Abstract
Background and Purpose:
Multiple sclerosis (MS) is an autoimmune disorder characterized by demyelination and axonal loss. Quantitative estimation of behavioral, locomotor, and histological changes following the use of alpha-tocopherol (AT) in the animal model of MS have not been reported. The present study was planned to evaluate whether AT can improve sensorimotor dysfunction and reduce demyelination in the cuprizone (CPZ)-induced rat model of MS.
Experimental approach:
Female Sprague-Dawley rats (8 weeks) were fed with cuprizone diet for 5 weeks followed by intraperitoneal injections of alpha-tocopherol (100 mg/Kg) or PBS for 2 weeks (groups E1 and E2, n = 8). Group C (n = 8) was fed with normal pellets followed by intraperitoneal doses of PBS. Open-field test and beam walking were carried out on every 10th day. The mean area of demyelination in the corpus callosum was quantified in Luxol® fast blue (LFB) stained histological sections of the forebrain. Qualitative grading for relative changes in the stains of myelinated fibers was also done.
Findings/Results:
During withdrawal of CPZ, AT treatment increased the average speed by 22% in group E1, compared to group E2 (P < 0.05). The mean time to walk the beam was reduced in group E1 by 2.6% compared to group E2 (P < 0.05). The rearing frequency was increased in group E1 during week 6-7 compared to that in the period of CPZ treatment. The mean area of demyelination in the corpus callosum showed a 12% reduction in group E1 compared to group E2 (P < 0.05).
Conclusion and implications:
Short-term AT therapy showed improvement in motor dysfunction and reduction of demyelination in the animal model of MS.
Keywords: Alpha-tocopherol, Cuprizone, Demyelination, Multiple sclerosis, Neuroprotection
INTRODUCTION
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS) characterized by a cascade of immunological changes leading to demyelination and neuronal dysfunction (1,2). Classical MS is the most common demyelinating disease and the key features are plaques of primary demyelination of varied size and shape in the cerebral cortex and subcortical white matter, cerebellar white matter, and spinal cord (3). Many methods are available that can induce demyelination in animal models such as experimental autoimmune encephalomyelitis (EAE), viral- induced demyelination, and toxin-induced demyelination through cuprizone (CPZ, bis- cyclohexanone-oxaldihydrazone), ethidium bromide, and lysolecithin (4). In comparison to the EAE model of MS which involves immunological reactions followed by T-cell activation (5), CPZ induces toxic demyelination caused by apoptosis of oligodendrocytes and microglial activation. Short-term treatment showed acute demyelination followed by spontaneous remyelination on withdrawal of treatment (6).
CPZ causes chelation of copper resulting in an alteration in the metabolic process of oligodendrocytes and induces selective loss of oligodendrocytes (7,8). Observation of early changes in MS patients has indicated apoptosis of oligodendrocytes in newly formed lesions (9,10,11). Both male and female rats were used to induce CPZ-induced demyelination in an electrophysiological study of the sciatic nerve (12). Oestrogen and progesterone were reported to delay the progress of demyelination and offered neuroprotection in female rodents (13).
Destruction of myelin leads to various signs and symptoms such as gait disturbance, impaired coordination, and sensory loss (14). An open-field test (OFT) is performed to measure locomotor activity, exploratory behavior, and anxiety-related behavior in rodents (15). Beam walking test (BWT) is used to assess sensorimotor balance and coordination ability (16).
Vitamin E, a fat-soluble 6-hydroxychroman compound, consists of eight subtypes which are alpha-, beta-, gamma-, and delta-tocotrienol and tocopherol. Among the tocopherols, alpha- tocopherol (AT) and gamma- tocopherols can be detected in the serum and erythrocytes, where AT remains in the highest amount. AT is reported to be the most active form of vitamin A. The synthetic form of AT is known as DL-alpha-tocopheryl acetate (DLTA), chemically known as all-rac-2, 5, 7, 8- tetramethyl-2-(4, 8, 12-trimethyltridecyl)-3,4- dihydro-2H-1 benzopyran-6-yl acetate (17). Ester forms of synthetic DLTA can form miscible micelles in water and are comparatively more water-soluble than naturally occurring AT (18). Preferential incorporation of DLTA to human brain tissue and binding of DLTA to tocopherol-binding protein facilitates its ability to cross the blood- brain barrier (19). The effect of AT on the EAE model of MS in mice has been explored in a previous study (20). A qualitative histological study was done to evaluate the change in demyelination. AT has also been used in a group of MS patients to evaluate the change in magnetic resonance imaging (MRI) lesions (21). However, histological, behavioral, and locomotor activities were not measured quantitatively in these studies and compared with similar changes in demyelination. The present study aimed to evaluate the beneficial effects of a synthetic form of AT in the CPZ model of demyelination in female rats using quantitative estimation of histological, open-field observation, and BWT.
MATERIALS AND METHODS
Experimental process
Female Sprague-Dawley rats, 8 weeks old (200-220 g), were divided into three groups (n = 8). Two rats were housed in one cage with automatic temperature control and a 12-12-h light/dark cycle. Control (group C) group of rats received standard rodent chow from week 1 to week 7 and daily intraperitoneal (IP) injections of 0.3 mL phosphate-buffered saline (PBS) on weeks 6 and 7. Both experimental groups (E1 and E2) received rodent chow impregnated with 0.2% CPZ (Envigo, US) from week 1 to week 5 followed by standard rodent chow on weeks 6 and 7. During weeks 6 and 7, when CPZ was withdrawn, group E1 was treated daily with IP injections of 0.3 mL DLTA emulsified with PBS (100 mg/kg body weight) (20). During withdrawal of CPZ, group E2 or vehicle-treated group was treated with IP injections of 0.3 mL of PBS on weeks 6 and 7. Water-miscible DLTA was purchased from Merck, Malaysia.
Ethical approval
Animal experiments were conducted according to the principles stated in the guidebook of the Laboratory Animal Care and Use Committee (ACUC). Ethical approval was taken from the University Joint Committee on Research and Ethics vide No. BP I-01/2019[09].
Open-field test (OFT) and beam walking test (BWT)
The OFT was performed to measure locomotor activity and anxiety-related behavior of the rats. The protocol described by Seibenhener and Wooten was followed in this study with slight modifications (15). The open box was 30 × 30 × 30 cm (L, W, H) and was evenly illuminated (7 lux). The base was colored black with a marked inner zone of 30 × 30 cm. The rats were placed at the center of the OF and the movement of rats was recorded for 10 min using a video camera (Panasonic, Japan) placed above the arena. Average speed (mm/sec) and total distance moved (cm) were analyzed by the ToxTrac software (22) from the recorded video. Rearing frequency and line crossing frequency in 10 min were calculated manually from the video. The frequency in which the rat stands on its hind legs lifting the forelegs upwards against the wall was termed as rearing frequency. Rearing is a stereotyped behavior and an increase in rearing frequency was interpreted as the increased exploratory activity or reduced anxiety (23). The floor of the arena was divided into squares of 5 × 5 cm. The frequency with which the rat crosses the grid lines in the floor of OF with all four paws was termed line crossing frequency and could be interpreted as a measure of locomotor activity (24). The beam walking apparatus was 122 cm long and elevated 75 cm above the ground level. The protocol for BWT as described by Luong et al. was used in this experiment with slight modifications (16). A LED light was installed at the starting point which served as the aversive stimulus. A black box was positioned at the opposite end. The time taken to traverse a length of 80 cm was calculated by manual observation. The mean values of three consecutive data taken 10 min apart were calculated. OFT and BWT were performed every 10 days for a total of 5 times (three times during weeks 1-5 and two times during weeks 6 and 7 having AT/vehicle treatment) during the experiment. BWT is a paradigm assessing sensorimotor balance and coordination ability (25).
Histological and histomorphometric studies
At the end of week 7, rats were anesthetized with ketamine/xylazine mixture of 100 mg/kg and 10 mg/kg, respectively via IP injections. Subsequently, trans-cardiac perfusion was done with 4% paraformaldehyde. In the end, rats were killed by cervical dislocation. Brains were post-fixed overnight followed by dissection of the forebrain area between optic chiasma and infundibulum. Following paraffin embedding, eight-micron coronal serial sections were stained with hematoxylin and eosin (H&E) and Luxol® fast blue (LFB). For quantitative analysis, randomly selected five LFB-stained slides (every 27th section between optic chiasma and infundibulum) in each rat were used. Six rats each in groups C, E1, and E2 were used for quantitative analysis. Nikon NIS-elements software was used to quantify the areas of demyelination at four random areas of corpus callosum from photomicrographs taken with a bright-field compound Nikon microscope YS 100 (26). For qualitative analysis five randomly selected LFB-stained slides from 6 animals per group were graded for relative changes in the stains of the myelinated corpus callosum. The observation was done at 400× magnification at three selected areas (midline and areas directly lateral to the midline of corpus callosum) consistent across all slides by two blinded, independent observers who were not associated with the study. The areas were graded on a scale of 1 to 3. Grade 1 indicated deep blue with the presence of no vacuoles; 2, pale blue with the presence of a small number of vacuoles; and 3, pale blue with the presence of a large number of vacuoles.
Statistical analysis
Mean ± SEM values of the parameters observed in OFT and BWT were subjected to repeated measure One-way ANOVA using SPSS 25 to find out significant (P < 0.05) difference in the values between the treatment groups. Post-hoc Bonferroni was used to find out the inter-group difference. Quantitative data of mean ± SEM area of demyelination was subjected to One-way ANOVA analysis and qualitative data of the count of grading of staining was subjected to the Pearson Chi-Square test.
RESULTS
Changes in the locomotor activity
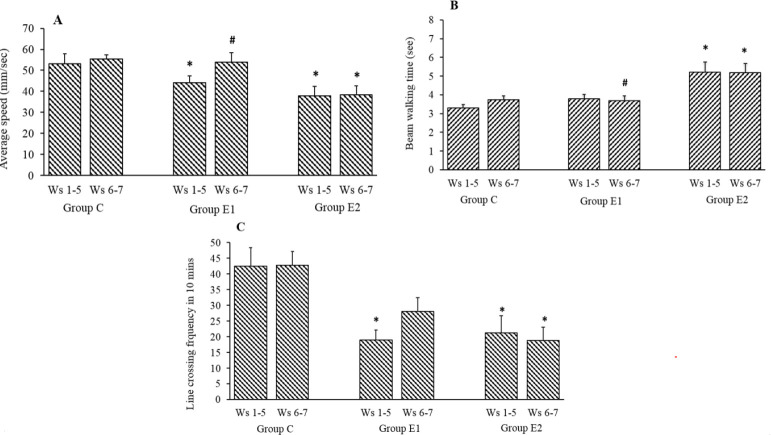
The mean body weight of the rats in all three groups was observed to be increasing across the duration of the experiment from week 1 to week 7. The intake of rodent chow impregnated with CPZ or its withdrawal did not affect the body weight of the rats. The average speed of movement in OFT of the CPZ-treated rats in group E1 and E2 during week 1 to week 5 reduced significantly (P < 0.05) compared to the group C. During withdrawal of CPZ (weeks 6 and 7), AT treatment increased the average speed by 22% in group E1 compared to the average speed in the same group during CPZ treatment (week 1-5). Vehicle-treated group E2 showed an increase of 1.78% only in mean speed with a similar comparison (Fig. 1A). A significant difference in the mean speed of movement between group E1 and group E2 P < 0.05) at the withdrawal period (week 6-7) was observed as shown in Fig. 1A. In the BWT, during withdrawal of CPZ (week 6-7), the mean time to walk the 80 cm distance of the beam was reduced in the group E1 by 2.6% compared to a similar time during CPZ treatment (week 1-5). In the vehicle-treated group E2, a similar reduction was only 0.64% (Fig. 1B). A significant difference in the mean time to cross the beam between group E1 and group E2 (P < 0.05) at the withdrawal period (week 6-7) was observed as shown in Fig. 1B. The mean frequency of crossing the lines in OFT, of the CPZ-treated rats in group E1 and E2 during week 1 to week 5 reduced significantly (P < 0.05) compared to the group C. During withdrawal of CPZ (week 6-7), AT treatment increased the line crossing frequency by 47.4% in group E1 compared to the similar frequency in the same group during CPZ treatment (week 1-5). Vehicle-treated group E2 showed a decrease of 11.1% with a similar comparison (Fig. 1C).
Fig. 1.
Effects of AT (group E1) on (A) average speed (mm/sec), (B) beam walking time, and (C) line crossing frequency in open field test and in CPZ-withdrawn rats compared to sham-treated control (group C) and PBS treatment (group E2). Values are mean ± SEM; n = 8. *P < 0.05 Indicates significant difference compared to group C and #P < 0.05 vs group E2. Weeks 1-5: CPZ treatment (groups E2 and E1); Weeks 6 and 7: AT treatment (E2) and PBS treatment (E1). CPZ, Cuprizone; AT, Alpha-tocopherol, Ws, weeks.
Changes in exploratory behavior
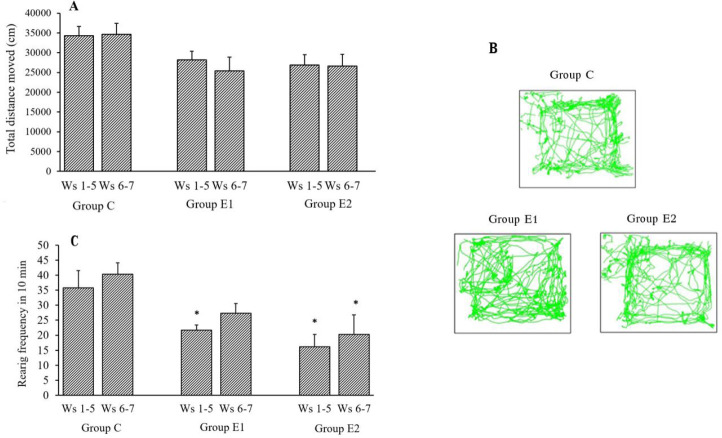
Total distance moved in OFT reduced in both group E1 and E2 following exposure to CPZ compared to group C. There is a reduction in mean total distance moved in cm, in group E1 during withdrawal of CPZ (week 5-6) compared to the distance moved during CPZ treatment (week 1-5) (Fig. 2A). However, comparison of track paths observed (Fig. 2B) in AT treated group E1 and vehicle-treated group E2 showed that the track in AT treated group crossed into the central part of the OF at regular intervals while the track in the vehicle-treated group remained in proximity to the walls of the maze. More movement in the proximity of walls indicated increased thigmotaxis or anxiety-related behavior.
Fig. 2.
Effects of AT (group E1) on (A) total distance moved (cm) and (B) rearing frequency in open field test in CPZ-withdrawn rats compared to control (group C) and PBS treatment (group E2). (C) Track of movement of rats of groups C, E1, and E2 is shown in the center and periphery of the open field. Values are mean ± SEM; n = 8. *P < 0.05 Indicates significant difference compared to group C. Weeks 1-5: CPZ treatment (groups E2 and E1); weeks 6 and 7: AT treatment (E2) and PBS treatment (E1). CPZ, Cuprizone; AT, alpha-tocopherol, Ws, weeks.
The mean frequency of rearing in OFT of the CPZ-treated rats in group E1 and E2 during week 1 to week 5 reduced significantly (P < 0.05) compared to the group C. During withdrawal of CPZ (week 6-7), AT treatment increased the rearing frequency by 26.1% in group E1 compared to the similar frequency in the same group during CPZ treatment (week 1-5). Vehicle-treated group E2 showed an increase of 25.7%, during withdrawal of CPZ, compared to the mean rearing frequency during CPZ treatment (Fig. 2C).
Histological changes in the corpus callosum
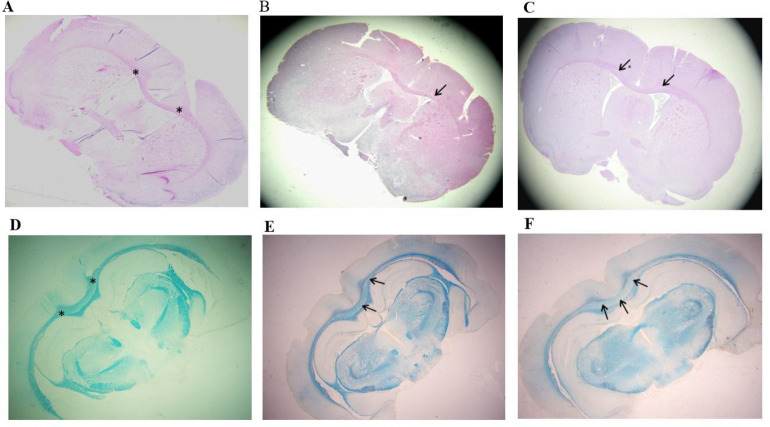
The histological changes in the myelinated white fiber of the corpus callosum were observed using H&E (Fig. 3A-C) and LFB stains (Fig. 3D-F). The demyelination was observed in the median and para-median areas of the pink-stained corpus callosum, by the appearance of pale areas under H&E stain Fig. 3A-C). The pale areas were comparatively more in PBS treated group E2 (Fig. 3C). In AT treated group E1, the pale area was seen in few areas only (Fig. 3B). Under LFB stain, group C showed blue myelinated areas of the corpus callosum (Fig. 3D). In PBS treated group E2, loss of myelination was observed by the appearance of wide pale areas in the corpus callosum (Fig. 3F). In AT treated group E1, the myelination appeared to be restored by the blue staining of the corpus callosum with few areas in median and paramedian locations of the corpus callosum showing patchy pale areas of demyelination (Fig. 3E).
Fig. 3.
Comparison of demyelination in the corpus callosum in control (group C), alpha-tocopherol -treatment (group E1), and vehicle-treatment (group E2). Haematoxylin and Eosin (H&E) sections of forebrain (A) group C, (B) group E1, and (C) group E2. Luxol fast blue (LFB) sections of forebrain (D) group C; (E) group E1, and (F) group E2. Asterisks show corpus callosum myelinated area; arrows show areas of demyelination which are more prominent in group E2 and less in group E1.
Qualitative changes in demyelination in the corpus callosum
Qualitative analysis of the grading of the staining of the myelinated corpus callosum in iso-cortex of group C, E1, and E2 was done using the Pearson Chi-Square test (X (4) = 11.75, phi = 0.885, P = 0.19). The result indicated a statistically significant association between three grades of staining and the three groups (C, E1, and E2). The count of grade 3 (pale blue stain with many vacuoles), signifying complete demyelination, was observed in 56% of sections of vehicle-treated group E2 and 28% of sections of alpha-tocopherol treated group E1.
The count of grade 2 (pale blue stain with a small number of vacuoles), signifying partial demyelination, was observed in 60% of sections of AT treated group E1and 32% sections of the vehicle-treated group E2. A qualitative shift towards partial demyelination was found in sections of AT treated group E1compared to those of group E2 (Fig. 4).
Fig. 4.
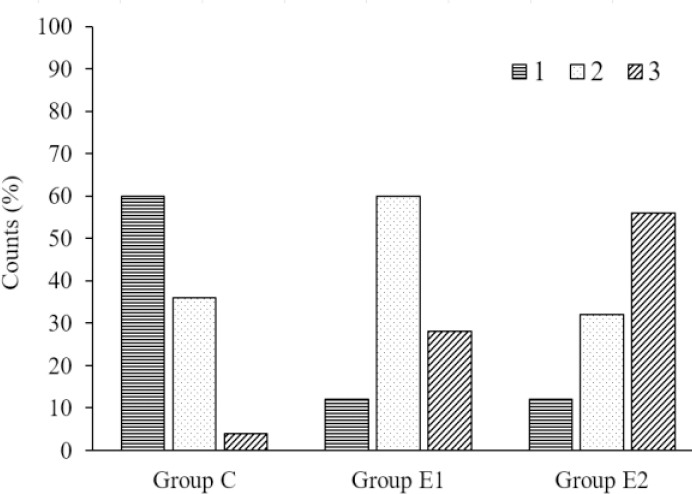
Bar chart showing the percentage of counts of grades 1, 2, and 3 in qualitative grading of luxol fast blue (LFB)-stained histological sections of groups C, E1, and E2. Grade 1, deep blue (myelinated); grade 2, pale blue with few vacuoles (intermediate); grade 3, pale blue with many vacuoles (demyelinated).
Quantitative changes in the area of demyelination in the corpus callosum
Quantitative analysis was done using the LFB-stained coronal sections of iso-cortex from group C, E1, and E2. The mean area of demyelination in square micron in the corpus callosum was highest in vehicle-treated group E2. Group E1, which received AT, during withdrawal of CPZ, showed a 12% reduction in the mean area of demyelination in the corpus callosum, compared to group E2 (Fig. 5). One-way ANOVA showed a significant difference between the mean area of demyelination in group C, E1 and E2 (P = 0.000). A Bonferroni post-hoc test showed a significant inter-group difference in the mean area of demyelination in corpus callosum between group E1 (23182.4 ± 6134.5 sq. micron) and E2 (26354.4 ± 6213.1 sq. micron) (P <0.05).
Fig. 5.
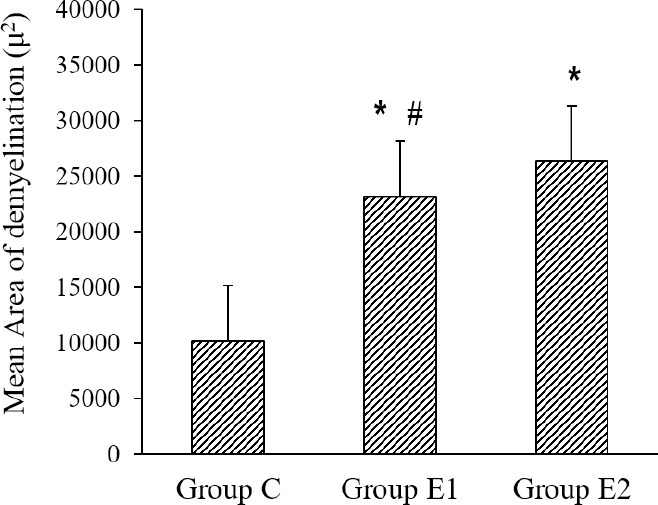
Effects of alpha-tocopherol (group E1) on the mean area of demyelination (μ2) of the corpus callosum in luxol fast blue (LFB)-stained histological sections of the forebrain. Group E2, PBS treatment; group C, Control. Values are mean ± SEM; n = 6. *P < 0.05 Indicates difference compared to group C and #P < 0.05 vs group E2.
DISCUSSION
The mean values of the average speed of movement and line crossing frequency in OFT were found to be reduced significantly in group E1 and E2, fed with 5 weeks of CPZ- impregnated pellets compared to group C. These parameters depend on the motor function and the limb strength of the rats. The copper chelator function of CPZ inhibited mitochondrial enzymes, leading to apoptosis of oligodendrocytes and subsequent demyelination (27,28). A previous study observed that the death of oligodendrocytes started at the end of the first week of CPZ exposure and lasted for up to 6 weeks (29). Demyelination occurring in the corpus callosum and cerebral cortex affect the functions of the iso-cortex, which regulates the motor function resulting in motor impairment (30,31). AT-treated group E1 showed an increase in the average speed by 22% during the withdrawal of CTZ, whereas the vehicle-treated group E2 showed an increase of 1.78% during a similar period. A similar increase in mean line crossing frequency in AT-treated group E1 during withdrawal of CTZ was 47.4% compared to an 11.1 % decrease in vehicle- treated group E2. Both the average speed of walking and line crossing frequency observed in OFT indicated spontaneous motor function. AT-treatment was found to improve the spontaneous motor function significantly in group E1 compared to the vehicle-treated group E2. Time to cross the 80 cm distance of the beam, was reduced by 2.6% in AT-treated group E1 during withdrawal of CTZ compared to the vehicle-treated group E2 showing a 0.64% reduction. In this study, we found that although AT had a significant treatment effect on the spontaneous motor function in 100 mg/kg dosage, such treatment had little effect on the improvement in motor coordination. The improved function in AT-treated group may attribute to either its neuroprotective effect against CPZ-induced demyelination or its neurorestorative effect on remyelination or a combination of both.
AT treatment reduced the mean total distance moved and increased mean rearing frequency observed in OFT, in group E1, during withdrawal of CTZ, compared to the similar mean values during CTZ treatment. Track of movement in AT-treated group crossed into the central part of the OF at regular intervals. In this study, AT-treatment improved exploratory behavior with a possible reduction in anxiety as evidenced by an increase in mean rearing frequency. Sustained long-term potentiation in the dentate gyrus of aged rats was observed following an all-rac alpha- tocopherol supplemented diet (32). Previous studies have observed a strong relationship between the hippocampus and rearing behavior. Hippocampal morphology and hippocampal intra- and infrapyramidal mossy fibers are directly linked to rearing behavior. Mice selectively bred for high-frequency rearing showed an increase in intra- and infrapyramidal mossy fiber terminal field size (33,34). Rearing increased when the environment became safer and reduced after exposure to acute swim or restraint stress (23). AT acts as a potent antioxidant, preventing superoxide free radicals from attacking myelin. The resultant metabolic effect causes a reduction in stress-induced anxiety leading to a potential increase in rearing frequency.
Quantitative analysis of the mean area of demyelination in the corpus callosum in LFB- stained sections showed a significant reduction in AT-treated group E1 compared to vehicle- treated group E2. Qualitative analysis showed a decrease in complete demyelination and an increase in partial demyelination in E1 compared to E2. After three to seven days of CPZ treatment, oligodendrocytes start to undergo apoptosis with evidence of big vacuoles, enlarged mitochondria, and dense nuclear chromatin. Following the withdrawal of CPZ, proliferation and aggregation of oligodendrocytic precursor cells in the subventricular zone and demyelinated areas are visible resulting at the beginning of a remyelination process (35). Acting on cell membranes and lipoproteins, AT entraps peroxide radicals and stops lipid peroxidation. It has also been proven to act as a free radical scavenger in the lipid-rich myelin sheath. AT facilitates remyelination by arresting the propagation of lipid peroxyl radicals and prevents apoptosis of oligodendrocytes (36). Oligodendrocyte apoptosis and myelin degradation in the CPZ-induced model of MS, involves the migration of various components of the immune system to the site of damage. Vitamin E reduces oxidative damage through suppression of circulating levels of various cytokines such as interleukin-1β, interleukin-6, and tumor necrosis factor-a (37). It has been proposed that the remyelinating properties of AT are due to its ability to promote the maturation of oligodendrocyte precursor cells by inhibiting the Notch signaling pathway (38). The reduction in the demyelination of the corpus callosum following the withdrawal of CPZ was facilitated by the AT-treatment in the dosage of 100 mg/kg IP. The improvement in the spontaneous motor function observed by analysis of OFT indicated improved functions of the iso-cortex.
CONCLUSION
AT was able to improve the locomotor functions of rats in the animal model of MS induced by the CPZ diet, as evidenced by the improvement of average speed and line crossing frequency. The myelo-protective and regenerative effects of alpha-tocopherol were also proven by its ability to reduce the areas of demyelination in corpus callosum during withdrawal from CPZ treatment in Sprague- Dawley rats.
CONFLICT OF INTEREST STATEMENT
All authors declared that there is no conflict of interest in this study.
AUTHORS' CONTRIBUTION
N.K. Mitra designed the project, developed the experimental protocol, secured the grant, submitted the project for ethical approval, and conceptualized the manuscript. K.Y. Xuan, C.C. Teo, and N. Xian-Zhuang carried out the experiment and collected the data. N.K. Mitra and J. Chellian analyzed the data. A. Singh helped the students in research work and helped to review the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank International Medical University Joint Committee on Research and Ethics and Institute for Research, Development, and Innovation (IRDI), International Medical University, for ethical approval and financial grant (Grant No. BP-I-01-2019) to carry out this project.
REFERENCES
- 1.Ghasemi N, Razavi S, Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017;19(1):1–10. doi: 10.22074/cellj.2016.4867. DOI: 10.22074/cellj.2016.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loma I, Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011;9(3):409–416. doi: 10.2174/157015911796557911. DOI: 10.2174/157015911796557911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Love S. Demyelinating diseases. J Clin Pathol. 2006;59(11):1151–1159. doi: 10.1136/jcp.2005.031195. DOI: 10.1136/jcp.2005.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133(2):223–244. doi: 10.1007/s00401-016-1631-4. DOI: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitra NK, Bindal U, Eng Hwa W, Chua CL, Tan CY. Evaluation of locomotor function and microscopic structure of the spinal cord in a mouse model of experimental autoimmune encephalomyelitis following treatment with syngeneic mesenchymal stem cells. Int J Clin Exp Pathol. 2015;8(10):12041–12052. [PMC free article] [PubMed] [Google Scholar]
- 6.Torkildsen O, Brunborg LA, Myhr KM, Bø L. The cuprizone model for demyelination. Acta Neurol Scand Suppl. 2008;188:72–76. doi: 10.1111/j.1600-0404.2008.01036.x. DOI: 10.1111/j. 1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 7.Sachs HH, Bercury KK, Popescu DC, Narayanan SP, Macklin WB. A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro. 2014;6(5):1759091414551955,1–16. doi: 10.1177/1759091414551955. DOI: 10.1177/1759091414551955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zatta P, Raso M, Zambenedetti P, Wittkowski W, Messori L, Piccioli F, et al. Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell Mol Life Sci. 2005;62(13):1502–1513. doi: 10.1007/s00018-005-5073-8. DOI: 10.1007/s00018-005-5073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. DOI: 10.1002/1531-8249(200006)47:6<707::aid- ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55(4):458–468. doi: 10.1002/ana.20016. DOI: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 11.Lassmann H. Multiple sclerosis: is there neurodegeneration independent from inflammation. J Neurol Sci. 2007;259(1-2):3–6. doi: 10.1016/j.jns.2006.08.016. DOI: 10.1016/j.jns.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Ünsal C, Özcan M. Neurotoxicity of cuprizone in female and male rats: electrophysiological observations. Neurophysiol. 2018;50:108–115. DOI: 10.1007/s11062-018-9724-4. [Google Scholar]
- 13.Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57(8):807–814. doi: 10.1002/glia.20806. DOI: 10.1002/glia.20806. [DOI] [PubMed] [Google Scholar]
- 14.Richards RG, Sampson FC, Beard SM, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6(10):1–73. doi: 10.3310/hta6100. DOI: 10.3310/hta6100. [DOI] [PubMed] [Google Scholar]
- 15.Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;96:e52434,1–6. doi: 10.3791/52434. DOI: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011;49:2376,1–3. doi: 10.3791/2376. DOI: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EDQM, Council of Europe. The European Pharmacopeia. 5th ed. 51. Strasbourg, France: 2005. p. 3024. [Google Scholar]
- 18.Abu-Fayyad A, Behery F, Sallam AA, Alqahtani S, Ebrahim H, El Sayed K, et al. PEGylated γ-tocotrienol isomer of vitamin E: Synthesis, characterization, in vitro cytotoxicity, and oral bioavailability. Eur J Pharm Biopharm. 2015;96:185–195. doi: 10.1016/j.ejpb.2015.07.022. DOI: 10.1016/j.ejpb.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Ranard KM, Erdman JW., Jr Effects of dietary RRR a-tocopherol vs all-racemic a-tocopherol on health outcomes. Nutr Rev. 2018;76(3):141–153. doi: 10.1093/nutrit/nux067. DOI: 10.1093/nutrit/nux067. [DOI] [PubMed] [Google Scholar]
- 20.Xue H, Ren H, Zhang L, Sun X, Wang W, Zhang S, et al. Alpha-tocopherol ameliorates experimental autoimmune encephalomyelitis through the regulation of Th1 cells. Iran J Basic Med Sci. 2016;19(5):561–566. [PMC free article] [PubMed] [Google Scholar]
- 21.Løken-Amsrud KI, Myhr KM, Bakke SJ, Beiske AG, Bjerve KS, Bjørnarå BT, et al. Alpha-tocopherol and MRI outcomes in multiple sclerosis-association and prediction. PLoS One. 2013;8(1):e54417,1–5. doi: 10.1371/journal.pone.0054417. DOI: 10.1371/journal.pone.0054417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez A, Zhang H, Klaminder J, Brodin T, Andersson PL, Andersson M. ToxTrac: a fast and robust software for tracking organisms. Methods Ecol Evol. 2018;9(3):460–464. DOI: 10.1111/2041-210X.12874. [Google Scholar]
- 23.Sturman O, Germain PL, Bohacek J. Exploratory rearing: a context-and stress-sensitive behavior recorded in the open-field test. Stress. 2018;21(5):443–452. doi: 10.1080/10253890.2018.1438405. DOI: 10.1080/10253890.2018.1438405. [DOI] [PubMed] [Google Scholar]
- 24.Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86(4):651–659. doi: 10.1016/j.pbb.2007.02.010. DOI: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J, Sun X, Ma C, Li BM, Luo F. Voluntary wheel running promotes myelination in the motor cortex through Wnt signaling in mice. Mol Brain. 2019;12:85–94. doi: 10.1186/s13041-019-0506-8. DOI: 10.1186/s13041-019-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra NK, Nadarajah VD, Siong HH. Effect of concurrent application of heat, swim stress and repeated dermal application of chlorpyrifos on the hippocampal neurons in mice. Folia Neuropathol. 2009;47(1):60–68. [PubMed] [Google Scholar]
- 27.Goldberg J, Daniel M, van Heuvel Y, Victor M, Beyer C, Clarner T, et al. Short-term cuprizone feeding induces selective amino acid deprivation with concomitant activation of an integrated stress response in oligodendrocytes. Cell Mol Neurobiol. 2013;33(8):1087–1098. doi: 10.1007/s10571-013-9975-y. DOI: 10.1007/s10571-013-9975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G. Animal models of multiple sclerosis. Eur J Pharmacol. 2015;759:182–191. doi: 10.1016/j.ejphar.2015.03.042. DOI: 10.1016/j.ejphar.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi-Rad M, Ghasemi N, Aliomrani M. Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Res Pharm Sci. 2019;14(5):424–431. doi: 10.4103/1735-5362.268203. DOI: 10.4103/1735-5362.268203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepper RE, Pitman KA, Cullen CL, Young KM. How do cells of the oligodendrocyte lineage affect neuronal circuits to influence motor function, memory and mood. Front Cell Neurosci. 2018;12:399–412. doi: 10.3389/fncel.2018.00399. DOI: 10.3389/fncel.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvestroff L, Bartucci S, Pasquini J, Franco P. Cuprizone-induced demyelination in the rat cerebral cortex and thyroid hormone effects on cortical remyelination. Exp Neurol. 2012;235(1):357–367. doi: 10.1016/j.expneurol.2012.02.018. DOI: 10.1016/j.expneurol.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem. 1998;273(20):12161–12168. doi: 10.1074/jbc.273.20.12161. DOI: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- 33.Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17(1-2):111–133. doi: 10.1515/revneuro.2006.17.1-2.111. DOI: 101515/revneuro2006171-2111. [DOI] [PubMed] [Google Scholar]
- 34.Crusio WE, Schwegler H, Van Abeelen JH. Behavioral responses to novelty and structural variation of the hippocampus in mice II Multivariate genetic analysis. Behav Brain Res. 1989;32(1):75–80. doi: 10.1016/s0166-4328(89)80075-0. DOI: 101016/s0166-4328(89)80074-9. [DOI] [PubMed] [Google Scholar]
- 35.Gudi V, Gingele S, Skripuletz T, Stangel M. Glial response during cuprizone-induced de-and remyelination in the CNS: lessons learned. Front Cell Neurosci. 2014;8:73–96. doi: 10.3389/fncel.2014.00073. DOI: 10.3389/fncel.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soltani R, Khorvash F, Meidani M, Badri S, Alaei S, Taheri S. Vitamin E in the prevention of vancomycin- induced nephrotoxicity. Res Pharm Sci. 2020;15(2):137–143. doi: 10.4103/1735-5362.283813. DOI: 10.4103/1735-5362.283813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54(5):333–338. doi: 10.1503/cjs.013610. DOI: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30(2):289–299. doi: 10.1007/s10571-009-9451-x. DOI: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]