ABSTRACT
The naturally derived flavonoids are well known to have anticarcinogenic effects. Flavonoids could be an alternative strategy for ovarian cancer treatment, due to existing platinum-based drugs are reported to develop resistance with low survival rates. Inhibition of antiapoptotic proteins, namely B-cell lymphoma (Bcl-2) and B-cell lymphoma-extra large (Bcl-xl), is the key target to stimulate apoptosis process in cancer cells. This study aimed to determine the binding interaction of five naturally derived flavonoids (biochanin A, myricetin, apigenin, galangin, and fisetin) with potential antiapoptotic target proteins (Bcl-2 and Bcl-xl). The molecular docking study was conducted using AutoDock Vina program. The binding affinity and the presence of hydrogen bonds between the flavonoids and target proteins were predicted. Our findings showed that all the flavonoids showed better binding affinity with Bcl-xl than that of Bcl-2 proteins. The highest binding affinity was recorded in fisetin–Bcl-xl protein complex (−8.8 kcal/mol). Meanwhile, the other flavonoids docked with Bcl-xl protein showed binding affinities, ranging from –8.0 to –8.6 kcal/mol. A total of four hydrogen bonds, four hydrophobic contacts, and one electrostatic interaction were detected in the docked fisetin–Bcl-xl complex, explaining its high binding affinity with Bcl-xl. The present results indicate that all flavonoids could potentially serve as Bcl-xl protein inhibitors, which would consequently lead to apoptotic process in ovarian cancers.
KEYWORDS: Antiapoptotic proteins, docking, flavonoid, ovarian cancer
INTRODUCTION
Ovarian cancer is the highest fatal type of gynecological cancer and ranked as the third of global top mortality rate among females.[1] Meanwhile, ovarian cancer in Malaysia was ranked 12th for both incidence and mortality rate in 2018.[2] Patients with ovarian cancer generally suffer from a recurrence and progressive disease due to the resistance with the existing chemotherapy treatment.[3] Most of the prescribed chemotherapy drugs are synthetically derived and have shown toxicity effects not only against the cancer cells, but also against the normal cells.[4] In contrast, the recent findings indicate that naturally extracted phytochemicals from plants significantly show selective cytotoxicity to cancer cells and minimal toxicity to normal cells.[5] This could be an alternative treatment for cancer therapy. One of the interesting phytochemical compounds are flavonoids that are considered to exert anticarcinogenic effects. Flavonoids will induce apoptosis in the cancer cells, in which the apoptosis induction can occur in B-cell lymphoma (Bcl-2) family members.[6]
Bcl-2 family members comprise both proapoptotic and antiapoptotic proteins, which play an important role in controlling cellular apoptosis.[6] Antiapoptotic proteins (Bcl-2 and B-cell lymphoma-extra large [Bcl-x]l) are involved in preserving mitochondrial integrity, preventing the loss of mitochondrial membrane potential, and preventing cell death.[7] In addition, the intrinsic apoptotic mechanism will be triggered by the suppression of Bcl-2 and Bcl-xl proteins in tumor cells, resulting from strong antagonizing apoptosis signals.[8] Antiapoptotic proteins are abundant in most cancer cells as compared with proapoptotic proteins which could be absent or less expressed.[9] Inhibition of Bcl-2 and Bcl-xl proteins is the key target to induce apoptosis process in cancer cells. The small molecules that could inhibit the function of Bcl-2 or Bcl-xl proteins have gathered attention in recent years to be studied as potential new anticancer drugs.[10]
In this study, we aimed to investigate the binding interaction between a total of five selected naturally derived flavonoids (biochanin A, myricetin, apigenin, galangin, and fisetin) and potential antiapoptotic target proteins (Bcl-2 and Bcl-xl). The selection of these flavonoids is based on the commonality of their structures with only the difference in hydroxyl group position. Besides, the availability of each flavonoid is required for further investigation. These flavonoids are known to exist naturally in plants and possess potential pharmacological properties in many reported studies. Docking studies are performed to examine the binding affinities and the presence of non-covalent interactions between the selected flavonoids and Bcl-2 and Bcl-xl proteins.
MATERIALS AND METHODS
All the molecular docking functions in this study were performed using AutoDock Vina.[11] This docking software has been reported to provide better results and explanations in docking analyses. The AutoDock Vina software is also recognized as a trusted and accurate methods due to its rapid operation.[12]
Preparation of ligands
The chemical structures of biochanin A, myricetin, apigenin, galangin, and fisetin were obtained from the Pubchem compound database (http://pubchem.ncbi.nlm.nih.gov/) with their respective IDs as follows: 5280373, 5281672, 5280443, 5281616, and 5281614 [Figure 1]. Polar hydrogen atoms were added to the ligands, whereas nonpolar hydrogen atoms were merged. The downloaded three-dimensional chemical structures in the .sdf format were then converted into the .pdbqt format using the AutoDock Tool (ADT) program, version 1.5.6. Local minimization of ligands was executed using Biovia Discovery studio to reduce any possible bad contacts, as well as to sustain the conformation ligand.
Figure 1.
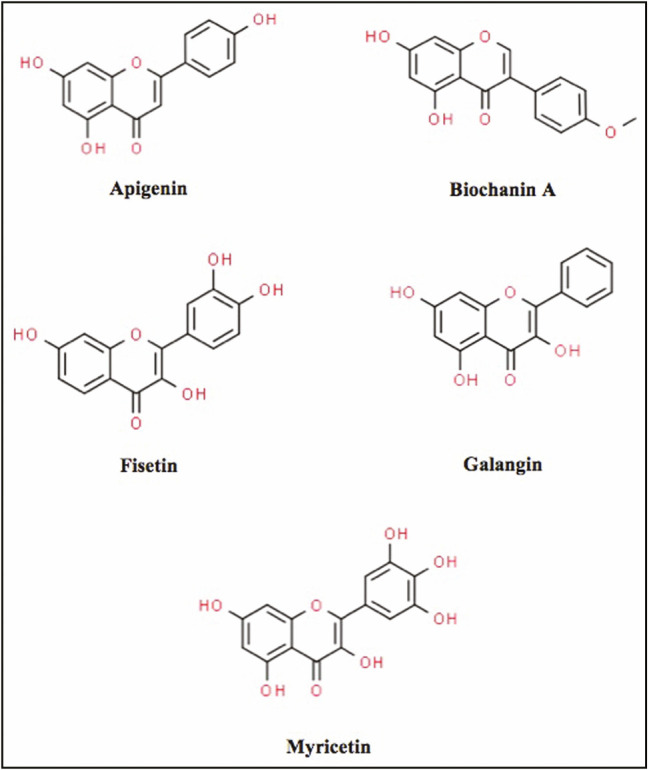
Two-dimensional structures of flavonoids
Preparation of proteins
Crystal structures of the Bcl-2 and Bcl-xl proteins with PDB IDs 4IEH[13] and 3ZK6,[14] respectively, were obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank. The crystal water molecules, ligand atoms, and ions that were bound to the proteins were removed. Hydrogen atoms were subsequently added using ADT.
Molecular docking
Molecular docking between flavonoids and antiapoptotic proteins (Bcl-2 and Bcl-xl) was performed using AutoDock Vina. The grid box for docking was positioned at the active binding site. The docking exhaustiveness was set up to 100 and repeated for 10 times. The results of hydrogen bond, hydrophobic, and electrostatic interactions were obtained using Biovia Discovery Studio Visualizer.[15]
RESULTS
Docking results were analyzed based on the binding affinity and non-covalent interactions that were present between the flavonoids and Bcl-2 or Bcl-xl proteins. The docking results were ranked according to the binding affinity of flavonoids–protein complex as presented in Table 1.
Table 1.
Docking results for Bcl-2 and Bcl-xl proteins with flavonoids
| Protein | Ligand | Binding affinity (kcal/mol) | Hydrogen bonding | Hydrophobic interaction | Electrostatic interaction |
|---|---|---|---|---|---|
| Bcl-2 | Myricetin | −7.3 | – | PHE63 | – |
| GLY104 | |||||
| Galangin | −7.3 | ARG66 | PHE63 | – | |
| TYR67 | |||||
| Apigenin | −7.2 | ARG105 | PHE63 | – | |
| Fisetin | −7.1 | – | – | PHE63 | |
| Biochanin A | −6.9 | – | TYR161 | ASP62 | |
| Bcl-xl | Fisetin | −8.8 | ASP133 | PHE105 | ARG139 |
| ARG139 | ARG139 | ||||
| GLU129 | ALA142 | ||||
| GLY138 | ALA104 | ||||
| Apigenin | −8.6 | ARG139 | ALA104 | – | |
| PHE105 | |||||
| Biochanin A | −8.5 | SER106 | LEU130 | – | |
| GLU129 | ALA104 | ||||
| ASN136 | PHE105 | ||||
| LEU108 | |||||
| ARG139 | |||||
| ALA142 | |||||
| Myricetin | −8.1 | ASP133 | PHE105 | ARG139 | |
| ARG139 | ARG139 | ||||
| GLU129 | ALA104 | ||||
| Galangin | −8.0 | LEU108 | LEU130 | – | |
| PHE105 | |||||
| ALA142 | |||||
| ARG139 | |||||
| ALA104 | |||||
| ARG102 | |||||
| LEU108 |
Docked with B-cell lymphoma 2 protein
The docked results showed that myricetin and galangin were found to have the highest binding affinities of −7.3 kcal/mol. In contrast, biochanin A showed the lowest binding affinity of −6.9 kcal/mol compared with other flavonoids. Hydrogen bonds were observed in the Bcl-xl–galangin complex but not in myricetin of two residues, that is Arg66 and Tyr67 [Table 1]. Based on the binding interactions, one critical residue (Phe63) of Bcl-2 protein interacted with all flavonoids except biochanin A either via hydrophobic or electrostatic interactions.
Docked with B-cell lymphoma-extra-large protein
Analyses of the docked flavonoids with Bcl-xl protein suggested that all ligands had good interaction with Bcl-xl protein with a binding affinity of less than −8.0 kcal/mol. Among them, fisetin showed the highest binding affinity (−8.8 kcal/mol), whereas galangin showed the lowest binding affinity (−8.0 kcal/mol). Four hydrogen bonds were formed in the Bcl-xl–fisetin complex of the Asp133, Arg139, Glu129, and Gly138 residues [Figure 2]. There were also four hydrophobic contacts contributed to the fisetin binding. Similarly, hydrogen bonds and hydrophobic contacts were also detected in other flavonoids with Bcl-xl protein. Two residues, namely, Phe105 and Ala104, were found to hydrophobically contacted with all flavonoids. Additional electrostatic interactions with Arg139 residue were observed in the docked myricetin and fisetin.
Figure 2.
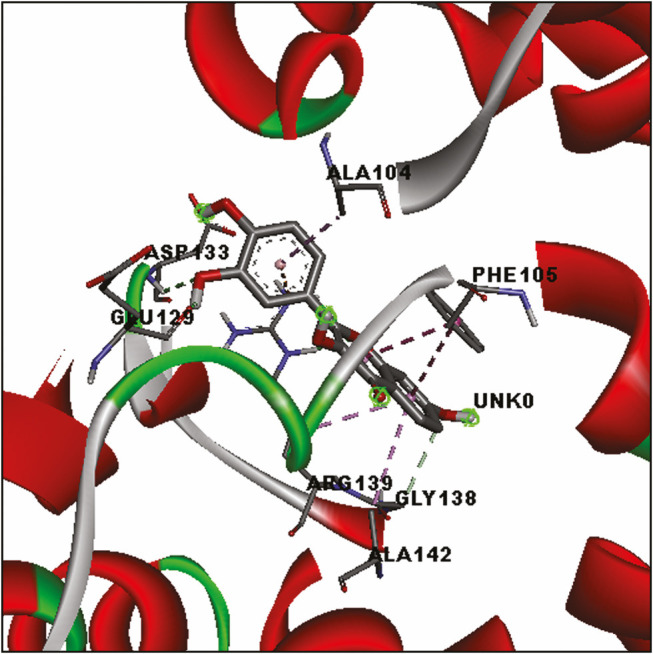
Three-dimensional illustration of docked Bcl-xl–fisetin complex. Green dashed lines represent hydrogen bonds. The hydrophobic interaction is depicted by purple dashed lines
DISCUSSION
The molecular docking in this study shows a vital role in predicting molecular interactions of flavonoids with targeted protein. This application is widely used in the pharmaceutical industry as a powerful tool, particularly in the analysis of structure–activity relationship.[16] The analysis of molecular docking outputs, such as binding affinity, are frequently applied in the determination of potential ligands.[17] Molecular docking also has the ability to predict small-molecule ligands binding toward appropriate target binding site.[18]
The targeted antiapoptotic proteins in this study are Bcl-2 and Bcl-xl, which are involved in the regulation of intrinsic apoptotic pathway. Bcl-2 and Bcl-xl proteins are said to be more crucial in apoptotic signaling pathway compared with other Bcl-2 family members.[19] Bcl-2 and Bcl-xl proteins have been investigated in various molecular modeling studies, including for apoptosis induction in cancer cells.[20,20,22] Those studies also involved small molecules, such as phenothiazine[21] and piperine,[22] which docked into Bcl-2 and Bcl-xl proteins, respectively.
According to our results, all flavonoids appear to have good docking interactions with both proteins. Similarly, two compounds as previously reported, ABT-737[23] and ABT-263,[24] interacted with both Bcl-2 and Bcl-xl proteins.[25] However, Bcl-xl protein in this study possessed better binding affinity with flavonoids, ranging from −8.0 to −8.8 kcal/mol, when compared with Bcl-2 protein, ranging from −6.9 to −7.3 kcal/mol [Table 1]. The binding interactions into the antiapoptotic binding pocket cause inhibitory activity and it eventually induces cell apoptosis via mitochondrial signaling pathway.[18,19]
The presence of hydrophobic interaction and hydrogen bond in the docked flavonoids with Bcl-xl protein was observed to contribute to the higher binding affinities compared with that of Bcl-2 protein [Table 1]. In the process of protein–ligand interaction, water forces the hydrophobic groups to aggregate and disrupt the hydrogen bond in water. This is known as the hydrophobic effect.[26] The presence of water molecules in hydrophobic site increases the binding affinity of protein and ligand.[27] These statements are also supported by Qian et al.,[28] where binding affinity values could associate with the hydrophobic interaction and the hydrogen bond. Although the water molecules were removed during preparation of proteins in this study, the chances of getting stronger binding affinities could possibly occur in a physiological condition.
In the docking between flavonoids and Bcl-2 protein, hydrophobic contact was detected at the same residue (Phe63) for myricetin, apigenin, and galangin. Meanwhile, the hydrophobic interaction between the docked flavonoids and Bcl-xl protein was facilitated via two critical residues, namely, Ala104 and Phe105. Phe105 gained contact with flavonoids due to the unfolding of α-helix 3 of Bcl-xl. It was also observed in A-1155463 along with other residues, such as Ser106 and Leu108.[25] In addition, Phe105 is located at the Bcl-xl region with BH3 peptide binding to it that causes a major conformational change in Bcl-xl.[29] Therefore, interaction with a critical residue, Phe105 as well as Ala104 in this study, is anticipated to be the key for the high binding affinities of flavonoid toward Bcl-xl protein. These findings suggest possible inhibitory interaction of flavonoids with essential residues in Bcl-xl located in the same binding site employed by the Bcl-xl inhibitors.
CONCLUSION
Molecular docking predicts the best orientation and conformation of flavonoids in Bcl-2 and Bcl-xl protein binding site to form stable complex for the inhibitory reaction. Our findings conclude that all flavonoids are possibly able to act as potential inhibitors for the targeted Bcl-2 and Bcl-xl proteins, supported by the high binding affinities. The binding of the flavonoids could, therefore, stimulate the apoptotic process in ovarian cancer cells. However, further confirmation, as well as more experiment studies, is required to validate the docking results in the development of anticancer drugs.
Financial support and sponsorship
This work was supported by the Universiti Sains Islam Malaysia under PPP grant (PPP/FPSK/0118/051000/14918).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.The Global Cancer Observatory 2019. [Last accessed on 2020 May 2. updated May 2019]. Available from: https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-sheets.pdf .
- 3.Tavsan Z, Kayali HA. Flavonoids showed anticancer effects on the ovarian cancer cells: involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed Pharmacother. 2019;116:109004. doi: 10.1016/j.biopha.2019.109004. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–20. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 5.Devi KP, Rajavel T, Habtemariam S, Nabavi SF, Nabavi SM. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015;142:19–25. doi: 10.1016/j.lfs.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Aaron NH, Jeffrey AE, Anthony CF. Targeted anti-cancer therapeutics. Cancer Discov. 2015;5:475–87. [Google Scholar]
- 7.Saxena N, Shashank P, Liu Y, Grover A, Gao R, Sundar D, et al. Molecular interactions of Bcl-2 and Bcl-xL with mortalin: identification and functional characterization. Biosci Rep. 2013;33:797–806. doi: 10.1042/BSR20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–24. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Qian J, Voorbach MJ, Huth JR, Coen ML, Zhang H, Ng SC, et al. Discovery of novel inhibitors of Bcl-xl using multiple high-throughput screening platforms. Anal Biochem. 2004;328:131–8. doi: 10.1016/j.ab.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Trott O, Olson AJ. Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro-Alvarez A, Costa AM, Vilarrasa J. The performance of several docking programs at reproducing protein–macrolide-like crystal structures. Molecules. 2017;22:136. doi: 10.3390/molecules22010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touré BB, Miller-Moslin K, Yusuff N, Perez L, Doré M, Joud C, et al. The role of the acidity of N-heteroaryl sulfonamides as inhibitors of Bcl-2 family protein-protein interactions. ACS Med Chem Lett. 2013;4:186–90. doi: 10.1021/ml300321d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9:390–7. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 15.Dassault Systèmes BIOVIA. Discovery Studio Visualizer, v17.2.0.16349. San Diego, CA: Dassault Systèmes; 2016. [Google Scholar]
- 16.Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–49. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalyaanamoorthy S, Chen YP. Structure-based drug design to augment hit discovery. Drug Discov Today. 2011;16:831–9. doi: 10.1016/j.drudis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. 2011;7:146–57. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooswinkel RW, van de Kooij B, de Vries E, Paauwe M, Braster R, Verheij M, et al. Antiapoptotic potency of Bcl-2 proteins primarily relies on their stability, not binding selectivity. Blood. 2014;123:2806–15. doi: 10.1182/blood-2013-08-519470. [DOI] [PubMed] [Google Scholar]
- 20.Tutumlu G, Dogan B, Avsar T, Orhan MD, Calis S, Durdagi S. Integrating ligand and target-driven based virtual screening approaches with in vitro human cell line models and time-resolved fluorescence resonance energy transfer assay to identify novel hit compounds against BCL-2. Front Chem. 2020;8:167. doi: 10.3389/fchem.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.do Carmo AL, Bettanin F, Oliveira Almeida M, Pantaleão SQ, Rodrigues T, Homem-de-Mello P, et al. Competition between phenothiazines and BH3 peptide for the binding site of the antiapoptotic BCL-2 protein. Front Chem. 2020;8:235. doi: 10.3389/fchem.2020.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinevicius VMAS, Andrade KS, Mota NSRS, Bretanha LC, Felipe KB, Ferreira SRS, et al. CDK2 and Bcl-xl inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem Toxicol. 2019;132:110644. doi: 10.1016/j.fct.2019.110644. [DOI] [PubMed] [Google Scholar]
- 23.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 24.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 25.Wakui N, Yoshino R, Yasuo N, Ohue M, Sekijima M. Exploring the selectivity of inhibitor complexes with Bcl-2 and Bcl-Xl: a molecular dynamics simulation approach. J Mol Graph Model. 2018;79:166–74. doi: 10.1016/j.jmgm.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Yunta MJR. Docking and ligand binding affinity: uses and pitfalls. American Journal of Modeling and Optimization. 2016;4:74–114. [Google Scholar]
- 27.Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS ONE. 2010;5:e12029. doi: 10.1371/journal.pone.0012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian SB, Waldron L, Choudhary N, Klevit RE, Chazin WJ, Patterson C. Engineering a ubiquitin ligase reveals conformational flexibility required for ubiquitin transfer. J Biol Chem. 2009;284:26797–802. doi: 10.1074/jbc.M109.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee EF, Fairlie WD. The structural biology of Bcl-xL. Int J Mol Sci. 2019;20:2234. doi: 10.3390/ijms20092234. [DOI] [PMC free article] [PubMed] [Google Scholar]


