Abstract
The CSPPT (China Stroke Primary Prevention Trial) demonstrated a significant risk reduction of first stroke in hypertensive patients treated with enalapril plus folic acid compared with those with enalapril alone, but the lifetime stroke-free survival associated with the treatment is unknown. By establishing adjusted models for competing risks and an age-based time scale using data from 19053 participants of the CSPPT, we estimated lifetime incremental stroke-free survival for enalapril-folic acid versus enalapril alone. Compared with enalapril alone, the enalapril plus folic acid treatment projected a mean lifetime stroke-free survival gain of 1.75 months, with an interquartile range from 0.73 to 2.39 months and the maximum gain up to 12.95 months. Subgroup analyses showed greater gain in stroke-free survival in younger, male patients, those with lower baseline folate levels, higher baseline systolic blood pressure, higher baseline total cholesterol and blood glucose, and with MTHFR (methylenetetrahydrofolate reductase) C677T CT or TT genotype. Overall, besides significant benefit in certain subgroups, enalapril plus folic acid treatment for hypertensive patients is associated with a modest gain in lifetime stroke-free survival, compared with enalapril alone.
Keywords: folic acid, hypertension, life expectancy, primary prevention, stroke
Stroke has been among the leading cause of mortality and serious, long-term disability in the world and China.1–4 Notably, as >75% of strokes are first occurrence,5 it is imperative to seek an effective strategy for primary prevention of stroke in China and around the world.
The role of supplemental folic acid therapy in cardiovascular disease (CVD) and stroke prevention has remained controversial.6–9 The CSPPT (China Stroke Primary Prevention Trial)10 demonstrated that enalapril-folic acid was more effective in first stroke prevention than enalapril alone among hypertensive patients, with a reduction of 21% in first stroke incidence. Recently, meta-analyses on various populations reported the consistent effect of folic acid in risk reduction of CVD, including stroke.11–13
Since the CSPPT and other trials only followed patients for a limited time period, it is unknown whether folic acid supplementation combined with antihypertensive treatment is a superior strategy than the antihypertensive strategy alone over a lifetime, given that hypertension is a chronic condition and requires a lifetime treatment. In addition, it is also of great interest to clarify whether the lifetime benefit varies by patients’ clinical and demographic characteristics. Such information is critically needed to inform clinical and public health practice and health policy, given millions of hypertensive patients who might benefit from this therapy. In this study, we performed a lifetime stroke-free survival projection of the treatment effect of enalapril plus folic acid compared with enalapril alone based on empirical data from the CSPPT.
Methods
Availability of Data and Materials
The data, analytic methods, and study materials that support the findings of this study will be available from the corresponding authors on request after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University.
Standard Protocol Approvals, Registrations, and Patient Consents
We used the data from the CSPPT, which was approved by the ethics committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number FWA00001263). All patients provided written informed consent. The CSPPT is registered at the National Institutes of Health website (http://www.clinical-trials.gov) as identifier NCT00794885.10
Summary of the CSPPT
The details of the CSPPT have been published elsewhere.10 Briefly, the CSPPT was a randomized, double-blind clinical trial with the primary focus of comparing the effects of 10 mg enalapril combined with 0.8 mg folic acid daily therapy (single-pill compound, the enalapril-folic acid group) with 10 mg enalapril daily therapy alone (the enalapril group) on first stroke prevention. The CSPPT enrolled 20702 hypertensive adults without history of stroke or MI (myocardial infarction) in 32 communities in Anhui and Jiangsu provinces in China between May 19, 2008 and August 24, 2013. The participants enrolled in this trial were aged 45 to 75 years with hypertension defined as seated resting systolic blood pressure (SBP) higher than 140 mm Hg, or diastolic blood pressure (DBP) higher than 90 mm Hg at both the screening and recruitment visits or, who were receiving antihypertensive medication. The primary outcome was the first nonfatal or fatal stroke (ischemic or hemorrhagic), excluding subarachnoid hemorrhage and silent stroke. After a mean follow-up of 4.5 years, the hazard ratio for occurrence of the primary outcome was 0.79 (95% CI, 0.68–0.93), favoring the enalapril-folic acid group.
Competing Risk Regression Model and Estimation of Gain in Stroke-Free Survival
We used a competing risk-adjusted lifetime model based on a left truncation and right censoring model, as well as adaptations from the Fine and Gray model, which yielded cause-specific estimates of the cumulative incidence of 2 competing end points, first stroke, and nonstroke death.14–19 To make a lifetime stroke-free survival projection, we used patient age as the time scale to account for the limited follow-up time of the CSPPT.19 By estimating survival functions and parameters (β-coefficients, baseline cumulative sub-hazard functions, age-based cumulative sub-hazard functions, cumulative incidence functions, etc), we projected annual rates of stroke-free survival for each patient, by which we could fit the predicted stroke-free survival curve for each patient throughout the patient’s lifetime. By calculating the area under the stroke-free survival curve, we were able to project stroke-free survival for each patient. We then established a prediction model which estimated the projection of a stroke-free survival for each patient according to their clinical and demographic characteristics.19 In our model, each patient performed one of the 2 treatment scenarios (enalapril versus enalapril-folic acid). The difference in each patient’s stroke-free survival under these 2 scenarios was considered as the patient’s clinical benefit. More details are described in the online-only Data Supplement.
Means and standard deviations and proportions were calculated for population characteristics by quartiles of the incremental stroke-free survival. Differences in population characteristics were compared using ANOVA for continuous variables or χ2tests for categorical variables, respectively. Multivariable linear regression models and logistic model were performed to determine the association between the incremental stroke-free survival with main risk factors. All analyses were adjusted for age, sex, MTHFR (methylenetetrahydrofolate reductase) C677T genotype, baseline SBP and DBP, mean SBP and DBP during treatment, body mass index, treatment group, study center, baseline serum folate, vitamin B12, fasting glucose, total cholesterol, triglycerides, HDL-C (high-density lipoprotein cholesterol), homocysteine, creatinine levels, smoking status, and alcohol drinking status.
Subgroup Analysis
We performed prespecified subgroup analysis of stroke-free survival according to a selected combination of risk factors strata, including age, sex, SBP at baseline, total cholesterol, fasting glucose, MTHFR C677T polymorphism, and folate levels.
The establishment of first stroke and nonstroke death competing model, calculation of baseline cumulative sub-hazard functions of 2 competing end points, and β-coefficients for the model were conducted using STATA (Version 14.0; StataCorp College Station, TX).20 Estimation of other survival functions and parameters, calculation of area under survival curve, and other calculations were all conducted using R software packages (version 3.5.3. http://www.r-project.org, packages: survival, LifeTables, and demography).
Results
Characteristics of the Study Population
A flow chart of participants who were included in the analyses is presented in the Figure S1 in the online-only Data Supplement. Among the original 20702 CSPPT participants, 588 were excluded due to missing data of one or more covariates. An additional 1061 individuals were removed due to age <47 years old. Baseline characteristics of the final sample of 19053 participants are shown in Table 1. The mean age of the participants was 60.8 (SD 7.0) years, and 41.1% were male, with 23.7% being active smoker and 24.0% consuming alcohol. The mean baseline SBP and DBP was 167.2 (SD 20.4) and 93.7 (SD 11.8) mm Hg, respectively, while mean SBP and DBP during treatment period was 139.4 (SD 10.8) and 82.6 (SD 7.2) mm Hg (Table 1).
Table 1.
Baseline Characteristics of Participants in the Final Analysis
| Characteristics | Total | Treatment Group | P Value | |
|---|---|---|---|---|
| Enalapril | Enalapril-Folic Acid | |||
| No. (%) with data | 19053 | 9507 | 9546 | |
| Age, mean (SD), y | 60.8 (7.0) | 60.8 (7.0) | 60.8 (6.9) | 0.870 |
| Male, No. (%) | 7829 (41.1) | 3919 (41.2) | 3910 (41.0) | 0.713 |
| Body mass index, mean (SD), kg/m2 | 24.9 (3.7) | 24.8 (3.7) | 24.9 (3.7) | 0.254 |
| Smoking status, No. (%) | 0.242 | |||
| Never | 1 3061 (68.6) | 6504 (68.4) | 6557 (68.7) | |
| Former | 1478 (7.8) | 768 (8.1) | 710 (7.4) | |
| Current | 4514 (23.7) | 2235 (23.5) | 2279 (23.9) | |
| Alcohol consumption, No. (%) | 0.5400 | |||
| Never | 1 3128 (68.9) | 6519 (68.6) | 6609 (69.2) | |
| Former | 1354 (7.1) | 691 (7.3) | 663 (6.9) | |
| Current | 4571 (24.0) | 2297 (24.2) | 2274 (23.8) | |
| MTHFR C677T polymorphism, No. (%) | 0.947 | |||
| CC genotype | 5221 (27.4) | 2611 (27.5) | 2610 (27.3) | |
| CT genotype | 9348 (49.1) | 4653 (48.9) | 4695 (49.2) | |
| TT genotype | 4484 (23.5) | 2243 (23.6) | 2241 (23.5) | |
| Blood pressure, mean (SD), mm Hg | ||||
| Baseline SBP | 167.2 (20.4) | 167.3 (20.4) | 167.1(20.3) | 0.504 |
| Baseline DBP | 93.7 (11.8) | 93.6 (11.9) | 93.7 (11.7) | 0.429 |
| Mean SBP during treatment period | 139.4 (10.8) | 139.5 (10.9) | 139.3 (10.7) | 0.239 |
| Mean DBP during treatment period | 82.6 (7.2) | 82.6 (7.3) | 82.6 (7.2) | 0.873 |
| Laboratory results | ||||
| TC, mean (SD), mmol/L | 5.5 (1.2) | 5.5 (1.2) | 5.5 (1.2) | 0.828 |
| HDL-C, mean (SD), mmol/L | 1.3 (0.4) | 1.3 (0.4) | 1.4 (0.4) | 0.677 |
| TG, mean (SD), mmol/L | 1.7 (1.2) | 1.7 (0.9) | 1.7 (1.3) | 0.937 |
| Fasting glucose, mean (SD), mmol/L | 5.8 (1.7) | 5.8 (1.7) | 5.8 (1.7) | 0.276 |
| Creatinine, mean (SD), μmol/L | 66.2 (19.5) | 66.1 (19.2) | 66.2 (19.7) | 0.977 |
| Homocysteine, mean (SD), μmol/L | 14.5 (8.3) | 14.6 (8.5) | 14.5 (8.1) | 0.394 |
| Folate, mean (SD), ng/mL | 8.6 (3.9) | 8.6 (3.9) | 8.6 (3.9) | 0.676 |
| Vitamin B12, mean (SD), pg/mL | 412.1 (155.7) | 414.5 (161.5) | 409.8 (149.6) | 0.035 |
DBP indicates diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure; TC, total cholesterol; and TG, triglycerides.
Model Establishment
We used the 19053 participants for the development of an age-based cumulative sub-hazard function of stroke and nonstroke death, respectively. Based on these function parameters, we established a projection model for participants at entry age up to an exit age of 80. In this model, we evaluated the lifetime benefit from the folic acid therapy versus without the folic acid therapy. The β-coefficients and P values of the projection models for first stroke-risk and nonstroke death are presented in Table 2.
Table 2.
Estimates of β-Coefficients of Projection Models for First Stroke-Risk and Nonstroke Death
| Variables | First Stroke | Nonstroke Death | ||
|---|---|---|---|---|
| β-Coefficients* | P Value | β Coefficients* | P Value | |
| Treatment (enalapril-folic acid vs enalapril alone) | −0.2439681 | 0.003 | 0.0082278 | 0.924 |
| Study center | 0.0465075 | 0.700 | −0.2311645 | 0.050 |
| Sex (male vs female) | −0.1251136 | 0.340 | −0.2258367 | 0.084 |
| Mean SBP during treatment period | 0.0069797 | 0.166 | 0.0290951 | <0.001 |
| Mean DBP during treatment period | 0.0324575 | 0.001 | 0.0157805 | 0.172 |
| Smoking status | 0.1598237 | 0.010 | 0.0820128 | 0.210 |
| Alcohol drinking status | −0.0669677 | 0.287 | 0.0155645 | 0.792 |
| Baseline SBP | 0.0136038 | <0.001 | −0.0051769 | 0.087 |
| Baseline DBP | 0.000034 | 0.995 | −0.0008798 | 0.884 |
| BMI | 0.009459 | 0.478 | −0.0630977 | <0.001 |
| TG | −0.1033082 | 0.071 | 0.0357859 | 0.004 |
| HDL-C | −0.1843994 | 0.187 | 0.221062 | 0.117 |
| MTHFR C677T polymorphism | 0.0119565 | 0.848 | 0.0206116 | 0.750 |
| Folate | −0.0246603 | 0.073 | −0.0141871 | 0.267 |
| Vitamin B12 | −0.0001864 | 0.516 | 0.0004509 | 0.083 |
| Homocysteine | 0.0032583 | 0.469 | 0.0108034 | 0.003 |
| Creatinine | −0.0027568 | 0.216 | 0.0059708 | <0.001 |
| TC | 0.1169953 | 0.002 | −0.0870128 | 0.040 |
| Fasting glucose | 0.1021834 | <0.001 | 0.1036063 | <0.001 |
BMI indicates body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure; TC, total cholesterol; and TG, triglycerides.
All analyses were adjusted for age, sex (female, male [reference]), MTHFR C677T genotype (TT, CT, CC [reference]), baseline SBP and DBP, mean SBP and DBP during treatment, BMI, treatment group, study center (Lianyungang, Anqing [reference]), baseline serum folate, vitamin B12, fasting glucose, total cholesterol, triglycerides, HDL-C, homocysteine, creatinine levels, smoking status (current, ever, never [reference]) and alcohol drinking status (current, ever, never [reference]), and the categorical variables are treated as continuous variables.
Baseline SBP (β=0.0136, P<0.001), fasting glucose (β=0.1022, P<0.001), mean DBP during treatment period (β=0.0325, P=0.001), together with smoking status and total cholesterol, were the statistically significant predictors of for first stroke to estimate stroke-free survival. The enalapril plus folic acid treatment was associated with a significantly lower risk for first stroke (β=−0.2440, P=0.003) and eventually increasing stroke-free survival.
For nonstroke death, the statistical significant predictor were mean SBP during treatment period (β=0.0291, P<0.001), body mass index (β=−0.0631, P<0.001), creatinine (β=0.0060, P<0.001), fasting glucose (β=0.1036, P<0.001), as well as homocysteine, total cholesterol, and triglycerides. It is noted that treatment with enalapril plus folic acid was not significantly associated with a lower risk for nonstroke death.
Gains in Stroke-Free Survival
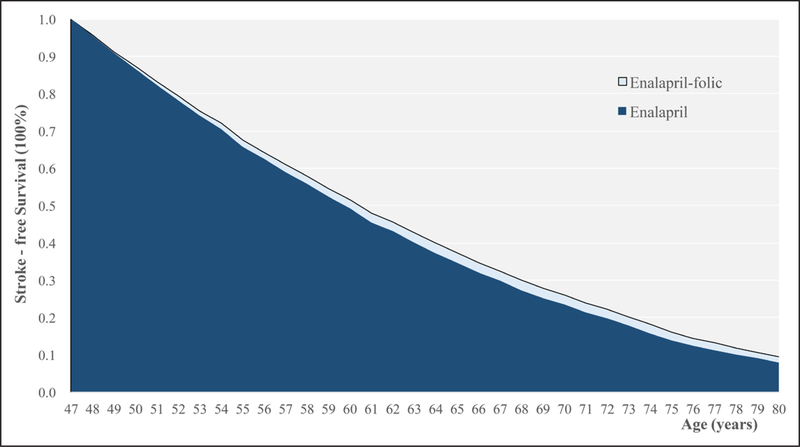
Compared with the enalapril alone therapy, the enalapril-folic acid therapy prolonged lifetime stroke-free survival by mean of 1.75 months (95% CI, 1.73–1.77 months), median gain of 1.42 months, an interquartile range from 0.73 months to 2.39 months with a maximum gain up to 12.95 months (Figure S2). As shown, Figure 1 illustrates the cumulative survival free of stroke and death for a 47-year-old male with diabetes mellitus (fasting blood glucose level of 7.86 mmol/L), total cholesterol level of 8.14 mmol/L, folate level of 2.74 ng/mL, and baseline SBP of 185 mm Hg. The effect of enalapril-folic acid compared with enalapril alone is represented by the size of the light blue area between the curves; for this patient, the gain is equal to 7.92 months.
Figure 1.
Predicted folic acid treatment effect for sampled patient. Cumulative survival free of stroke and nonstroke death for 47-year–old male with diabetes mellitus (with fasting blood glucose level of 7.86 mmol/L), total cholesterol level of 8.14 mmol/L, folate level of 2.74 ng/mL, and systolic blood pressure of 185 mm Hg. The effect of enalapril-folic acid is represented by the size of the light blue area between the curves, equal to 7.92 mo.
Baseline characteristics of the participants according to quartiles of incremental stroke-free survival are presented in Table 3. Participants with higher predicted folic acid treatment effect tended to have younger age, higher body mass index, higher SBP and DBP, higher total cholesterol, higher glucose and lower folate concentrations, and were more likely to be current smokers and alcohol drinkers and the MTHFR C677T CT or TT genotype carrier (Table 3). These patterns remain after adjusting for potential covariates (Table S1 and Figure S3).
Table 3.
Comparison of the Characteristics of Patients With Different Predicted Folic Acid Treatment Effect
| Characteristics | Incremental Stroke-Free Survival, Mo | P Value | |||
|---|---|---|---|---|---|
| Q1 (<0.73) | Q2 (≥0.73, <1.42) | Q3 (≥1.42, <2.39) | Q4 (≥2.39) | ||
| No. (%) with data | 4763 | 4763 | 4763 | 4764 | |
| Age, mean (SD), y | 68.9 (4.0) | 62.2 (4.6) | 57.7 (4.5) | 54.4 (4.3) | <0.001 |
| BMI, mean (SD), kg/m2 | 23.4 (3.5) | 24.4 (3.5) | 25.4 (3.5) | 26.3 (3.6) | <0.001 |
| Current smoking (male), No. (%) | 1066 (49.1) | 969 (51.2) | 909 (51.4) | 1189 (59.6) | <0.001 |
| Current alcohol drinking (male), No. (%) | 1038 (47.8) | 984 (52.0) | 950 (53.7) | 1137 (57.0) | <0.001 |
| MTHFRC677T polymorphisms, No. (%) | <0.001 | ||||
| CC genotype | 1477 (31.0) | 1383 (29.0) | 1253 (26.3) | 1108 (23.3) | |
| CT genotype | 2289 (48.1) | 2335 (49.0) | 2325 (48.8) | 2399 (50.4) | |
| TT genotype | 997 (20.9) | 1045 (21.9) | 1185 (24.9) | 1257 (26.4) | |
| BP, mean (SD), mm Hg | |||||
| Baseline SBP | 164.0 (18.9) | 164.1 (19.6) | 165.1 (18.7) | 175.7 (21.8) | <0.001 |
| Baseline DBP | 86.9 (10.9) | 91.1 (10.0) | 94.9 (9.8) | 101.8 (11.1) | <0.001 |
| Laboratory results (baseline) | |||||
| TC, mean (SD), mmol/L | 5.3 (1.1) | 5.4 (1.2) | 5.6 (1.1) | 5.9 (1.3) | <0.001 |
| Fasting glucose, mean (SD), mmol/L | 5.5 (1.3) | 5.6 (1.5) | 5.8 (1.5) | 6.3 (2.3) | <0.001 |
| Folate, mean (SD), ng/mL | 9.3 (4.7) | 9.0 (4.0) | 8.3 (3.5) | 7.8 (3.1) | <0.001 |
BMI indicates body mass index; BP, blood pressure; DBP, diastolic blood pressure; MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure; TC, total cholesterol; and TG, triglycerides.
Subgroup Analysis by Risk Factors
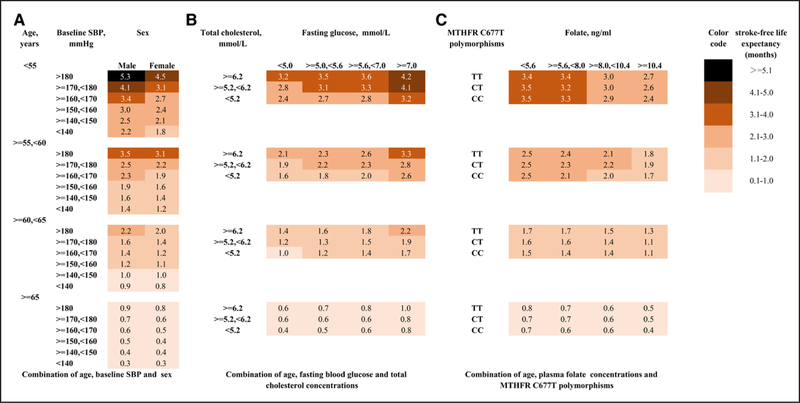
The gains in stroke-free survival by the enalapril-folic acid therapy were categorized according to combined selected risk factors (Figure 2). Up to a mean of 5.3 months of stroke-free life years could be achieved in male patients with baseline SBP higher than 180 and age younger than 55. Up to a mean of 4.2 months of stroke-free life years could be gained in patients with glucose higher than 7.0 mmol/L while total cholesterol higher than 6.2 mmol/L as well as age younger than 55. A mean of 3.4 months of stroke-free life years could be gained in patients whose folate level were <5.6 ng/mL with MTHFR C677T TT genotype as well as age younger than 55.
Figure 2.
Predicted lifetime gain in stroke-free survival from enalapril-folic acid compared with enalapril treatment alone. A zero means no treatment effect. A 3.0 means that lifetime enalapril-folic acid treatment is expected to prolong the patient’s stroke-free survival by 3 months compared with enalapril alone. A, The lifetime stroke-free survival of enalapril-folic acid treatment is higher for younger male individuals with higher systolic blood pressure. B, The lifetime benefits of enalapril-folic acid treatment are higher for younger individuals with higher fasting blood glucose and higher total cholesterol. C, Younger individuals with lower folate levels and the CT/TT genotype gain more in stroke-free survival. CC indicates MTHFR C677T CC genotype; CT, MTHFR C677T CT genotype; CT, MTHFR C677T CT genotype; MTHFR, methylenetetrahydrofolate reductase; and SBP, systolic blood pressure.
Discussion
This study attempted to address a critically needed public health and policy question related to primary prevention of stroke in China, a country with the largest burden of stroke in the world. First, the CSPPT primary results were limited to the estimation of folic acid efficacy during the clinical trial period (an average of 4.5 years).10 We, for the first time, estimated the lifetime benefit of enalapril plus folic acid treatment compared with enalapril alone in the primary prevention of stroke via assessing stroke-free survival. This is important, given hypertension requires life-long treatment. Second, the primary results focused on the risk of incident stroke, while this study focused on stroke-free survival, an important parameter of health economics. Third, our analyses lent further support for the CSPPT primary findings and post hoc analyses10,21–23 that certain subgroups appeared to have greater reduction in stroke risk in response to folic acid therapy. Consistently, this study showed a greater gain in lifetime stroke-free survival with the folic acid therapy in certain subgroups, including younger males, those with lower baseline folate levels, and those with higher levels of SBP, total cholesterol, and fasting glucose, or those with MTHFR C677T CT or TT genotype.
Our subgroup analyses suggested that patients with low folate baseline level may have longer lifetime stroke-free survival from folate therapy. This helps to explain why most folate trials among western countries such as the United States were negative, where the proportion of individuals with very low folate levels (<3 ng/mL) has decreased substantially from 22% to 1.7% and mean folate levels increased from 4.6 ng/mL to 11.9 ng/mL after the mandatory folate fortification of grain products (implemented since 1998 to reduce incidence of neural tube defects).24,25 However, even in the United States, Asian Americans have a higher mortality, prevalence, and incidence of stroke and more severe stroke complications than whites.26,27 We speculate that folic acid therapy may benefit those Asian Americans, especially among first-generation immigrants, with MTHFR 677 TT genotype and low folate levels, even though United States is the country with mandatory folic acid fortification. More importantly, besides people living in China, large parts of the world’s population in Bangladesh and Scandinavia,28 whose mean folate level may be as low as 6.1 nmol/L with 89.2% of local males in spring suffering from inadequate folate intake,29 and those populations in low folate regions may potentially benefit from folate supplementation. However, whether our findings can be readily extrapolated to other populations with different genetic, nutritional, and clinical characteristics will require further research.
Our findings seem to be biologically plausible. Previous biochemical and pathological studies have showed that elevated total homocysteine could induce vascular disease, and the main mechanisms involve impaired endothelial function, increased oxidative stress, alterations of lipid metabolism, and induction of thrombosis.30 Hypertension and elevated homocysteine are both independent, modifiable risk factors, which were proven to act additively to increase the risk of first stroke and stroke mortality.31,32 Folate is considered as one of the most important dietary determinants of homocysteine,33 which acts as substrates in the metabolism of methionine and homocysteine.34 The C→T mutation of MTHFR C677T leads to a reduction in enzyme activity for folate metabolism, resulting in decreased blood folate levels and increased blood homocysteine levels.34 The CSPPT primary results and its post hoc studies have also demonstrated that baseline serum folate levels and the MTHFR C677T genotype could jointly modify the folic acid therapeutic effect on serum homocysteine lowering and primary prevention of stroke.10
While our estimated incremental stroke-free survival for enalapril-folic acid appears to be modest, it is comparable to previous studies in CVD. Using the same method as our study, Dorresteijn19 showed that most of participants from the Women’s Health Study35,36 (94%) had a predicted aspirin treatment effect of <2 months gain in CVD-free survival compared with those without aspirin treatment. Ferket et al37 predicted that the mean increased CHD/stroke-free survival was 8.4 months with statin therapy compared with no therapy for asymptomatic individuals. Of note, the aforementioned studies on the lifetime benefits of aspirin and statin were all based on comparison to no therapy, our research on folic acid therapy was compared with standard antihypertensive therapy (enalapril). As enalapril was proven to have significant primary CVD preventive effect than no therapy,38 it may well be that folic acid therapy coupled with enalapril may have a much longer lifetime stroke-free survival when compared with no therapy among hypertensive patients, but such a study is not possible due to ethical concern.
As for the subgroup analysis of lifetime benefit stratified by patients’ characteristics, our findings illustrated those by the Joint British Societies’ consensus recommendations for the prevention of CVD,17 which found that younger patients who had been evaluated with a low 10-year risk were nevertheless proven to have a high lifetime event risk. Additionally, aspirin and statins were proven to have a greater lifetime benefit when the medication was applied at a younger age.19,37 These findings are consistent with our results in that a younger population could benefit more than an elder population from the folic acid therapy.
Our study has a number of strengths. Methodologically, based on a previously validated method,19 we converted the original CSPPT data (by far, the world largest randomized double-blind clinical trial for the primary prevention of stroke) into an evaluation of patient-level lifetime stroke-free survival benefit. Our model took competing risks into consideration for long-term projection. Ignoring competing risks may cause an overestimation of the final result.39 Our study has important clinical and public health implications. We for the first time estimated the lifetime benefit of enalapril plus folic acid treatment compared with enalapril alone in the primary prevention of stroke via assessing stroke-fee survival. Chronic diseases such as hypertension generally require lifetime medications, and evidence for decision-making should reflect the long-term benefit.17 Such information can facilitate physicians, patients, and policy makers to make informed decisions according to individual characteristics, risk, and benefits of life long hypertension treatment for the prevention of stroke.
Some limitations in our study are noted. We did not consider the changes in risk factor levels over time, such as age-related changes in blood pressure and cholesterol levels. However, bias may be mitigated because large fluctuations in these biomarkers or risk factors are usually not seen until first CVD event.17 Second, the effect of folic acid therapy was modeled based on the CSPPT data with stringent inclusion and exclusion criteria that may constrain generalizability of our findings. Due to the fact that there was no occurrence of the end point before age 47 in the CSPPT, our prediction model can only apply to patients aged 47 to 80 years old. Third, although our model for prediction of stroke-free survival is useful, our findings need further validation in independent data sets. Lastly, our study only included Chinese hypertensive patients, whether the findings can be extrapolated to other populations requires further verification.
Perspectives
Based on the CSPPT data, we estimated patient-level lifetime stroke-free survival for the enalapril-folic acid therapy compared with enalapril alone for primary prevention of stroke in hypertensive patients. We projected that enalapril plus folic acid treatment will have a modest lifetime stroke-free survival gain compared with enalapril alone in overall sample, and the benefit will be greater in some specific subgroups. From population health perspective, even a moderate gain may translate into a gain of millions of stroke-free months and will provide critically needed evidence to facilitate clinicians, patients, and policy holders to make informed decisions on stroke prevention.
Supplementary Material
Novelty and Significance.
What Is New?
The CSPPT (China Stroke Primary Prevention Trial) demonstrated a significant risk reduction of first stroke in hypertensive patients treated with enalapril plus folic acid compared with those with enalapril alone, but the life time stroke-free survival associated with the treatment is unknown. By establishing adjusted models for competing risks and an age-based time scale using data from 19053 participants of the CSPPT, we estimated lifetime incremental stroke-free survival for enalapril-folic acid versus enalapril alone.
What Is Relevant?
Compared with enalapril alone, the enalapril plus folic acid treatment projected a mean lifetime stroke-free survival gain of 1.75 months, with an interquartile range from 0.73 to 2.39 months and the maximum gain up to 12.95 months. From population health perspective, even a moderate gain may translate into a gain of millions of stroke-free months and will provide critically needed evidence to facilitate clinicians, patients, and policy holders to make informed decisions on stroke prevention.
Summary
Generally, besides significant benefit in certain subgroups, Enalapril plus folic acid treatment for hypertensive patients is associated with a modest gain in lifetime stroke-free survival, compared with enalapril alone.
Acknowledgments
Sources of Funding
The study was supported by funding from the following: the National Natural Science Foundation of China (grant number 71704064), the Natural Science Foundation of Guangdong Province, China (grant number 2017A030310174), the National Key Research and Development Program (2016YFC0903103), the Presidential Foundation of Nanfang Hospital, Southern Medical University (grant number 2017C007); the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009); Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (grant number U54-GM104941 United States).
Disclosures
T. Zhang reports grants from the National Natural Science Foundation of China (grant number 71704064) and the Natural Science Foundation of Guangdong Province, China (grant number 2017A030310174). Y. Huo reports grants from the National Key Research and Development Program (2016YFC0903103). X. Qin reports grants from the Presidential Foundation of Nanfang Hospital, Southern Medical University (grant number 2017C007) and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009). Z. Zhang reports grants from Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (grant number U54-GM104941 United States). The other authors report no conflicts.
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.14102.
Contributor Information
Tiantian Zhang, College of Pharmacy and International Cooperative Laboratory of Traditional Chinese Medicine Modernization and Innovative Drug Development of Chinese Ministry of Education (MOE), Jinan University, Guangzhou, China; Guangzhou Huabo Biopharmaceutical Research Institute, China.
Tengfei Lin, College of Pharmacy and International Cooperative Laboratory of Traditional Chinese Medicine Modernization, Jinan University, Guangzhou, China; Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food Science and Nutritional Engineering, China Agricultural University, China.
Yang Wang, Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, CA.
Binyan Wang, National Clinical Research Study Center for Kidney Disease; the State Key Laboratory for Organ Failure Research; Renal Division, Nanfang Hospital, Southern Medical University, Guangzhou, China; Shenzhen Evergreen Medical Institute, China.
Xianhui Qin, National Clinical Research Study Center for Kidney Disease; the State Key Laboratory for Organ Failure Research; Renal Division, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Feng Xie, Department of Clinical Epidemiology and Biostatistics and Centre for Health Economics and Policy Analysis, McMaster University, Hamilton, Ontario, Canada; Program for Health Economics and Outcomes Research, Hamilton, Ontario, Canada.
Yimin Cui, Department of Pharmacy, Peking University First Hospital, Beijing, China.
Yong Huo, Department of Cardiology, Peking University First Hospital, Beijing, China.
Xiaobin Wang, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland.
Zugui Zhang, Christiana Care Health System, Newark, Delaware.
Jie Jiang, College of Pharmacy and International Cooperative Laboratory of Traditional Chinese Medicine Modernization, Jinan University, Guangzhou, China.
References
- 1.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, Barker-Collo S, Moran AE, Sacco RL, Truelsen T, et al. ; GBD 2013 Writing Group; GBD 2013 Stroke Panel Experts Group. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 Study. Neuroepidemiology. 2015;45:161–176. doi: 10.1159/000441085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, et al. ; NESS-China Investigators. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X [DOI] [PubMed] [Google Scholar]
- 7.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, et al. ; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 8.Hankey GJ, Eikelboom JW, Baker RI, Gelavis A, Hickling SC, Jamrozik K, van Bockxmeer FM, Vasikaran S, Chen C, Eikelboom JW, et al. ; VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–865. doi: 10.1016/S1474-4422(10)70187-3 [DOI] [PubMed] [Google Scholar]
- 9.Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, Cooper J, Breteler MM, Bautista LE, Sharma P, Whittaker JC, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584–594. doi: 10.1016/S0140-6736(11)60872-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, et al. ; CSPPT Investigators. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. doi: 10.1001/jama.2015.2274 [DOI] [PubMed] [Google Scholar]
- 11.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71:2570–2584. doi: 10.1016/j.jacc.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Wu G, Li Y, Wang X, Hou FF, Xu X, Qin X, Cai Y. Meta-analysis of folic acid efficacy trials in stroke prevention: insight into effect modifiers. Neurology. 2017;88:1830–1838. doi: 10.1212/WNL.0000000000003909 [DOI] [PubMed] [Google Scholar]
- 13.Wang WW, Wang XS, Zhang ZR, He JC, Xie CL. A meta-analysis of folic acid in combination with anti-hypertension drugs in patients with hypertension and hyperhomocysteinemia. Front Pharmacol. 2017;8:585. doi: 10.3389/fphar.2017.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 15.Beiser A, D’Agostino RB Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: [DOI] [PubMed] [Google Scholar]
- 16.Wolkewitz M, Cooper BS, Bonten MJ, Barnett AG, Schumacher M. Interpreting and comparing risks in the presence of competing events. BMJ. 2014;349:g5060. doi: 10.1136/bmj.g5060 [DOI] [PubMed] [Google Scholar]
- 17.Board JBS. Joint british societies’ consensus recommendations for the prevention of cardiovascular disease(JBS3). Heart. 2014;100(suppl 2):ii1–ii67. doi: 10.1136/heartjnl-2014-305693 [DOI] [PubMed] [Google Scholar]
- 18.Hippisley-Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ. 2010;341:c6624. doi: 10.1136/bmj.c6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorresteijn JA, Kaasenbrood L, Cook NR, van Kruijsdijk RC, van der Graaf Y, Visseren FL, Ridker PM. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ. 2016;352:i1548. doi: 10.1136/bmj.i1548 [DOI] [PubMed] [Google Scholar]
- 20.Survival Analysis Reference Manual. Stata Press; 2017. https://www.stata.com/bookstore/survival-analysis-reference-manual/. [Google Scholar]
- 21.Qin X, Li J, Spence JD, Zhang Y, Li Y, Wang X, Wang B, Sun N, Chen F, Guo J, et al. Folic acid therapy reduces the first stroke risk associated with hypercholesterolemia among hypertensive patients. Stroke. 2016;47:2805–2812. doi: 10.1161/STROKEAHA.116.014578 [DOI] [PubMed] [Google Scholar]
- 22.Xu RB, Kong X, Xu BP, Song Y, Ji M, Zhao M, Huang X, Li P, Cheng X, Chen F, et al. Longitudinal association between fasting blood glucose concentrations and first stroke in hypertensive adults in China: effect of folic acid intervention. Am J Clin Nutr. 2017;105:564–570. doi: 10.3945/ajcn.116.145656 [DOI] [PubMed] [Google Scholar]
- 23.Kong X, Huang X, Zhao M, Xu B, Xu R, Song Y, Yu Y, Yang W, Zhang J, Liu L, et al. Platelet count affects efficacy of folic acid in preventing first stroke. J Am Coll Cardiol. 2018;71:2136–2146. doi: 10.1016/j.jacc.2018.02.072 [DOI] [PubMed] [Google Scholar]
- 24.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901 [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr. 2007;86:718–727. doi: 10.1093/ajcn/86.3.718 [DOI] [PubMed] [Google Scholar]
- 26.Palaniappan LP, Araneta MR, Assimes TL, Barrett-Connor EL, Carnethon MR, Criqui MH, Fung GL, Narayan KM, Patel H, Taylor-Piliae RE, et al. ; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; Council on Cardiovascular Nursing. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. doi: 10.1161/CIR.0b013e3181f22af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jose PO, Frank AT, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, Cullen MR, Palaniappan LP. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64:2486–2494. doi: 10.1016/j.jacc.2014.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(2 suppl):S38–S51. doi: 10.1177/15648265080292S107 [DOI] [PubMed] [Google Scholar]
- 29.Hao L, Zheng JC, Tian YH, Fan DW, Li Z. [Comparative study of the detection of plasma folate with microbial assay and radioimmunoassay]. Beijing Da Xue Xue Bao Yi Xue Ban. 2004;36:210–214. [PubMed] [Google Scholar]
- 30.Spence JD. Homocysteine-lowering therapy: a role in stroke prevention? Lancet Neurol. 2007;6:830–838. doi: 10.1016/S1474-4422(07)70219-3 [DOI] [PubMed] [Google Scholar]
- 31.Towfighi A, Markovic D, Ovbiagele B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: a nationwide study. J Neurol Sci. 2010;298:153–157. doi: 10.1016/j.jns.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Jiang S, Zhang Y, Tang G, Wang Y, Mao G, Li Z, Xu X, Wang B, Huo Y. H-type hypertension and risk of stroke in chinese adults: a prospective, nested case-control study. J Transl Int Med. 2015;3:171–178. doi: 10.1515/jtim-2015-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, et al. ; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900 [DOI] [PubMed] [Google Scholar]
- 34.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the nutrition committee, American Heart Association. Circulation. 1999;99:178–182. doi: 10.1161/01.cir.99.1.178 [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 36.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferket BS, van Kempen BJ, Heeringa J, Spronk S, Fleischmann KE, Nijhuis RL, Hofman A, Steyerberg EW, Hunink MG. Personalized prediction of lifetime benefits with statin therapy for asymptomatic individuals: a modeling study. PLoS Med. 2012;9:e1001361. doi: 10.1371/journal.pmed.1001361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savarese G, Costanzo P, Cleland JG, Vassallo E, Ruggiero D, Rosano G, Perrone-Filardi P. A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013;61:131–142. doi: 10.1016/j.jacc.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 39.Nelson CL, Sun JL, Tsiatis AA, Mark DB. Empirical estimation of life expectancy from large clinical trials: use of left-truncated, right-censored survival analysis methodology. Stat Med. 2008;27:5525–5555. doi: 10.1002/sim.3355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods, and study materials that support the findings of this study will be available from the corresponding authors on request after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University.