Abstract
Genetic nephropathies represent a challenging class of disorders to be treated by gene therapy. This is primarily due to the filtering properties of the kidney itself, which does not allow the vehicle carrying the transgene of interest to remain long enough in the organ to penetrate efficiently into the nephrotic cells. Also, the kidney has a complex anatomical structure composed of different cell types compartmentalized within isolated anatomic structures that limit their access. Here, we describe a simple surgical procedure to deliver recombinant adeno-associated virus (rAAV) to the whole kidney based on the hydraulic force of the retrograde renal vein injection. In its clinical form, this procedure would correspond to a renal venography where a catheter is threaded retrograde from the femoral vein under fluoroscopic guidance.
Keywords: Mouse, Kidney, Gene therapy, Genetic, Nephropathies, Recombinant adeno-associated virus, Renal vein injection
1. Introduction
1.1. General Introduction
In the past decades, a lot of effort has been dedicated to develop efficient and safe gene transfer technologies. Gene replacement therapy appears to be an attractive approach for monogenetic disorders. However, getting a gene expressed in a physiological manner, in a specific location without risk of oncogenic insertion in the genome, is not trivial. First of all, it requires a good vehicle. There are two ways to deliver a gene: (a) Nonviral approach: naked DNA is delivered to the tissue using physical or chemical methods to enhance gene delivery. This strategy presents with the advantage of having simple large-scale production and low host immunogenicity. However, there is low efficiency rate due to difficulty crossing physical body barriers. (b) The viral approach, on other hand, uses the infective properties of viruses to deliver a payload gene into cells and is consequently more efficient but with some risk of immunogenicity and/or insertional mutagenesis. However, genetic manipulations of the viral vectors considerably improve safety for in vivo usage. That said, it is crucial to opt for an adequate delivery method to achieve tissue-specific gene expression without off-site vector sequestration and viral inactivation. Furthermore, it is also important that the chosen delivery method does not artificially exacerbate the disease state by inducing, for instance, ischemia or traumatic injury of the organ. In this chapter, we describe an efficient and safe method for kidney-specific gene transfer in mice.
1.2. Kidney Gene Transfer
Inherited kidney diseases constitute at least 150 different disorders and they have an overall prevalence of about 60–80 cases per 100, 000 in Europe and the USA. At least 10% of adults and nearly all children who progress to renal-replacement therapy have an inherited kidney disease [1]. As with every other type of organ transplant, there are consequent disadvantages associated with renal allografts: (a) severe shortage of donor organs and average waiting time of 3–5 years [2]; (b) short average lifetime of renal allografts (10–15 years) [3]; (c) significant morbidity and mortality associated with transplant rejection [4]; and (d) patients have to take lifelong immunosuppressive drugs that lead to hypertension, increased cholesterol levels, tremors, headache, etc. For monogenic hereditary nephropathies, gene replacement therapy may represent an idealistic alternative. However, this organ is particularly difficult to target in comparison to others (brain, eye, or liver), because of its complex anatomical structure limiting the successful delivery of genetic material. So far, few studies have been performed to deliver genes into the kidney using nonviral and viral vectors, with different routes of injection (see Table 1). Recombinant adeno-associated viral (rAAV) seems to be the most promising vehicle for kidney-gene delivery, although the expression is always limited to a restricted part of the kidney. Therefore, rAAVs are the vectors of choice for targeted gene therapy because they overcome many of the problems associated with other vector systems. rAAVs are non-pathogenic and minimally immunogenic, can infect both dividing and nondividing cells, persist after infection, and have extensive cell and tissue tropism. Their main limitation is the size of the transgene capacity (<5 kb).
Table 1.
Examples of kidney gene delivery
| Vehicle | Route of injection | Results | Paper |
|---|---|---|---|
| LacZ expression plasmid | Retrograde renal vein injection | Expression only in interstitial fibroblasts near proximal tubular cells | [7] |
| Adenoviral vector | – Intrarenal ureteral route. – Intrarenal arterial route. |
– Expression in distal tubular and pyelic epithelial cells. – Expression in cortical interstitial cells. |
[8] |
| rAAV2 | Parenchymal injection | Low transgene expression in the tubular structures near the point of injection | [9] |
| rAAV2 | Renal arterial injection | Limited transduction of the S3 segments of proximal tubular cells, straight segments of the proximal tubule descending into the outer medulla, for only 6 weeks. Significant inflammation and renal injury were noted and attributed to the procedure | [10] |
| rAAV serotype 1–5 | Catheter inserted into the renal artery | Only rAAV2 could transduce the kidney but only the tubular epithelial cells | [11] |
| rAAV2, 8, and 9 mutants | Microinjection into the renal cortex | Only rAAV2 mutant led to a robust transgene expression in the distal tubular cells | [12] |
1.3. Summary
We published, in 2014, a comprehensive study for kidney-specific gene delivery using a procedure and vectors relevant for clinical application in mice [5]. We modified a technique that has first been described by Maruyama et al. in rats [4]. Postinjection, the levels of kidney transduction were investigated in both the cortex and medulla using two different reporter genes analyzed by both qualitative and quantitative assays. Our results showed that retrograde renal vein injection of rAAV9 represents a promising strategy for the treatment of kidney diseases. We also showed that we could significantly improve the specific expression of transgenes to the kidney by using a kidney-specific promoter. This is translationally relevant because there is already a clinical equivalent procedure that is well established in humans: renal venography, a minimally invasive and readily performed outpatient procedure [6]. We already investigated the efficacy of this technique in a mouse model of Glycogen storage disease Ia (GSD-Ia), which improved the fasting blood glucose levels of the mice but also led to normal renal function for 52 weeks postinjection, last time-point tested (https://doi.org/10.1016/S1525-0016(16)33159-8). Here, we describe step by step how to perform this surgical procedure in mice, in order to efficiently and specifically deliver a gene to the kidney. We think that this procedure is scalable and could be performed in larger animals.
2. Materials
Mice: C57BL/6 mice aged 8–10 weeks purchased from Jackson Laboratories and bred continuously at UCSD vivarium.
rAAV-CMV-Luciferase or rAAV-P1-Luciferase (P1 is a parathyroid hormone receptor “kidney-specific” promoter) serotype 9 particles were produced by and purchased from the University of North Carolina Gene Therapy Center.
Phosphate Buffer Saline 1X.
BD insulin syringe with the BD Ultra-Fine™ needle 1 mL 31 G × 5/16 in. (8 mm).
Anesthetic: Isoflurane.
Complete rodent anesthesia system: anesthesia machine, vaporizer, non-rebreathing circuit and mask, evacuation cartridges, dual diverter valves, and induction chamber.
Small animal electric clipper.
Ethanol 70%.
- Standard surgical instrument including:
- Surgical scissors.
- Retractor.
- Forceps.
- Vascular clamps 11 mm.
- Clamp applicator.
- Needle holder.
- 5–0 absorbable vicryl suture.
- 5–0 nonabsorbable surgical Ethilon suture.
Cotton Q-tip.
Tissue adhesive (Vetbond, 3 M).
Analgesic: Buprenorphine.
Heat pad.
IVIS Imaging System 200 Series (Caliper Life Sciences).
Luciferin.
3. Methods
During the whole procedure, the surgeon will wear scrubs, sterile latex gloves, Tyvek sleeves, surgical mask, and foot protection. All the surgical instruments will be sterilized with povidone iodine prior each surgery.
3.1. Virus Preparation
Dilute the rAAV stock solution in order to obtain 10E11 virus particles in a final volume of 100 μL of PBS-1X (see Note 1).
3.2. Mouse Preparation
Put the mouse in an induction box that delivers 2.5–3% isoflurane and also 5% oxygen by a precision vaporizer to put the mouse in a deeply sleeping state. Then, maintain the mouse in a deeply sleeping state throughout the whole duration of the procedure by using a nose cone associated with the vaporizer to continue delivering isoflurane (see Note 2).
Place the mouse on a clean blue pad. Remove hair on the left ventral part of the animal using an electric clipper and disinfect the skin with 70% ethanol.
3.3. Surgical Procedure
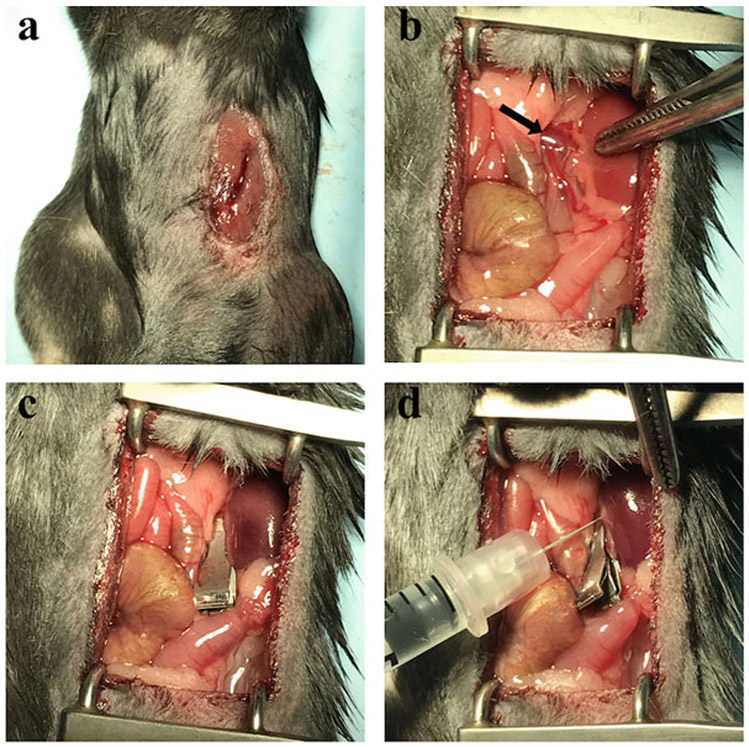
Perform a small incision in the median section of the abdomen on the left side of the anesthetized mice (Fig. 1a; see Note 3).
Expose the left kidney in order to see the renal vein. Use the retractor to gently push all the organs covering the kidney on each side (Fig. 1b).
Prepare the 31G needle syringe with 100 μL of rAAV particles (see Note 4).
Delicately, isolate the vein from the fatty tissue surrounding it using forceps with round points to avoid damaging the vein. Clamp the vein at a location close to the kidney to allow the base of the vein to inflate a little bit (Fig. 1c).
Pierce the inflated vein slowly and then quickly and forcefully inject the solution in order to create a hydraulic force that will infiltrate the solution into all the different structures of the kidney (Fig. 1d; see Note 5).
Remove the needle while applying compression for 30 s with cotton Q tip for hemostasis.
Leave the vein clamp for 15 min to allow the rAAV particles to infect the renal cells and also prevent the rAAV from being flushed out through blood flow. Monitor the mouse for normal breathing, gagging, and for any bleeding (see Note 6).
Gently remove the clamp and continue to monitor the mouse for any bleeding for a couple of minutes (see Note 7).
If no bleeding is observed, suture the peritoneum using 5–0 absorbable vicryl suture and suture the skin using 5–0 nonabsorbable surgical Ethilon suture. Clean the skin with a cotton Q tip to remove any blood and apply tissue adhesive on the wound (see Note 8).
At this point, remove the anesthesia nose cone, place the mouse on a heat pad, and inject analgesic for recovery. When the mouse wakes up, it can be then return to its cage.
Fig. 1.
Different steps of the retrograde renal vein injection. (a) Incision in the upper-left quadrant of the mouse abdomen. (b) Exposure of the kidney and identification of the renal vein (arrow). (c) Positioning of the clamp with the kidney appearing darker after a proper clamping. (d) Injection of the rAAV solution in the segment of the vein between the clamp and the kidney
3.4. Visualization of the rAAV9-Luciferase Animals
As early as one week postinjection, animals injected with rAAV9-Luciferase can be visualized by bioluminescence using an In Vivo imaging system 10 min after intraperitoneal injection of luciferin (75 μL of a 30 mg/mL solution), (Fig. 2; see Note 9).
Fig. 2.
Comparison of mice after retrograde renal vein injection of rAAV9-CMV-luciferase and rAAV9-P1-luciferase visualized by IVIS. We can observe that even if the retrograde renal vein injection was performed in the left kidney, both left and right kidneys express the transgene, as well as other organs like the liver and spleen. In contrast, using a kidney-specific promoter (P1 = parathyroid hormone receptor “kidney-specific” promoter), we can significantly restrict luciferase expression in kidneys (right panel) compared to a ubiquitous promoter (left panel)
Footnotes
Prepare the 100 μL virus preparation in a 1.5 mL Eppendorf tube because the needle does not fit in a 0.5 mL tube. Keep the tube on ice until the injection.
It is important to modulate the rate of anesthetic delivered during the surgery to make sure it is only enough to maintain the mouse in deep sleep. Monitor how the mouse is breathing. If it is too slow, remove the nose cone temporarily in order for the animal to get fresh air and return to a normal breathing. Then, replace the nose cone to keep the mouse anesthetized.
After the incision, separate the skin from the peritoneum to facilitate the sutures.
Make sure there is no bubble.
At this point, it is possible to see if the injection was successfully done. One indication is that the kidney turns pale because the blood inside the organ has been flushed out by the rAAV-containing solution. However, if the liquid incorrectly went into the capsule instead of the kidney, it will form a bubble around the kidney. Also, if there is too much bleeding at this point, it might affect the efficiency because of the loss of the rAAV solution into the blood.
During the 15 min, it is normal for the kidney to turn very dark because of the blood flow interruption. Again, if breathing is too slow, remove the nose cone in order for the animal to get fresh air and go back to normal breathing.
If the mouse is bleeding after removing the clamp, apply compression with cotton Q tip until the bleeding stops.
Monitor the sutured skin for a couple of days because the animals have a tendency to scratch and reopen the wound.
Luciferase expression gives a global view of where the transgene is expressed. For a cell-specific expression analysis, it is recommended to use a fluorescent transgene (i.e., eGFP), and analyze the tissues by microscopy post-processing.
References
- 1.Devuyst O, Knoers NV, Remuzzi G et al. (2014) Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet 383(9931):1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knoll G (2008) Trends in kidney transplantation over the past decade. Drugs 68(Suppl 1):3–10 [DOI] [PubMed] [Google Scholar]
- 3.Davis CL, Delmonico FL (2005) Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol 16(7):2098–2110 [DOI] [PubMed] [Google Scholar]
- 4.Cecka JM (2008) Kidney transplantation in the United States. Clin Transpl 1–18 [PubMed] [Google Scholar]
- 5.Rocca CJ, Ur SN, Harrison F et al. (2014) rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther 21 (6):618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckmann CF, Abrams HL (1980) Renal venography: anatomy, technique, applications, analysis of 132 venograms, and a review of the literature. Cardiovasc Intervent Radiol 3 (1):45–70 [DOI] [PubMed] [Google Scholar]
- 7.Maruyama H, Hiquchi N, Nishikawa Y et al. (2002) Kidney-targeted naked DNA transfer by retrograde renal vein injection in rats. Hum Gene Ther 13(3):455–468 [DOI] [PubMed] [Google Scholar]
- 8.Chetboul V, Klonjkowski B, Lefebvre HP et al. (2001) Short-term efficiency and safety of gene delivery into canine kidneys. Nephrol Dial Transplant 16(3):608–614 [DOI] [PubMed] [Google Scholar]
- 9.Lipkowitz MS, Hanss B, Tulchin N et al. (1999) Transduction of renal cells in vitro and in vivo by adeno-associated virus gene therapy vectors. J Am Soc Nephrol 10(9):1908–1915 [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Agarwal A, Glushakova OY et al. (2003) Gene delivery in renal tubular epithelial cells using recombinant adeno-associated viral vectors. J Am Soc Nephrol 14(4):947–958 [DOI] [PubMed] [Google Scholar]
- 11.Takeda S, Takahashi M, Mizukami H et al. (2004) Successful gene transfer using adeno-associated virus vectors into the kidney: comparison among adeno-associated virus serotype 1-5 vectors in vitro and in vivo. Nephron Exp Nephrol 96(4):e119–e126 [DOI] [PubMed] [Google Scholar]
- 12.Qi YF, Li QH, Shenoy V et al. (2013) Comparison of the transduction efficiency of tyrosine-mutant adeno-associated virus serotype vectors in kidney. Clin Exp Pharmacol Physiol 40 (1):53–55 [DOI] [PMC free article] [PubMed] [Google Scholar]