Abstract
Nuclear receptors represent a large family of ligand-activated transcription factors which sense the physiological environment and make long-term adaptations by mediating changes in gene expression. In this review, we will first discuss the fundamental mechanisms by which nuclear receptors mediate their transcriptional responses. We will focus on the PPAR family of adopted orphan receptors paying special attention to PPARγ, the isoform with the most compelling evidence as an important regulator of arterial blood pressure. We will review genetic data showing that rare mutations in PPARγ cause severe hypertension and clinical trial data which show that PPARγ activators have beneficial effects on blood pressure. We will detail the tissue- and cell-specific molecular mechanisms by which PPARs in the brain, kidney, vasculature and immune system modulates blood pressure and related phenotypes such as endothelial function. Finally, we will discuss the role of placental PPARs in preeclampsia, a life-threatening form of hypertension during pregnancy. We will close with a viewpoint on future research directions and implications for developing novel therapies.
Keywords: PPARγ, blood pressure, hypertension
Introduction to Nuclear Receptors and PPARs
The human body is continuously working to sustain homeostasis. For the purpose of this review, homeostasis is defined as the preservation of blood pressure (BP) within a narrow range to ensure effective perfusion of body tissues. Disruption of these homeostatic systems can cause hypertension, and during pregnancy, can cause preeclampsia. The sensors designed to preserve homeostasis come in many flavors including receptors that sense and respond to mechanical or chemical signals, among others. This review will focus on a category of sensors called nuclear receptor transcription factors which detect signals produced by the body in the form of ligands and exert effects through changes in gene expression. They represent a translationally important group of proteins, because like G protein coupled receptors, they are targets of a wide range of pharmacological agents which treat disease. We will focus on peroxisome proliferator activated receptors (PPAR), spotlighting PPARγ as one member of the nuclear receptor superfamily for which there is extensive evidence for a major participation in BP regulation, hypertension, and preeclampsia.
In humans, the nuclear receptor superfamily includes 48 evolutionarily conserved and structurally homologous members which control the expression of a repertoire of target genes. Each carries a DNA binding domain which provides specificity to define its targets, a ligand binding domain (LBD) which provides specificity to the response, and transactivation domains which mediate interactions with other members of the transcriptional machinery. Although there are formally 6 classes of nuclear receptors, it is expedient to divide them into 4 groups: 1) steroid hormone receptors (e.g., glucocorticoid receptor), 2) non-steroid hormone receptors (e.g., thyroid hormone receptor), 3) orphan receptors whose ligands remain unidentified, and 4) adopted orphan receptors, previous orphan receptors which have since been associated with at least one ligand. The PPARs are members of this latter group.
There are 3 PPAR isoforms, α, βδ and γ, which differ in their ligand selectivity, tissue-specific expression, and repertoire of target genes. PPARα (NR1C1) is expressed in liver, brown adipose tissue, heart, and kidney, and uses arachidonic acid metabolites and polyunsaturated fatty acids as natural ligands, and fibrates as synthetic ligands. Among its myriad of effects, PPARα has been implicated to regulate arterial BP and modulate endothelial function through its effects as a regulator of anti-oxidant and anti-inflammatory gene expression.1 PPARβδ (NR1C2) is expressed in adipose tissue, skin and brain, and is associated with development and oxidative capability in muscle.2 PPARβδ is the least recognized PPAR family member evidenced to regulate BP.3 PPARγ (NR1C3) is ubiquitously expressed and is best known for its essential role in adipogenesis.4 Fatty acids and eicosanoids can act as PPARγ ligands, but the primary physiologically relevant ligand(s) for this receptor remain unclear.5 PPARγ’s physiological and medical significance was highlighted by the recognition that the thiazolidinediones (TZD) drugs which improve insulin sensitivity are high affinity ligands of PPARγ.6
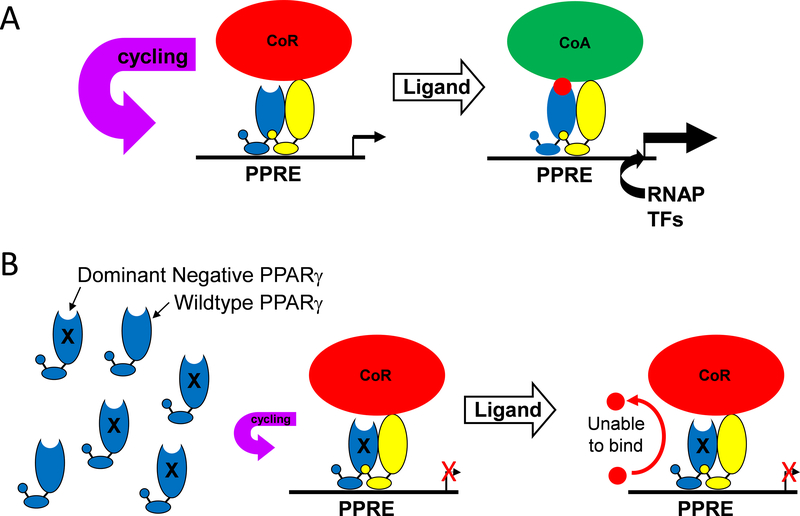
To understand how the PPARs function, it is instructive to contrast their mechanism of action with other members of the superfamily (Figure 1A). Steroid hormone receptors, such as glucocorticoid receptor, are localized in the cytoplasm and are coupled to cytoplasmic chaperones. When they bind ligand, they homodimerize, translocate to the nucleus, bind DNA as homodimers, and recruit a co-activator protein complex to transactivate transcription of their target genes. Alternatively, PPARγ resides in the nucleus already bound to its cognate DNA binding sites as obligate heterodimers with retinoid X receptor (RXR). In the absence of ligand, they interact with co-repressors and histone deacetylases to form a co-repressor complex which promotes gene repression. In response to ligand binding, co-repressors are dismissed, and co-activators and histone acetyltransferases are recruited to form a co-activator complex which promotes transcription. Genome wide chromatin immunoprecipitation studies revealed that PPARγ/RXR can bind to over 5000 sites in the genome.7 However, transcriptome analysis revealed that the number of genes activated are substantially smaller.8 Thus, whereas the repertoire of potential target genes is large, the actual alterations in gene expression are smaller and cell-specific.
Figure 1: Mechanisms Distinguishing Steroid Hormone Receptors from Adopted Orphan Receptors.
Figure 1: A) Steroid hormone receptors are localized in the cytoplasm coupled to cytoplasmic chaperones under baseline conditions. In response to endogenous or exogenous ligand, the chaperone is dismissed, the receptor/ligand dimerizes and translocates into the nucleus. The dimerized nuclear receptor finds its cognate binding sites (Nuclear Receptor Response Element, NRE) in the genome and recruits a series of co-activator (CoA) proteins which facilitates the opening of chromatin and activation of transcription of its repertoire of genes in that cell type. Adopted orphan receptors such as PPARγ are localized in the nucleus complexed to its cognate binding site as a heterodimer with RXR. Co-repressor (CoR) and histone deacetylases maintain a repressive condition on transcription. In response to ligand binding, corepressors are dismissed, transcriptional coactivators are recruited which facilitates transactivation. B-D) Alternative PPARγ’s mechanism of actions includes: a trans-repression mechanism requiring SUMOylation of a lysine reside in the ligand binding domain, ubiquitination of the NF-κB p65 subunit, and shuttling the NF-κB complex off the nucleus. ER: estrogen receptor, GR: glucocorticoid receptor, PPRE: PPAR response element, RNAP: RNA polymerase. (Illustration credit: Ben Smith).
In additional to the classical mechanism of transactivation, PPARγ represses inflammatory gene expression via a transrepression mechanism (Figure 1B).9 This mechanism involves SUMOylation of a lysine reside in the LBD which targets SUMOylated-PPARγ to a co-repressor complex on the promoter of inflammatory genes such as inducible nitric oxide synthase. The formation of this complex, which interestingly, occurs independently of PPARγ ligand and PPARγ binding to a PPAR response element (PPRE), prevents ubiquitination and proteasomal activity of the co-repressor complex and prevents the activation of transcription.
PPARγ has also been reported to contain a “really interesting new gene” (RING) domain (Figure 1C).10 These domains are present in E3 RING ubiquitin ligases which function to regulate the stability of proteins by targeting them for ubiquitination and proteasomal degradation. PPARγ has been reported to function as an E3 RING ligase for the p65 subunit of NK-кB.10 In our studies in vascular smooth muscle cells, PPARγ limited NK-кB activity, not by functioning as an E3 ligase, but by binding to p65 to shuttle it out of the nucleus (Figure 1D).11 Interestingly, a mutant form of PPARγ which causes human hypertension prevents its binding to p65 and enhances inflammatory gene expression.11
Genetic Evidence for PPARγ as a Regulator of BP
Of the three PPAR isoforms, the strongest genetic evidence implicating a member of the family as a major regulator of blood pressure is for PPARγ. This is largely based on the seminal finding that mutations in PPARγ (V290M and P467L) not only caused diabetes, and insulin resistance, but also severe hypertension.12 The mutations caused a loss of basal transcriptional activity and acted in a dominant negative fashion, meaning they inhibited the activity of the wildtype PPARγ (Figure 2). A “knock in” mouse model in which the mouse PPARγ gene was mutated to emulate the human P467L mutation (mouse P465L) exhibited modest hypertension, but incompletely phenocopied the dyslipidemia phenotypes of the human mutation.13 Two additional rare mutations in PPARγ, R165T and L339X (X = termination codon) were identified in familial partial lipodystrophy (FPLD3) associated with early-onset severe hypertension.14 Increased activity of the renin-angiotensin system genes, exaggerated responses to Ang-II stimulation, and increased NF-кB activity all of which was dependent on angiotensin-II (Ang-II) type-1 receptor (AT1R) signaling were identified in cultured cells derived from patients carrying these mutations.
Figure 2: Mechanism of Dominant Negative PPARγ Mutations.
A) As illustrated in Figure 1, PPARγ forms a heterodimer with RXR and binds to a PPAR response element (PPRE) in chromatin in the regulatory region of a PPARγ target gene even in the unliganded state. This is not a static process, because the complex cycles through DNA bound and unbound states. Consequently, the rate of transcription under these circumstances reflects the occupancy of the site by the PPARγ-RXR-corepressor complex and the binding of other stimulatory transcription factors and RNA polymerase to other sequences in the regulatory region of the gene. Ligand binding causes dismissal of the corepressors (CoR) and a recruitment of coactivators (CoA). The coactivator complex facilitates a conformation of chromatin favorable for transcription. B) Replacement of wild-type PPARγ with P467L PPARγ causes increased occupancy (reduced cycling) of the PPRE by the PPARγ-RXR-corepressor complex, which further reduces baseline transcription of actively repressed PPARγ target genes. Figure was previously reported in 46.
In additional to rare variants which have profound physiological effects, more frequent variants of PPARγ have been examined for association with hypertension. A meta-analysis examining 21 studies looking at the association between the P12A polymorphism, the most extensively studied polymorphism, and BP suggested the variant could influence hypertension risk in Asians.15 More recently, PPARγ was identified as a locus which interacts at the level of chromatin and thus is a distal associated gene associated with BP traits in a study of 1 million people.16
Clinical Evidence for PPARs as Regulators of BP
PPARα agonists are primary used to treat dyslipidemia. Consequently, there are only a few clinical studies and trials of PPARα agonists where blood pressure was an outcome or measure. Fenofibrate, a PPARα agonist, reduced blood pressure in salt-sensitive, but not salt-resistant volunteers in a small study of 37 hypertensive subjects fed high salt.17 The beneficial effects of fenofibrate on lowering BP were amplified by the AT1R blocker candesartan in hypertensive patients with hypertriglyceridemia suggesting a potential link between PPARα and the renin-angiotensin system.18 The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study evaluated the effect of fenofibrate in 9,795 type-2 diabetes patients. There was no significant benefit from major coronary events, although there was significant reduction in total cardiovascular events when compared with placebo.19 Blood pressure was lowered and indices of renal function were improved in the Helsinki sub-study of FIELD where intensive 5-year fenofibrate treatment was studied.20
There are many more clinical studies and trials of PPARγ agonists that included BP as an outcome. Early studies in humans revealed that the TZDs, in addition to improving glycemic control and improving insulin sensitivity, also decreased blood pressure. Troglitazone decreased BP in nondiabetic obese subjects and outpatients with essential hypertension and mild diabetes.21,22 Similar modest BP lowering effects were observed with rosiglitazone and pioglitazone, findings replicated in many small clinical trials.23 In the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes (RECORD) trial, addition of rosiglitazone to metformin or a sulfonylurea reduced ambulatory BP to a greater level than when metformin and a sulfonylurea were combined without TZD.24 Similarly, pioglitazone reduced diastolic BP in 522 patients with type 2 diabetes mellitus (T2DM) with a high risk for stroke in the Primary Prevention of High Risk Type 2 Diabetes in Japan study (PROFIT-J).25 Finally, pioglitazone reduced the composite of all-cause mortality, non-fatal myocardial infarction, and stroke in 5238 patients with T2DM in the Prospective Pioglitazone Clinical Trial In Macrovascular Events (PROactive) trial.26 BP was modestly but significantly lowered in the pioglitazone group.
It is important to point out that this discussion is not designed to suggest that TZDs are effective antihypertensives or have major cardiovascular benefit. In fact, the use of some TZDs, particularly rosiglitazone, has markedly declined in recent years due to adverse effects, some severe, although some of these adverse effects may be drug specific.27 Instead, our goal was to highlight some of the evidence for the physiological and translational relevance of the PPARγ pathway. As illustrated below, there are tissue-specific benefits to PPARγ that merit further attention in the development of therapies that preserve its beneficial effects while minimizing detrimental effects.
Tissue-Specific Mechanisms of PPAR Action that Regulate Arterial BP
Early evidence for the importance of PPARs as regulators of BP was obtained from studies of PPAR agonists in animals. Activation of PPARα with clofibrate or fenofibrate was shown to lower blood pressure in a number of hypertensive models including the spontaneously hypertensive rat (SHR), and in hypertension models induced by DOCA-salt, Ang-II, and endothelin, among others.28–33 PPARγ activation by TZD administration lowered BP in a variety of rat models including Dahl salt-sensitive (Dahl-S), one-kidney, one-clip Sprague-Dawley rats, obese Zucker rats, diet-induced obese rats, and in hypertensive transgenic mice, hyperlipidemic rabbits, and spontaneously obese, insulin-resistant rhesus monkeys.34–40 Similar to the other isoforms, activation of PPARβδ reduced blood pressure in a number of animals models of hypertension (reviewed in 3). These observations have led many investigators to examine the tissue-specific mechanisms by which this occurs. Here we review evidence for the beneficial tissue-specific effects of the PPARs (with a focus on PPARγ) in the vasculature, brain, kidney, and immune system on blood pressure.
A. Vasculature
That PPARγ activators consistently lower BP in the face of volume expansion (see Kidney section) suggests that many of the protective effects of PPARγ are likely to be extra-renal. Indeed, there has been a long-standing hypothesis that many of the cardioprotective effects are mediated by PPARγ activity in vascular endothelium and smooth muscle. This has led to studies defining these protective mechanisms with the goal of identifying clinically therapeutic pathways that preserve the beneficial aspects of PPARγ activation while limiting adverse effects.
Early studies revealed that rosiglitazone prevented the development of hypertension and partially protected endothelium-dependent vasodilation in the mesenteric arteries in insulin-resistant Zucker fatty rats.40 Similar effects were observed in models of hypertension that occur independent of hyperlipidemia and hyperglycemia. For example, both rosiglitazone and pioglitazone attenuated hypertension induced by Ang-II in male Sprague Dawley rats.41 Rosiglitazone blunted hypertension and improved vascular remodeling and endothelium-dependent vasodilation in deoxycorticosterone acetate (DOCA)-salt treated rats and had similar effects in hypertensive transgenic mice expressing the human renin and angiotensinogen genes.33,37 Of course, the beneficial effects on the vasculature could be mediated by decreases in arterial pressure. Thus, more direct early evidence for a role of vascular PPARγ came from studies showing it was expressed in both endothelium and vascular smooth muscle and regulated the expression of vasoactive agents such as endothelin and Ang-II and pro- and antioxidant genes such as copper-zinc superoxide dismutase and subunits of the nicotinamide adenine dinucleotide phosphate oxidase enzyme.42–45
It is now generally accepted that PPARγ in the endothelium is required to maintain nitric oxide (NO) bioavailability and restrain oxidative stress (reviewed in 46). This is based on studies of endothelial-specific PPARγ-deficient mice and mice selectively expressing hypertension-causing dominant negative PPARγ mutation (V290M) in the endothelium (so called E-V290M mice). In endothelial-specific PPARγ-deficient mice, NO production was significantly decreased in aorta while serum reactive oxygen metabolites were significantly elevated.47 Concomitantly, these mice exhibited an impaired endothelium-dependent vasodilation response and an augmented pressor response to Ang-II. Endothelial-specific PPARγ-deficient mice were also more sensitive to high-fat diet induced hypertension and exhibited accelerated vascular aging.48,49 E-V290M mice displayed a modest increase in BP at baseline, an augmented pressor response to Ang-II, and impaired endothelial-dependent vasodilation response to acetylcholine and Ang-(1–7).50,51 The endothelial dysfunction was reversed by antioxidants. Oxidative stress-mediated endothelial dysfunction was also observed in the aorta of E-V290M mice treated with IL-1β, in the cerebral artery of E-V290M mice challenged with a low-salt diet to induce the endogenous renin-angiotensin system, and in E-V290M mice treated with either a sub-pressor dose of Ang-II or a high-fat diet.50,52–54 Consistent with this, gene expression profiling revealed altered expression of both pro-oxidant and anti-oxidant genes in response to dominant negative PPARγ.8
We identified the retinol binding protein 7 (RBP7, previously known as CRBP-III) gene as an endothelial-specific PPARγ target gene.8,55 RBP7-deficient mice exhibited impaired vasodilation in cerebral vessels that was normalized by antioxidant treatment suggesting its protective role against oxidative stress.56 In fact, RBP7-deficient mice exhibited the same impaired endothelial function phenotype as E-V290M mice suggesting that RBP7 may mediate the anti-oxidant effects of PPARγ. Further studies revealed that another PPARγ target gene, adiponectin, was required to mediate the antioxidant effects of RBP7. Recombinant adiponectin reduced oxidative stress and restored normal endothelial function in RBP7-deficient mice treated with either high-fat diet or Ang-II.
It is notable that RBP7 belongs to the family of fatty acid-binding proteins.57 Other members of this family act as co-factors for other nuclear receptors and have been shown to shuttle ligands through the cytoplasm or nucleus to nuclear receptor transcription factors.58 Based on its similarity with other members of this family, we hypothesized a mechanism where PPARγ stimulates expression of RBP7, which then acts as a vehicle to deliver a naturally occurring endogenous ligand to PPARγ in the nucleus where it further promotes expression of RBP7 and adiponectin (reviewed in 59). We termed this the PPARγ:RBP7 regulatory hub (Figure 3). Indeed, RBP7-deficiency prevented the induction of several PPARγ target genes by rosiglitazone.56
Figure 3: Model Illustrating the PPARγ/RBP7 Transcriptional Hub.
PPARγ induces expression of RBP7, an endothelium-specific PPARγ target gene. RBP7 facilitates transcriptional activation of RBP7-dependent PPARγ target genes including adiponectin. Expression of these genes is impaired in the absence of RBP7. Expression of adiponectin mediates an antioxidant response when the mice are challenged with either high-fat diet or Ang-II. Impairment of this protective response under conditions where either RBP7 or PPARγ is impaired causes oxidative stress and endothelial dysfunction in the presence of cardiovascular stressors. Adapted from 56.
Mice with vascular smooth muscle specific PPARγ-deficiency exhibited elevated vasoconstriction, vascular remodeling, oxidative stress, and inflammation upon Ang-II infusion, whereas the vasodilation response to acetylcholine was significantly reduced.60 Interestingly, smooth muscle PPARγ was reported to protect vessels from Ang-II-induced abdominal aortic aneurysms.61 It is notable that unlike vascular smooth muscle specific PPARγ-deficiency, the phenotypes of smooth muscle specific expression of a different dominant negative PPARγ mutation (P467L, in S-P467L mice) appeared independent of oxidative stress.
S-P467L mice exhibited elevated BP at baseline, accompanied by arterial stiffness, enhanced vasoconstriction, and severely impaired vasodilation, and develop augmented hypertension when fed a high salt diet.62,63 They also exhibited increased formation and rupture of cerebral aneurysms when challenged with Ang-II.64 Importantly, interference with smooth muscle PPARγ not only impaired endothelium-dependent vasodilation to agonists such as acetylcholine and Ang-(1–7), but also endothelium-independent agonists such as sodium nitroprusside, suggesting a state of NO-resistance.65,66 Concomitant with a loss of vasodilatory tone, was a gain in vasoconstrictor tone. Vessels from S-P467L mice exhibited marked elevated contraction to endothelin, Ang-II, and serotonin, among others.62,66 Similarly, myogenic tone in both mesenteric and cerebral vessels was markedly augmented.67,68 Mechanistic studies revealed the augmented myogenic tone was mediated by a loss of Regulator of G Protein Signaling 5 (RGS5), a PPARγ (and PPARβδ) target gene. RGS5-deficiency exacerbated hypertension and vasoconstriction in mice subjected to Ang-II infusion.69 Smooth muscle PPARγ also restrains vascular remodeling by regulating expression of Tissue Inhibitor of Metalloproteinases 4 (TIMP4).70 This is consistent with studies reporting that PPARγ agonists prevent cell-cycle progression and telomerase expression.71,72
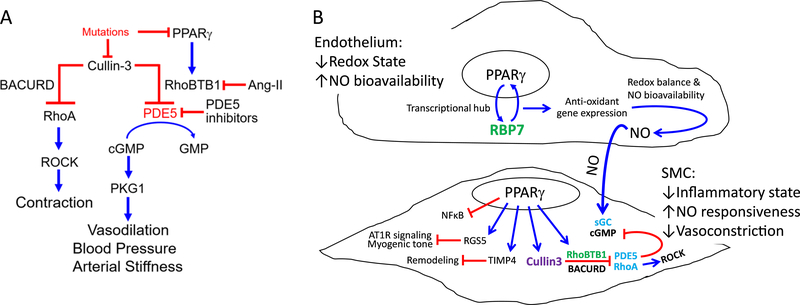
RhoA and Rho Kinase are well-known to mediate many vasoconstrictor signals. The ROCK inhibitor Y27632 blocked the hypercontractile phenotype of vessels from S-P467L.66 Notably, the level of RhoA protein but not mRNA was increased in the aorta from S-P467L leading us to hypothesize there was a defect in the RhoA turnover. Indeed, Cullin-3 E3 RING ubiquitin ligase mediates the ubiquitination and degradation of RhoA, and the levels of Cullin-3 protein was significantly decreased in aorta from S-P467L.66,73 Further studies revealed that Cullin-3 plays a critical role as a downstream mediator of the protective effects of PPARγ in vascular smooth muscle.74–78 Mutations in Cullin-3 cause a Mendelian form of hypertension.79
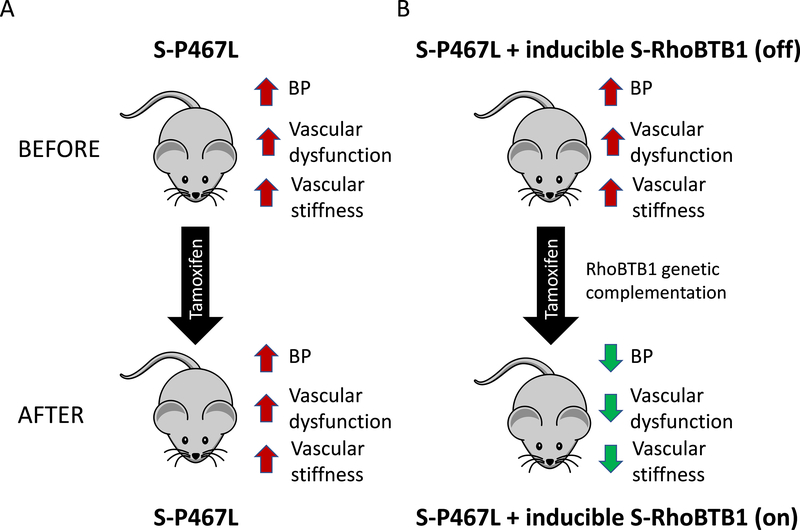
In terms of PPARγ-dependent mechanisms regulating vasodilation, we identified Rho Related BTB Domain Containing 1 (RhoBTB1) as a novel PPARγ target gene.66 Genetic complementation (e.g. restoration) of RhoBTB1 in smooth muscle cells of S-P467L mice normalized blood pressure, arterial stiffness, and impaired vasodilation response to both acetylcholine and sodium nitroprusside (Figure 4).80 Surprisingly, restoration of RhoBTB1 did not reduce the enhanced vasoconstriction, indicating that its protective effect is mainly mediated by promoting the vasodilation pathway. That the improvement in arterial stiffness occurred in the face of preserved vasoconstriction is a remarkable finding that requires further study. Experiments revealed that RhoBTB1 controlled the level of cGMP in smooth muscle cells via phosphodiesterase 5 (PDE5). RhoBTB1 and PDE5 directly interact and RhoBTB1 functions as a substrate adaptor for PDE5 to the Cullin-3-RING dependent E3 ubiquitin ligase to restrain PDE5 activity. Thus, PPARγ resides upstream of two Cullin-3 pathways which control vasoconstriction through RhoA and vasodilation through PDE5 (Figure 5A).
Figure 4. RhoBTB1 Regulates BP and Arterial Stiffness.
RhoBTB1 is a PPARγ target gene in vascular smooth muscle. Its expression is decreased in S-P467L mice due to the action of the dominant negative mutation in PPARγ. A) S-P467L mice exhibited hypertension, vascular dysfunction and arterial stiffness which was not improved by tamoxifen. B) We generated a transgene which inducibly re-activates RhoBTB1 in response to Cre-recombinase. In the absence of Tamoxifen (Top), RhoBTB1 remains silent and the mice exhibit the same phenotype as S-P467L. In response to Tamoxifen, the transgene is induced and RhoBTB1 expression is restored to normal levels. This resulted in decreased BP, improved vascular function and a regression of arterial stiffness back to normal within 2 weeks of treatment. Data were previously reported in 80.
Figure 5. PPARγ-dependent Pathways in the Vasculature.
A) Mutations in either Cullin-3 or PPARγ cause hypertension. Cullin-3 regulates both vasoconstriction and vasodilation pathways: Cullin-3 suppresses the RhoA/ROCK pathway via adaptor protein BACURD; Cullin-3 targets PDE5, a negative regulator of the NO/cGMP pathway, using a PPARγ target gene, RhoBTB1, as the adaptor. RhoBTB1 regulates the activity of PDE5 by ensuring that excess PDE5 is targeted for Cullin-3 dependent ubiquitination and proteasomal degradation. Mutations in PPARγ or treatment with Ang-II causes RhoBTB1-deficiency. Experimental data suggest that arterial stiffness correlates better with the state of vasodilation rather than contraction. Previously reported in 80. B) Precise BP regulation requires coordination between vasodilator and vasoconstrictor signals in the endothelium and smooth muscle (SMC). Endothelium-derived nitric oxide (NO) is among the key signals which instruct the SMC to dilate or contract. The NO pathway is coordinately regulated through transcriptional and post-translational pathways initiated by PPARγ. Our data support the concepts that PPARγ: 1) acts as a sensor in the endothelium to regulate redox state, and through this, bioavailability of NO, and 2) regulates the responsiveness of SMC to NO by independently controlling a RhoA/Rho kinase (ROCK) activity that promotes constriction, and the production and stability of cyclic GMP (cGMP), a critical mediator of vasodilation.
Appropriate regulation of arterial BP requires coordination between vasodilator and vasoconstrictor signals in the endothelium and smooth muscle (Figure 5B). Evidence suggests that the NO pathway is coordinately regulated through transcriptional and post-translational pathways initiated by PPARγ. PPARγ acts as a sensor in the endothelium to regulate redox state, and through this, bioavailability of NO. It also regulates the responsiveness of smooth muscle to NO by independently controlling a RhoA/Rho kinase activity that promotes constriction, and production and stability of cyclic GMP (cGMP), a critical mediator of vasodilation. The range of PPARγ-dependent molecular mechanisms in both cell types is surprisingly complex; requiring novel transcriptional co-factors (e.g. RBP7) which form a transcriptional regulatory hub with PPARγ, and post-translational regulation of critical SMC mediators (RhoA and PDE5) by Cullin-3 E3 ubiquitin ligase-mediated protein turnover (Figure 5B). Importantly, this PPARγ initiated “final common pathway” has profound effects on vasomotor function, BP and vascular stiffness, and the studies proposed below have potential implications for the treatment of these disorders.
PPARα and PPARβδ are also expressed in endothelial and vascular smooth muscle cells. PPARα agonists induce expression of eNOS in endothelium which increases NO bioavailability and promotes vasodilation.81 PPARα can also suppress agonist-induced ET-1 secretion from endothelial cells which could blunt vasoconstriction.82
The PPARβδ agonist GW501516 normalized the impairment in vasodilation caused by palmitic acid by activating the antioxidant gene dihydrofolate reductase.83 Similarly, PPARβδ agonist improves the vasodilation response in high glucose fed mice and db/db mice.84 PPARβδ activation abolishes NADPH oxidase-dependent vascular oxidative stress, normalizes endothelial dependent vasodilation, and lowers blood pressure in NZBWF1 lupus mice.85 The beneficial anti-oxidant and pro-vasodilatory effects of PPARβδ activation were also observed in spontaneous hypertensive rats.86 Moreover, the protective effects of PPARβδ in endothelial cells were reported to be mediated by Nrf2 and endoplasmic reticulum stress.87,88 Other beneficial effects such as exercise also improve the vasodilation response in a PPARβδ-dependent manner.89
B. Brain
The autonomic nervous system plays a fundamental role in the maintenance of cardiovascular function. Its importance in both the short-term and long-term regulation of BP via the baroreflex is accepted.90,91 Increased activity of the sympathetic nervous system is associated with elevated BP in animal models of hypertension and hypertensive obese patients.92–94 Moreover, dysfunctional autonomic control is particularly important in the genesis of obesity-induced hypertension and salt-sensitive hypertension.93,95 In the brain, PPARγ is expressed in both neuronal and non-neuronal cells, is distributed in several anatomical structures regulating BP, and is involved in brain development.96,97
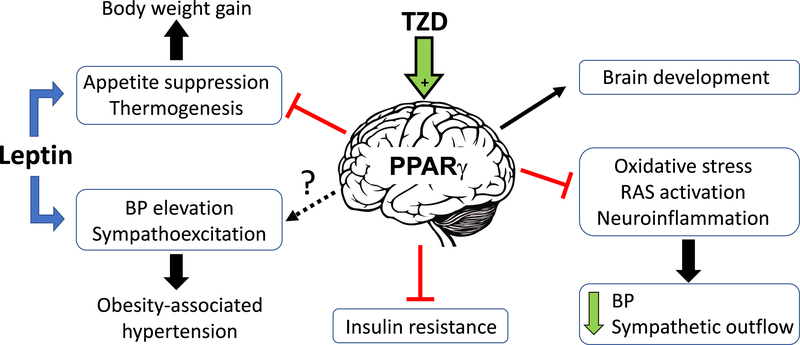
Studies on brain PPARγ were initially designed to understand the mechanisms causing some of the undesirable effects of TZDs such as weight gain.98 Studies in neuron-specific PPARγ knockout mice revealed that the metabolic effects of TZDs, including insulin-sensitizing actions and weight gain, are attributable to their actions in the central nervous system. Many phenotypic characteristics of neuron-specific PPARγ knockout mice were recapitulated in transgenic mice that conditionally expressed the hypertension-causing dominant-negative PPARγ mutant (P467L) in neurons.97 A summary of PPARγ effects in the brain is illustrated in Figure 6.
Figure 6: Involvement of PPARγ in Neural Mechanisms of Metabolic and Cardiovascular Control.
Activation of PPARγ by TZD decreases BP and sympathetic outflow in part by attenuating oxidative stress, brain renin-angiotensin system (RAS) activation, and suppressing neuroinflammation in specific cardiovascular centers of the brain. The metabolic effects of TZDs (insulin-sensitization and obesogenic side-effects) were attributed to a direct effect of PPARγ ligands in the pro-opiomelanocortin neurons located in the arcuate nucleus of the hypothalamus. PPARγ activation can blunt the thermogenic and anorexigenic effects of leptin in the central nervous system. However, whether PPARγ can also attenuate leptin-induced sympathoexcitation and pressor responses, a pathophysiological mechanism that has been linked to obesity-associated hypertension, is still unknown.
Leptin acts on receptors located in the arcuate nucleus of the hypothalamus where it influences both metabolic and cardiovascular function.99 Intracerebroventricular infusion of PPARγ antagonist, GW9662, or genetic ablation of neuronal PPARγ enhances leptin elicited appetite suppression and body weight loss in diet-induced obesity.98,100 These data implicate a mechanistic link between PPARγ and leptin and suggest that central PPARγ may play a role in leptin resistance. Leptin resistance may play an important role in some forms of hypertension.101 Activation of brain PPARγ diminishes the firing rate of anorexigenic pro-opiomelanocortin (POMC) neurons while promoting the activity of obesogenic agouti-related peptide (AgRP) neurons.102 Ablation of PPARγ in POMC neurons augments the anorexic effects of leptin when mice are exposed to a high-fat diet, an effect that was surprisingly not observed in mice conditionally expressing dominant-negative PPARγ (P467L) in POMC neurons.103,104
In addition to its metabolic effects, leptin can also induce renal sympathetic nerve activation and BP elevation.105 In healthy individuals, leptin exerts protective effects by suppressing appetite and promoting thermogenesis. In contrast, in obese subjects, despite increased circulating leptin, the beneficial metabolic effects of leptin are impaired, whereas leptin-induced sympathoexcitation and increased BP is preserved.106 Given the importance of the selective resistance to leptin in the genesis of obesity-induced hypertension, it will be important to assess whether PPARγ in POMC neurons also regulates the cardiovascular actions of leptin.101
Studies in prediabetic rats, SHR, and patients with acute myocardial infarction and T2DM revealed that the activation of PPARγ can mitigate autonomic dysfunction in certain pathological conditions.107–109 It has been hypothesized that the anti-hypertensive effects TZDs might be attributable to their effects on the central nervous system because TZDs can cross the brain-blood barrier, and models of hypertension exhibit lower levels of PPARγ in the brain.110,111 Supporting this hypothesis, intracerebroventricular infusion of pioglitazone prevents Ang-II-induced hypertension and attenuates neuroinflammation in the subfornical organ and the paraventricular nucleus.112 Other studies support a mechanistic link between PPARγ and the renin-angiotensin system in the brain. Intracerebroventricular infusion of pioglitazone attenuated sympathoexcitation and a concomitant decrease in hypothalamic expression of AT1R in rats subjected to coronary artery ligation.113 Another interaction between AT1R signaling and PPARγ has been identified in the brainstem. Superoxide production elicited by the activation of the renin-angiotensin system in the rostral ventrolateral medulla, a key brainstem nucleus controlling vasomotor sympathetic nerve activity, induces sympathoexcitation and BP elevation, an effect abrogated by coadministration of rosiglitazone.114–116 The upregulation of mitochondrial uncoupling protein 2 (UCP2) represents a protective mechanism against oxidative stress in the rostral ventrolateral medulla (RVLM). Ang-II induces UCP2 via p38 MAPK-mediated phosphorylation of PPARγ coactivator 1α (PGC-1α) and promotes the formation of PCG-1α/PPARγ complexes.116 Notably, oral rosiglitazone is sufficient to induce UCP2 in the RVLM, and the anti-hypertensive/antioxidant effects of oral rosiglitazone can be partially abrogated when UCP2 is ablated in the RVLM.108 This PPARγ-mediated UCP2-dependent antioxidant mechanism is not unique to neurons but is also active in cerebral vasculature.117 Together, these studies support a protective role of PPARγ against central Ang-II signaling, oxidative stress, and sympathoexcitation in experimental hypertension.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key regulator of redox hemostasis in the RVLM and the targeted ablation of Nrf2 in the RVLM leads to hypertension.118 PPARγ can induce expression of Nrf2 at the transcriptional level. However, cellular protein levels of Nrf2 are tightly regulated by the Kelch-like-ECH-associated-protein 1 (Keap)/Cullin-3 ubiquitin E3 ligase complex.119 Since mice expressing dominant negative PPARγ exhibited impaired Cullin-3 activity we hypothesize PPARγ might restrain Nrf2 activity in the RVLM by promoting its ubiquitination and proteasomal degradation.66 Thus, whether Nrf2 plays a role as an alternative mechanism mediating the antioxidant effects of PPARγ agonists in the brainstem requires additional studies.
Evidence has been reported that some of the beneficial BP effects of PPARα activation may be mediated by improving baroreflex afferent function via PPARα-mediated transactivation of uncoupling protein 2 and reduction of oxidative stress in several regions of the brain regulating BP.120 In Ang-II hypertension, GW0742, a PPARβδ agonist decreased BP and restored sympathetic tone, through a mechanism involving decreased Ang-II AT1R signaling as a result of up-regulation of the PPARβδ target gene RGS5.121
C. Kidney
There are several beneficial effects elicited by PPARγ activation within the kidney. For example, PPARγ activation improves glomerular endothelial NO bioavailability through increased eNOS expression and activity and Nrf2-dependent antioxidant responses.122 In human glomerular endothelial cells, knockdown of PPARγ reduces Nrf2 expression and Nrf2-dependent upregulation of eNOS expression, phosphorylation and activity.122 Interestingly, PPARγ itself is a Nrf2 target gene. Activated Nrf2 binds to the antioxidant response element in the PPARγ promotor region to induce its transcription, an effect abolished by Nrf2 knockdown. Thus, PPARγ-Nrf2 reciprocal interaction supports an antioxidant defense required to maintain renal NO bioavailability in response to pro-oxidant stimuli such as excess salt intake. Consistent with this, mice selectively expressing the human hypertension-causing mutation P467L in vascular smooth muscle (S-P467L) exhibit severely impaired vasodilation in renal vessels, renal NO deficiency, and augmented hypertension responses to high salt diet.63 The renal NO deficiency in HSD-fed S-P467L mice is associated with blunted renal blood flow, elevated Na+-K+−2Cl− cotransporter (NKCC2) protein, and increased sodium retention compared to littermate controls. The impaired natriuresis/diuresis and salt-induced increase in BP are corrected by pharmacological inhibition of NKCC2. These observations suggest that the antioxidant effects of PPARγ in the renal vasculature may contribute to the overall cardiovascular protection mediated by global PPARγ activation.
One of the adverse effects of global PPARγ activation by TZDs is systemic fluid retention, edema, and weight gain. Because of this and other potential adverse effects, TZDs were withdrawn as frontline medications for T2DM, especially in those complicated with congestive heart failure.123 TZD-induced fluid retention could be recapitulated in rodents, and could be reversed by treatment with the collecting duct-targeted diuretic amiloride implicating a role of the epithelial sodium channel (ENaC).124,125 PPARγ is most abundantly expressed in the collecting duct among nephron segments; and in the collecting duct, Scnn1g which encodes ENaCγ is thought to be a PPARγ-target gene (Figure 7).124 Consistent with this, rosiglitazone-induced increases in ENaCγ mRNA expression, sodium reabsorption and plasma volume were abolished by collecting duct-specific ablation of PPARγ in mice.124,125 These studies provided evidence supporting a role of ENaC in the distal nephron in mediating PPARγ-dependent fluid retention.
Figure 7: Mechanism of PPARγ-mediated Salt/Fluid Reabsorption in Kidney.
In the renal collecting duct, TZDs induce PPARγ-dependent expression of Scnn1g encoding the γ subunit of the epithelial sodium channel (ENaCγ). This promotes sodium reabsorption in the distal nephron, which is inhibitable by ENaC-targeted inhibitors such as Amiloride. This was initially thought to be a primary mechanism for the adverse fluid retention, edema and weight gain associated with TZDs. However, further studies have found conflicting results including the expression of a non-selective cation channel (23ps-C) in response to TZDs which provided an alternative explanation for the sodium and fluid retention after functional ENaC knockdown. The molecular identity of this non-selective cation channel remains unknown. The diagram also illustrates key molecules in the collecting duct, including Na+-K+-ATPase on the basolateral membrane and the renal outer medullary potassium channel (ROMK) on the apical membrane. (Illustration credit: Ben Smith).
Another study revealed that conditional deletion of Scnn1a which encodes ENaCα failed to prevent rosiglitazone-induced weight gain, fluid retention, and plasma volume expansion despite functional knockdown of ENaC-mediated Li+/Na+ uptake in the collecting duct.126 In the littermate controls, rosiglitazone induced weight gain and fluid retention in the absence of any change in ENaC α, β, or γ protein. Rosiglitazone and pioglitazone failed to alter the density of membrane bound ENaC channels or its Na+ conductance in patch clamp studies of primary cortical collecting duct. Instead, a 23-pS nonselective cation channel, which is biophysically distinct from the highly Na+-selective ENaC (approximately 6–8 pS), was identified in inner medullary collecting duct, providing a possible explanation for TZD-induced sodium and fluid retention after functional ENaC knockdown (Figure 7). Moreover, increased expression of NKCC2, sodium hydrogen exchanger (NHE3), and aquaporin 2/3 have been reported in the whole kidney of rats treated with rosiglitazone, questioning a primary role of ENaC in TZD-induced sodium and fluid retention.127 Further, TZDs have been shown to suppress ENaC mRNA and protein in kidney cortex or collecting duct cell cultures.128 Thus, although renal sodium retention and fluid expansion have been well documented as adverse effects associated with TZD use, the underlying molecular mechanisms remain controversial.
PPARα activation by clofibrate lowers BP and sodium retention through renal cytochrome P4504A (CYP4A) / 20-Hydroxyeicosatetraenoic acid (20-HETE) pathway in DOCA-salt hypertension, an effect abolished by PPARα deficiency.29,129 Similarly, the BP lowering effect of clofibrate requires renal induction of CYP4A/2C and 20-HETE-mediated sodium excretion in salt-induced hypertensive Sprague-Dawley rats.130 Clofibrate decreased BP, glomerular filtration rate and cumulative sodium balance which was correlated with increased CYP4A expression and decreased NHE3 in the proximal tubule in high fat diet fed rats.131 PPARα agonists prevent, but do not reverse salt-sensitive hypertension in Dahl SS rats.132
PPARβδ is ubiquitously expressed in all nephron segments. PPARβδ agonists protect the kidney from acute ischemic/reperfusion injury and systemic lupus erythematosus (SLE) associated albuminuria and chronic renal damage.85,133 In renal microvessels, PPARβδ mediates prostacyclin-induced vasodilation which is essential for the maintenance of renal blood flow.134
D. Immune System
The involvement of the immune system in the development of hypertension was initially proposed in 1967 when it was observed that transferring lymphoid cells from hypertensive rats to normotensive recipients increased blood pressure.135 Later it was demonstrated that the lack of lymphocytes in mice caused an attenuated response to Ang-II, but their responses to Ang-II were restored when T cells were adoptively transferred to these mice.136 Similarly, depletion of monocytes from the innate arm of the immune system dampens the responses to Ang-II.137
PPARγ is expressed in almost every immune cell type and plays multiples roles in the regulation of the immune function (reviewed in 138). Generally, PPARγ is considered anti-inflammatory since PPARγ activators have been shown to suppress the recruitment of immune cells in the vasculature, kidney, and heart.139,140 As discussed above, the anti-inflammatory actions of PPARγ are reported to be mediated by trans-repression, and termination of NF-кB signaling by facilitating either p65 turnover or nuclear export.9–11 Some natural and synthetic ligands of PPARγ can suppress cytokine release even in macrophages lacking PPARγ indicating that part of their anti-inflammatory actions may be PPARγ-independent.141 A schematic showing the effects of PPARγ in the immune system is shown in Figure 8.
Figure 8: PPARγ Modulation of the Innate and Adaptive Immunity.
PPARγ suppresses the innate immune response by preventing the activation of the master inflammatory transcription factor NF-κB via a trans-repression mechanism resulting in a downregulation of proinflammatory cytokines interleukin (IL)-1β and IL-6, inducible nitric oxide synthase (iNOS), and chemokine receptor (CCR) 2. PPARγ is also involved in the differentiation of macrophages towards the anti-inflammatory M2 macrophages expressing IL-10 and arginase 1. Formation of neoantigens (Ag) such as isoketals are captured, processed and presented by the immunogenic dendritic cells (DC) to T cells resulting in the differentiation of naïve T cells to effector T cells. Among the wide variety of T helper cell subsets interferon (IFN)-γ expressing TH1 and IL-17 expressing TH17 are key players in the development of autoimmune disease and hypertension. PPARγ suppresses the adaptive immune response by preventing TH17 and B cells, but also by inducing regulatory T cells (Tregs), presumably via induction of tolerogenic DC. (Illustration credit: Ben Smith).
Studies using mice lacking PPARγ in macrophages and the myeloid lineage confirmed the immunoregulatory role of PPARγ.139 Macrophage-specific PPARγ knockout mice exhibited larger vascular lesions, higher macrophage uptake of oxidized LDL and elevated immune cell infiltration in models of atherosclerosis. Myeloid-specific ablation of PPARγ also results in aggravated cardiac injury in myocardial infarction induced by coronary artery ligation.142 Although PPARγ appears to elicit protection from vascular, cardiac and renal injury in several animal models, studies attempting to elucidate whether PPARγ in immune cells provides protection in models of hypertension are still scarce. PPARγ modulates the innate immune response by suppressing the expression of pro-inflammatory cytokines (interleukin (IL)-1β and IL-6), chemokine receptor 2, inducible nitric oxide synthase among other mediators.142–145 Notably, the blockade of IL-1β with neutralizing antibodies blunts the inflammatory response in myeloid-PPARγ-deficient mice subjected to myocardial infarction.142 In addition, PPARγ is involved in the polarization of macrophages to the alternative M2 phenotype, which are considered anti-inflammatory.146 We observed that endothelium-specific overexpression of wildtype PPARγ or treatment with rosiglitazone ameliorated endothelial dysfunction in response to IL-1β, whereas endothelium-specific overexpression of dominant negative PPARγ (E-V290M) aggravated it.52 This indicates that PPARγ might modulate the immune response both by suppressing NF-кB in immune cells and by reducing oxidative stress.
Synthetic PPARγ agonists have shown promising results in several immune diseases such as SLE, psoriasis, arthritis, and autoimmune encephalomyelitis among others.147–150 TZDs can attenuate the production of autoreactive antibodies and ameliorate vascular and kidney injury in a mouse model resembling human SLE. Some of these protective effects were attributed to adiponectin, a PPARγ target gene, and the induction of tolerogenic dendritic cells.151,152 In female NZBWF1 mice, a model of SLE hypertension, rosiglitazone reduced albuminuria, renal inflammatory macrophage infiltration and glomerulosclerosis, and also blunted high blood pressure.153 Similarly, rosiglitazone ameliorated salt sensitivity of BP in ovariectomized female Dahl salt-sensitive rats and this was associated with reduced renal resident macrophages and inflammation.154
In addition to its role in innate immunity, PPARγ also protects from aberrant adaptive immune responses. PPARγ suppresses effector T cell activation and stimulates the differentiation and migration of regulatory T cells, which are known to protect from hypertension and vascular injury elicited by Ang-II.155–157 In addition, activation of PPARγ in T cells suppresses autoimmunity and prevents the differentiation of naïve T cells to the interleukin 17-producing T helper 17 cells by controlling retinoic acid receptor-related orphan receptor γδ.158 Together, these studies suggest that suppression of both innate and adaptive immune responses may contribute to the overall anti-hypertensive effects of global PPARγ activation. However, whether the selective modulation of PPARγ in different subset of T cells is sufficient to attenuate hypertension and end organ damage remains unclear.
PPARα is the dominant PPAR isoform in T and B lymphocytes.159 In aged animals, PPARα-deficiency leads to a proinflammatory phenotype in immune cells evidenced by elevated NF-kB activity and inflammatory cytokine production.160 Treatment of T cells with PPARα agonist induces repression of NF-kB resulting in attenuated secretion of pro-atherogenic cytokines including IFN-γ, TNF-α, and IL-2, and activation of other transcription factors, although some of the actions of fibrates might be PPARα independent.159,161,162
PPARβδ agonist GW0742 decreases plasma levels of pro-inflammatory cytokines and autoantibodies against double-stranded DNA, alleviates splenomegaly and reduces lupus disease activity in a mouse model of SLE.85 These anti-inflammatory actions may contribute to the anti-hypertensive effects of PPARβδ activation.
Hypertension in Women: Evidence for a Role for PPAR in Preeclampsia
Preeclampsia is a pregnancy-specific disorder of hypertension and end-organ dysfunction that poses serious clinical problems for women and their offspring that extend beyond the peripartum period. Hypertensive disorders of pregnancy, including preeclampsia, are one of the leading causes of maternal mortality and complicates up to 8% of all pregnancies.163 The effects of preeclampsia appear to be long lasting for both the mother and offspring. Women with a history of preeclampsia have an increased risk of cardiovascular disease, cerebrovascular events and cardiovascular mortality.163 Infants born to mothers with preeclampsia also have lasting complications, not only those related to placental dysfunction, which include preterm birth, prematurity, and growth restriction, but also lifelong consequences unrelated to these antepartum and intrapartum events.
A. Placental Development
Abnormal placentation is at the core of preeclampsia. Both PPARγ and PPARβδ deficiency in mice are lethal providing evidence that they play an important regulatory role during development.4,164 PPARγ-deficient mice exhibit numerous placental defects including malformed labyrinth zone, lack of infiltration of fetal blood vessels, and rupture of maternal blood sinuses, while PPARβδ-deficient placentas exhibited defects in the placental-decidua interface.164
All three PPAR isoforms are expressed in the amnion, decidua, and villous placenta, but PPARγ remains the most robustly studied isoform in placenta.165 In early placentation, PPARγ inhibits extravillous cytotrophoblast invasion, promotes trophoblast differentiation and may control expression of human chorionic gonadotrophin.166–168 Trophoblast differentiation is important as the cell characteristics change. For one, the syncytiotrophoblasts are a multinucleated layer that lines the placental villi. Interestingly, syncytiotrophoblasts are more resistant than cytotrophoblasts to hypoxic injury, so PPARγ-mediated differentiation may make the cells more resistant to hypoxia-induced apoptosis.169 When human cytotrophoblasts differentiate into syncytiotrophoblasts, they accumulate lipid droplets. PPARγ plays a role in this as PPARγ-deficient placentas lack lipid droplets.4,168 Studies in a cell line carrying PPAR response element-luciferase reporter demonstrated that sera from pregnant women are capable of activating PPARγ.170 This suggests that PPARγ activating factors (presumable ligands) are present throughout pregnancy. It was reported that serum from preeclamptic women had reduced levels of PPARα and PPARγ ligands even prior to the onset of clinical features of the disease suggesting that downregulation of PPAR signaling might participate in the pathogenesis of preeclampsia.171,172 This is consistent with plasma from preeclamptic patients being capable of stimulating an inflammatory response in monocyte culture resulting in decreased levels of PPARγ and increased IL-1a, IL-6, and TNF-α.173
B. Genetics
Perhaps one of the most convincing links of PPARγ and preeclampsia comes from the description of human patients with known dominant negative PPARγ mutations. One of the female probands had two pregnancies which were both affected by preeclampsia.12 On the contrary, there was no association between PPARγ polymorphism and the occurrence of preeclampsia in a Finnish cohort.174 Several other reports have been published with some conflicting results. There was no difference in PPARγ protein expression between normal and preeclamptic pregnancies and those with growth restricted fetuses.175 But, in a subsequent study, preeclampsia with co-existing growth restriction of the fetus demonstrated increased PPARγ expression and DNA binding activity.176 Decreased PPARγ expression, independent of hypoxia-inducible factor and histone deacetylases, was found in hypoxia, a common finding in preeclamptic placenta.177
C. Animal Models
In a rat model of preeclampsia caused by daily treatment with an inhibitor of nitric oxide synthase, vascular endothelial growth factor (VEGF) gene expression was lower in the preeclampsia cohort, whereas omega 3 fatty acids, potent activators of PPARγ, improved levels of VEGF as well as PPARγ expression in late-onset preeclampsia.178 Similarly, rats treated with PPARγ inhibitors in the second half of pregnancy exhibited a decrease in fetal weights and impaired vasodilation of uterine arteries in response to agonists.179 Chronic administration of a PPARγ antagonist to pregnant rats resulted in elevated blood pressure, proteinuria, endothelial dysfunction, reduced pup weight, increased platelet aggregation and reduced plasma VEGF.180 Defect in endothelial PPARγ in offspring from vasopressin-exposed mice exhibiting a preeclampsia-like pregnancy infers an increased risk of endothelial dysfunction when exposed to a cardiovascular stressor in that offspring’s adulthood.181 As indicated above RGS5 is a PPARγ and PPARβδ target gene. Studies in pregnant mice revealed that PPARβδ and PPARγ agonist treatments blunted the hypertensive response to Ang-II infusion. That the protective effects of these agonists were absent in homozygous RGS5-deficient mice suggests the RGS5 is required to mediate these responses.182
Conclusion and Perspectives
Herein, we provided substantial evidence from genetic, clinical and mechanistic studies that PPARs, particularly PPARγ are critical to achieving and maintaining BP homeostasis. Impaired PPARγ function has serious implications for BP control, renal regulation, vascular function, immunity, and importantly, a healthy pregnancy. Despite this, PPARγ activators, particularly the TZD class of drugs, remain on the decline due to adverse effects. The drugs are particularly contraindicated for heart failure patients where edema could worsen the condition and be life threatening. Investigators have used a two-pronged approach to deal with this. First, new non-agonist PPARγ activators continue to be explored as drugs which could modulate PPARγ activity without fully activating it in all cell types. As an analogy, consider a stereo system, as the volume increases, the music eventually becomes noise. TZDs fully activate PPARγ and cause massive changes in gene expression throughout the body. Drugs which could modulate the PPARγ “rheostat” appropriately, might provide beneficial effects without the adverse effects.183 By way of example, SR1664 is a non-agonist PPARγ ligand which serves to prevent the phosphorylation of PPARγ (which inactivates PPARγ) and thus preserves its basal activity.184,185 SR1664 was reported to have potent antidiabetic actions but did not cause fluid retention or inhibit bone formation. It is unknown if it has the same beneficial effect on blood pressure as the TZDs. Moreover, the selective PPARγ modulators such as MRL24, INT-131, amorfrutins, and telmisartan (also an angiotensin receptor blocker), which act as partial PPARγ agonists with lower affinity, might also offer fewer adverse effects.186 Whether another PPARγ targeted drug will ever be approved for treatment remains unclear.
The second line of research is to identify PPARγ target genes with the goal of identifying new druggable targets. From our research alone, we identified RGS5, TIMP4, RBP7, RhoBTB1 as PPARγ targets, and through RhoBTB1, PDE5, each of which provides protection from hypertension. RhoBTB1 has remarkable effects on arterial stiffness. Future studies will need to be performed to assess if these can be targets of drugs to improve cardiovascular control and to reduce the risk of cardiovascular disease.
PPARα and PPARβδ have also emerged as potential regulators of BP, but the mechanisms governing their action are not as well defined. Whether some of the molecular mechanisms we defined for PPARγ are shared by other members of the family and whether the benefits of activating multiple PPAR isoform is additive would need to be investigated in the future.
Acknowledgments
Sources of funding
This work was supported through research grants from the National Institutes of Health (NIH) to CDS (HL084207, HL144807), PN (K01 HL153101), JW was supported by an AHA postdoctoral fellowship (17POST33660685) and K01 (DK126792). SF was supported by an AHA predoctoral fellowship (20PRE35120137).
Non-standard Abbreviations and Acronyms
- AgRP
agouti-related peptide
- Ang
angiotensin
- AT1R
Ang-II type-1 receptor
- BP
blood pressure
- Dahl-S
Dahl salt-sensitive
- DOCA
deoxycorticosterone acetate
- ENaC
epithelial sodium channel
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- FPLD3
familial partial lipodystrophy 3
- Keap
Kelch-like-ECH-associated-protein 1
- IFN
interferon
- IL
interleukin
- LBD
ligand binding domain
- NHE3
sodium hydrogen exchanger
- NKCC2
Na+-K+−2Cl− co-transporter
- NRE
Nuclear Receptor, Response Element
- NO
nitric oxide
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PDE5
phosphodiesterase 5
- PPAR
Peroxisome proliferator activated receptor
- PPRE
PPAR response element
- POMC
pro-opiomelanocortin
- PROactive
Prospective Pioglitazone Clinical Trial In Macrovascular Events
- PROFIT-J
Primary prevention of high-risk Type 2 diabetes in Japan
- RECORD
Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes
- RBP7
retinol binding protein 7
- RING
really interesting new gene
- RhoBTB1
Rho Related BTB Domain Containing 1
- RGS5
Regulator of G Protein Signaling 5
- RVLM
rostral ventral lateral medulla
- RXR
Retinoid X Receptor
- SHR
Spontaneously Hypertensive Rats
- SLE
systemic lupus erythematosus
- T2DM
Type 2 Diabetes Mellitus
- TIMP4
Tissue Inhibitor of Metalloproteinases-4
- TNF-α
Tumor necrosis factor-α
- TZD
thiazolidinediones
- UCP2
uncoupling protein 2
- 20-HETE
20-Hydroxyeicosatetraenoic acid
Footnotes
Disclosures
CDS is a member of a Scientific Advisory Board for Ionis Pharmaceuticals. His contributions to that board are unrelated to the content of this manuscript. There are no other conflicts of interest.
References
- 1.Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF and Schiffrin EL. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002;40:866–71. [DOI] [PubMed] [Google Scholar]
- 2.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M and Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. Faseb j. 2003;17:2299–301. [DOI] [PubMed] [Google Scholar]
- 3.Toral M, Romero M, Perez-Vizcaino F, Duarte J and Jimenez R. Antihypertensive effects of peroxisome proliferator-activated receptor-beta/delta activation. Am J Physiol Heart Circ Physiol. 2017;312:H189–H200. [DOI] [PubMed] [Google Scholar]
- 4.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A and Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. [DOI] [PubMed] [Google Scholar]
- 5.Schupp M and Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem. 2010;285:40409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM and Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270:12953–6. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Børgesen M, Francoijs KJ, Mandrup S and Stunnenberg HG. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keen HL, Halabi CM, Beyer AM, de Lange WJ, Liu X, Maeda N, Faraci FM, Casavant TL and Sigmund CD. Bioinformatic analysis of gene sets regulated by ligand-activated and dominant-negative peroxisome proliferator-activated receptor gamma in mouse aorta. Arterioscler Thromb Vasc Biol. 2010;30:518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG and Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou Y, Moreau F and Chadee K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat Commun. 2012;3:1300. [DOI] [PubMed] [Google Scholar]
- 11.Mukohda M, Lu KT, Guo DF, Wu J, Keen HL, Liu X, Ketsawatsomkron P, Stump M, Rahmouni K, Quelle FW and Sigmund CD. Hypertension-Causing Mutation in Peroxisome Proliferator-Activated Receptor γ Impairs Nuclear Export of Nuclear Factor-κB p65 in Vascular Smooth Muscle. Hypertension. 2017;70:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK and O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. [DOI] [PubMed] [Google Scholar]
- 13.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK and Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest. 2004;114:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auclair M, Vigouroux C, Boccara F, Capel E, Vigeral C, Guerci B, Lascols O, Capeau J and Caron-Debarle M. Peroxisome proliferator-activated receptor-γ mutations responsible for lipodystrophy with severe hypertension activate the cellular renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2013;33:829–38. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Zhang J, Li L, Wang Q and Feng L. Association between peroxisome proliferator-activated receptor γ−2 gene Pro12Ala polymorphisms and risk of hypertension: an updated meta-analysis. Biosci Rep. 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, Ng FL, Evangelou M, Witkowska K, Tzanis E, Hellwege JN, Giri A, Velez Edwards DR, Sun YV, Cho K, Gaziano JM, Wilson PWF, Tsao PS, Kovesdy CP, Esko T, Mägi R, Milani L, Almgren P, Boutin T, Debette S, Ding J, Giulianini F, Holliday EG, Jackson AU, Li-Gao R, Lin WY, Luan J, Mangino M, Oldmeadow C, Prins BP, Qian Y, Sargurupremraj M, Shah N, Surendran P, Thériault S, Verweij N, Willems SM, Zhao JH, Amouyel P, Connell J, de Mutsert R, Doney ASF, Farrall M, Menni C, Morris AD, Noordam R, Paré G, Poulter NR, Shields DC, Stanton A, Thom S, Abecasis G, Amin N, Arking DE, Ayers KL, Barbieri CM, Batini C, Bis JC, Blake T, Bochud M, Boehnke M, Boerwinkle E, Boomsma DI, Bottinger EP, Braund PS, Brumat M, Campbell A, Campbell H, Chakravarti A, Chambers JC, Chauhan G, Ciullo M, Cocca M, Collins F, Cordell HJ, Davies G, de Borst MH, de Geus EJ, Deary IJ, Deelen J, Del Greco MF, Demirkale CY, Dörr M, Ehret GB, Elosua R, Enroth S, Erzurumluoglu AM, Ferreira T, Frånberg M, Franco OH, Gandin I, Gasparini P, Giedraitis V, Gieger C, Girotto G, Goel A, Gow AJ, Gudnason V, Guo X, Gyllensten U, Hamsten A, Harris TB, Harris SE, Hartman CA, Havulinna AS, Hicks AA, Hofer E, Hofman A, Hottenga JJ, Huffman JE, Hwang SJ, Ingelsson E, James A, Jansen R, Jarvelin MR, Joehanes R, Johansson Å, Johnson AD, Joshi PK, Jousilahti P, Jukema JW, Jula A, Kähönen M, Kathiresan S, Keavney BD, Khaw KT, Knekt P, Knight J, Kolcic I, Kooner JS, Koskinen S, Kristiansson K, Kutalik Z, Laan M, Larson M, Launer LJ, Lehne B, Lehtimäki T, Liewald DCM, Lin L, Lind L, Lindgren CM, Liu Y, Loos RJF, Lopez LM, Lu Y, Lyytikäinen LP, Mahajan A, Mamasoula C, Marrugat J, Marten J, Milaneschi Y, Morgan A, Morris AP, Morrison AC, Munson PJ, Nalls MA, Nandakumar P, Nelson CP, Niiranen T, Nolte IM, Nutile T, Oldehinkel AJ, Oostra BA, O’Reilly PF, Org E, Padmanabhan S, Palmas W, Palotie A, Pattie A, Penninx B, Perola M, Peters A, Polasek O, Pramstaller PP, Nguyen QT, Raitakari OT, Ren M, Rettig R, Rice K, Ridker PM, Ried JS, Riese H, Ripatti S, Robino A, Rose LM, Rotter JI, Rudan I, Ruggiero D, Saba Y, Sala CF, Salomaa V, Samani NJ, Sarin AP, Schmidt R, Schmidt H, Shrine N, Siscovick D, Smith AV, Snieder H, Sõber S, Sorice R, Starr JM, Stott DJ, Strachan DP, Strawbridge RJ, Sundström J, Swertz MA, Taylor KD, Teumer A, Tobin MD, Tomaszewski M, Toniolo D, Traglia M, Trompet S, Tuomilehto J, Tzourio C, Uitterlinden AG, Vaez A, van der Most PJ, van Duijn CM, Vergnaud AC, Verwoert GC, Vitart V, Völker U, Vollenweider P, Vuckovic D, Watkins H, Wild SH, Willemsen G, Wilson JF, Wright AF, Yao J, Zemunik T, Zhang W, Attia JR, Butterworth AS, Chasman DI, Conen D, Cucca F, Danesh J, Hayward C, Howson JMM, Laakso M, Lakatta EG, Langenberg C, Melander O, Mook-Kanamori DO, Palmer CNA, Risch L, Scott RA, Scott RJ, Sever P, Spector TD, van der Harst P, Wareham NJ, Zeggini E, Levy D, Munroe PB, Newton-Cheh C, Brown MJ, Metspalu A, Hung AM, O’Donnell CJ, Edwards TL, Psaty BM, Tzoulaki I, Barnes MR, Wain LV, Elliott P and Caulfield MJ. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert K, Nian H, Yu C, Luther JM and Brown NJ. Fenofibrate lowers blood pressure in salt-sensitive but not salt-resistant hypertension. J Hypertens. 2013;31:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Kim JA, Lee Y and Shin EK. Additive beneficial effects of fenofibrate combined with candesartan in the treatment of hypertriglyceridemic hypertensive patients. Diabetes Care. 2006;29:195–201. [DOI] [PubMed] [Google Scholar]
- 19.Steiner G How can we improve the management of vascular risk in type 2 diabetes: insights from FIELD. Cardiovasc Drugs Ther. 2009;23:403–8. [DOI] [PubMed] [Google Scholar]
- 20.Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop PH and Taskinen MR. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care. 2010;33:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan JJ, Ludvik B, Beerdsen P, Joyce M and Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–93. [DOI] [PubMed] [Google Scholar]
- 22.Ogihara T, Rakugi H, Ikegami H, Mikami H and Masuo K. Enhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensives. Am J Hypertens. 1995;8:316–20. [DOI] [PubMed] [Google Scholar]
- 23.Füllert S, Schneider F, Haak E, Rau H, Badenhoop K, Lübben G, Usadel KH and Konrad T. Effects of pioglitazone in nondiabetic patients with arterial hypertension: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2002;87:5503–6. [DOI] [PubMed] [Google Scholar]
- 24.Komajda M, Curtis P, Hanefeld M, Beck-Nielsen H, Pocock SJ, Zambanini A, Jones NP, Gomis R and Home PD. Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study). Cardiovasc Diabetol. 2008;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshii H, Onuma T, Yamazaki T, Watada H, Matsuhisa M, Matsumoto M, Kitagawa K, Kitakaze M, Yamasaki Y and Kawamori R. Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke: the PROFIT-J study. J Atheroscler Thromb. 2014;21:563–73. [PubMed] [Google Scholar]
- 26.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U and Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. [DOI] [PubMed] [Google Scholar]
- 27.Nissen SE and Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. [DOI] [PubMed] [Google Scholar]
- 28.Yousefipour Z and Newaz M. PPARα ligand clofibrate ameliorates blood pressure and vascular reactivity in spontaneously hypertensive rats. Acta Pharmacol Sin. 2014;35:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Luo P, Chang HH, Huang H, Yang T, Dong Z, Wang CY and Wang MH. Colfibrate attenuates blood pressure and sodium retention in DOCA-salt hypertension. Kidney Int. 2008;74:1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglarz M, Touyz RM, Viel EC, Paradis P, Amiri F, Diep QN and Schiffrin EL. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension. 2003;42:737–43. [DOI] [PubMed] [Google Scholar]
- 31.Banks T and Oyekan A. Peroxisome proliferator-activated receptor alpha activation attenuated angiotensin type 1-mediated but enhanced angiotensin type 2-mediated hemodynamic effects to angiotensin II in the rat. J Hypertens. 2008;26:468–77. [DOI] [PubMed] [Google Scholar]
- 32.Diep QN, Benkirane K, Amiri F, Cohn JS, Endemann D and Schiffrin EL. PPAR alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J Mol Cell Cardiol. 2004;36:295–304. [DOI] [PubMed] [Google Scholar]
- 33.Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN and Schiffrin EL. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol. 2003;23:45–51. [DOI] [PubMed] [Google Scholar]
- 34.Dubey RK, Zhang HY, Reddy SR, Boegehold MA and Kotchen TA. Pioglitazone attenuates hypertension and inhibits growth of renal arteriolar smooth muscle in rats. Am J Physiol. 1993;265:R726–32. [DOI] [PubMed] [Google Scholar]
- 35.Pershadsingh HA, Szollosi J, Benson S, Hyun WC, Feuerstein BG and Kurtz TW. Effects of ciglitazone on blood pressure and intracellular calcium metabolism. Hypertension. 1993;21:1020–3. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman LN, Peterson MM and DeGrange LM. Pioglitazone attenuates diet-induced hypertension in rats. Metabolism. 1995;44:1105–9. [DOI] [PubMed] [Google Scholar]
- 37.Ryan MJ, Didion SP, Mathur S, Faraci FM and Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–6. [DOI] [PubMed] [Google Scholar]
- 38.Saku K, Zhang B, Ohta T and Arakawa K. Troglitazone lowers blood pressure and enhances insulin sensitivity in Watanabe heritable hyperlipidemic rabbits. Am J Hypertens. 1997;10:1027–33. [DOI] [PubMed] [Google Scholar]
- 39.Kemnitz JW, Elson DF, Roecker EB, Baum ST, Bergman RN and Meglasson MD. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes. 1994;43:204–11. [DOI] [PubMed] [Google Scholar]
- 40.Walker AB, Chattington PD, Buckingham RE and Williams G. The thiazolidinedione rosiglitazone (BRL-49653) lowers blood pressure and protects against impairment of endothelial function in Zucker fatty rats. Diabetes. 1999;48:1448–53. [DOI] [PubMed] [Google Scholar]
- 41.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF and Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–302. [DOI] [PubMed] [Google Scholar]
- 42.Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP and Hsueh WA. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–8. [DOI] [PubMed] [Google Scholar]
- 43.Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, Maekawa H, Isoo N, Kimura S and Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757–63. [DOI] [PubMed] [Google Scholar]
- 44.Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T and Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. [DOI] [PubMed] [Google Scholar]
- 45.Takeda K, Ichiki T, Tokunou T, Funakoshi Y, Iino N, Hirano K, Kanaide H and Takeshita A. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102:1834–9. [DOI] [PubMed] [Google Scholar]
- 46.Sigmund CD. Endothelial and vascular muscle PPARgamma in arterial pressure regulation: lessons from genetic interference and deficiency. Hypertension. 2010;55:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL and Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol. 2009;297:H1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicol CJ, Adachi M, Akiyama TE and Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–56. [DOI] [PubMed] [Google Scholar]
- 49.De Silva TM, Li Y, Kinzenbaw DA, Sigmund CD and Faraci FM. Endothelial PPARγ (Peroxisome Proliferator-Activated Receptor-γ) Is Essential for Preventing Endothelial Dysfunction With Aging. Hypertension. 2018;72:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM and Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Silva TM, Hu C, Kinzenbaw DA, Modrick ML, Sigmund CD and Faraci FM. Genetic Interference With Endothelial PPAR-γ (Peroxisome Proliferator-Activated Receptor-γ) Augments Effects of Angiotensin II While Impairing Responses to Angiotensin 1–7. Hypertension. 2017;70:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukohda M, Stump M, Ketsawatsomkron P, Hu C, Quelle FW and Sigmund CD. Endothelial PPAR-γ provides vascular protection from IL-1β-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2016;310:H39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair AR, Agbor LN, Mukohda M, Liu X, Hu C, Wu J and Sigmund CD. Interference With Endothelial PPAR (Peroxisome Proliferator-Activated Receptor)-γ Causes Accelerated Cerebral Vascular Dysfunction in Response to Endogenous Renin-Angiotensin System Activation. Hypertension. 2018;72:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM and Sigmund CD. Interference with PPARγ in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genomics. 2016;48:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caprioli A, Zhu H and Sato TN. CRBP-III:lacZ expression pattern reveals a novel heterogeneity of vascular endothelial cells. Genesis. 2004;40:139–45. [DOI] [PubMed] [Google Scholar]
- 56.Hu C, Keen HL, Lu KT, Liu X, Wu J, Davis DR, Ibeawuchi SC, Vogel S, Quelle FW and Sigmund CD. Retinol-binding protein 7 is an endothelium-specific PPARγ cofactor mediating an antioxidant response through adiponectin. JCI Insight. 2017;2:e91738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel S, Mendelsohn CL, Mertz JR, Piantedosi R, Waldburger C, Gottesman ME and Blaner WS. Characterization of a new member of the fatty acid-binding protein family that binds all-trans-retinol. J Biol Chem. 2001;276:1353–60. [DOI] [PubMed] [Google Scholar]
- 58.Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W and Noy N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woll AW, Quelle FW and Sigmund CD. PPARγ and retinol binding protein 7 form a regulatory hub promoting antioxidant properties of the endothelium. Physiol Genomics. 2017;49:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchesi C, Rehman A, Rautureau Y, Kasal DA, Briet M, Leibowitz A, Simeone SM, Ebrahimian T, Neves MF, Offermanns S, Gonzalez FJ, Paradis P and Schiffrin EL. Protective role of vascular smooth muscle cell PPARγ in angiotensin II-induced vascular disease. Cardiovasc Res. 2013;97:562–70. [DOI] [PubMed] [Google Scholar]
- 61.Subramanian V, Golledge J, Ijaz T, Bruemmer D and Daugherty A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPAR{gamma}. Circ Res. 2010;107:953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM and Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu J, Agbor LN, Fang S, Mukohda M, Nair AR, Nakagawa P, Sharma A, Morgan DA, Grobe JL, Rahmouni K, Weiss RM, McCormick JA and Sigmund CD. Failure to Vasodilate in Response to Salt Loading Blunts Renal Blood Flow and Causes Salt-Sensitive Hypertension. Cardiovasc Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasan DM, Starke RM, Gu H, Wilson K, Chu Y, Chalouhi N, Heistad DD, Faraci FM and Sigmund CD. Smooth Muscle Peroxisome Proliferator-Activated Receptor γ Plays a Critical Role in Formation and Rupture of Cerebral Aneurysms in Mice In Vivo. Hypertension. 2015;66:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Silva TM, Modrick ML, Ketsawatsomkron P, Lynch C, Chu Y, Pelham CJ, Sigmund CD and Faraci FM. Role of peroxisome proliferator-activated receptor-γ in vascular muscle in the cerebral circulation. Hypertension. 2014;64:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM and Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase. Cell Metab. 2012;16:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ketsawatsomkron P, Lorca RA, Keen HL, Weatherford ET, Liu X, Pelham CJ, Grobe JL, Faraci FM, England SK and Sigmund CD. PPARγ regulates resistance vessel tone through a mechanism involving RGS5-mediated control of protein kinase C and BKCa channel activity. Circ Res. 2012;111:1446–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Silva TM, Ketsawatsomkron P, Pelham C, Sigmund CD and Faraci FM. Genetic interference with peroxisome proliferator-activated receptor γ in smooth muscle enhances myogenic tone in the cerebrovasculature via A Rho kinase-dependent mechanism. Hypertension. 2015;65:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holobotovskyy V, Manzur M, Tare M, Burchell J, Bolitho E, Viola H, Hool LC, Arnolda LF, McKitrick DJ and Ganss R. Regulator of G-protein signaling 5 controls blood pressure homeostasis and vessel wall remodeling. Circ Res. 2013;112:781–91. [DOI] [PubMed] [Google Scholar]
- 70.Ketsawatsomkron P, Keen HL, Davis DR, Lu KT, Stump M, De Silva TM, Hilzendeger AM, Grobe JL, Faraci FM and Sigmund CD. Protective Role for Tissue Inhibitor of Metalloproteinase-4, a Novel Peroxisome Proliferator-Activated Receptor-γ Target Gene, in Smooth Muscle in Deoxycorticosterone Acetate-Salt Hypertension. Hypertension. 2016;67:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA and Law RE. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1--> S transition in vascular smooth muscle cells. J Biol Chem. 2000;275:22435–41. [DOI] [PubMed] [Google Scholar]
- 72.Ogawa D, Nomiyama T, Nakamachi T, Heywood EB, Stone JF, Berger JP, Law RE and Bruemmer D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ Res. 2006;98:e50–9. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB and Shao F. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35:841–55. [DOI] [PubMed] [Google Scholar]
- 74.Agbor LN, Ibeawuchi SC, Hu C, Wu J, Davis DR, Keen HL, Quelle FW and Sigmund CD. Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight. 2016;1:e91015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferdaus MZ, Miller LN, Agbor LN, Saritas T, Singer JD, Sigmund CD and McCormick JA. Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornelius RJ, Zhang C, Erspamer KJ, Agbor LN, Sigmund CD, Singer JD, Yang CL and Ellison DH. Dual gain and loss of cullin 3 function mediates familial hyperkalemic hypertension. Am J Physiol Renal Physiol. 2018;315:F1006–f1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agbor LN, Nair AR, Wu J, Lu KT, Davis DR, Keen HL, Quelle FW, McCormick JA, Singer JD and Sigmund CD. Conditional deletion of smooth muscle Cullin-3 causes severe progressive hypertension. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdel Khalek W, Rafael C, Loisel-Ferreira I, Kouranti I, Clauser E, Hadchouel J and Jeunemaitre X. Severe Arterial Hypertension from Cullin 3 Mutations Is Caused by Both Renal and Vascular Effects. J Am Soc Nephrol. 2019;30:811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Välimäki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B and Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukohda M, Fang S, Wu J, Agbor LN, Nair AR, Ibeawuchi SC, Hu C, Liu X, Lu KT, Guo DF, Davis DR, Keen HL, Quelle FW and Sigmund CD. RhoBTB1 protects against hypertension and arterial stiffness by restraining phosphodiesterase 5 activity. J Clin Invest. 2019;129:2318–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, Saito H, Kouhara H, Kasayama S and Kawase I. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–63. [DOI] [PubMed] [Google Scholar]
- 82.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P and Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Z, Xie X, Yao Q, Liu J, Tian Y, Yang C, Xiao L and Wang N. PPARδ agonist prevents endothelial dysfunction via induction of dihydrofolate reductase gene and activation of tetrahydrobiopterin salvage pathway. Br J Pharmacol. 2019;176:2945–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian XY, Wong WT, Wang N, Lu Y, Cheang WS, Liu J, Liu L, Liu Y, Lee SS, Chen ZY, Cooke JP, Yao X and Huang Y. PPARδ activation protects endothelial function in diabetic mice. Diabetes. 2012;61:3285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romero M, Toral M, Robles-Vera I, Sánchez M, Jiménez R, O’Valle F, Rodriguez-Nogales A, Pérez-Vizcaino F, Gálvez J and Duarte J. Activation of Peroxisome Proliferator Activator Receptor β/δ Improves Endothelial Dysfunction and Protects Kidney in Murine Lupus. Hypertension. 2017;69:641–650. [DOI] [PubMed] [Google Scholar]
- 86.Zarzuelo MJ, Jiménez R, Galindo P, Sánchez M, Nieto A, Romero M, Quintela AM, López-Sepúlveda R, Gómez-Guzmán M, Bailón E, Rodríguez-Gómez I, Zarzuelo A, Gálvez J, Tamargo J, Pérez-Vizcaíno F and Duarte J. Antihypertensive effects of peroxisome proliferator-activated receptor-β activation in spontaneously hypertensive rats. Hypertension. 2011;58:733–43. [DOI] [PubMed] [Google Scholar]
- 87.Jimenez R, Toral M, Gómez-Guzmán M, Romero M, Sanchez M, Mahmoud AM and Duarte J. The Role of Nrf2 Signaling in PPARβ/δ-Mediated Vascular Protection against Hyperglycemia-Induced Oxidative Stress. Oxid Med Cell Longev. 2018;2018:5852706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toral M, Jiménez R, Romero M, Robles-Vera I, Sánchez M, Salaices M, Sabio JM and Duarte J. Role of endoplasmic reticulum stress in the protective effects of PPARβ/δ activation on endothelial dysfunction induced by plasma from patients with lupus. Arthritis Res Ther. 2017;19:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheang WS, Wong WT, Zhao L, Xu J, Wang L, Lau CW, Chen ZY, Ma RC, Xu A, Wang N, Tian XY and Huang Y. PPARδ Is Required for Exercise to Attenuate Endoplasmic Reticulum Stress and Endothelial Dysfunction in Diabetic Mice. Diabetes. 2017;66:519–528. [DOI] [PubMed] [Google Scholar]
- 90.Cowley AW Jr., Liard JF and Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–76. [DOI] [PubMed] [Google Scholar]
- 91.DeLalio LJ, Sved AF and Stocker SD. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can J Cardiol. 2020;36:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.King AJ, Novotny M, Swain GM and Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1262–7. [DOI] [PubMed] [Google Scholar]
- 93.Lim K, Barzel B, Burke SL, Armitage JA and Head GA. Origin of Aberrant Blood Pressure and Sympathetic Regulation in Diet-Induced Obesity. Hypertension. 2016;68:491–500. [DOI] [PubMed] [Google Scholar]
- 94.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN and Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. [DOI] [PubMed] [Google Scholar]
- 95.Terker AS, Yang CL, McCormick JA, Meermeier NP, Rogers SL, Grossmann S, Trompf K, Delpire E, Loffing J and Ellison DH. Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension. 2014;64:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD and Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stump M, Guo DF, Lu KT, Mukohda M, Cassell MD, Norris AW, Rahmouni K and Sigmund CD. Nervous System Expression of PPARγ and Mutant PPARγ Has Profound Effects on Metabolic Regulation and Brain Development. Endocrinology. 2016;157:4266–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW and Olefsky JM. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL and Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ryan KK, Li B, Grayson BE, Matter EK, Woods SC and Seeley RJ. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med. 2011;17:623–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, Suyama S, Kelly K, Gyengesi E, Arbiser JL, Belsham DD, Sarruf DA, Schwartz MW, Bennett AM, Shanabrough M, Mobbs CV, Yang X, Gao XB and Horvath TL. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Long L, Toda C, Jeong JK, Horvath TL and Diano S. PPARγ ablation sensitizes proopiomelanocortin neurons to leptin during high-fat feeding. J Clin Invest. 2014;124:4017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stump M, Guo DF, Lu KT, Mukohda M, Liu X, Rahmouni K and Sigmund CD. Effect of selective expression of dominant-negative PPARγ in pro-opiomelanocortin neurons on the control of energy balance. Physiol Genomics. 2016;48:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y and Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI and Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–42. [DOI] [PubMed] [Google Scholar]
- 107.Hong LZ, Chan YC, Wang MF, Wang JY, Hung SW, Tsai CI and Tseng CJ. Modulation of baroreflex function by rosiglitazone in prediabetic hyperglycemic rats. Physiol Res. 2012;61:443–52. [DOI] [PubMed] [Google Scholar]
- 108.Chan SH, Wu KL, Kung PS and Chan JY. Oral intake of rosiglitazone promotes a central antihypertensive effect via upregulation of peroxisome proliferator-activated receptor-gamma and alleviation of oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertension. 2010;55:1444–53. [DOI] [PubMed] [Google Scholar]
- 109.Yokoe H, Yuasa F, Yuyama R, Murakawa K, Miyasaka Y, Yoshida S, Tsujimoto S, Sugiura T and Iwasaka T. Effect of pioglitazone on arterial baroreflex sensitivity and sympathetic nerve activity in patients with acute myocardial infarction and type 2 diabetes mellitus. J Cardiovasc Pharmacol. 2012;59:563–9. [DOI] [PubMed] [Google Scholar]
- 110.Grommes C, Karlo JC, Caprariello A, Blankenship D, Dechant A and Landreth GE. The PPARγ agonist pioglitazone crosses the blood-brain barrier and reduces tumor growth in a human xenograft model. Cancer Chemother Pharmacol. 2013;71:929–36. [DOI] [PubMed] [Google Scholar]
- 111.Li HB, Li X, Huo CJ, Su Q, Guo J, Yuan ZY, Zhu GQ, Shi XL, Liu JJ and Kang YM. TLR4/MyD88/NF-κB signaling and PPAR-γ within the paraventricular nucleus are involved in the effects of telmisartan in hypertension. Toxicol Appl Pharmacol. 2016;305:93–102. [DOI] [PubMed] [Google Scholar]
- 112.Yu Y, Xue BJ, Wei SG, Zhang ZH, Beltz TG, Guo F, Johnson AK and Felder RB. Activation of central PPAR-γ attenuates angiotensin II-induced hypertension. Hypertension. 2015;66:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu Y, Zhang ZH, Wei SG, Weiss RM and Felder RB. Peroxisome proliferator-activated receptor-γ regulates inflammation and renin-angiotensin system activity in the hypothalamic paraventricular nucleus and ameliorates peripheral manifestations of heart failure. Hypertension. 2012;59:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu D, Gao L, Roy SK, Cornish KG and Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res. 2008;103:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC and Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–80. [DOI] [PubMed] [Google Scholar]
- 116.Chan SH, Wu CA, Wu KL, Ho YH, Chang AY and Chan JY. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ Res. 2009;105:886–96. [DOI] [PubMed] [Google Scholar]
- 117.Wang P, Li B, Cai G, Huang M, Jiang L, Pu J, Li L, Wu Q, Zuo L, Wang Q and Zhou P. Activation of PPAR-γ by pioglitazone attenuates oxidative stress in aging rat cerebral arteries through upregulating UCP2. J Cardiovasc Pharmacol. 2014;64:497–506. [DOI] [PubMed] [Google Scholar]
- 118.Gao L, Zimmerman MC, Biswal S and Zucker IH. Selective Nrf2 Gene Deletion in the Rostral Ventrolateral Medulla Evokes Hypertension and Sympathoexcitation in Mice. Hypertension. 2017;69:1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Furukawa M and Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guan J, Zhao M, He C, Li X, Li Y, Sun J, Wang W, Cui YL, Zhang Q, Li BY and Qiao GF. Anti-Hypertensive Action of Fenofibrate via UCP2 Upregulation Mediated by PPAR Activation in Baroreflex Afferent Pathway. Neurosci Bull. 2019;35:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Romero M, Jiménez R, Toral M, León-Gómez E, Gómez-Gúzman M, Sánchez M, Zarzuelo MJ, Rodríguez-Gómez I, Rath G, Tamargo J, Pérez-Vizcaíno F, Dessy C and Duarte J. Vascular and Central Activation of Peroxisome Proliferator-Activated Receptor-β Attenuates Angiotensin II-Induced Hypertension: Role of RGS-5. J Pharmacol Exp Ther. 2016;358:151–63. [DOI] [PubMed] [Google Scholar]
- 122.Luo Z, Aslam S, Welch WJ and Wilcox CS. Activation of nuclear factor erythroid 2-related factor 2 coordinates dimethylarginine dimethylaminohydrolase/PPAR-gamma/endothelial nitric oxide synthase pathways that enhance nitric oxide generation in human glomerular endothelial cells. Hypertension. 2015;65:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH and Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–63. [DOI] [PubMed] [Google Scholar]
- 124.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y and Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–6. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ and Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci U S A. 2005;102:9406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R and Stockand J. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol. 2009;20:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song J, Knepper MA, Hu X, Verbalis JG and Ecelbarger CA. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J Pharmacol Exp Ther. 2004;308:426–33. [DOI] [PubMed] [Google Scholar]
- 128.Borsting E, Cheng VP, Glass CK, Vallon V and Cunard R. Peroxisome proliferator-activated receptor-γ agonists repress epithelial sodium channel expression in the kidney. Am J Physiol Renal Physiol. 2012;302:F540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee DL, Wilson JL, Duan R, Hudson T and El-Marakby A. Peroxisome Proliferator-Activated Receptor-α Activation Decreases Mean Arterial Pressure, Plasma Interleukin-6, and COX-2 While Increasing Renal CYP4A Expression in an Acute Model of DOCA-Salt Hypertension. PPAR Res. 2011;2011:502631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sankaralingam S, Desai KM, Glaeser H, Kim RB and Wilson TW. Inability to upregulate cytochrome P450 4A and 2C causes salt sensitivity in young Sprague-Dawley rats. Am J Hypertens. 2006;19:1174–80. [DOI] [PubMed] [Google Scholar]
- 131.Zhou Y, Huang H, Chang HH, Du J, Wu JF, Wang CY and Wang MH. Induction of renal 20-hydroxyeicosatetraenoic acid by clofibrate attenuates high-fat diet-induced hypertension in rats. J Pharmacol Exp Ther. 2006;317:11–8. [DOI] [PubMed] [Google Scholar]
- 132.Roman RJ, Ma YH, Frohlich B and Markham B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension. 1993;21:985–8. [DOI] [PubMed] [Google Scholar]
- 133.Letavernier E, Perez J, Joye E, Bellocq A, Fouqueray B, Haymann JP, Heudes D, Wahli W, Desvergne B and Baud L. Peroxisome proliferator-activated receptor beta/delta exerts a strong protection from ischemic acute renal failure. J Am Soc Nephrol. 2005;16:2395–402. [DOI] [PubMed] [Google Scholar]
- 134.Kirkby NS, Sampaio W, Etelvino G, Alves DT, Anders KL, Temponi R, Shala F, Nair AS, Ahmetaj-Shala B, Jiao J, Herschman HR, Wang X, Wahli W, Santos RA and Mitchell JA. Cyclooxygenase-2 Selectively Controls Renal Blood Flow Through a Novel PPARβ/δ-Dependent Vasodilator Pathway. Hypertension. 2018;71:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okuda T and Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25:257–64. [PubMed] [Google Scholar]
- 136.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A and Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–81. [DOI] [PubMed] [Google Scholar]
- 138.Széles L, Töröcsik D and Nagy L. PPARgamma in immunity and inflammation: cell types and diseases. Biochim Biophys Acta. 2007;1771:1014–30. [DOI] [PubMed] [Google Scholar]
- 139.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S and Linton MF. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–53. [DOI] [PubMed] [Google Scholar]
- 140.Ito H, Nakano A, Kinoshita M and Matsumori A. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates myocardial ischemia/reperfusion injury in a rat model. Lab Invest. 2003;83:1715–21. [DOI] [PubMed] [Google Scholar]
- 141.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P and Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. [DOI] [PubMed] [Google Scholar]
- 142.Shen ZX, Yang QZ, Li C, Du LJ, Sun XN, Liu Y, Sun JY, Gu HH, Sun YM, Wang J and Duan SZ. Myeloid peroxisome proliferator-activated receptor gamma deficiency aggravates myocardial infarction in mice. Atherosclerosis. 2018;274:199–205. [DOI] [PubMed] [Google Scholar]
- 143.Han KH, Chang MK, Boullier A, Green SR, Li A, Glass CK and Quehenberger O. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J Clin Invest. 2000;106:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ricote M, Li AC, Willson TM, Kelly CJ and Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. [DOI] [PubMed] [Google Scholar]
- 145.Zhang WY, Schwartz EA, Permana PA and Reaven PD. Pioglitazone inhibits the expression of inflammatory cytokines from both monocytes and lymphocytes in patients with impaired glucose tolerance. Arterioscler Thromb Vasc Biol. 2008;28:2312–8. [DOI] [PubMed] [Google Scholar]
- 146.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW and Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aprahamian TR, Bonegio RG, Weitzner Z, Gharakhanian R and Rifkin IR. Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus. Immunology. 2014;142:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chi CC, Lee CY, Liu CY, Wang SH, Tien O’Donnell F and Tung TH. Effects of antidiabetic drugs on psoriasis: A meta-analysis. Eur J Clin Invest. 2020:e13377. [DOI] [PubMed] [Google Scholar]
- 149.Chedrawe MAJ, Holman SP, Lamport AC, Akay T and Robertson GS. Pioglitazone is superior to quetiapine, clozapine and tamoxifen at alleviating experimental autoimmune encephalomyelitis in mice. J Neuroimmunol. 2018;321:72–82. [DOI] [PubMed] [Google Scholar]
- 150.Byun SH, Lee JH, Jung NC, Choi HJ, Song JY, Seo HG, Choi J, Jung SY, Kang S, Choi YS, Chung JH and Lim DS. Rosiglitazone-mediated dendritic cells ameliorate collagen-induced arthritis in mice. Biochem Pharmacol. 2016;115:85–93. [DOI] [PubMed] [Google Scholar]
- 151.Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang LK, Sato K, Walsh K and Rifkin IR. The peroxisome proliferator-activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol. 2009;182:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Obreque J, Vega F, Torres A, Cuitino L, Mackern-Oberti JP, Viviani P, Kalergis A and Llanos C. Autologous tolerogenic dendritic cells derived from monocytes of systemic lupus erythematosus patients and healthy donors show a stable and immunosuppressive phenotype. Immunology. 2017;152:648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR Jr., Jones AV, Reckelhoff JF and Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sartori-Valinotti JC, Venegas-Pont MR, Lamarca BB, Romero DG, Yanes LL, Racusen LC, Jones AV, Ryan MJ and Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt-sensitive rats. Steroids. 2010;75:794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C and Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P and Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–76. [DOI] [PubMed] [Google Scholar]
- 157.Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L and Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–71. [DOI] [PubMed] [Google Scholar]
- 158.Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, Langhans B, Klockgether T, Waisman A, Eberl G, Schultze J, Famulok M, Kolanus W, Glass C, Kurts C and Knolle PA. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones DC, Ding X and Daynes RA. Nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. J Biol Chem. 2002;277:6838–45. [DOI] [PubMed] [Google Scholar]
- 160.Poynter ME and Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–41. [DOI] [PubMed] [Google Scholar]
- 161.Marx N, Kehrle B, Kohlhammer K, Grüb M, Koenig W, Hombach V, Libby P and Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Selim E, Frkanec JT and Cunard R. Fibrates upregulate TRB3 in lymphocytes independent of PPAR alpha by augmenting CCAAT/enhancer-binding protein beta (C/EBP beta) expression. Mol Immunol. 2007;44:1218–29. [DOI] [PubMed] [Google Scholar]
- 163.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260. [DOI] [PubMed] [Google Scholar]
- 164.Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R and Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Berry EB, Eykholt R, Helliwell RJ, Gilmour RS, Mitchell MD and Marvin KW. Peroxisome proliferator-activated receptor isoform expression changes in human gestational tissues with labor at term. Mol Pharmacol. 2003;64:1586–90. [DOI] [PubMed] [Google Scholar]
- 166.Fournier T, Handschuh K, Tsatsaris V and Evain-Brion D. Involvement of PPARgamma in human trophoblast invasion. Placenta. 2007;28 Suppl A:S76–81. [DOI] [PubMed] [Google Scholar]
- 167.Fournier T, Pavan L, Tarrade A, Schoonjans K, Auwerx J, Rochette-Egly C and Evain-Brion D. The role of PPAR-gamma/RXR-alpha heterodimers in the regulation of human trophoblast invasion. Ann N Y Acad Sci. 2002;973:26–30. [DOI] [PubMed] [Google Scholar]
- 168.Tarrade A, Schoonjans K, Guibourdenche J, Bidart JM, Vidaud M, Auwerx J, Rochette-Egly C and Evain-Brion D. PPAR gamma/RXR alpha heterodimers are involved in human CG beta synthesis and human trophoblast differentiation. Endocrinology. 2001;142:4504–14. [DOI] [PubMed] [Google Scholar]
- 169.Crocker IP, Barratt S, Kaur M and Baker PN. The in-vitro characterization of induced apoptosis in placental cytotrophoblasts and syncytiotrophoblasts. Placenta. 2001;22:822–30. [DOI] [PubMed] [Google Scholar]
- 170.Waite LL, Person EC, Zhou Y, Lim KH, Scanlan TS and Taylor RN. Placental peroxisome proliferator-activated receptor-gamma is up-regulated by pregnancy serum. J Clin Endocrinol Metab. 2000;85:3808–14. [DOI] [PubMed] [Google Scholar]
- 171.Waite LL, Louie RE and Taylor RN. Circulating activators of peroxisome proliferator-activated receptors are reduced in preeclamptic pregnancy. J Clin Endocrinol Metab. 2005;90:620–6. [DOI] [PubMed] [Google Scholar]
- 172.Arbogast BW, Leeper SC, Merrick RD, Olive KE and Taylor RN. Which plasma factors bring about disturbance of endothelial function in pre-eclampsia? Lancet. 1994;343:340–1. [DOI] [PubMed] [Google Scholar]
- 173.Rahardjo B, Widjajanto E, Sujuti H and Keman K. Different levels of IL-1α, IL-6, TNF-α, NF-κB and PPAR-γ in monocyte cultures exposed by plasma preeclampsia and normotensive pregnancy. Pregnancy Hypertens. 2014;4:187–93. [DOI] [PubMed] [Google Scholar]
- 174.Laasanen J, Heinonen S, Hiltunen M, Mannermaa A and Laakso M. Polymorphism in the peroxisome proliferator-activated receptor-gamma gene in women with preeclampsia. Early Hum Dev. 2002;69:77–82. [DOI] [PubMed] [Google Scholar]
- 175.Rodie VA, Young A, Jordan F, Sattar N, Greer IA and Freeman DJ. Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig. 2005;12:320–9. [DOI] [PubMed] [Google Scholar]
- 176.Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M and Lappas M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta. 2010;31:222–9. [DOI] [PubMed] [Google Scholar]
- 177.Tache V, Ciric A, Moretto-Zita M, Li Y, Peng J, Maltepe E, Milstone DS and Parast MM. Hypoxia and trophoblast differentiation: a key role for PPARγ. Stem Cells Dev. 2013;22:2815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kasture V, Sundrani D, Dalvi S, Swamy M, Kale A and Joshi S. Maternal omega-3 fatty acids and vitamin E improve placental angiogenesis in late-onset but not early-onset preeclampsia. Mol Cell Biochem. 2019;461:159–170. [DOI] [PubMed] [Google Scholar]
- 179.Gokina NI, Chan SL, Chapman AC, Oppenheimer K, Jetton TL and Cipolla MJ. Inhibition of PPARγ during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Front Physiol. 2013;4:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC and Walsh SK. Evidence implicating peroxisome proliferator-activated receptor-γ in the pathogenesis of preeclampsia. Hypertension. 2011;58:882–7. [DOI] [PubMed] [Google Scholar]
- 181.Nair AR, Silva SD Jr., Agbor LN, Wu J, Nakagawa P, Mukohda M, Lu KT, Sandgren JA, Pierce GL, Santillan MK, Grobe JL and Sigmund CD. Endothelial PPARγ (Peroxisome Proliferator-Activated Receptor-γ) Protects From Angiotensin II-Induced Endothelial Dysfunction in Adult Offspring Born From Pregnancies Complicated by Hypertension. Hypertension. 2019;74:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Holobotovskyy V, Chong YS, Burchell J, He B, Phillips M, Leader L, Murphy TV, Sandow SL, McKitrick DJ, Charles AK, Tare M, Arnolda LF and Ganss R. Regulator of G protein signaling 5 is a determinant of gestational hypertension and preeclampsia. Sci Transl Med. 2015;7:290ra88. [DOI] [PubMed] [Google Scholar]
- 183.Norris AW and Sigmund CD. A second chance for a PPARgamma targeted therapy? Circ Res. 2012;110:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, Griffin PR and Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schürer SC, Vidović D, Shulman GI, Spiegelman BM and Griffin PR. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sauer S Ligands for the Nuclear Peroxisome Proliferator-Activated Receptor Gamma. Trends Pharmacol Sci. 2015;36:688–704. [DOI] [PubMed] [Google Scholar]