOI: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Barraclough KA, Isbel NM, Staatz CE. Evaluation of the mycophenolic acid exposure estimation methods used in the APOMYGERE, FDCC, and Opticept trials. Transplantation. 2010;90(1):44–51. doi: 10.1097/TP.0b013e3181e06584. DOI: 10.1097/TP.0b013e3181e06584. [DOI] [PubMed] [Google Scholar]
- 25.Chrystyn H, Ellis JW, Mulley BA, Peake MD. The accuracy and stability of Bayesian theophylline predictions. Ther Drug Monit. 1988;10(3):299–305. doi: 10.1097/00007691-198803000-00011. DOI: 10.1097/00007691-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Hurley SF, McNeil JJ. A comparison of the accuracy of a least squares regression, a Bayesian, Chiou's and the steady-state clearance method of individualising theophylline dosage. Clin Pharmacokinet. 1988;14(5):311–320. doi: 10.2165/00003088-198814050-00003. DOI: 10.2165/00003088-198814050-00003. [DOI] [PubMed] [Google Scholar]
- 27.Burton ME, Chow MS, Platt DR, Day RB, Brater DC, Vasko MR. Accuracy of Bayesian and Sawchuk- Zaske dosing methods for gentamicin. Clin Pharm. 1986;5(2):143–149. [PubMed] [Google Scholar]
- 28.Kraus DM, Dusik CM, Rodvold KA, Campbell MM, Kecskes SA. Bayesian forecasting of gentamicin pharmacokinetics in pediatric intensive care unit patients. Pediatr Infect Dis J. 1993;12(9):713–718. doi: 10.1097/00006454-199309000-00002. DOI: 10.1097/00006454-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Rodvold KA, Pryka RD, Kuehl PG, Blum RA, Donahue P. Bayesian forecasting of serum gentamicin concentrations in intensive care patients. Clin Pharmacokinet. 1990;18(5):409–418. doi: 10.2165/00003088-199018050-00005. DOI: 10.2165/00003088-199018050-00005. [DOI] [PubMed] [Google Scholar]
- 30.Kuranari M, Chiba S, Ashikari Y, Kodama Y, Sakata T, Takeyama M. Clearance of phenytoin and valproic acid is affected by a small body weight reduction in an epileptic obese patient: a case study. J Clin Pharm Ther. 1996;21(2):83–87. doi: 10.1111/j.1365-2710.1996.tb00005.x. DOI: 10.1111/j.1365-2710.1996.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 31.Boucher BA, Rodman JH, Fabian TC, Cupit GC, Ludden TM, West ME, et al. Disposition of phenytoin in critically ill trauma patients. Clin Pharm. 1987;6(11):881–887. [PubMed] [Google Scholar]
- 32.el Desoky E, Meinshausen J, Buhl K, Engel G, Harings-Kaim A, Drewelow B, et al. Generation of pharmacokinetic data during routine therapeutic drug monitoring: Bayesian approach vs pharmacokinetic studies. Ther Drug Monit. 1993;15(4):281–288. doi: 10.1097/00007691-199308000-00004. DOI: 101097/00007691-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher GE, Barr JT. Bayesian approaches in pharmacokinetic decision making. Clin Pharm. 1984;3(5):525–530. [PubMed] [Google Scholar]
- 34.Sheiner LB, Rosenberg B, Marathe VV. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5(5):445–479. doi: 10.1007/BF01061728. DOI: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- 35.Duffull SB, Kirkpatrick CM, Begg EJ. Comparison of two Bayesian approaches to dose-individualization for once-daily aminoglycoside regimens. Br J Clin Pharmacol. 1997;43(2):125–135. doi: 10.1046/j.1365-2125.1997.05341.x. DOI: 10.1046/j.1365-2125.1997.05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda K, Miyakawa T, Katanoda T, Hashiguchi Y, Iwamura K, Nosaka K, et al. A case of recovery from aphasia following dose reduction of cefepime by bayesian prediction-based therapeutic drug monitoring. J Infect Chemother. 2020;26(5):498–501. doi: 10.1016/j.jiac.2019.10.006. DOI: 10.1016/j.jiac.2019.10.006. [DOI] [PubMed] [Google Scholar]