Abstract
Cancer-associated thrombosis is a leading cause of non-cancer death in cancer patients and is comprised of both arterial and venous thromboembolism. There are multiple risk factors for developing VTE, including cancer type, stage, treatment, and other medical comorbidities, which suggests that the etiology of thrombosis is multifactorial. While cancer-associated thrombosis can be treated with anticoagulation, benefits of therapy must be balanced with the increased bleeding risks seen in patients with cancer. Although risk models exist for primary and recurrent VTE, additional predictors are needed to improve model performance and discrimination of high-risk patients. This review will outline the diverse mechanisms driving thrombosis in cancer patients, as well as provide an overview of biomarkers studied in thrombosis risk and important considerations when selecting candidate biomarkers.
Introduction
Cancer-associated thrombosis (CAT), which includes both arterial and venous thromboembolism (VTE), is a significant complication and leading cause of non-cancer death in ambulatory cancer patients receiving systemic therapy.1 VTE has been more widely studied than arterial thromboembolism (ATE), with cancer patients having 4–7 times the risk of VTE compared to the general populace, generally concentrated in the first few months after cancer diagnosis.2,3 Interestingly, the timing of ATE risk appears to be more uniform, with a cumulative incidence of 1–5% at 6-months after cancer diagnosis.4,5 More than half of deaths from pulmonary embolism are potentially preventable through appropriate prophylaxis, but risks of VTE must be balanced with increased bleeding risks in cancer patients while on anticoagulation.6,7 Known risk factors for VTE include cancer type, stage, treatment regimen, and comorbid disease with rates being highest in pancreatic, brain, and stomach cancer.8 This variety of risk factors likely reflects the multifactorial etiology of thrombosis in cancer. New advances in research have deepened the mechanistic knowledge driving CAT and have identified new targets for study.
Management of CAT is an evolving field, especially given the availability of new direct oral anticoagulants, and there is continued research on what are the best practices for improving CAT outcomes. Risk assessment models can guide clinical decision-making and are available for predicting primary VTE and recurrent VTE and include the Khorana, Vienna CATS, PROTECHT, CONKO, ONKOTEV, COMPASS-CAT, TiC-Onco, CATS nomogram, and Ottawa scores. 9–17 However, these models have mixed performances and require further refinement to improve discrimination and identification of high-risk patients.
Biomarkers are measurable, biologic parameters that can be used to improve prediction, diagnostic, and prognostic models of disease. In CAT, they have been extensively studied for prediction of primary VTE risk (Table 1), with fewer studies on recurrent VTE (Table 2) and primary ATE (Table 3). No studies have assessed biomarker use in predicting risk of recurrent ATE.
Table 1.
Biomarker studies assessing primary venous thromboembolism risk in cancer patients
| Biomarker | Study Design | Cancer Type | Number of patients | Biomarker Cutoff or Numeric Variable | Statistic | 95% CI | Reference |
|---|---|---|---|---|---|---|---|
| Leukocyte count | |||||||
| Prospective | Mixed | 2701 | >11 × 109/| | OR 2.2 | 1.2–4 | 9 | |
| Prospective | Mixed | 4405 | >11 × 109/l | HR 2.10 | 1.30–3.40 | 209 | |
| Prospective | Solid tumor | 665 | Per 2-fold increase | HR 1.15 | 0.63–2.11 | 210 | |
| Platelet count | |||||||
| Prospective | Mixed | 2701 | ≥350 × 109/l | OR 1.8 | 1.1–3.2 | 9 | |
| Prospective | Mixed | 4405 | ≥350 × 109/l | HR 1.83 | 1.19–2.83 | 209 | |
| Prospective | Solid tumor | 665 | ≥443 × 109/l | HR 3.50 | 1.52–8.06 | 210 | |
| Prospective | Mixed | 1023 | ≥350 × 109/l | OR 2.53 | 1.35–4.74 | 14 | |
| D-dimer | |||||||
| Prospective | Colorectal | 176 | >0.3 μg/ml | HR 6.53 | 1.58–27.0 | 211 | |
| Prospective | Gynecologic | 267 | >5 μg/ml | OR 1.19 | 1.04–1.37 | 212 | |
| Prospective | Mixed | 124 | >0.65 μg/ml | HR 4.04 | 1.22–13.3 | 213 | |
| Prospective | Mixed | 112 | Per 2-fold increase | HR 1.76* | 1.32–2.35 | 214 | |
| Prospective | Mixed | 821 | ≥1.44 μg/ml | HR 1.8 | 1.0–3.2 | 215 | |
| Retrospective | Lung | 108 | >1.50 μg/ml | HR 11.0 | 2.62–46.2 | 216 | |
| Prospective | Pancreatic | 140 | ≥2.16 μg/ml | HR 4.9 | 1.0–23.1 | 81 | |
| Prospective | Mixed | 946 | Per 10 μg/ml increase | sHR 1.31 | 1.00–1.73 | 112 | |
| Soluble P-selectin | |||||||
| Prospective | Mixed | 112 | Per 2-fold increase | HR 2.44* | 1.31–4.53 | 214 | |
| Prospective | Glioma | 76 | n/a | HR 1.068 | 1.017–1.122 | 132 | |
| Prospective | Mixed | 797 | Per 10 ng/ml increase | HR 1.19 | 1.07–1.33 | 134 | |
| Prospective | Mixed | 687 | ≥53.1 ng/ml | HR 2.6 | 1.4–4.9 | 221 | |
| Prospective | Mixed | 946 | Per 10 ng/ml increase | sHR 1.05 | 1.00–1.73 | 112 | |
| Extracellular vesicles | |||||||
| Prospective | Mixed | 728 | ≥4.62 nM PS | HR 0.95 | 0.55–1.64 | 33 | |
| Tissue factor-positive extracellular vesicles | Prospective | Pancreas | 60 | Per 2-fold increase | HR 1.5 | 1.0–2.4 | 55 |
| Brain | 119 | HR 0.9 | 0.7–1.3 | ||||
| Stomach | 43 | n/a | n/a | ||||
| Colorectal | 126 | HR 0.9 | 0.6–1.6 | ||||
| Prospective | Multiple myeloma | 122 | >11.8 fM Xa/min | OR 1.4 | 0.4–4.7 | 79 | |
| Prospective | Mixed | 648 | ≥13% | sHR 1.0 | 0.99–3.8 | 80 | |
| Retrospective | Pancreaticobiliary | 117 | ≥2.5 pg/ml | OR 4.78 | 1.64–13.98 | 56 | |
| Prospective | Pancreatic | 140 | ≥2.37 pg/ml | HR 10.5 | 1.5–72.4 | 81 | |
| Meta-analysis | Solid tumor | n/a | n/a | OR 1.76 | 1.21–2.56 | 82 | |
| Neutrophil extracellular traps | |||||||
| H3Cit | Prospective | Mixed | 946 | Per 100 ng/ml increase | sHR 1.11 | 1.03–1.20 | 112 |
| Cell free DNA | Prospective | Mixed | 946 | Per 100 ng/ml increase | sHR 1.03* | 0.96–1.10 | 112 |
| Nucleosome | Prospective | Mixed | 946 | Per 1 unit increase | sHR 0.95* | 0.89–1.02 | 112 |
| Neutrophil-to-lymphocyte ratio | Prospective | Solid tumor | 810 | >3 | HR 1.37 | 0.52–3.57 | 113 |
| Prospective | Solid tumor | 1469 | Per 2-fold increase | sHR 1.2 | 1.0–1.4 | 114 | |
| Retrospective | Gastric | 112 | >3 | HR 0.8* | 0.3–2.5 | 6 | |
| Inflammatory molecules | |||||||
| IL-1β | Prospective | Mixed | 726 | Per 2-fold increase | HR 0.98* | 0.80–1.20 | 131 |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 1.046* | 0.732–1.495 | 132 | |
| IL-3 | Prospective | Mixed | 726 | Per 2-fold increase | HR 0.99* | 0.84–1.18 | 131 |
| IL-4 | Prospective | Mixed | 726 | Per 2-fold increase | HR 1.11* | 0.86–1.42 | 131 |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.907* | 0.634–1.298 | 132 | |
| IL-6 | Retrospective | Ovarian | 200 | 5–19.9 pg/ml | HR 7.98 | 0.99–64.0 | 129 |
| ≥20 pg/ml | HR 8.90 | 1.04–76.0 | |||||
| Prospective | Diffuse large B cell lymphoma | 322 | >Detection limit | sHR 1.07 | 0.56–2.06 | 130 | |
| Prospective | Mixed | 726 | Per 2-fold increase | HR 1.08* | 0.98–1.20 | 131 | |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.894* | 0.602–1.328 | 132 | |
| IL-8 | Prospective | Mixed | 726 | Per 2-fold increase | HR 1.06* | 0.94–1.20 | 131 |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.918* | 0.585–0.585 | 132 | |
| IL-10 | Prospective | Diffuse large B cell lymphoma | 322 | ≥Median value | sHR 1.66 | 0.84–3.26 | 130 |
| Prospective | Mixed | 726 | Per 2-fold increase | HR 1.01* | 0.83–1.22 | 131 | |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.822* | 0.505–1.340 | 132 | |
| IL-11 | Prospective | Mixed | 726 | Per 2-fold increase | HR 0.90* | 0.76–1.06 | 131 |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.621* | 0.284–1.357 | 132 | |
| CCL3 | Prospective | Mixed | 726 | Per 2-fold increase | HR 0.99* | 0.90–1.10 | 131 |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.348 | 0.146–0.830 | 132 | |
| TNF-α | Prospective | Diffuse large B cell lymphoma | 322 | ≥0.285 pg/ml | sHR 0.77* | 0.46–1.29 | 130 |
| Retrospective | Metastatic colorectal | 45 | <6.6 pg/ml | HR 0.17 | 0.04–0.75 | 133 | |
| Prospective | Glioma | 76 | Per doubling | HR 1.097* | 0.697–1.727 | 132 | |
| Soluble VEGF | Prospective | Mixed | 797 | Per 10 pg/ml increase | HR 1.04 | 1.00–1.09 | 134 |
| Prospective | Glioma | 76 | Per 2-fold increase | HR 0.995* | 0.640–1.548 | 132 | |
| Podoplanin (tissue) | Prospective | Brain | 213 | <50% expression | HR 2.44 | 0.73–8.17 | 143 |
| 50–70% expression | HR 3.28 | 0.91–11.75 | |||||
| >70% expression | HR 5.71 | 1.52–21.36 | |||||
| Retrospective | Glioma | 165 | ≥30% expression | OR 3.423 | 1.083–10.814 | 151 | |
| Isocitrate dehydrogenase 1 | Prospective | Brain | 213 | IDH1 R132H mutation | HR 0.11 | 0.01–0.83 | 150 |
| Retrospective | Glioma | 165 | IDH1 R132H mutation | OR 0.101 | 0.010–0.975 | 151 | |
Denotes a univariable analysis. All other statistics are given for multivariable analyses. CCL3, chemockine ligand 3; CI, confidence interval; HR, hazard ratio; IL, interleukin; OR, odds ratio; PS, phosphatidylserine; sHR, subdistribution hazard ratio; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Table 2.
Biomarker studies assessing recurrent venous thromboembolism risk in cancer patients
| Biomarker | Study Design | Cancer Type | Number of patients | Biomarker Cutoff or Numeric Variable | Statistic | 95% CI | Reference |
|---|---|---|---|---|---|---|---|
| Tissue factor | |||||||
| Prospective | Mixed | 805 | >64.6 pg/ml | sHR 3.4 | 2.1–5.5 | 225 | |
| C-reactive protein | |||||||
| Prospective | Mixed | 482 | >75 mg/l | sHR 2.3 | 1.2–4.4 | 225 | |
| Prospective | Mixed | 99 | >4.5 mg/l | sHR 9.82 | 1.86–51.7 | 227 | |
| Soluble P-selectin | |||||||
| Prospective | Mixed | 117 | >136 ng/ml | sHR 4.4 | 1.3–16 | 226 | |
| D-dimer | |||||||
| Prospective | Mixed | 117 | >4.0 μg/ml | sHR 2.5* | 0.76–7.9 | 226 | |
| Prospective | Mixed | 111 | >600 ng/ml >age × 10 ng/ml |
sHR 5.81 sHR 5.11 |
1.06–31.72 1.032–25.3 |
227 | |
Denotes a univariable analysis. All other statistics are given for multivariable analyses. CI, confidence interval; sHR, subdistribution hazard ratio.
Table 3.
Biomarker studies assessing primary arterial thromboembolism risk in cancer patients
| Biomarker | Study Design | Cancer Type | Number of patients | Biomarker Cutoff or Numeric Variable | Statistic | 95% CI | Reference |
|---|---|---|---|---|---|---|---|
| Neutrophil count | Prospective | Mixed | 1883 | >4.9 × 109/l | sHR 2.4 | 1.3‐4.6 | 228 |
| Soluble P-selectin | Prospective | Mixed | 1883 | >46.3 ng/ml | sHR 2.1 | 1.2‐3.8 | 228 |
| D-dimer | Prospective | Mixed | 1883 | Per 2-fold increase | sHR 1.1 | 0.9‐1.4 | 228 |
| Neutrophil-to-lymphocyte ratio | Prospective | Mixed | 1469 | Per 2-fold increase | sHR 1.2 | 0.9–1.6 | 114 |
| Platelet-to-lymphocyte ratio | Prospective | Mixed | 1469 | Per 2-fold increase | sHR 1.1 | 0.7–1.5 | 114 |
| Citrullinated histone H3 | Prospective | Mixed | 957 | Per 100 ng/ml increase | sHR 1.0* | 0.7–1.4 | 229 |
| Cell free DNA | Prospective | Mixed | 957 | Per 100 ng/ml increase | sHR 1.0* | 0.9–1.2 | 229 |
| Nucleosome | Prospective | Mixed | 957 | Per unit increase | sHR 1.1* | 1.0–1.2 | 229 |
Denotes a univariable analysis. All other statistics are given for multivariable analyses. CI, confidence interval; sHR, subdistribution hazard ratio.
While many potential biomarkers exist, selection of candidate biomarkers require consideration of scalability and practicality as their use transitions from research to clinical practice. Ideal biomarkers are noninvasive, low cost, simple to perform, accurate, and discriminative.18 During clinical practice, physicians may avoid ordering invasive or costly tests to reduce physical and fiscal patient burdens. Complex tests that rely heavily on skilled laboratory personnel or expensive technology can limit accuracy of biomarker measurement and reduce testing availability. Finally, biomarkers should be able to adequately separate patients with different risks. Biomarkers and their associated models must balance both simplicity, which encourages clinical use, with complexity and enhanced model performance to improve clinical outcomes.19
This review will cover key mechanisms underlying CAT and relevant biomarkers for risk assessment, along with associated challenges in biomarker assays (Table 4). Where appropriate, both mechanisms and biomarker roles will be described, but some topics will be limited to mechanistic or biomarker research. Detailed descriptions of CAT epidemiology, risk factors, and treatment have been previously described elsewhere and are beyond this scope of this review.8,20–24
Table 4.
Comparison of biomarker assays and challenges for clinical use
| Biomarker | Assay | Clinical Assay | Comments | Reference |
|---|---|---|---|---|
| Leukocyte count | ||||
| CBC | Yes | Routinely acquired in clinical practice | ||
| Platelet count | ||||
| CBC | Yes | Routinely acquired in clinical practice | ||
| D-dimer | ELISA | Yes | ||
| ELFA | Yes | Reference units not standardized | 217 | |
| Latex-based assay | Yes | (1 FEU ~ 2 DDU) | ||
| Whole blood assay | Yes | |||
| Soluble P-selectin | ELISA | No | ||
| EVs | Flow cytometry | No | ||
| Electron microscopy | No | Subject to varied pre-analytical challenges (blood | ||
| Confocal microscopy | No | collection trauma, centrifugation protocol) | 25,34,36 | |
| Atomic force microscopy | No | See ISEV and ERCC for more details and protocols | ||
| TF+ EV | ||||
| Flow cytometry | No | Monocytes may be contaminating TF source | 67,71,85,86 | |
| ELISA | No | Poor anti-TF antibody specificity Monocytes may be contaminating TF source | ||
| Factor Xa generation | No | Variable specificity depending on exact methods May be labor intensive with high interassay variability | 67,71,83 | |
| Fibrin generation | No | Monocytes may be contaminating TF source | 67,71,84 | |
| H3Cit | ||||
| ELISA | No | 116 | ||
| Inflammatory markers | ELISA ELISPOT |
Yes Yes |
||
| Subject to pre-analytical challenges (blood | ||||
| PCR | Yes | collection tubes, processing time, freeze-thaw use, | 136 | |
| Multiplexed assay | Yes | storage) | ||
| Podoplanin (tissue) | ||||
| Immunohistochemistry | Yes | Requires invasive tissue collection | ||
| IDH1 (tissue) | ||||
| Immunohistochemistry | Yes | Requires invasive tissue collection | ||
| miRNAs | ||||
| qRT-PCR | Yes | Biased RNA isolation and library kits can change | ||
| Hybridization assay | Yes | results | 36,164–166 | |
| NGS | Yes | Lack of endogenous normalization controls See ERCC for recommended protocols | ||
CBC, complete blood count; DDU, D-dimer unit; ELFA, enzyme-linked immunofluorescence assay; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immune spot assay; EV, extracellular vesicle; FEU, fibrinogen equivalent unit; IDH1, isocitrate dehydrogenase 1; miRNA, micro RNA; NGS, next-generation sequencing; qRT-PCR, quantitative reverse transcription polymerase chain reaction; TF+ EV, tissue factor-positive extracellular vesicles
Extracellular Vesicles
Mechanism
Extracellular vesicles (EVs) are defined by the International Society for Extracellular Vesicles (ISEV) as “the generic term for naturally released particles from the cell that are delimited by a lipid bilayer and cannot replicate”.25 Historically, the literature has used the terms microvesicles or microparticles to generally refer to EVs that are ≤1000 nm in size and derived from plasma membranes of cells. However, this classification is controversial and we will refer to these particles as EVs in this review, in accordance with ISEV guidelines. Cancer cells are capable of shedding EVs which can contain cell-specific surface proteins and cargo such as RNAs, DNAs, proteins, and lipid rafts.26–28
Intrinsic procoagulant activity of EVs stem from their negatively charged plasma membrane which serves as a catalytic surface for the vitamin K-dependent clotting factors: VII, IX, X, and prothrombin. This negative charge results from the asymmetric externalization of aminophospholipids, such as phosphatidylserine, from the inner plasma membrane leaflet during the formation of EVs by calcium-dependent scramblases, such as TMEM16F.29–31 Phosphatidylserine on EVs binds clotting factors through their positively charged γ-carboxyglutamic acid domains and increases catalytic efficiency of phospholipid-dependent coagulation reactions such as the tenase and prothrombinase complexes by 3 orders of magnitude.32
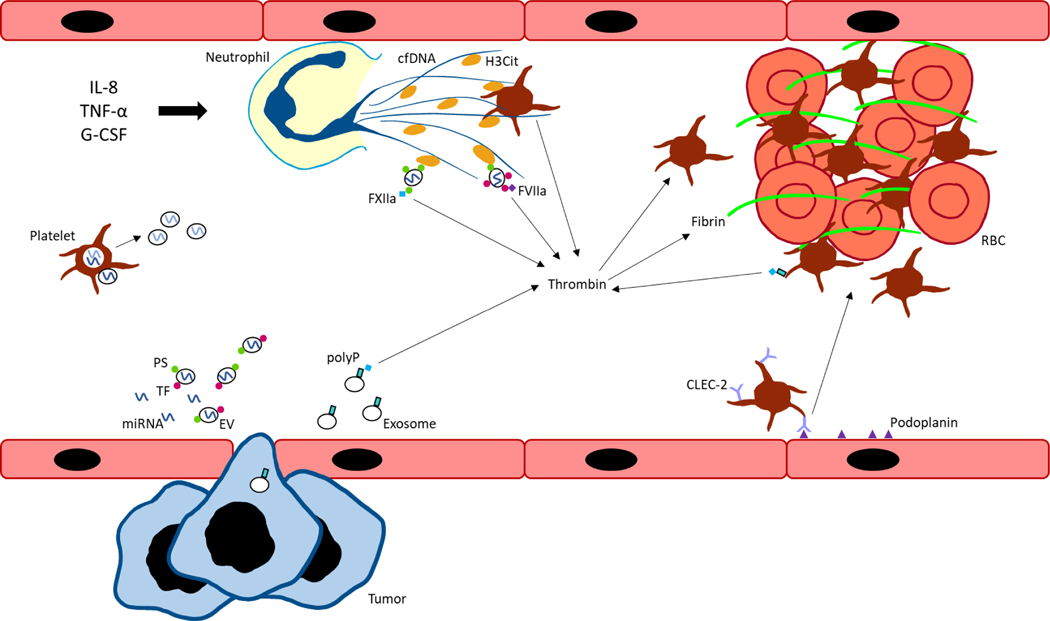
EVs also contain differential procoagulant activity depending on their mechanism of formation and cargo contents. Prothrombotic surface proteins and cargo include tissue factor (TF), inflammatory molecules, and podoplanin. A simplified model of key mechanisms occurring in blood are presented in Figure 1.
Figure 1. Graphical summary of key mechanisms driving cancer-associated thrombosis.
Interleukin-8 (IL-8), tumor necrosis factor-α (TNF- α), and granulocyte-colony stimulating factor (G-CSF) stimulate neutrophils to form neutrophil extracellular traps (NETs) containing cell free DNA (cfDNA) and citrullinated histone H3 (H3Cit). Extracellular vesicles (EVs), derived from tumor or host cells, express negatively charged phosphatidylserine (PS) which bind histones in NETs. EVs also express tissue factor (TF) which binds factor VIIa (FVIIa) to initiate the extrinsic pathway of coagulation and generate thrombin. Tumor cells can also secrete exosomes expressing polyphosphate (polyP) to activate the contact pathway. Similarly, platelets can express polyP to initiate contact pathway activation, as well as interact with cfDNA and histones present in NETs for thrombin and thrombus formation. Podoplanin-expressing activated endothelial cells can bind C-type lectin-like receptor 2 (CLEC-2) on platelets to induce platelet activation and aggregation. Inhibitory microRNAs (miRNAs) can be secreted extracellularly in a protein- or EV-bound form. Platelets and other host cells can take up miRNAs which regulate gene expression of coagulation factors and re-secrete miRNAs into plasma. FXIIa, factor XIIa; RBC, red blood cell.
Biomarker Use in Primary VTE Risk
Studies on VTE risk with total EV quantities are limited. In a prospective study of newly diagnosed and progressive disease cancer patients, procoagulant activity of EVs due to the exposure of negatively charged phospholipids, as quantified by a prothrombinase assay, was not significantly associated with VTE risk (HR [hazard ratio] 0.95, 95% CI [confidence interval] 0.55–1.64).33
Testing of EVs falls broadly into phenotypic or functional assays and include flow cytometry, electron microscopy, confocal microscopy, and atomic force microscopy in addition to function-specific assays. Phenotypic assays allow for quantification and assessment of morphology and cellular origin of EVs. Flow cytometry is the most common assay for EV quantification and assessment of cellular origin. Electron microcopy can study membrane morphology and composition, while confocal microscopy can evaluate cellular origin. Atomic force microscopy measures interaction forces between a cantilever tip and the test sample, allowing for three-dimensional topography construction as well as quantification.34 Functional assays commonly require extraction of EVs by capture techniques or ultracentrifugation prior to study.
While research on EVs as biomarkers has rapidly grown, reproducible and reliable testing of EVs has been hampered by both pre-analytical and analytical obstacles. Differences in pre-analytical steps, such as blood collection, sample processing, and handling and storage can severely impact the testing results. For example, traumatic blood collection can activate platelets and falsely increase EV quantity, while different centrifugation speeds will impact extraction efficiency of EVs.34,35
Analytical challenges are intrinsic to the assay itself, such as older flow cytometers having limited capability to detect smaller EVs.35 These obstacles, combined with the sheer diversity of assay options makes EV testing for biomarkers candidacy difficult as they invoke problems of assay accuracy and limited comparability of results depending on assay selection. Given the complicated landscape of EV experimentation, ISEV has published guidelines for studying EVs with the most recent update in 2018 and protocols for biofluid collection and EV isolation/enrichment are available through the Extracellular RNA Communication Consortium (ERCC).25,36
Tissue Factor/Tissue Factor-Positive Extracellular Vesicles
Mechanism
TF is a procoagulant protein that initiates the extrinsic pathway of the coagulation cascade, leading to thrombin and fibrin clot formation. TF is expressed on perivascular cells such as fibroblasts and vascular smooth muscle cells and binds factor VII to stabilize its catalytic domain for downstream activation of factors IX and X. TF expression can also be induced in monocytes and endothelial cells upon activation with ligands such as lipopolysaccharide and released in TF extracellular vesicles (TF+ EVs), although small subsets of resting monocytes have also been shown to express TF.37–41 In contrast, malignant tissues can constitutively express TF and release TF+ EVs.42–48
Clinical studies of cancer patient plasma have shown elevated TF and TF+ EV expression and activity, including in breast, colorectal, prostate, gastric, brain, and pancreatic cancers.49–56 However, the source of TF+ EVs in cancer patients is unclear and likely results from multiple etiologies, including host and malignant cells.51,52,57 Studies in nonmalignant settings have shown generation of TF+ EVs from endothelial cells, monocytes, and macrophages.58 Platelet TF expression and TF+ EV generation is controversial with discrepant results of expression and function being reported.59–66 Some of these conflicting results may be attributed to the use of non-specific anti-TF antibodies and Xa functional assays.67 This issue is further complicated by monocyte-derived TF which can be transferred to platelets via EVs and can represent a contaminating source of TF if not adequately removed from the samples of interest.67–71 Together, these findings suggest that while platelets may not have intrinsic expression of TF, they can acquire TF through external sources. Notably, although TF may be present on platelet-derived EVs, the thrombogenicity of these EVs appears to be driven primarily by exposed phosphatidylserine, rather than TF.72 In contrast, thrombin formation from EVs derived from lipopolysaccharide-stimulated monocytes was effectively inhibited by anti-TF antibodies, suggesting that TF on monocyte-derived EVs plays a greater thrombogenic role than on platelet-derived EVs. Similar to platelets, granulocytes have been shown to take up external TF from monocytes.73,74
Despite elevated expression and activity in malignancy, consistent association of TF or TF+ EVs with VTE have only clearly been shown in pancreatic cancer.75,76 While studies of thrombotic risk in cancer patients with TF or TF+ EVs have primarily focused on VTE, a recent study has shown that TF+ EVs were elevated in patients with cancer-related stroke compared to those with cancer-unrelated stroke.77 However, TF+ EVs did not mediate an association between cancer-cell derived EVs and D-dimer levels by statistical path analysis; effects of TF+ EVs on cancer-related stroke were not modeled.
In pancreatic cancer mouse models, platelet-dependent and independent pathways have been proposed for TF+ EV induced thrombosis. In platelet-dependent thrombosis, TF+ EVs localize at thrombus sites by binding markers such as P-selectin to activate and aggregate platelets via a TF-dependent pathway.45,47 Recently, a platelet- and leukocyte-independent mechanism was shown that requires host TF to initiate thrombus formation for propagation by phosphatidylethanolamine-dependent TF+ EVs.78
Biomarker Use in Primary VTE Risk
In prospective studies of mixed cancer and multiple myeloma patients, TF+ EV activity as measured by a TF-dependent factor Xa generation assay did not significantly correlate with VTE risk.55,79 Similarly, a prospective study of mixed cancer patients using a fibrin generation assay for TF+ EV activity showed no association with increased VTE risk (sHR [subdistribution hazard ratio] 1.94, 95% CI 0.99–3.80).80 However, a retrospective study showed that pancreaticobiliary patients had 4.78 times the risk of developing VTE (95% CI 1.64–13.98) with TF+ EV activity ≥ 2.5 pg/ml, as measured by TF-dependent factor Xa generation.56 In a prospective study of pancreatic cancer patients, TF+ EV activity ≥ 2.37 pg/ml by TF-dependent factor Xa generation increased VTE risk by 10.5-fold, although precision of the estimated risk was low (95% CI 1.5–72.4).81 A meta-analysis of cancer patients showed an increased 76% risk of VTE with elevated TF+ EV quantity or function (95% CI 1.21–2.56).82 Subgroup analyses revealed that the increased risk was driven by elevated TF+ EV levels on flow cytometry assays (OR [odds ratio] 2.97, 95% CI 1.81–4.86) rather than activity assays (OR 1.25, 95% CI 0.95–1.65).82
TF+ EV assays are often based on factor Xa generation using a chromogenic substrate after incubation with factors VII and X in the presence of anti-TF antibodies. These assays can incorporate exogenous addition of negatively charged phospholipids, which may increase total factor Xa generation in a TF-independent manner.83 Another TF+ EV functional assay is the fibrin generation test which measures fibrin formation using optical densitometry in the presence of anti-factor VIIa antibodies.84
When studying specific sources of TF+ EV, a serious pre-analytical challenge includes monocytes which can be a major source of contaminating TF and care should be taken to purify samples, such as by using microbeads bound to anti-CD14 antibodies.67,71 In regards to analytical challenges, flow cytometry assays are not recommended as they suffer from poor specificity of anti-TF antibodies, which bind non-specifically at high concentrations and may cross-react with phosphatidylserine on cell membranes.67,85,86 Enzyme-linked immunosorbent assays (ELISA) suffer from similar poor antibody specificity and selectivity.67 Functional assays of TF+ EV activity have variable specificity depending on the methods used, as TF-independent mechanisms can also generate factor Xa. One method to limit TF-independent Xa generation in these assays is to add prothrombin, FVa, and phospholipids and reduce incubation time to 3–4 minutes, rather than the standard minimum incubation time of 60 minutes.67,87 An alternative method avoids adding exogenous phospholipids, but is time consuming with high interassay variability and therefore not appropriate for clinical use.83,88
Neutrophils and Neutrophil Extracellular Traps
Mechanism
Activated neutrophils can form neutrophil extracellular traps (NETs), which are extracellular fibers containing histones, DNA, and proteins derived from neutrophilic granules.89 While they were originally discovered in the role of host defense, neutrophils and NET formation, also known as NETosis, have since been proposed to be a mediator of CAT. During thrombus formation, neutrophils are the first leukocyte to arrive at the site of vessel injury, only secondary to TF.90,91 Stimulation of neutrophils by multiple factors including activated endothelial cells, activated platelets, interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and granulocyte-colony stimulating factor (G-CSF) can induce NETosis, which serves as a scaffold for platelets, red blood cells, and other procoagulants.92–94 During NETosis, neutrophils swell and lose their nuclear membrane integrity leading to mixing of chromatin, histones, and granule-derived proteins.95 Peptidylarginine deiminase 4 is a required nuclear enzyme for NETosis that citrullinates arginine residues of histones H3 and H4 and causes dissociation of chromatin from histones due to the loss of electrostatic force.96
Histones present in NETs activate platelets through Toll-like receptors 2 and 4, leading to thrombin generation.97 Furthermore, histones induce endothelial cells to release von Willebrand factor (vWF) which binds glycoprotein Ib (GPIb) on platelets, leading to vessel wall adhesion and clot initiation.98,99 Histone-independent activation of platelets can also occur via direct binding of platelets to cell free DNA (cfDNA), as well as cathepsin G activity.100 Cathepsin G and neutrophil elastase are present on NETs and inhibit TF pathway inhibitor to enhance TF and factor XII-dependent fibrin and thrombus formation.101,102 Neutrophils have also been shown to both release TF and EVs.103–106 Neutrophil- and tumor-derived EVs scaffold to NETs through phosphatidylserine-histone interactions and contribute to thrombosis.106–108
In mouse studies of cancer, tumor-bearing mice were associated with increased neutrophil counts, G-CSF, cfDNA, citrullinated histone H3 (H3Cit), and NET formation.94,109 Thrombi from a mouse model of human pancreatic cancer also contained elevated levels of H3Cit, cfDNA, and the neutrophil marker Ly6G, and were reduced in size with neutrophil depletion and DNase treatment.109 Tumor-derived EVs also increased NET formation from mouse neutrophils treated with G-CSF and injection of these EVs increased venous thrombus formation in vivo.108
NET markers such as nucleosomes, cfDNA, and H3Cit are also elevated in cancer patients.110–112 In a case-control study of patients with ischemic stroke, elevated post-stroke levels of H3Cit were associated with positive cancer status, elevated thrombin-antithrombin complexes, and soluble P-selectin levels.111
Biomarker Use in Primary VTE Risk
Research on NET-related biomarkers in CAT has just begun with a prospective study of cancer patients showing that each 100 ng/ml increase in plasma H3Cit was associated with an 11% increase in VTE (95% CI 1.03–1.20).112 Upon subgroup analysis by cancer type, only pancreatic and lung cancers showed a significantly increased risk of VTE with elevated H3Cit levels. However, these results should be cautiously interpreted as the study was underpowered for subgroup analysis. Elevated nucleosome and cfDNA levels were not significantly correlated with VTE. Studies on neutrophil-to-lymphocyte ratios (NLR) have also been explored in cancer patients but have failed to show significance.113–115 Plasma H3Cit can be measured by ELISA, but clinical access to this assay is not yet widely available.116
Inflammatory Molecules
Mechanism
Cancer cells synthesize and secrete a variety of inflammatory molecules that have diverse procoagulant capabilities, including TNF-α, IL-1β, IL-6, IL-8, and vascular endothelial growth factor (VEGF). TNF-α, IL-1β, and IL-6 can induce TF expression and activity from endothelial cells.117,118 TNF-α has also been shown to increase EV release from cancer cells, with elevated TF activity seen on EVs derived from lung, pancreatic, and colon cancer.119 TNF-α and IL-1β can also upregulate plasminogen activator inhibitor-1 (PAI-1), although fibrinolysis was not completely abolished in cancer patients after TNF-α infusion.120,121 IL-1β has been shown to be synthesized by activated platelets from pre-messenger RNA stored in polysomes which are spliced to messenger RNA (mRNA) upon activation and can be released in platelet EVs to interact with other inflammatory cells.122 Platelets can also express IL-1 receptor and respond to IL-1β in an autocrine loop to amplify platelet activation.123 IL-8 expression can be induced by fibrin and has been shown to induce granulocyte chemotaxis and NET formation.124–126 Finally, VEGF can upregulate TF expression in endothelial cells via the transcription factor early growth response 1.127 TF has also been shown to induce VEGF expression in tumor cells and may represent a potential positive procoagulant feedback loop.128
Biomarker Use in Primary VTE Risk
Data on the use of inflammatory molecules for VTE risk have been mixed. A retrospective study demonstrated ovarian cancer patients with plasma IL-6 levels ≥20 pg/ml had 8.9 times the risk of developing VTE, although precision of the increased VTE risk is low (95% CI 1.04–76.0).129 In contrast, a prospective study on diffuse large B cell lymphoma patients showed no association VTE risk with IL-6, IL-10, or TNF-α.130 Similarly, a prospective study of solid tumor patients showed no association of IL-1β, IL-3, IL-4, IL-6, IL-8, IL-10, IL-11, or chemokine ligand 3 (CCL3) with VTE.131 In glioma patients, for each two-fold increase in pg/ml of CCL3, VTE risk was reduced approximately 60–80%, depending on other variables in the Cox regression model.132 In the same study, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-11, TNF-α, and VEGF were not found to be significantly associated with VTE.132 However in a study of metastatic colorectal cancer patients, low pre-chemotherapy serum TNF-α levels (<6.6 pg/ml) decreased VTE risk by 83% (95% CI 0.04–0.75), but the estimated effect is imprecise due to the wide CI.133 Another study showed that for every 10 pg/ml increase in soluble VEGF, VTE risked increased by 4% (95% CI 1.00–1.09) in cancer patients.134
Inflammatory markers are common candidates for biomarkers and can be assayed with ELISA, enzyme-linked immune spot assays (ELISPOT), polymerase chain reaction (PCR), and multiplexed bead- or protein-based arrays.135 These markers are advantageous as quantitative assays are readily available in clinical practice, and many markers can be assayed at once through multiplexing. However, cytokines have short half-lives, diurnal variation, and are prone to degradation with multiple freeze-thaw cycles. Furthermore, sample preparation, such as collecting serum or plasma and anticoagulation choice can affect cytokine synthesis and release after blood collection.136 When considering these markers for risk models in clinical practice, blood collection procedures should be developed in accordance with the assays used. EDTA-treated tubes provide consistent results for most cytokine immunoassays, although citrate can also be considered. If using bioassays, blood should be collected in tubes for serum preparation or low-level preservative free-heparin tubes for plasma preparation. Blood should be processed immediately within two hours after collection and samples divided into small aliquots to minimize need for repeated freezing and thawing. Freeze-thaw cycles should be limited to no more than 3 cycles and stored samples should be used within approximately 4 years, depending on the specific cytokines of interest.136,137
Podoplanin and Isocitrate Dehydrogenase 1
Mechanism
Podoplanin is a transmembrane glycoprotein that is normally present in a variety of normal tissue, including lymphatic endothelial cells, kidney podocytes, type 1 alveolar lung cells, and lymph node fibroblastic reticular cells, but not vascular endothelial cells.138,139 Expression is also seen in cancer cells such as squamous cell carcinomas, germinal tumors, brain tumors, mesotheliomas and acute promyelocytic leukemia.138–140 Podoplanin binds to the C-type lectin-like receptor 2 (CLEC-2) on platelets to induce platelet activation and aggregation.141,142
Elevated expression of podoplanin has been observed in primary brain cancer tissue as well as in podoplanin-containing EVs in the blood of pancreatic cancer patients.143,144 A mouse melanoma (B16F10) model has shown podoplanin-dependent thrombus formation through CLEC-2-podoplanin interaction.145 While podoplanin can arise from cancer cells, it can also be abnormally induced by thromboinflammation in host vascular cells. In a mouse model of inferior vena cava stenosis, podoplanin was expressed in the inferior vena cava vessel wall after 48 hours of stenosis.146
Isocitrate dehydrogenase 1 (IDH1) is commonly mutated in gliomas and the mutant form has been associated with decreased VTE in glioma patients compared to the IDH1 wild-type.147 Mutated IDH1 can produce D-2-hydroxyglutarate to reduce platelet aggregation and clotting activity in vitro.147 IDH1 mutants are also associated with increased F3 promoter hypermethylation and decreased F3 mRNA transcripts, leading to decreased TF expression in vitro, which could be reversed with a demethylating agent.147,148 Notably, IDH1 mutations are associated with hypermethylation in human gliomas, but not in acute myeloid leukemia, cholangiocarcinoma, or melanoma, suggesting that the protective effects of IDH1 mutation are specific to the brain cancer setting.149
Biomarker Use in Primary VTE Risk
Studies using brain cancer patients from the Vienna CATS cohort have shown increased risk of VTE with >70% podoplanin expression in tumor tissue (HR 5.71, 95% CI 1.52–21.36) and a score ≥3 based on a combined scoring system of podoplanin tissue expression and IDH1 mutant status (3 points: HR 8.4, 95% CI 1.04–67.58; 4 points: HR 13.28, 95% CI 1.65–106.97).143,150 However, the reported CIs are wide and demonstrate low precision of estimated VTE risk. Furthermore, because the studies do not report the timing of VTE events relative to acquisition of tumor sample for podoplanin expression and IDH1 genotyping, causative conclusions about the effect of podoplanin and IDH1 on VTE risk cannot be made. In a separate study of newly diagnosed glioma patients, podoplanin tissue positivity (≥30% expression) and IDH1 mutant status were significantly correlated with subsequent VTE risk with low precision of estimated risk (OR 3.423, 95% CI 1.083–10.814; OR 0.101, 95% CI 0.010–0.975, respectively).151
Podoplanin and IDH1 tissue expression are assessed histologically after immunostaining of tumor tissue. While these assays can be completed in the clinical setting, the invasive nature of tissue collection, especially in the setting of brain cancer, can delay and limit use of risk models.
Extracellular microRNAs
Mechanism
MicroRNAs (miRNAs) are small noncoding RNAs that are about 20–22 nucleotides in length and function as inhibitors of gene expression. miRNAs bind to complementary sequences on target mRNAs at 3’ untranslated regions and form miRNA-induced silencing complexes with Argonaute proteins leading to mRNA degradation.152 miRNAs can be released into plasma bound to Argonaute proteins or in vesicles, but the relative proportions of protein- and vesicle-bound miRNAs are unclear.153 Interestingly, miRNAs have also been found in anucleate platelets; these can be shed in EVs and transferred to other cells for gene regulation, suggesting a potential role for intercellular signaling.154–156
Dysregulation of miRNAs are associated with increased procoagulant phenotypes. In patients with systemic lupus erythematosus and antiphospholipid syndrome, miR19-b and miR-20a, which inhibit TF, were significantly decreased compared to healthy controls and may contribute to their hypercoagulable states.157 Case-control studies of acute, non-cancerous deep vein thrombosis and recurrent VTE patients also showed distinct miRNA profiles in cases compared to controls.158,159 Similarly, in a case-control study of colorectal cancer patients, 9 miRNAs were differentially expressed in patients who developed VTE compared to controls.160
Biomarker Use in Primary VTE Risk
Studies assessing miRNA biomarkers for cancer VTE risk are limited. Recently, a study on newly diagnosed pancreatic and cholangiocarcinoma patients assessed the predictive capabilities of miRNAs for primary VTE. Study subjects were split into screening (n = 10) and confirmatory groups (n = 32) and 7 miRNAs (miR-486–5p, miR-106b-5p, let-7i-5p, let-7g-5p, miR-144–3p, miR-19a-3p and miR-103a-3p) were identified for inclusion in a predictive model. Model testing in the confirmatory group demonstrated a receiver operating characteristic (ROC) curve area under the curve of 0.95 (95% CI 0.87–1).161 However, the number of events included in this study is small for the number of predictors included in regression modeling and patients from the screening cohort were also included in the confirmatory group, leading to a highly overfit model and results. Nevertheless, miRNAs are a growing field of study in CAT and further studies are needed.
New advances in miRNA assays are appearing rapidly, but quantification is most commonly done through quantitative reverse transcription polymerase chain reaction (qRT-PCR), hybridization assays such as microarrays, and next-generation sequencing. Hybridization platforms such as microarrays are often low-cost and have fast turnaround times with high-throughput but have the lowest specificity. qRT-PCR performs similarly with improved specificity. Next-generation sequencing is the most costly, but is the only assay that can identify novel miRNAs, and with continued technological advances, cost has dropped dramatically over time.162
While miRNAs are exciting candidate biomarkers, there are considerable challenges for research and clinical use, similar to EVs. Though miRNAs are quite stable both at room temperature and upon multiple freeze-thaw cycles, study of miRNAs remain challenging due to their multiple transport mechanisms (RNA-binding proteins, EVs), technical difficulties in isolation and data analysis, and lack of widely used standards.163 Prior studies have shown there are significant biases in RNA isolation and library preparation kits that can impact results.164,165 Furthermore, data analysis is hampered by the lack of endogenous normalization controls which has generated controversy on the most appropriate normalization methods.166 The ERCC was formed to combat these challenges and create guidelines for standardization of testing.36,167
Polyphosphate and the Contact Pathway
Mechanism
Polyphosphates (polyPs) are linear polymers of anionic phosphate units that promote length-dependent mechanisms of thrombosis. On microbes, polyPs are found in a long chain form ranging from tens to thousands of units which activates the contact pathway through generation of factor XIIa and kallikrein.168–170 Short chain forms of polyP are released by platelets from dense granules which function to accelerate factor XI and V activation for enhanced thrombin generation.171–175 Short chain polyP also inhibits TF pathway inhibitor and remodels fibrin clots to increase resistance to fibrinolysis.169,174,176,177 Activated platelets can also express membrane-associated polyPs as spherical nanoparticles which potently activate factor XII and the contact pathway.178
The contact pathway, or contact activation system, is a proteolytic pathway including factors XII, XI, prekallikrein (PK), and high molecular weight kininogen (HK) that is activated upon contact of factor XII with a negatively charged surface, such as polyP or cfDNA, leading to cleavage of PK into kallikrein, which generates bradykinin from HK.179 Factor XII-independent contact activation can also occur via HK-PK complexes on endothelial cells leading to PK activation and cleavage.179 The principal inhibitor of the pathway is C1 esterase inhibitor which inhibits factor XIIa and kallikrein.180
Human studies investigating the role of polyP in cancer and CAT have not yet been reported. However, prostasomes or prostate-cancer derived EVs express long chain polyP that potently induces pulmonary embolism in mouse models through factor XII-dependent mechanisms, suggesting a potential role in prostate CAT.181
Current studies of contact pathway function in cancer patients are limited, but suggest that activation of the contact pathway leads to consumption and decrease of factor XII in gastrointestinal, colorectal, and lung cancer patients.182–184 In prostate cancer, patient-derived exosomes showed significantly more thrombin generation potential and factor XIIa activity compared to controls.181
Plasminogen Activator Inhibitor-1
Mechanism
PAI-1 is a serine protease that inhibits tissue plasminogen activator and urokinase, which are activators of plasminogen and fibrinolysis. Elevated levels of PAI-1 have been found in glioma, non-small cell lung, multiple myeloma, and pancreatic cancer patients.185–188 However, studies of PAI-1 in CAT are limited and even in nonmalignant settings, there has been limited evidence to support PAI-1-associated VTE.189 An in vitro study of human umbilical vein endothelial cells treated with PAI-1 lead to increased EV release, which were associated with increased anionic phospholipid expression and thrombin generation.190 Interestingly, a mouse lung cancer model showed increased expression of PAI-1 in tumor tissue and plasma after treatment with bevacizumab, an antiangiogenic VEGF antibody that is associated with increased VTE in cancer patients, suggesting that PAI-1 may play a more significant procoagulant role in antiangiogenic therapy-based VTE.191,192
Platelet Activators
Mechanism
Platelets are key actors in thrombus formation and can interact with cancer cells for enhanced activation and aggregation. Resting platelets adhere to and are activated at sites of endothelial damage upon binding of exposed vWF through GPIb, leading to rise of intracellular calcium which drives shape change and degranulation of adenosine diphosphate (ADP), fibrinogen, GPIb, and glycoprotein IIb/IIIa (GPIIb/IIIa). Further activation is driven by newly released ADP, thromboxane A2 (TxA2), and CD40 ligand (CD40L). ADP is stored in dense granules and upon release binds receptors P2Y1 and P2Y12 on platelets for activation. TxA2 is synthesized by thromboxane synthase after platelet activation and diffuses readily across the plasma membrane for binding of TxA2 receptor on platelets. CD40 is expressed on activated platelets and binds soluble CD40L which is released after platelet activation. Thrombin, a serine protease, can also activate platelets by cleaving the extracellular domains of protease-activated receptors 1 and 4 (PAR1/PAR4) on platelet membranes. After activation, platelets aggregate through crosslinking of fibrinogen and vWF with GPIIb/IIIa, forming the hemostatic plug.193,194
Cancer cells are capable of secreting these platelet activating factors and in vitro studies have shown release of tumor-derived ADP, TxA2, and CD40L, leading to tumor cell-induced platelet aggregation.195–198 Thromboxane synthase overexpression with increased TxA2 metabolites have also been demonstrated in cancer cell lines and human tissue.199–203 Additionally, large multimers of vWF have been found in the plasma of patients with disseminated cancer and vWF expression has been reported in both patient-derived glioblastoma and glioma and osteosarcoma cell lines.204,205 Platelet-tumor cell interactions using these activating factors have well-established roles in metastasis, where platelets coat tumor cells to provide a protective covering against host immune cells and allow tumor cell adhesion to endothelium for extravasation at distal tissue sites.206–208 These same interactions may also play a role in CAT.
Blood Count
Biomarker Use in Primary VTE Risk
Blood counts such as leukocyte and platelet count have been widely studied as biomarkers of primary VTE and are used broadly across many risk models. In prospective studies of cancer patients, both elevated leukocyte (>11 × 109/l) and platelet (≥350 × 109/l) counts increased risk of initial VTE.9,14,209 This elevated risk remained when assessing leukocyte counts per 2-fold increase and elevated platelet counts as ≥443 × 109/l.210 Leukocyte and platelet counts feature in the Khorana, Vienna CATS, PROTECHT, CONKO, and COMPASS-CAT scores.9–12,14 Leukocyte and platelet counts are assayed through the complete blood count (CBC) and are ubiquitously available in clinical practice. Furthermore, CBC tests are routinely acquired during typical management of patients, which reduces testing burden of patients during risk modeling.
D-dimer
Biomarker Use in Primary VTE Risk
D-dimer is a degradation product of plasmin-induced fibrinolysis and is one of the most common biomarkers studied for primary VTE development in cancer patients. Prospective studies of newly diagnosed cancer patients have shown that elevated pre-treatment levels of D-dimer increase the risk of primary VTE in colorectal, gynecologic, and mixed cancer populations.211–214 Another prospective study on newly diagnosed cancer patients and those with disease progression also found increased levels of D-dimer with elevated risk of primary VTE (HR 1.8, 95% CI 1.0–3.2).215 In a retrospective study of lung cancer patients who were categorized as intermediate risk by the Khorana score, elevated prechemotherapy D-dimer levels were associated with increased risk for VTE (HR 11.0, 95% CI 2.62–46.2), suggesting that D-dimer levels may help discriminate between intermediate Khorana score patients.216 However, due to the wide CI, the precision of estimated VTE risk is low. Borderline associations of D-dimer levels with VTE risk have also been demonstrated.81,112 D-dimer has been incorporated into the Vienna CATS and CATS nomogram risk models.10,16
Assays for D-dimer quantification include ELISA, enzyme-linked immunofluorescence assays (ELFA), latex-based assays, and whole-blood assays. While ELISA-based values represent the reference assay for D-dimer, ELFA and latex-based assays provide faster turnaround times and are fully automated. Whole-blood is often used for point-of-care testing and suffers from low sensitivity.217
A major obstacle with D-dimer quantification is the lack of transparency on unit measurement in laboratory reports, which can be expressed as purified D-dimer units (DDUs) or fibrinogen equivalent units (FEUs), with 1 FEU being equivalent to approximately 2 DDUs.217,218 This discrepancy makes development of standard D-dimer cut-offs difficult, as chosen cut-off limits in different studies may not be comparable. In clinical practice, physicians may input incorrect units of D-dimer into risk models and reduce validity of their results. We recommend that D-dimer biomarker research clearly state which assay and D-dimer unit type is used and that risk calculators specify D-dimer unit type when allowing users to input data. Furthermore, D-dimer levels increase with age, contributing to the difficulty in developing standard reference levels.219 Finally, levels of D-dimer may be elevated in a variety of inflammatory settings, not directly linked to activation of the coagulation system.220
P-selectin
Biomarker Use in Primary VTE Risk
P-selectin is a cell adhesion molecule found on activated endothelial cells and platelets and can be released into plasma, including on EVs. Multiple prospective studies derived from the Vienna CATS cohort have shown that increased soluble P-selectin levels are correlated with VTE risk, with one study estimating that a soluble P-selectin level ≥53.1 ng/ml increased the risk of VTE by 2.6-fold (95% CI 1.4–4.9).132,134,214,221 However, in another study based on the Vienna CATS cohort, soluble P-selectin levels were not significantly correlated with increased risk.112 Soluble P-selectin is measured by ELISA, and while assays are available for research purposes, clinical assays are not widely available. Although the Vienna CATS risk model may improve VTE prediction, the lack of access to soluble P-selectin assays limit its proposed clinical function.10
Biomarkers in Recurrent VTE Risk
The Ottawa score is a risk model for recurrent VTE and includes predictors for sex, primary tumor site (lung or breast), stage, and prior VTE.17 However, subsequent validation studies have shown mixed results on its discriminative capability, suggesting need for additional predictors.222–225 A study of cancer patients treated with either tinzaparin or warfarin demonstrated that elevated pre-anticoagulant TF levels (>64.6 pg/ml) and C-reactive protein (CRP; >75 mg/l) increased risk of recurrent VTE (sHR, 3.4; 95% CI, 2.1–5.5; sHR, 2.3; 95% CI, 1.2–4.4).225 Another study of cancer patients on low molecular weight heparin for 6 months showed that pre-anticoagulant soluble P-selectin (>136 ng/ml) but not D-dimer (>4.0 μg/ml) levels increased risk of recurrent VTE (sHR, 4.4, 95% CI 1.3–16; sHR 2.5, 95% CI 0.76–7.9).226 Elevated CRP (>4.5 mg/l) 21-days after 3 months of low molecular weight heparin treatment was also associated with increased risk for recurrent VTE (sHR 9.8,; 95% CI 1.86–51.7).227 Similarly, elevated D-dimer, as determined by ROC curve analysis (>600 ng/ml) and age adjustment (>age*10 ng/ml) at 21days post-anticoagulation increased risk of recurrent VTE (sHR 5.81, 95% CI 1.06–31.72; sHR 5.11, 95% CI 1.032–25.3).227 However, precision of estimated effects of CRP and D-dimer with VTE risk are low.
Biomarkers in Primary ATE Risk
Currently, no risk models are available for primary arterial thromboembolism and biomarker studies are sparse. Elevated neutrophil counts (>4.9 × 109/l) and soluble P-selectin levels (>46.3 ng/ml) have been shown to increase arterial thromboembolic risk (sHR 2.4, 95% CI 1.3‐4.6; sHR 2.1, 95% CI 1.2–3.8).228 However, D-dimer, NLR, platelet-to-lymphocyte ratio, H3Cit, cfDNA, and nucleosome levels failed to show significance.114,228,229
Future Directions and Considerations
Improvements in understanding CAT and its mechanisms have opened up a variety of potential biomarker targets for improving risk models and clinical decision making. While TF and TF+ EV have been extensively studied, research on NET-related proteins, polyphosphate, podoplanin, and miRNAs has just begun. Currently, risk models exist for predicting primary and recurrent VTE, but few have studied risk models and biomarkers for arterial thromboembolism—the other component of CAT. Furthermore, the role of biomarkers in guiding duration of treatment and predicting bleeding complications from anticoagulation has yet to be assessed. However, selection of candidate biomarkers must also balance predictive potential with clinical feasibility to ensure usage of the biomarker in practice. Awareness of these challenges can advance biomarker study to generate improved risk tools for CAT.
Acknowledgements
This work was supported by the U01 HL143402 research grant to KRM and AAK from the National Heart, Lung and Blood Institute CLOT Consortium, as well as the North American Society on Thrombosis and Hemostasis/Hemostasis and Thrombosis Research Society research fellowship to ASK. AAK additionally acknowledges research support from the Sondra and Stephen Hardis Chair in Oncology Research. All authors have read and approve the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. The authors declare no conflicts of interest. No sources of editorial support were used in preparation of this manuscript.
Abbreviations
- ADP
adenosine diphosphate
- ATE
arterial thromboembolism
- CAT
cancer-associated thrombosis
- CBC
complete blood count
- CCL3
chemokine ligand 3
- CD40L
CD40 ligand
- cfDNA
cell free DNA
- CI
confidence interval
- CLEC-2
C-type lectin-like receptor 2
- CRP
C-reactive protein
- DDU
D-dimer unit
- ELFA
enzyme-linked immunofluorescence assay
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme linked immune spot assays
- ERCC
Extracellular RNA Communication Consortium
- EV
extracellular vesicle
- FEU
fibrinogen equivalent unit
- G-CSF
granulocyte-colony stimulating factor
- GPIb
glycoprotein Ib
- GPIIb/IIIa
glycoprotein IIb/IIIa
- H3Cit
citrullinated histone H3
- HK
high molecular weight kininogen
- HR
hazard ratio
- IDH1
isocitrate dehydrogenase 1
- IL
interleukin
- ISEV
International Society for Extracellular Vesicles
- miRNA
microRNA
- mRNA
messenger RNA
- NET
neutrophil extracellular trap
- NLR
neutrophil-to-lymphocyte ratio
- OR
odds ratio
- PAI-1
plasminogen activator inhibitor-1
- PAR1
protease-activated receptor 1
- PAR4
protease-activated receptor 4
- PCR
polymerase chain reaction
- PK
prekallikrein
- polyP
polyphosphate
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- ROC
receiver operating characteristic
- sHR
subdistribution hazard ratio
- TF
tissue factor
- TF+ EV
tissue factor-positive extracellular vesicle
- TNF-α
tumor necrosis factor-α
- TxA2
thromboxane A2
- VEGF
vascular endothelial growth factor
- VTE
venous thromboembolism
- vWF
von Willebrand factor
References
- 1.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism: A Population-Based Case-Control Study. Arch Intern Med. 2000;160(6):809–815. doi: 10.1001/archinte.160.6.809 [DOI] [PubMed] [Google Scholar]
- 3.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715 [DOI] [PubMed] [Google Scholar]
- 4.Grilz E, Königsbrügge O, Posch F, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103(9):1549–1556. doi: 10.3324/haematol.2018.192419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navi BB, Reiner AS, Kamel H, et al. Risk of Arterial Thromboembolism in Patients With Cancer. Journal of the American College of Cardiology. 2017;70(8):926–938. doi: 10.1016/j.jacc.2017.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarvelis D, Anderson J, Davis L, et al. Hospital mortality due to pulmonary embolism and an evaluation of the usefulness of preventative interventions. Thromb Res. 2010;125(2):166–170. doi: 10.1016/j.thromres.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108 [DOI] [PubMed] [Google Scholar]
- 8.Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Practice & Research Clinical Haematology. 2009;22(1):9–23. doi: 10.1016/j.beha.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. doi: 10.1182/blood-2010-02-270116 [DOI] [PubMed] [Google Scholar]
- 11.Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291–292. doi: 10.1007/s11739-012-0784-y [DOI] [PubMed] [Google Scholar]
- 12.Pelzer U, Sinn M, Stieler J, Riess H. [Primary pharmacological prevention of thromboembolic events in ambulatory patients with advanced pancreatic cancer treated with chemotherapy?]. Dtsch Med Wochenschr. 2013;138(41):2084–2088. doi: 10.1055/s-0033-1349608 [DOI] [PubMed] [Google Scholar]
- 13.Cella CA, Minno GD, Carlomagno C, et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. The Oncologist. 2017;22(5):601–608. doi: 10.1634/theoncologist.2016-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerotziafas GT, Taher A, Abdel‐Razeq H, et al. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS–Cancer‐Associated Thrombosis Study. The Oncologist. 2017;22(10):1222–1231. doi: 10.1634/theoncologist.2016-0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín AJM, Ortega I, Font C, et al. Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer. British Journal of Cancer. 2018;118(8):1056. doi: 10.1038/s41416-018-0027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. The Lancet Haematology. 2018;5(7):e289–e298. doi: 10.1016/S2352-3026(18)30063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louzada Martha L, Carrier Marc, Lazo-Langner Alejandro, et al. Development of a Clinical Prediction Rule for Risk Stratification of Recurrent Venous Thromboembolism in Patients With Cancer-Associated Venous Thromboembolism. Circulation. 2012;126(4):448–454. doi: 10.1161/CIRCULATIONAHA.111.051920 [DOI] [PubMed] [Google Scholar]
- 18.Jain KK. The Handbook of Biomarkers. Second edition. Humana Press; 2017. [Google Scholar]
- 19.Khorana AA. Simplicity versus complexity: an existential dilemma as risk tools evolve. The Lancet Haematology. 2018;5(7):e273–e274. doi: 10.1016/S2352-3026(18)30067-X [DOI] [PubMed] [Google Scholar]
- 20.Mahajan A, Brunson A, White R, Wun T. The Epidemiology of Cancer-Associated Venous Thromboembolism: An Update. Seminars in Thrombosis and Hemostasis. 2019;45(04):321–325. doi: 10.1055/s-0039-1688494 [DOI] [PubMed] [Google Scholar]
- 21.Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel). 2018;10(10). doi: 10.3390/cancers10100380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes CJ, Morinaga LTK, Alves JL, et al. Cancer-associated thrombosis: the when, how and why. European Respiratory Review. 2019;28(151). doi: 10.1183/16000617.0119-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimpton M, Carrier M. What’s new in the prevention and treatment of cancer-associated thrombosis? Hematology Am Soc Hematol Educ Program. 2019;2019(1):158–166. doi: 10.1182/hematology.2019000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Research and Practice in Thrombosis and Haemostasis. 2018;2(3):429–438. doi: 10.1002/rth2.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorak HF, Quay SC, Orenstein NS, et al. Tumor shedding and coagulation. Science. 1981;212(4497):923–924. doi: 10.1126/science.7195067 [DOI] [PubMed] [Google Scholar]
- 27.Rak J Microparticles in Cancer. Semin Thromb Hemost. 2010;36(08):888–906. doi: 10.1055/s-0030-1267043 [DOI] [PubMed] [Google Scholar]
- 28.Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. New England Journal of Medicine. 2018;379(10):958–966. doi: 10.1056/NEJMra1704286 [DOI] [PubMed] [Google Scholar]
- 29.Olivier Morel, Laurence Jesel, Freyssinet Jean-Marie Toti Florence. Cellular Mechanisms Underlying the Formation of Circulating Microparticles. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(1):15–26. doi: 10.1161/ATVBAHA.109.200956 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468(7325):834–838. doi: 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- 31.Fujii T, Sakata A, Nishimura S, Eto K, Nagata S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. PNAS. 2015;112(41):12800–12805. doi: 10.1073/pnas.1516594112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhotra OP, Nesheim ME, Mann KG. The kinetics of activation of normal and gamma-carboxyglutamic acid-deficient prothrombins. J Biol Chem. 1985;260(1):279–287. [PubMed] [Google Scholar]
- 33.Thaler J, Ay C, Weinstabl H, et al. Circulating procoagulant microparticles in cancer patients. Ann Hematol. 2011;90(4):447–453. doi: 10.1007/s00277-010-1111-1 [DOI] [PubMed] [Google Scholar]
- 34.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105(03):396–408. doi: 10.1160/TH10-09-0595 [DOI] [PubMed] [Google Scholar]
- 35.Mooberry MJ, Key NS. Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry Part A. 2016;89(2):111–122. doi: 10.1002/cyto.a.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Extracellular RNA Communication Consortium. exRNA Protocols. exRNA Portal. Accessed May 13, 2020. https://exrna.org/resources/protocols/ [Google Scholar]
- 37.Egorina Elena M, Sovershaev Mikhail A, Bjørkøy Geir, et al. Intracellular and Surface Distribution of Monocyte Tissue Factor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(7):1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Molecular and Cellular Biology. 1989;9(6):2752–2755. doi: 10.1128/MCB.9.6.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franco RF, de Jonge E, Dekkers PEP, et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96(2):554–559. doi: 10.1182/blood.V96.2.554 [DOI] [PubMed] [Google Scholar]
- 40.Bode M, Mackman N. Regulation of tissue factor gene expression in monocytes and endothelial cells: Thromboxane A2 as a new player. Vascular Pharmacology. 2014;62(2):57–62. doi: 10.1016/j.vph.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier MEW, Akinmolayan A, Goodall AH. Comparison of tissue factor expression and activity in foetal and adult endothelial cells. Blood Coagul Fibrinolysis. 2017;28(6):452–459. doi: 10.1097/MBC.0000000000000621 [DOI] [PubMed] [Google Scholar]
- 42.Dvorak HF, DeWater LV, Bitzer AM, et al. Procoagulant Activity Associated with Plasma Membrane Vesicles Shed by Cultured Tumor Cells. Cancer Res. 1983;43(9):4434–4442. [PubMed] [Google Scholar]
- 43.Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–1741. doi: 10.1182/blood-2004-05-2042 [DOI] [PubMed] [Google Scholar]
- 44.Davila M, Amirkhosravi A, Coll E, et al. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. Journal of Thrombosis and Haemostasis. 2008;6(9):1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x [DOI] [PubMed] [Google Scholar]
- 45.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell–derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206(9):1913–1927. doi: 10.1084/jem.20082297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J-G, Geddings JE, Aleman MM, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119(23):5543–5552. doi: 10.1182/blood-2012-01-402156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geddings JE, Hisada Y, Boulaftali Y, et al. Tissue factor–positive tumor microvesicles activate platelets and enhance thrombosis in mice. Journal of Thrombosis and Haemostasis. 2016;14(1):153–166. doi: 10.1111/jth.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hisada Y, Ay C, Auriemma AC, Cooley BC, Mackman N. Human pancreatic tumors grown in mice-release tissue factor-positive microvesicles that increase venous clot size. Journal of Thrombosis and Haemostasis. 2017;15(11):2208–2217. doi: 10.1111/jth.13809 [DOI] [PubMed] [Google Scholar]
- 49.Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. British Journal of Cancer. 2000;83(2):164–170. doi: 10.1054/bjoc.2000.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilley RE, Holscher T, Belani R, Nieva J, Mackman N. Tissue factor activity is increased in a combined platelet and microparticle sample from cancer patients. Thrombosis Research. 2008;122(5):604–609. doi: 10.1016/j.thromres.2007.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer. Thrombosis and Haemostasis. 2007;97(01):119–123. doi: 10.1160/TH06-03-0141 [DOI] [PubMed] [Google Scholar]
- 52.Tesselaar MET, Romijn FPHTM, Linden IKVD, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? Journal of Thrombosis and Haemostasis. 2007;5(3):520–527. doi: 10.1111/j.1538-7836.2007.02369.x [DOI] [PubMed] [Google Scholar]
- 53.Haubold K, Rink M, Spath B, et al. Tissue factor procoagulant activity of plasma microparticles is increased in patients with early-stage prostate cancer. Thrombosis and Haemostasis. 2009;101(06):1147–1155. doi: 10.1160/TH08-10-0654 [DOI] [PubMed] [Google Scholar]
- 54.Trappenburg MC, van Schilfgaarde M, Bredewold EO, et al. Elevated numbers and altered subsets of procoagulant microparticles in breast cancer patients using endocrine therapy. Thrombosis Research. 2011;127(4):363–369. doi: 10.1016/j.thromres.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 55.Thaler J, Ay C, Mackman N, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. Journal of Thrombosis and Haemostasis. 2012;10(7):1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x [DOI] [PubMed] [Google Scholar]
- 56.Bharthuar A, Khorana AA, Hutson A, et al. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thrombosis Research. 2013;132(2):180–184. doi: 10.1016/j.thromres.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 57.Langer F, Spath B, Haubold K, et al. Tissue factor procoagulant activity of plasma microparticles in patients with cancer-associated disseminated intravascular coagulation. Ann Hematol. 2008;87(6):451–457. doi: 10.1007/s00277-008-0446-3 [DOI] [PubMed] [Google Scholar]
- 58.Grover Steven P, Mackman Nigel. Tissue Factor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(4):709–725. doi: 10.1161/ATVBAHA.117.309846 [DOI] [PubMed] [Google Scholar]
- 59.Zillmann A, Luther T, Müller I, et al. Platelet-Associated Tissue Factor Contributes to the Collagen-Triggered Activation of Blood Coagulation. Biochemical and Biophysical Research Communications. 2001;281(2):603–609. doi: 10.1006/bbrc.2001.4399 [DOI] [PubMed] [Google Scholar]
- 60.Siddiqui FA, Desai H, Amirkhosravi A, Amaya M, Francis JL. The presence and release of tissue factor from human platelets. Platelets. 2002;13(4):247–253. doi: 10.1080/09537100220146398 [DOI] [PubMed] [Google Scholar]
- 61.Müller I, Klocke A, Alex M, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. The FASEB Journal. 2003;17(3):476–478. doi: 10.1096/fj.02-0574fje [DOI] [PubMed] [Google Scholar]
- 62.Marina Camera, Marta Frigerio, Vincenzo Toschi, et al. Platelet Activation Induces Cell-Surface Immunoreactive Tissue Factor Expression, Which Is Modulated Differently by Antiplatelet Drugs. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(9):1690–1696. doi: 10.1161/01.ATV.0000085629.23209.AA [DOI] [PubMed] [Google Scholar]
- 63.Panes O, Matus V, Sáez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109(12):5242–5250. doi: 10.1182/blood-2006-06-030619 [DOI] [PubMed] [Google Scholar]
- 64.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105(7):2764–2770. doi: 10.1182/blood-2004-09-3567 [DOI] [PubMed] [Google Scholar]
- 65.Bouchard BA, Mann KG, Butenas S. No evidence for tissue factor on platelets. Blood. 2010;116(5):854–855. doi: 10.1182/blood-2010-05-285627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouchard BA, Krudysz-Amblo J, Butenas S. Platelet tissue factor is not expressed transiently after platelet activation. Blood. 2012;119(18):4338–4339. doi: 10.1182/blood-2012-01-403469 [DOI] [PubMed] [Google Scholar]
- 67.Østerud B, Bouchard BA. Detection of tissue factor in platelets: why is it so troublesome? Platelets. 2019;30(8):957–961. doi: 10.1080/09537104.2019.1624708 [DOI] [PubMed] [Google Scholar]
- 68.Rauch U, Bonderman D, Bohrmann B, et al. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood. 2000;96(1):170–175. doi: 10.1182/blood.V96.1.170 [DOI] [PubMed] [Google Scholar]
- 69.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095 [DOI] [PubMed] [Google Scholar]
- 70.Sovershaev MA, Egorina EM, Østerud B, Hansen J-B. Evidence for direct transfer of tissue factor from monocytes to platelets in whole blood: Blood Coagulation & Fibrinolysis. 2012;23(4):345–350. doi: 10.1097/MBC.0b013e328350bf2f [DOI] [PubMed] [Google Scholar]
- 71.Østerud B, Olsen JO. Human platelets do not express tissue factor. Thrombosis Research. 2013;132(1):112–115. doi: 10.1016/j.thromres.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 72.Tripisciano C, Weiss R, Eichhorn T, et al. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Scientific Reports. 2017;7(1):1–11. doi: 10.1038/s41598-017-03262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waard V de, Hansen HR, Spronk HHM, et al. Differential expression of tissue factor mRNA and protein expression in murine sepsis. Thromb Haemost. 2006;95(02):348–353. doi: 10.1160/TH05-07-0512 [DOI] [PubMed] [Google Scholar]
- 74.Egorina EM, Sovershaev MA, Olsen JO, Østerud B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: evidence for a direct transfer. Blood. 2008;111(3):1208–1216. doi: 10.1182/blood-2007-08-107698 [DOI] [PubMed] [Google Scholar]
- 75.Almeida VH, Rondon AMR, Gomes T, Monteiro RQ. Novel Aspects of Extracellular Vesicles as Mediators of Cancer-Associated Thrombosis. Cells. 2019;8(7):716. doi: 10.3390/cells8070716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lacroix R, Vallier L, Bonifay A, et al. Microvesicles and Cancer Associated Thrombosis. Semin Thromb Hemost. 2019;45(06):593–603. doi: 10.1055/s-0039-1693476 [DOI] [PubMed] [Google Scholar]
- 77.Bang OY, Chung J-W, Lee MJ, et al. Cancer Cell-Derived Extracellular Vesicles Are Associated with Coagulopathy Causing Ischemic Stroke via Tissue Factor-Independent Way: The OASIS-CANCER Study. PLOS ONE. 2016;11(7):e0159170. doi: 10.1371/journal.pone.0159170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Konstantin Stark, Irene Schubert, Urjita Joshi, et al. Distinct Pathogenesis of Pancreatic Cancer Microvesicle–Associated Venous Thrombosis Identifies New Antithrombotic Targets In Vivo. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(4):772–786. doi: 10.1161/ATVBAHA.117.310262 [DOI] [PubMed] [Google Scholar]
- 79.Auwerda J, Yuana Y, Osanto S, et al. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thrombosis and Haemostasis. 2011;105(01):14–20. doi: 10.1160/TH10-03-0187 [DOI] [PubMed] [Google Scholar]
- 80.van Es N, Hisada Y, Di Nisio M, et al. Extracellular vesicles exposing tissue factor for the prediction of venous thromboembolism in patients with cancer: A prospective cohort study. Thrombosis Research. 2018;166:54–59. doi: 10.1016/j.thromres.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 81.Faille D, Bourrienne M-C, de Raucourt E, et al. Biomarkers for the risk of thrombosis in pancreatic adenocarcinoma are related to cancer process. Oncotarget. 2018;9(41):26453–26465. doi: 10.18632/oncotarget.25458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui C, Wang G, Yang S, Huang S, Qiao R, Cui W. Tissue Factor-bearing MPs and the risk of venous thrombosis in cancer patients: A meta-analysis. Scientific Reports. 2018;8(1):1–8. doi: 10.1038/s41598-018-19889-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owens AP, Mackman N. Microparticles in Hemostasis and Thrombosis. Circ Res. 2011;108(10):1284–1297. doi: 10.1161/CIRCRESAHA.110.233056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berckmans RJ, Sturk A, van Tienen LM, Schaap MCL, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117(11):3172–3180. doi: 10.1182/blood-2010-06-290460 [DOI] [PubMed] [Google Scholar]
- 85.Basavaraj MG, Olsen JO, Østerud B, Hansen J-B. Differential ability of tissue factor antibody cloneson detection of tissue factor in blood cells and microparticles. Thrombosis Research. 2012;130(3):538–546. doi: 10.1016/j.thromres.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 86.Gardiner C, Harrison P, Belting M, et al. Extracellular vesicles, tissue factor, cancer and thrombosis – discussion themes of the ISEV 2014 Educational Day. Journal of Extracellular Vesicles. 2015;4(1):26901. doi: 10.3402/jev.v4.26901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Engstad CS, Lia K, Rekdal Ø, Olsen JO, Østeoid B. A novel biological effect of platelet factor 4 (PF4): enhancement of LPS-induced tissue factor activity in monocytes. Journal of Leukocyte Biology. 1995;58(5):575–581. doi: 10.1002/jlb.58.5.575 [DOI] [PubMed] [Google Scholar]
- 88.Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Research and Practice in Thrombosis and Haemostasis. 2019;3(1):44–48. doi: 10.1002/rth2.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 90.Gross PL, Furie BC, Merrill‐Skoloff G, Chou J, Furie B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. Journal of Leukocyte Biology. 2005;78(6):1318–1326. doi: 10.1189/jlb.0405193 [DOI] [PubMed] [Google Scholar]
- 91.Darbousset R, Thomas GM, Mezouar S, et al. Tissue factor–positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120(10):2133–2143. doi: 10.1182/blood-2012-06-437772 [DOI] [PubMed] [Google Scholar]
- 92.Guimarães-Costa AB, Nascimento MTC, Wardini AB, Pinto-da-Silva LH, Saraiva EM. ETosis: A Microbicidal Mechanism beyond Cell Death. Journal of Parasitology Research. Published online 2012. doi: 10.1155/2012/929743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. PNAS. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. PNAS. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laridan E, Martinod K, Meyer SFD. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin Thromb Hemost. 2019;45(01):086–093. doi: 10.1055/s-0038-1677040 [DOI] [PubMed] [Google Scholar]
- 96.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. PNAS. 2013;110(21):8674–8679. doi: 10.1073/pnas.1301059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generatio nhrough platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–1961. doi: 10.1182/blood-2011-03-343061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. Journal of Thrombosis and Haemostasis. 2012;10(1):136–144. doi: 10.1111/j.1538-7836.2011.04544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lam FW, Cruz MA, Parikh K, Rumbaut RE. Histones stimulate von Willebrand factor release in vitro and in vivo. Haematologica. 2016;101(7):e277–e279. doi: 10.3324/haematol.2015.140632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elaskalani O, Razak NBA, Metharom P. Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. Cell Commun Signal. 2018;16(1):1–15. doi: 10.1186/s12964-018-0235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Massberg S, Grahl L, von Bruehl M-L, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nature Medicine. 2010;16(8):887–896. doi: 10.1038/nm.2184 [DOI] [PubMed] [Google Scholar]
- 102.von Brühl M-L, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–835. doi: 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kambas K, Mitroulis I, Apostolidou E, et al. Autophagy Mediates the Delivery of Thrombogenic Tissue Factor to Neutrophil Extracellular Traps in Human Sepsis. PLoS One. 2012;7(9). doi: 10.1371/journal.pone.0045427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Y-M, Wang H, Wang C, Chen M, Zhao M-H. Promotion of Hypercoagulability in Antineutrophil Cytoplasmic Antibody–Associated Vasculitis by C5a-Induced Tissue Factor–Expressing Microparticles and Neutrophil Extracellular Traps. Arthritis & Rheumatology. 2015;67(10):2780–2790. doi: 10.1002/art.39239 [DOI] [PubMed] [Google Scholar]
- 105.Stakos DA, Kambas K, Konstantinidis T, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405–1414. doi: 10.1093/eurheartj/ehv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y, Luo L, Braun OÖ, et al. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Scientific Reports. 2018;8(1):1–14. doi: 10.1038/s41598-018-22156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas GM, Brill A, Mezouar S, et al. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. Journal of Thrombosis and Haemostasis. 2015;13(7):1310–1319. doi: 10.1111/jth.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leal AC, Mizurini DM, Gomes T, et al. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Scientific Reports. 2017;7(1):1–12. doi: 10.1038/s41598-017-06893-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hisada Y, Grover SP, Maqsood A, et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica. 2020;105(1):218–225. doi: 10.3324/haematol.2019.217083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oklu R, Sheth RA, Wong KHK, Jahromi AH, Albadawi H. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovascular Diagnosis and Therapy. 2017;7(3):S140-S149-S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thålin C, Demers M, Blomgren B, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thrombosis Research. 2016;139:56–64. doi: 10.1016/j.thromres.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mauracher L-M, Posch F, Martinod K, et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. Journal of Thrombosis and Haemostasis. 2018;16(3):508–518. doi: 10.1111/jth.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferroni P, Riondino S, Formica V, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: Clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. International Journal of Cancer. 2015;136(5):1234–1240. doi: 10.1002/ijc.29076 [DOI] [PubMed] [Google Scholar]
- 114.Grilz E, Posch F, Königsbrügge O, et al. Association of Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio with the Risk of Thromboembolism and Mortality in Patients with Cancer. Thromb Haemost. 2018;118(11):1875–1884. doi: 10.1055/s-0038-1673401 [DOI] [PubMed] [Google Scholar]
- 115.Fuentes HE, Paz LH, Wang Y, Oramas DM, Simons CR, Tafur AJ. Performance of Current Thromboembolism Risk Assessment Tools in Patients With Gastric Cancer and Validity After First Treatment. Clin Appl Thromb Hemost. 2018;24(5):790–796. doi: 10.1177/1076029617726599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thålin C, Daleskog M, Göransson SP, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of citrullinated histone H3 as a marker for neutrophil extracellular traps in human plasma. Immunol Res. 2017;65(3):706–712. doi: 10.1007/s12026-017-8905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Szotowski Björn Antoniak Silvio, Wolfgang Poller, Schultheiss Heinz-Peter Rauch Ursula. Procoagulant Soluble Tissue Factor Is Released From Endothelial Cells in Response to Inflammatory Cytokines. Circulation Research. 2005;96(12):1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa [DOI] [PubMed] [Google Scholar]
- 118.Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR. Interleukin-1β induced vascular permeability is dependent on induction of endothelial Tissue Factor (TF) activity. Journal of Translational Medicine. 2005;3(1). doi: 10.1186/1479-5876-3-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gieseler F, Plattfaut C, Quecke T, Freund A, Ungefroren H, Ender F. Heterogeneity of microvesicles from cancer cell lines under inflammatory stimulation with TNF-α. Cell Biology International. 2018;42(11):1533–1544. doi: 10.1002/cbin.11040 [DOI] [PubMed] [Google Scholar]
- 120.van Hinsbergh V, Kooistra T, van den Berg E, Princen H, Fiers W, Emeis J. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood. 1988;72(5):1467–1473. doi: 10.1182/blood.V72.5.1467.bloodjournal7251467 [DOI] [PubMed] [Google Scholar]
- 121.van Hinsbergh VW, Bauer KA, Kooistra T, et al. Progress of fibrinolysis during tumor necrosis factor infusions in humans. Concomitant increase in tissue-type plasminogen activator, plasminogen activator inhibitor type-1, and fibrin(ogen) degradation products. Blood. 1990;76(11):2284–2289. doi: 10.1182/blood.V76.11.2284.2284 [DOI] [PubMed] [Google Scholar]
- 122.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. J Cell Biol. 2001;154(3):485–490. doi: 10.1083/jcb.200105058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown GT, Narayanan P, Li W, Silverstein RL, McIntyre TM. Lipopolysaccharide Stimulates Platelets through an IL-1β Autocrine Loop. The Journal of Immunology. 2013;191(10):5196–5203. doi: 10.4049/jimmunol.1300354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qi J, Kreutzer DL. Fibrin activation of vascular endothelial cells. Induction of IL-8 expression. The Journal of Immunology. 1995;155(2):867–876. [PubMed] [Google Scholar]
- 125.Lalla RV, Goralnick SJ, Tanzer ML, Kreutzer DL. Fibrin induces IL-8 expression from human oral squamous cell carcinoma cells. Oral Oncology. 2001;37(3):234–242. doi: 10.1016/S1368-8375(00)00090-7 [DOI] [PubMed] [Google Scholar]
- 126.Alfaro C, Teijeira A, Oñate C, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res. 2016;22(15):3924–3936. doi: 10.1158/1078-0432.CCR-15-2463 [DOI] [PubMed] [Google Scholar]
- 127.Mechtcheriakova D, Wlachos A, Holzmüller H, Binder BR, Hofer E. Vascular Endothelial Cell Growth Factor–Induced Tissue Factor Expression in Endothelial Cells Is Mediated by EGR-1. Blood. 1999;93(11):3811–3823. doi: 10.1182/blood.V93.11.3811 [DOI] [PubMed] [Google Scholar]
- 128.Abe K, Shoji M, Chen J, et al. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. PNAS. 1999;96(15):8663–8668. doi: 10.1073/pnas.96.15.8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsuo K, Hasegawa K, Yoshino K, et al. Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma. European Journal of Cancer. 2015;51(14):1978–1988. doi: 10.1016/j.ejca.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim SH, Woo S, Kim S, Ko YH, Kim WS, Kim SJ. Cross-sectional Study of Patients with Diffuse Large B-Cell Lymphoma: Assessing the Effect of Host Status, Tumor Burden, and Inflammatory Activity on Venous Thromboembolism. Cancer Res Treat. 2016;48(1):312–321. doi: 10.4143/crt.2014.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reitter E-M, Ay C, Kaider A, et al. Interleukin levels and their potential association with venous thromboembolism and survival in cancer patients. Clinical & Experimental Immunology. 2014;177(1):253–260. doi: 10.1111/cei.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mir Seyed Nazari P, Marosi C, Moik F, et al. Low Systemic Levels of Chemokine C-C Motif Ligand 3 (CCL3) are Associated with a High Risk of Venous Thromboembolism in Patients with Glioma. Cancers. 2019;11(12):2020. doi: 10.3390/cancers11122020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferroni P, Riondino S, Portarena I, et al. Association between increased tumor necrosis factor alpha levels and acquired activated protein C resistance in patients with metastatic colorectal cancer. Int J Colorectal Dis. 2012;27(12):1561–1567. doi: 10.1007/s00384-012-1493-8 [DOI] [PubMed] [Google Scholar]
- 134.Posch F, Thaler J, Zlabinger G-J, et al. Soluble Vascular Endothelial Growth Factor (sVEGF) and the Risk of Venous Thromboembolism in Patients with Cancer: Results from the Vienna Cancer and Thrombosis Study (CATS). Clinical Cancer Research. 2016;22(1):200–206. doi: 10.1158/1078-0432.CCR-14-3358 [DOI] [PubMed] [Google Scholar]
- 135.Stenken JA, Poschenrieder AJ. Bioanalytical Chemistry of Cytokines-A Review. Anal Chim Acta. 2015;853:95–115. doi: 10.1016/j.aca.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13(5):541–547. doi: 10.1097/MCO.0b013e32833cf3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hennø LT, Storjord E, Christiansen D, et al. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines – Consequences for defining reference values in healthy humans. Cytokine. 2017;97:86–95. doi: 10.1016/j.cyto.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 138.Suzuki‐Inoue K, Osada M, Ozaki Y. Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: partners from in utero to adulthood. Journal of Thrombosis and Haemostasis. 2017;15(2):219–229. doi: 10.1111/jth.13590 [DOI] [PubMed] [Google Scholar]
- 139.Takemoto A, Miyata K, Fujita N. Platelet-activating factor podoplanin: from discovery to drug development. Cancer Metastasis Rev. 2017;36(2):225–234. doi: 10.1007/s10555-017-9672-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lavallée V-P, Chagraoui J, MacRae T, et al. Transcriptomic landscape of acute promyelocytic leukemia reveals aberrant surface expression of the platelet aggregation agonist Podoplanin. Leukemia. 2018;32(6):1349–1357. doi: 10.1038/s41375-018-0069-1 [DOI] [PubMed] [Google Scholar]
- 141.Suzuki-Inoue K, Kato Y, Inoue O, et al. Involvement of the Snake Toxin Receptor CLEC-2, in Podoplanin-mediated Platelet Activation, by Cancer Cells. J Biol Chem. 2007;282(36):25993–26001. doi: 10.1074/jbc.M702327200 [DOI] [PubMed] [Google Scholar]
- 142.Kato Y, Kaneko MK, Kunita A, et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Science. 2008;99(1):54–61. doi: 10.1111/j.1349-7006.2007.00634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riedl J, Preusser M, Nazari PMS, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129(13):1831–1839. doi: 10.1182/blood-2016-06-720714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mege D, Panicot‐Dubois L, Ouaissi M, et al. The origin and concentration of circulating microparticles differ according to cancer type and evolution: A prospective single-center study. International Journal of Cancer. 2016;138(4):939–948. doi: 10.1002/ijc.29837 [DOI] [PubMed] [Google Scholar]
- 145.Shirai T, Inoue O, Tamura S, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. Journal of Thrombosis and Haemostasis. 2017;15(3):513–525. doi: 10.1111/jth.13604 [DOI] [PubMed] [Google Scholar]
- 146.Payne H, Ponomaryov T, Watson SP, Brill A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 2017;129(14):2013–2020. doi: 10.1182/blood-2016-09-742999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132(6):917–930. doi: 10.1007/s00401-016-1620-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Unruh D, Mirkov S, Wray B, et al. Methylation-dependent Tissue Factor Suppression Contributes to the Reduced Malignancy of IDH1-mutant Gliomas. Clin Cancer Res. 2019;25(2):747–759. doi: 10.1158/1078-0432.CCR-18-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Unruh D, Zewde M, Buss A, et al. Methylation and transcription patterns are distinct in IDH mutant gliomas compared to other IDH mutant cancers. Scientific Reports. 2019;9(1):1–11. doi: 10.1038/s41598-019-45346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nazari PMS, Riedl J, Preusser M, et al. Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism. Journal of Thrombosis and Haemostasis. 2018;16(6):1121–1127. doi: 10.1111/jth.14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Watanabe J, Natsumeda M, Okada M, et al. Podoplanin Expression and IDH-Wildtype Status Predict Venous Thromboembolism in Patients with High-Grade Gliomas in the Early Postoperative Period. World Neurosurgery. 2019;128:e982–e988. doi: 10.1016/j.wneu.2019.05.049 [DOI] [PubMed] [Google Scholar]
- 152.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nature Reviews Molecular Cell Biology. 2019;20(1):21. doi: 10.1038/s41580-018-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9. doi: 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nature Structural and Molecular Biology. 2009;16(9):961. doi: 10.1038/nsmb.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Risitano A, Beaulieu LM, Vitseva O, Freedman JE. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood. 2012;119(26):6288–6295. doi: 10.1182/blood-2011-12-396440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laffont B, Corduan A, Rousseau M, et al. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost. 2016;115(02):311–323. doi: 10.1160/th15-05-0389 [DOI] [PubMed] [Google Scholar]
- 157.Teruel R, Pérez‐Sánchez C, Corral J, et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. Journal of Thrombosis and Haemostasis. 2011;9(10):1985–1992. doi: 10.1111/j.1538-7836.2011.04451.x [DOI] [PubMed] [Google Scholar]
- 158.Qin J, Liang H, Shi D, et al. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. J Thromb Thrombolysis. 2015;39(2):215–221. doi: 10.1007/s11239-014-1131-0 [DOI] [PubMed] [Google Scholar]
- 159.Wang X, Sundquist K, Svensson PJ, et al. Association of recurrent venous thromboembolism and circulating microRNAs. Clinical Epigenetics. 2019;11(1):28. doi: 10.1186/s13148-019-0627-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim AS, Kalady MF, DeVecchio J, et al. Identifying miRNA Biomarkers and Predicted Targets Associated with Venous Thromboembolism in Colorectal Cancer Patients. Blood. 2019;134(Supplement_1):3643–3643. doi: 10.1182/blood-2019-127585 [DOI] [Google Scholar]
- 161.Oto J, Navarro S, Larsen AC, et al. MicroRNAs and Neutrophil Activation Markers Predict Venous Thrombosis in Pancreatic Ductal Adenocarcinoma and Distal Extrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences. 2020;21(3):840. doi: 10.3390/ijms21030840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13(5):358–369. doi: 10.1038/nrg3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grasedieck S, Schöler N, Bommer M, et al. Impact of serum storage conditions on microRNA stability. Leukemia. 2012;26(11):2414–2416. doi: 10.1038/leu.2012.106 [DOI] [PubMed] [Google Scholar]
- 164.Tanriverdi K, Kucukural A, Mikhalev E, et al. Comparison of RNA isolation and associated methods for extracellular RNA detection by high-throughput quantitative polymerase chain reaction. Analytical Biochemistry. 2016;501:66–74. doi: 10.1016/j.ab.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 165.Giraldez MD, Spengler RM, Etheridge A, et al. Comprehensive multi-center assessment of small RNA-seq methods for quantitative miRNA profiling. Nature Biotechnology. 2018;36(8):746–757. doi: 10.1038/nbt.4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grasedieck S, Sorrentino A, Langer C, et al. Circulating microRNAs in hematological diseases: principles, challenges, and perspectives. Blood. 2013;121(25):4977–4984. doi: 10.1182/blood-2013-01-480079 [DOI] [PubMed] [Google Scholar]
- 167.Das S, Abdel-Mageed AB, Adamidi C, et al. The Extracellular RNA Communication Consortium: Establishing Foundational Knowledge and Technologies for Extracellular RNA Research. Cell. 2019;177(2):231–242. doi: 10.1016/j.cell.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rao NN, Gómez-García MR, Kornberg A. Inorganic Polyphosphate: Essential for Growth and Survival. Annual Review of Biochemistry. 2009;78(1):605–647. doi: 10.1146/annurev.biochem.77.083007.093039 [DOI] [PubMed] [Google Scholar]
- 169.Smith SA, Choi SH, Davis-Harrison R, et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116(20):4353–4359. doi: 10.1182/blood-2010-01-266791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Engel R, Brain CM, Paget J, Lionikiene AS, Mutch NJ. Single-chain factor XII exhibits activity when complexed to polyphosphate. Journal of Thrombosis and Haemostasis. 2014;12(9):1513–1522. doi: 10.1111/jth.12663 [DOI] [PubMed] [Google Scholar]
- 171.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human Platelet Dense Granules Contain Polyphosphate and Are Similar to Acidocalcisomes of Bacteria and Unicellular Eukaryotes. J Biol Chem. 2004;279(43):44250–44257. doi: 10.1074/jbc.M406261200 [DOI] [PubMed] [Google Scholar]
- 172.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118(26):6963–6970. doi: 10.1182/blood-2011-07-368811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Geng Y, Verhamme IM, Smith SB, et al. The dimeric structure of factor XI and zymogen activation. Blood. 2013;121(19):3962–3969. doi: 10.1182/blood-2012-12-473629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103(4):903–908. doi: 10.1073/pnas.0507195103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Choi S, Smith S, Morrissey J. Polyphosphate accelerates factor V activation by factor XIa. Thrombosis and Haemostasis. 2015;113(03):599–604. doi: 10.1160/TH14-06-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112(7):2810–2816. doi: 10.1182/blood-2008-03-145755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariëns RAS. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115(19):3980–3988. doi: 10.1182/blood-2009-11-254029 [DOI] [PubMed] [Google Scholar]
- 178.Verhoef JJF, Barendrecht AD, Nickel KF, et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129(12):1707–1717. doi: 10.1182/blood-2016-08-734988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Campello E, Henderson MW, Noubouossie DF, Simioni P, Key NS. Contact System Activation and Cancer: New Insights in the Pathophysiology of Cancer-Associated Thrombosis. Thromb Haemost. 2018;118(2):251–265. doi: 10.1160/TH17-08-0596 [DOI] [PubMed] [Google Scholar]
- 180.van Montfoort ML, Meijers JCM. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematology Am Soc Hematol Educ Program. 2014;2014(1):60–65. doi: 10.1182/asheducation.V2014.1.60.3882400 [DOI] [PubMed] [Google Scholar]
- 181.Nickel KF, Ronquist G, Langer F, et al. The polyphosphate–factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood. 2015;126(11):1379–1389. doi: 10.1182/blood-2015-01-622811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Roeise O, Sivertsen S, Ruud TE, Bouma BN, Stadaas JO, Aasen AO. Studies on components of the contact phase system in patients with advanced gastrointestinal cancer. Cancer. 1990;65(6):1355–1359. doi: [DOI] [PubMed] [Google Scholar]
- 183.Battistelli S, Stefanoni M, Lorenzi B, et al. Coagulation Factor Levels in Non-Metastatic Colorectal Cancer Patients. Int J Biol Markers. 2008;23(1):36–41. doi: 10.1177/172460080802300106 [DOI] [PubMed] [Google Scholar]
- 184.Pan J, Qian Y, Weiser P, et al. Glycosaminoglycans and Activated Contact System in Cancer Patient Plasmas. In: Zhang L, ed. Progress in Molecular Biology and Translational Science. Vol 93. Glycosaminoglycans in Development, Health and Disease. Academic Press; 2010:473–495. doi: 10.1016/S1877-1173(10)93020-2 [DOI] [PubMed] [Google Scholar]
- 185.Sciacca FL, Ciusani E, Silvani A, et al. Genetic and Plasma Markers of Venous Thromboembolism in Patients with High Grade Glioma. Clin Cancer Res. 2004;10(4):1312–1317. doi: 10.1158/1078-0432.CCR-03-0198 [DOI] [PubMed] [Google Scholar]
- 186.Sotiropoulos G, Kotopouli M, Karampela I, et al. Circulating plasminogen activator inhibitor-1 activity: a biomarker for resectable non-small cell lung cancer? J BUON. 2019;24(3):943–954. [PubMed] [Google Scholar]
- 187.Nomura S, Ito T, Yoshimura H, et al. Evaluation of thrombosis-related biomarkers before and after therapy in patients with multiple myeloma. J Blood Med. 2018;9:1–7. doi: 10.2147/JBM.S147743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Andrén‐Sandberg Å, Lecander I, Martinsson G, Åstedt B. Peaks in plasma plasminogen activator inhibitor-1 concentration may explain thrombotic events in cases of pancreatic carcinoma. Cancer. 1992;69(12):2884–2887. doi: [DOI] [PubMed] [Google Scholar]
- 189.Prins MH, Hirsh J. A Critical Review of the Evidence Supporting a Relationship Between Impaired Fibrinolytic Activity and Venous Thromboembolism. Arch Intern Med. 1991;151(9):1721–1731. doi: 10.1001/archinte.1991.00400090023006 [DOI] [PubMed] [Google Scholar]
- 190.Brodsky SV, Malinowski K, Golightly M, Jesty J, Goligorsky MS. Plasminogen Activator Inhibitor-1 Promotes Formation of Endothelial Microparticles With Procoagulant Potential. Circulation. Published online October 29, 2002. doi: 10.1161/01.CIR.0000033972.90653.AF [DOI] [PubMed] [Google Scholar]
- 191.Chen N, Ren M, Li R, et al. Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer. 2015;14(1):140. doi: 10.1186/s12943-015-0418-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of Venous Thromboembolism With the Angiogenesis Inhibitor Bevacizumab in Cancer Patients: A Meta-analysis. JAMA. 2008;300(19):2277–2285. doi: 10.1001/jama.2008.656 [DOI] [PubMed] [Google Scholar]
- 193.Rivera J, Lozano ML, Navarro-Nunez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94(5):700–711. doi: 10.3324/haematol.2008.003178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Plantureux L, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Impacts of Cancer on Platelet Production, Activation and Education and Mechanisms of Cancer-Associated Thrombosis. Cancers. 2018;10(11):441. doi: 10.3390/cancers10110441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Haemmerle M, Bottsford-Miller J, Pradeep S, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885–1896. doi: 10.1172/JCI85086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boukerche H, Berthier‐Vergnes O, Penin F, et al. Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. British Journal of Haematology. 1994;87(4):763–772. doi: 10.1111/j.1365-2141.1994.tb06736.x [DOI] [PubMed] [Google Scholar]
- 197.de Leval X, Benoit V, Delarge J, et al. Pharmacological evaluation of the novel thromboxane modulator BM-567 (II/II). Effects of BM-567 on osteogenic sarcoma-cell-induced platelet aggregation. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;68(1):55–59. doi: 10.1016/S0952-3278(02)00235-1 [DOI] [PubMed] [Google Scholar]
- 198.Bussolati B, Russo S, Deambrosis I, et al. Expression of CD154 on renal cell carcinomas and effect on cell proliferation, motility and platelet-activating factor synthesis. International Journal of Cancer. 2002;100(6):654–661. doi: 10.1002/ijc.10545 [DOI] [PubMed] [Google Scholar]
- 199.Sakai H, Suzuki T, Takahashi Y, et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Letters. 2006;580(14):3368–3374. doi: 10.1016/j.febslet.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 200.Nie D, Che M, Zacharek A, et al. Differential Expression of Thromboxane Synthase in Prostate Carcinoma. Am J Pathol. 2004;164(2):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cathcart M-C, Gately K, Cummins R, Kay E, O’Byrne KJ, Pidgeon GP. Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer. Mol Cancer. 2011;10(1):25. doi: 10.1186/1476-4598-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kajita S, Ruebel KH, Casey MB, Nakamura N, Lloyd RV. Role of COX-2, thromboxane A 2 synthase, and prostaglandin I 2 synthase in papillary thyroid carcinoma growth. Modern Pathology. 2005;18(2):221–227. doi: 10.1038/modpathol.3800285 [DOI] [PubMed] [Google Scholar]
- 203.Moussa O, Yordy JS, Abol-Enein H, et al. Prognostic and Functional Significance of Thromboxane Synthase Gene Overexpression in Invasive Bladder Cancer. Cancer Res. 2005;65(24):11581–11587. doi: 10.1158/0008-5472.CAN-05-1622 [DOI] [PubMed] [Google Scholar]
- 204.Oleksowicz L, Bhagwati N, DeLeon-Fernandez M. Deficient Activity of von Willebrand’s Factor-cleaving Protease in Patients with Disseminated Malignancies. Cancer Res. 1999;59(9):2244–2250. [PubMed] [Google Scholar]
- 205.Mojiri A, Stoletov K, Lorenzana Carrillo MA, et al. Functional assessment of von Willebrand factor expression by cancer cells of non-endothelial origin. Oncotarget. 2016;8(8):13015–13029. doi: 10.18632/oncotarget.14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143(7):819–826. doi: 10.1038/sj.bjp.0706013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Contursi A, Sacco A, Grande R, Dovizio M, Patrignani P. Platelets as crucial partners for tumor metastasis: from mechanistic aspects to pharmacological targeting. Cell Mol Life Sci. 2017;74(19):3491–3507. doi: 10.1007/s00018-017-2536-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131(16):1777–1789. doi: 10.1182/blood-2017-05-743187 [DOI] [PubMed] [Google Scholar]
- 209.Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thrombosis Research. 2010;126(2):113–118. doi: 10.1016/j.thromres.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Journal of Thrombosis and Haemostasis. 2010;8(1):114–120. doi: 10.1111/j.1538-7836.2009.03680.x [DOI] [PubMed] [Google Scholar]
- 211.Stender MT, Frøkjær JB, Larsen TB, Lundbye-Christensen S, Thorlacius-Ussing O. Preoperative Plasma D-Dimer Is a Predictor of Postoperative Deep Venous Thrombosis in Colorectal Cancer Patients: A Clinical, Prospective Cohort Study with One-Year Follow-Up. Diseases of the Colon & Rectum. 2009;52(3):446–451. doi: 10.1007/DCR.0b013e318197e2b2 [DOI] [PubMed] [Google Scholar]
- 212.Kodama J, Seki N, Masahiro S, et al. D-dimer level as a risk factor for postoperative venous thromboembolism in Japanese women with gynecologic cancer. Annals of Oncology. 2010;21(8):1651–1656. doi: 10.1093/annonc/mdq012 [DOI] [PubMed] [Google Scholar]
- 213.Arpaia G, Carpenedo M, Verga M, et al. D-dimer before chemotherapy might predict venous thromboembolism. Blood Coagulation & Fibrinolysis. 2009;20(3):170–175. doi: 10.1097/MBC.0b013e32831bc2de [DOI] [PubMed] [Google Scholar]
- 214.Reitter E-M, Kaider A, Ay C, et al. Longitudinal analysis of hemostasis biomarkers in cancer patients during antitumor treatment. Journal of Thrombosis and Haemostasis. 2016;14(2):294–305. doi: 10.1111/jth.13218 [DOI] [PubMed] [Google Scholar]
- 215.Ay C, Vormittag R, Dunkler D, et al. D-Dimer and Prothrombin Fragment 1 + 2 Predict Venous Thromboembolism in Patients With Cancer: Results From the Vienna Cancer and Thrombosis Study. JCO. 2009;27(25):4124–4129. doi: 10.1200/JCO.2008.21.7752 [DOI] [PubMed] [Google Scholar]
- 216.Ferroni P, Martini F, Portarena I, et al. Novel High-Sensitive D-Dimer Determination Predicts Chemotherapy-Associated Venous Thromboembolism in Intermediate Risk Lung Cancer Patients. Clinical Lung Cancer. 2012;13(6):482–487. doi: 10.1016/j.cllc.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 217.Linkins L-A, Lapner ST. Review of D-dimer testing: Good, Bad, and Ugly. International Journal of Laboratory Hematology. 2017;39(S1):98–103. doi: 10.1111/ijlh.12665 [DOI] [PubMed] [Google Scholar]
- 218.Riley RS, Gilbert AR, Dalton JB, Pai S, McPherson RA. Widely Used Types and Clinical Applications of D-Dimer Assay. Lab Med. 2016;47(2):90–102. doi: 10.1093/labmed/lmw001 [DOI] [PubMed] [Google Scholar]
- 219.Haase C, Joergensen M, Ellervik C, Joergensen MK, Bathum L. Age- and sex-dependent reference intervals for D-dimer: Evidence for a marked increase by age. Thrombosis Research. 2013;132(6):676–680. doi: 10.1016/j.thromres.2013.09.033 [DOI] [PubMed] [Google Scholar]
- 220.Kolkhir P, André F, Church MK, Maurer M, Metz M. Potential blood biomarkers in chronic spontaneous urticaria. Clin Exp Allergy. 2017;47(1):19–36. doi: 10.1111/cea.12870 [DOI] [PubMed] [Google Scholar]
- 221.Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112(7):2703–2708. doi: 10.1182/blood-2008-02-142422 [DOI] [PubMed] [Google Scholar]
- 222.Exter PL den, Kooiman J, Huisman MV. Validation of the Ottawa prognostic score for the prediction of recurrent venous thromboembolism in patients with cancer-associated thrombosis. Journal of Thrombosis and Haemostasis. 2013;11(5):998–1000. doi: 10.1111/jth.12192 [DOI] [PubMed] [Google Scholar]
- 223.Ahn S, Lim KS, Lee Y-S, Lee J-L. Validation of the clinical prediction rule for recurrent venous thromboembolism in cancer patients: the Ottawa score. Support Care Cancer. 2013;21(8):2309–2313. doi: 10.1007/s00520-013-1792-9 [DOI] [PubMed] [Google Scholar]
- 224.Astruc N, Ianotto J-C, Metges J-P, Lacut K, Delluc A. External validation of the modified Ottawa score for risk stratification of recurrent cancer-associated thrombosis. European Journal of Internal Medicine. 2016;36:e11–e12. doi: 10.1016/j.ejim.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 225.Khorana AA, Kamphuisen PW, Meyer G, et al. Tissue Factor As a Predictor of Recurrent Venous Thromboembolism in Malignancy: Biomarker Analyses of the CATCH Trial. Journal of Clinical Oncology. 2017;35(10):1078–1085. doi: 10.1200/JCO.2016.67.4564 [DOI] [PubMed] [Google Scholar]
- 226.van Es N, Louzada M, Carrier M, et al. Predicting the risk of recurrent venous thromboembolism in patients with cancer: A prospective cohort study. Thrombosis Research. 2018;163:41–46. doi: 10.1016/j.thromres.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 227.Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, et al. D-dimer and high-sensitivity C-reactive protein levels to predict venous thromboembolism recurrence after discontinuation of anticoagulation for cancer-associated thrombosis. British Journal of Cancer. 2018;119(8):915–921. doi: 10.1038/s41416-018-0269-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grilz E, Marosi C, Königsbrügge O, et al. Association of complete blood count parameters, d-dimer, and soluble P-selectin with risk of arterial thromboembolism in patients with cancer. Journal of Thrombosis and Haemostasis. 2019;17(8):1335–1344. doi: 10.1111/jth.14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Grilz E, Mauracher L-M, Posch F, et al. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. British Journal of Haematology. 2019;186(2):311–320. doi: 10.1111/bjh.15906 [DOI] [PMC free article] [PubMed] [Google Scholar]