Abstract
Background:
Primary ciliary dyskinesia (PCD) is a rare genetic disease arising from motile ciliary dysfunction and associated with recurrent and chronic upper and lower respiratory tract infections. Pediatric otolaryngologists may see these patients prior to the development of lung disease. Features of PCD may overlap with other suppurative respiratory diseases, creating diagnostic challenges. A simple screening tool would be beneficial to identify potential patients who have chronic upper respiratory tract disease requiring further specialist evaluation.
Objective:
To test a simple screening tool consisting of four questions to detect PCD in children with chronic otitis media and chronic rhinosinusitis seen in a tertiary otolaryngology clinic.
Methods:
A prospective, single site, observational study in a tertiary care pediatric otolaryngology clinic. Children aged 3 to 17 years diagnosed with chronic otitis media or rhinosinusitis with onset at less than 2 years of age were recruited. All study subjects had at least one of four key clinical features for PCD as determined by answers to screening questions, while control subjects had none. All participants completed a medical history questionnaire and nasal nitric oxide measurements. Those with reduced nasal nitric oxide levels were referred to our PCD center for further evaluation.
Results:
A total of 153 patients were screened and 62 subjects were enrolled. Of those, 35 were enrolled as study subjects and 27 as matched controls. Study subjects had mean age of 7.5 years (3.2–16.5) with pre-screening diagnosis of chronic otitis media (n=29) or chronic rhinosinusitis (n=6). Control subjects (n=27) had mean age 7.2 years (3.0–16.3) with pre-screening diagnosis of chronic otitis media (n=25), and chronic rhinosinusitis (n=2). There were no differences in subject demographics or mean nasal nitric oxide values between the two groups (179.8 vs 210.8 nl/min). Ten individuals had low nasal nitric oxide values, 7 of which were normal on repeat testing. Three subjects failed to return for follow up evaluations. Four referrals were made for further evaluation on the basis of clinical symptoms and nasal nitric oxide results. While no new cases of PCD were detected, a subject and his sibling with recurrent sinopulmonary infections were referred for immunologic evaluation.
Conclusion:
The use of standardized screening questions can be used in an otolaryngology clinic to identify patients who require further evaluation for PCD or primary immunodeficiency.
Keywords: children, early diagnosis, primary ciliary dyskinesia, primary immunodeficiency, nasal nitric oxide
1. INTRODUCTION
With an estimated of 1:10,000 to 1:20,000 live births [1], primary ciliary dyskinesia (PCD) is a rare genetic disease caused by defective motile cilia function. The upper respiratory tract is often involved in PCD, characterized by chronic rhinosinusitis and chronic otitis media [2], and thus initial referrals are usually directed to otolaryngologists. Symptoms associated with PCD are not unique to the disease, and can overlap with other suppurative respiratory diseases, such as cystic fibrosis (CF) and primary immunodeficiencies (PID). Failure to identify these diseases can have serious consequences. Early identification and initiation of therapy for PCD is important to avoid long term sequelae such as hearing loss, reduced olfaction, bronchiectasis, and ultimately chronic respiratory failure.
Determining which cases of persistent middle ear or paranasal sinus disease warrant additional testing for PCD is challenging, as recurrent otitis media and rhinosinusitis are among the most common illnesses of childhood [3,4]. The peak prevalence of otitis media occurs at 12 months of age, but only about a quarter of patients experience at least 3 episodes [5]. Some of these children may have PCD, which first manifests as middle ear or sinus disease [2], while others may have CF or PID. Some individuals may have severe and recurrent middle ear and sinus involvement for unknown reasons [6–8].
In recent years, several groups have developed clinical screening tools to aid in the diagnosis of PCD [9,10], and a retrospective, multicenter study of clinical features of children who were evaluated at eight North American centers was incorporated into the American Thoracic Society diagnostic guidelines for PCD [11]. These guidelines were developed in a specialty pulmonary clinic and include four key clinical features (Table 1). Guidelines were established in conjunction with nasal nitric oxide testing, transmission electron microscopy analysis of cilia, high speed video microscopy, and genetic testing as a means to diagnose PCD [10,11]. Measurement of nasal nitric oxide has emerged as a simple and sensitive test, but remains under investigation. Most patients with PCD have values <77 nL/min however, values below this cutoff have also been seen in individuals with CF, a disease that needs to be excluded prior to testing for PCD. [12,13].
Table 1:
Screening questions for primary ciliary dyskinesia used in this study [9].
| Clinical screening questions |
|---|
|
Notably, diagnostic criteria for PCD were previously developed by retrospectively analysis of patient populations referred to PCD centers, but these criteria have not been prospectively studied. Our goal was to determine the utility of a simple screening tool that could be incorporated into an otolaryngology practice. We proposed that a set of four basic clinical questions could be added to the evaluation of children with chronic otitis media or chronic rhinosinusitis in a pediatric otolaryngology clinic to identify those who require further evaluation for PCD. We also explored whether the addition of nasal nitric oxide measurement would improve the identification of affected patients.
2. METHODS
2.1. Subjects
The study was approved by the Institutional Review Board of Washington University and the guardians of all subjects provided consent. Assent was obtained in children of appropriate age. Children between the ages 3 and 17 years presenting with chronic otitis or sinusitis to the pediatric otolaryngology clinic at St. Louis Children’s Hospital between November 2018 and February 2020 were eligible. The subjects were evaluated by the treating otolaryngologist for the following study inclusion criteria: diagnosis of chronic or recurrent otitis media with placement of tympanostomy tubes, or diagnosis of chronic rhinosinusitis as confirmed by the treating physician. Patients were excluded if they had a previous diagnosis of PCD, CF, PID, were treated with immunosuppressive medications, had craniofacial abnormality associated with increased risk of developing sinusitis or otitis media, or were actively using combustible or electronic cigarettes.
2.2. Screening algorithm
Screening questions were derived from the clinical features for PCD in the ATS guideline [9] (Table 1). To qualify as a study subject, an individual must fulfill at least one clinical feature (Table 1), which contrasts from guideline criteria that recommended at least two clinical features to be considered for further evaluation. This approach was used to increase identification of individuals with PCD who may have milder disease. If an individual was born at less than 37 weeks estimated gestational age, the second question (unexplained respiratory distress at birth?) was excluded from the screening tool. Age and sex matched controls were selected from the group of patients with diagnoses of chronic oitis media or chronic rhinosinusitis who did not answer “yes” to any of the screening questions. To identify additional patient characteristics independent of the screening questions, control and study subjects were administered a more comprehensive questionnaire, modified from Amirav et al. [14] (Appendix A). Both screening and comprehensive questionnaires were administered by one of the study investigators (SKB).
2.3. PCD prevalence in otolaryngology clinic
To estimate the number of patients diagnosed with PCD through referral from the otolaryngology clinic, patient data from the previous ten years was analyzed. The number of children seen in the otolaryngology clinic who met study inclusion criteria was determined from the electronic medical record system. The number of patients diagnosed with PCD who were first assessed in otolaryngology clinic and then referred to the Washington University PCD and Rare Airways Disease Center was obtained from pulmonary clinic records. The ratio of these two numbers was taken as indicative of the disease prevalence in the otolaryngology clinic.
2.4. Nasal nitric oxide measurement
Nasal nitric oxide measurement was conducted in accordance to published ATS operational guidelines [15] and performed by a single trained investigator (SKB). This test is not yet approved by the FDA for the diagnosis of PCD. As such, the test was performed at no charge under the research protocol. All measurements were conducted using an Eco Physics CLD 88 SP chemiluminescence nitric oxide gas analyzer and Spiroware (3.0) data acquisition software (Eco Medics, Duerenten, Switzerland). Before each test, the sampling flow rate and ambient air nitric oxide measurements were recorded. The flow rate was constant at 0.32 L/min for every test performed. If able, children were asked to clear their nose to remove any obstructive mucus prior to testing. For those children who were minimally cooperative with the procedure, only one measurement per nare was used rather than two measurements per nares as in the more cooperative subjects. For younger children who could not perform the test by blowing against the resistor, the tidal breathing method was used. Total testing time per subject was 3 to 5 minutes, regardless of age. The final value was calculated using the validated Genetic Disorders of Mucociliary Clearance Consortium calculation sheet (Appendix B). Those patients who had an initial nNO value <77 nL/min were asked to return in 4–6 weeks’ time to repeat the test to confirm that the value was persistently reduced.
2.5. Clinical Evaluation
Patients whose screening results, questionnaire answers, and nasal nitric oxide results were suggestive of a diagnosis of PCD were referred to the Washington University PCD and Rare Airways Disease Center. Patients then underwent clinically indicated testing including laboratory screening for immunodeficiencies, cystic fibrosis, and genetic testing for primary ciliary dyskinesia via 36-gene targeted panel (Invitae Corp., San Francisco, CA).
2.6. Statistical analysis
All statistical analysis was conducted using SPSS Statistics for Windows, version 25.0 (SPSS Inc., Chicago, USA). For descriptive analysis, central tendency and dispersion values were calculated. For univariate analysis chi square test, Mann-Whitney, and Kruskal Wallis tests were used as appropriate.
3. RESULTS
3.1. Estimate of PCD prevalence in tertiary otolaryngology clinic
A 10-year review of patient referral data from our otolaryngology clinic identified 4,451 patients who met study inclusion criteria. Analysis of PCD center data over the same period identified 3 patients referred from the otolaryngology clinic and subsequently diagnosed with PCD. Thus, disease prevalence in our center was calculated as 1:1484, or approximately 7-fold higher than the estimated lower bound of the population prevalence of 1:10,000 [1].
3.2. Screening and enrollment
We screened 153 patients, and enrolled 35 study subjects and 27 control subjects (Table 1, Figure 1). One patient declined to enroll after screening positive and another did not return to complete the nNO testing and questionnaire after consent was obtained. Of 35 study subjects, 6 answered yes to two of four questions screening questions.. No subjects enrolled had situs inversus or laterality defects, however the patient who declined to enroll was screen positive for a laterality defect.
Figure 1:
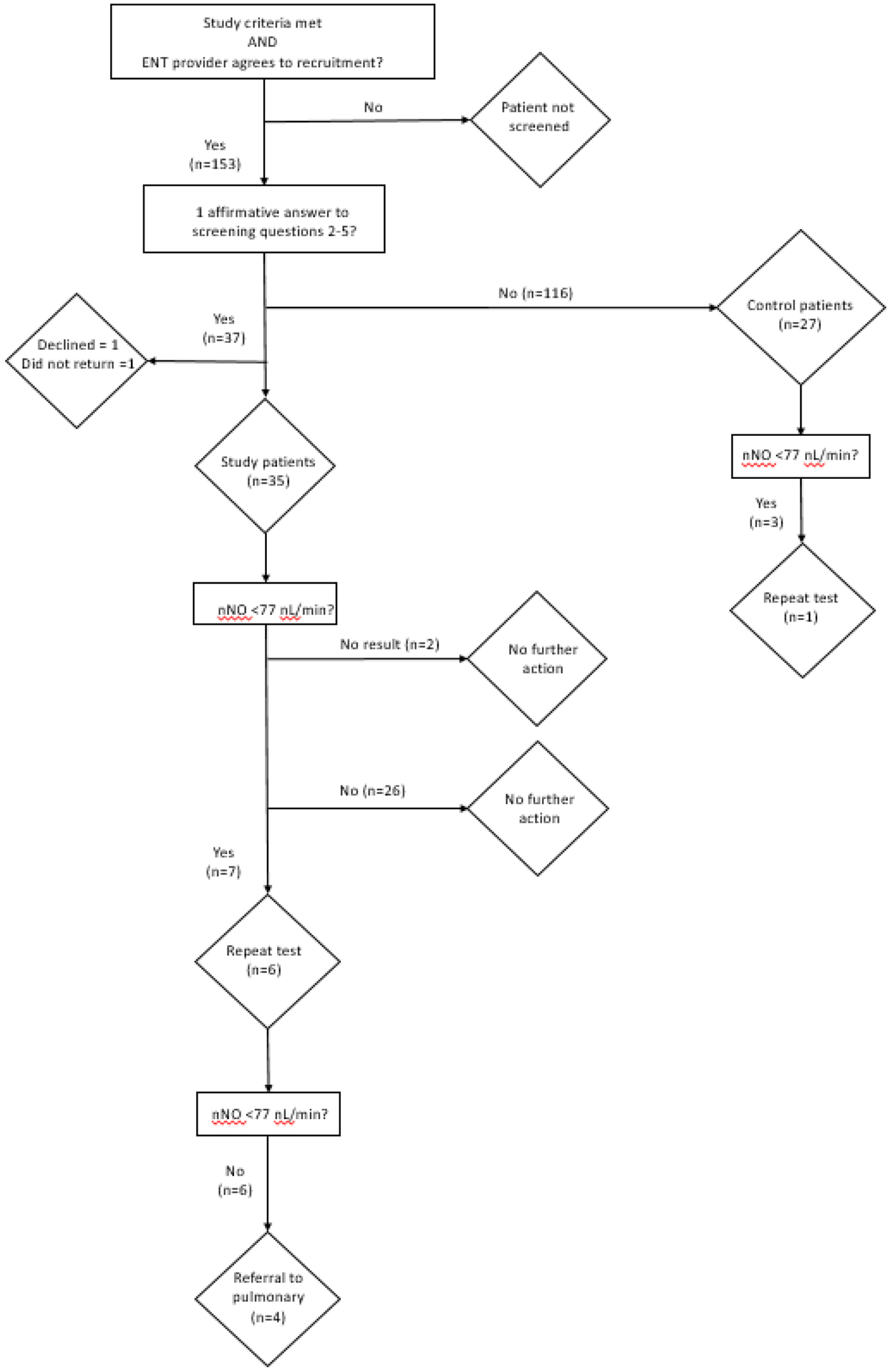
Patient screening and enrollment diagram. The number of subjects meeting each criterion is indicated.
3.3. Nasal nitric oxide testing
Nasal nitric oxide results were obtained for 60 of 62 individuals (Figure 2A). Two study subjects, both 3 years old, were non-cooperative with testing procedures and could not produce reliable results. A total of ten results (7 study subjects, 3 control subjects) fell below the previously determined cutoff of 77 nL/min, a level found to be consistent with PCD in subjects 5 years and older [13]. Seven children (6 study subjects, 1 control subject) underwent repeat testing, while three (1 study subject, 2 control subjects) did not return for follow up evaluations (Figure 2B). All repeated measurements were above 77 nL/min. Of those who had repeated measurements, values increased to the non-diagnostic range after three weeks of antibiotic treatment in two subjects, multiple weeks of nasal irrigation and nasal steroid treatment in two subjects, and no treatment in three subjects.
Figure 2: Nasal nitric oxide levels of subjects.
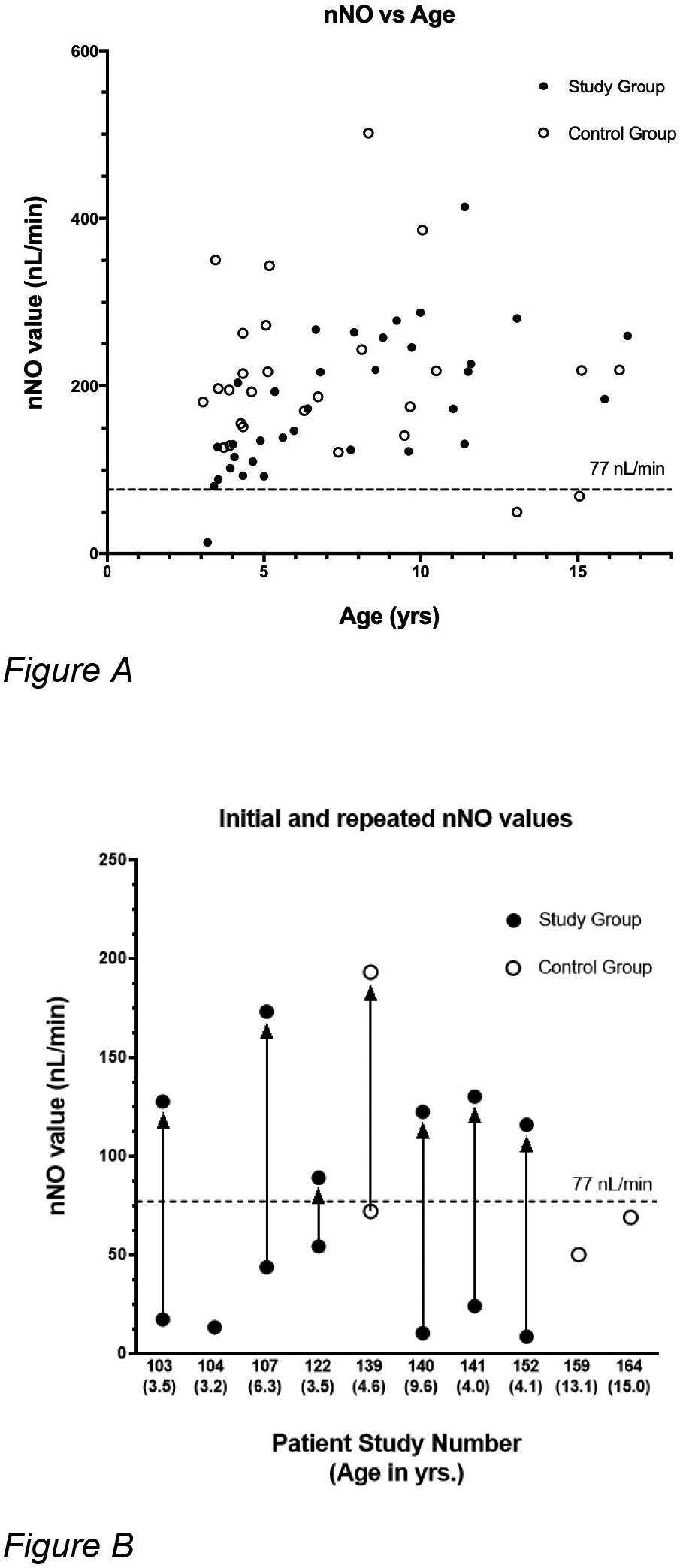
A: Scatterplot of nasal nitric oxide values versus patient age. The upper limit measure of 77 nL/min used for further evaluation of PCD is denoted by the dashed line. Low initial levels are not shown if retested. B: Repeat testing of low nNO tests (less than 77 nL/min)). Initial and repeated nasal nitric oxide (nNO) levels according to patient study number and corresponding age. Initial and repeat tests for each patient are joined by a line with the arrowhead indicating the repeat test result. Points with no adjoining arrow indicate patients who did not return for follow-up testing.
Mean nasal nitric oxide values did not differ significantly between study and control groups. No statistically significant correlation between nasal nitric oxide values and increasing age was observed (Table 2, Figure 2A). Univariate analysis revealed no statistically significant correlations between the rate of otitis media, sinusitis, hearing loss, speech delay, or tympanostomy tube placement in comparison between study and control groups. Correlations were not observed between these factors or levels of measured nasal nitric oxide.
Table 2:
Patient enrollment characteristics.
| Characteristics | Control Group n (%) | Study Group n (%) | p value |
|---|---|---|---|
| Sex | 0.42 | ||
| Male | 14 (52) | 22 (61) | |
| Female | 13 (48) | 13 (39) | |
| Total | 27 (100) | 35 (100) | |
| Ethnicity | 0.42 | ||
| Caucasian | 22 (81) | 28 (81) | |
| African American | 5 (19) | 7 (19) | |
| Age (yrs) | |||
| Mean | 7.2 | 7.5 | 0.61 |
| SD | 3.9 | 3.6 | |
| Range | 3.0 – 16.3 | 3.2 – 16.5 | |
| Pre-Screen Diagnosis | 0.53 | ||
| Otitis Media | 25 (93) | 29 (83) | |
| Rhinosinusitis | 2 (7) | 6 (17) | |
| Number of positive responses to screening questions | |||
| 1 | 0 | 29 (90) | |
| 2 | 0 | 6 (10) | |
| 3 | 0 | 0 | |
| 4 | 0 | 0 | |
| nNO values (nL/min) | |||
| Mean | 210.8 | 178.3 | 0.22 |
| Std dev | 96.9 | 82.3 | |
| Range | 50.1 – 501.1 | 13.2 – 413.8 |
3.4. Pulmonary evaluations
Four subjects were referred to the PCD center for further evaluation (Figure 1) based on clinical criteria and an initial low nasal nitric oxide results. No patients were found to be homozygous for mutations in PCD-associated genes. Two patients (#107 and #140) who had low initial nasal nitric oxide values (43.8 nL/min and 10.3 nL/min) that rose above the diagnostic cut-off upon repeat testing (173.2 nL/min and 122.3 nl/min, respectively) were subsequently found to have reduced specific IgG antibodies to Streptococcus pneumoniae and Hemophilus influenzae B. These patients were re-vaccinated and showed appropriate responses to vaccines on serologic retesting. The sibling of patient #140 was found to have similar symptoms and also tested negative for PCD and primary immunodeficiency. She too showed initially decreased specific IgG antibodies which responded appropriately to revaccination. Patient #140 and his sister continued to report recurrent sinus and pulmonary infections, despite demonstrating normal vaccine titers.
4. DISCUSSION
Using criteria modified from diagnostic guidelines, we prospectively evaluated children referred to a tertiary otolaryngology clinic where PCD would be expected to be more prevalent than in the general population. Although we did not identify new cases of PCD using this screening approach, implementation led to further evaluation of four subjects for possible PCD or immunodeficiency. One subject and his sibling continue to have recurrent infections, which remain undiagnosed at this time.
Chronic upper respiratory infections commonly seen in a pediatric ENT clinics are often early features of PCD. The majority (83%) of patients enrolled in our study had ear disease as their primary complaint, while none had both ear and sinus complaints, perhaps due to our stringent diagnostic criteria for chronic sinus disease. Prior studies of ENT complications of PCD have noted significant morbidity from sinonasal disease in adults as symptoms of otitis media decrease with age [16]. However, in the pediatric population the morbidity is often a result of otitis media, which may require intervention to prevent permanent hearing damage [17]. Although patients with PCD frequently present with serious and recurrent otitis media, this was not found to be a sensitive characteristic for disease diagnosis in the North American study and thus was not included in the ATS criteria [11]. In contrast, a large study of children with PCD in Europe concluded that otitis media was a sensitive factor in disease diagnosis [10]. Reasons for the difference may be due to lower use of tympanostomy tube insertion in Europe compared to the United States [18].
Although definitive diagnosis is the end goal of our screening tool, there remains a high degree of merit in the identification of patients who require further testing for an underlying genetic disease. PCD has a spectrum of presenting features, including symptoms restricted to the upper airway [2]. Recent genetic studies indicated that prototypical aspects of PCD may not be present in all patients. For example, individuals with homozygous recessive mutations of DNAH9, CCDC103, TTC12, RSPH1, GAS8, STK36, CFAP221, or NEK10 genes have presented with recurrent respiratory tract infections but normal nNO values [2, 19–25]. Therefore, family history, medical history and nasal nitric oxide measurement in addition to comprehensive evaluation of suspected cases in specialized pulmonary centers is helpful. The symptom overlap between PCD, PID, and cystic fibrosis makes identification of patients with risk factors for any of these diseases crucial as they all have different natural histories and treatment approaches.
A limitation of our study is the small number of patients we were able to recruit. PCD is a rare disease, and only through large scale, multicenter, and prolonged screening would our proposed study design identify new cases. Small sample size also limited our ability to adequately evaluate the detailed medical history questionnaire data (Supplement 2) allowing only univariate analysis rather than an ability to construct a multivariate model. In addition, three patients were lost to follow up who did not provide repeated nNO results after testing <77 nL/min. Nonetheless, we were able to initiate a simple screening system for assessment of a subpopulation of patients hypothesized to be at increased risk for PCD.
Another limitation is related to our definition of diseases used for enrollment. By setting the evaluation of patients with one rather than two affirmative responses to our set of questions, we potentially captured some patients who had less severe disease and perhaps created a more heterogenous group than desired. Finally, the key clinical features derived by the American Thoracic Society guidelines focused on identifying patients who had “classic” forms of PCD, and not on those with atypical disease. As a result, we may have missed identifying children with motile cilia defects but milder clinical manifestations. It is possible that the chronic otitis media and rhinitis of children evaluated in otolaryngology clinics are due to a yet undefined genetic basis, affecting cilia function or not.
5. CONCLUSION
We show of the feasibility of physicians in an academic ENT clinic to screen children using four questions adapted from American Thoracic Society guidelines for diagnosis of PCD [11]. Clinical features of PCD can overlap with PID or cystic fibrosis so referral of children with persistent upper respiratory tract disease may require specialized testing for these diseases. We found that reduced nasal nitric oxide values as a sole diagnostic test for PCD was not specific for the disease in the absence of any of the positive key clincal features for PCD, and strongly advise against use of the test in such a manner. Thus, asking four specific questions about features of upper airway disease could be useful in otolaryngology clinics to identify individuals who require more targeted evaluation and treatment.
Highlights:
A clinical screening tool can be used in an ENT clinic to identify children requiring further evaluation for primary ciliary dyskinesia or immunodeficiency.
Acknowledgements:
We thank Jane Quante, RN for training and support with nasal nitric oxide measurements.
Funding Sources:
National Institutes of Health (NIH) K08HL150223 (AH) and R01HL146601 (SLB), and the Barnes Jewish Hospital Research Foundation (SLB is the Dorothy R. and Hubert C. Moog Professor of Pulmonary Medicine)
Appendix A. Study Questionnaire



Appendix B: Nasal Nitric Oxide Measurement

6. References
- [1].Horani A, Ferkol TW, Advances in the genetics of primary ciliary dyskinesia: Clinical Implications, Chest. (2018). 10.1016/j.chest.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Loges NT, Antony D, Maver A, Deardorff MA, Güleç EY, Gezdirici A, Nöthe-Menchen T, Höben IM, Jelten L, Frank D, Werner C, Tebbe J, Wu K, Goldmuntz E, Čuturilo G, Krock B, Ritter A, Hjeij R, Bakey Z, Pennekamp P, Dworniczak B, Brunner H, Peterlin B, Tanidir C, Olbrich H, Omran H, Schmidts M, Recessive DNAH9 loss-of-function mutations cause laterality defects and subtle respiratory ciliary-beating defects, Am. J. Hum. Genet (2018). 10.1016/j.ajhg.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schilder AGM, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP, Otitis media., Nat. Rev. Dis. Prim 2 (2016) 16063. 10.1038/nrdp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Magit A, Pediatric rhinosinusitis, Otolaryngol. Clin. North Am (2014). 10.1016/j.otc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- [5].Kaur R, Morris M, Pichichero ME, Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era, Pediatrics. (2017). 10.1542/peds.2017-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoo F, Suh JD, What is the evidence for genetics in chronic rhinosinusitis?, Curr. Opin. Otolaryngol. Head Neck Surg. (2017). 10.1097/MOO.0000000000000329. [DOI] [PubMed] [Google Scholar]
- [7].Hsu J, Avila PC, Kern RC, Hayes MG, Schleimer RP, Pinto JM, Genetics of chronic rhinosinusitis: state of the field and directions forward., J. Allergy Clin. Immunol 131 (2013) 977–93, 993.e1–5. 10.1016/j.jaci.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tian C, Hromatka BS, Kiefer AK, Eriksson N, Noble SM, Tung JY, Hinds DA, Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections., Nat. Commun 8 (2017) 599. 10.1038/s41467-017-00257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leigh MW, Ferkol TW, Davis SD, Lee H-S, Rosenfeld M, Dell SD, Sagel SD, Milla C, Olivier KN, Sullivan KM, Zariwala MA, Pittman JE, Shapiro AJ, Carson JL, Krischer J, Hazucha MJ, Knowles MR, Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents., Ann. Am. Thorac. Soc 13 (2016) 1305–1313. 10.1513/AnnalsATS.201511-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Behan L, Dimitrov BD, Kuehni CE, Hogg C, Carroll M, Evans HJ, Goutaki M, Harris A, Packham S, Walker WT, Lucas JS, {PICADAR}: A diagnostic predictive tool for primary ciliary dyskinesia, Eur Respir J. 47 (2016) 1103–1112. 10.1183/13993003.01551-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD, Chilvers MA, Ferkol TW, Zariwala MA, Sagel SD, Josephson M, Morgan L, Yilmaz O, Olivier KN, Milla C, Pittman JE, Daniels MLA, Jones MH, Janahi IA, Ware SM, Daniel SJ, Cooper ML, Nogee LM, Anton B, Eastvold T, Ehrne L, Guadagno E, Knowles MR, Leigh MW, Lavergne V, Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline, Am J Respir Crit Care Med. 197 (2018) e24–e39. 10.1164/rccm.201805-0819ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zysman-Colman ZN, Kaspy KR, Alizadehfar R, NyKamp KR, Zariwala MA, Knowles MR, Vinh DC, Shapiro AJ, Nasal nitric oxide in primary immunodeficiency and primary ciliary dyskinesia: Helping to distinguish between clinically similar diseases., J. Clin. Immunol 39 (2019) 216–224. 10.1007/s10875-019-00613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, Lavange LM, Horton BJ, Qaqish B, Carson JL, Davis SD, Dell SD, Ferkol TW, Atkinson JJ, Olivier KN, Sagel SD, Rosenfeld M, Milla C, Lee H-S, Krischer J, Zariwala MA, Knowles MR, Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia., Ann. Am. Thorac. Soc 10 (2013) 574–581. 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amirav I, Roduta Roberts M, Mussaffi H, Mandelberg A, Roth Y, Abitbul R, Luder A, Blau H, Alkrinawi S, Aviram M, Ben-Ami M, Rotschild M, Bentur L, Shoseyov D, Cohen-Cymberknoh M, Kerem E, Avital A, Springer C, Hevroni A, Dabbah H, Elizur A, Picard E, Goldberg S, Rivlin J, Livnat G, Lavie M, Alias N, Soferman R, Olbrich H, Raidt J, Wallmeier J, Werner C, Loges NT, Omran H, Collecting clinical data in primary ciliary dyskinesia- challenges and opportunities., F1000Research. 5 (2016) 2031. 10.12688/f1000research.9323.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shapiro AJ, Dell SD, Gaston B, O’Connor M, Marozkina N, Manion M, Hazucha MJ, Leigh MW, Nasal nitric oxide measurement in primary ciliary dyskinesia. A technical paper on standardized testing protocols., Ann. Am. Thorac. Soc 17 (2020) e1–e12. 10.1513/AnnalsATS.201904-347OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bequignon E, Dupuy L, Zerah-Lancner F, Bassinet L, Honoré I, Legendre M, Devars du Mayne M, Escabasse V, Crestani B, Maitre B, Escudier E, Coste A, J-F. Papon, Critical evaluation of sinonasal disease in 64 adults with primary ciliary dyskinesia, Journal of Clinical Medicine. 8 (2019) 619. doi: 10.3390/jcm8050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Campbell R, Birman C, Morgan L, Management of otitis media with effusion in children with primary ciliary dyskinesia: A literature review, Int. J. Pediatr. Otorhinolaryngol 73 (2009) 1630–1638. doi: 10.1016/j.ijporl.2009.08.024. [DOI] [PubMed] [Google Scholar]
- [18].Parker DM, Schang L, Wasserman JR, Viles WD, Bevan G, Goodman DC, Variation in utilization and need for tympanostomy tubes across England and New England, J Pediatr. 179 (2016). doi: 10.1016/j.jpeds.2016.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shoemark A, Moya E, Hirst RA, Patel MP, Robson EA, Hayward J, Scully J, Fassad MR, Lamb W, Schmidts M, Dixon M, Patel-King RS, V Rogers A, Rutman A, Jackson CL, Goggin P, Rubbo B, Ollosson S, Carr S, Walker W, Adler B, Loebinger MR, Wilson R, Bush A, Williams H, Boustred C, Jenkins L, Sheridan E, Chung EMK, Watson CM, Cullup T, Lucas JS, Kenia P, O’Callaghan C, King SM, Hogg C, Mitchison HM, High prevalence of CCDC103 p.His154Pro mutation causing primary ciliary dyskinesia disrupts protein oligomerisation and is associated with normal diagnostic investigations., Thorax. 73 (2018) 157–166. 10.1136/thoraxjnl-2017-209999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thomas L, Bouhouche K, Whitfield M, Thouvenin G, Coste A, Louis B, Szymanski C, Bequignon E, Papon J-F, Castelli M, Lemullois M, Dhalluin X, Drouin-Garraud V, Montantin G, Tissier S, Duquesnoy P, Copin B, Dastot F, Couvet S, Barbotin A-L, Faucon C, Honore I, Maitre B, Beydon N, Tamalet A, Rives N, Koll F, Escudier E, Tassin A-M, Touré A, Mitchell V, Amselem S, Legendre M, TTC12 loss-of-function mutations cause primary ciliary dyskinesia and unveil distinct dynein assembly mechanisms in motile cilia versus flagella., Am. J. Hum. Genet 106 (2020) 153–169. 10.1016/j.ajhg.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, Hazucha MJ, Carson JL, Olivier KN, Sagel SD, Rosenfeld M, Ferkol TW, Dell SD, Milla CE, Randell SH, Yin W, Sannuti A, Metjian HM, Noone PG, Noone PJ, Olson CA, V Patrone M, Dang H, Lee H-S, Hurd TW, Gee HY, Otto EA, Halbritter J, Kohl S, Kircher M, Krischer J, Bamshad MJ, Nickerson DA, Hildebrandt F, Shendure J, Zariwala MA, Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype., Am. J. Respir. Crit. Care Med 189 (2014) 707–717. 10.1164/rccm.201311-2047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Olbrich H, Cremers C, Loges NT, Werner C, Nielsen KG, Marthin JK, Philipsen M, Wallmeier J, Pennekamp P, Menchen T, Edelbusch C, Dougherty GW, Schwartz O, Thiele H, Altmüller J, Rommelmann F, Omran H, Loss-of-function GAS8 mutations cause primary ciliary dyskinesia and disrupt the dexin-dynein regulatory complex., Am. J. Hum. Genet 97 (2015) 546–554. 10.1016/j.ajhg.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Edelbusch C, Cindrić S, Dougherty GW, Loges NT, Olbrich H, Rivlin J, Wallmeier J, Pennekamp P, Amirav I, Omran H, Mutation of serine/threonine protein kinase 36 (STK36) causes primary ciliary dyskinesia with a central pair defect., Hum. Mutat 38 (2017) 964–969. 10.1002/humu.23261. [DOI] [PubMed] [Google Scholar]
- [24].Bustamante-Marin XM, Shapiro A, Sears PR, Charng W-L, Conrad DF, Leigh MW, Knowles MR, Ostrowski LE, Zariwala MA, Identification of genetic variants in CFAP221 as a cause of primary ciliary dyskinesia., J. Hum. Genet 65 (2020) 175–180. 10.1038/s10038-019-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chivukula RR, Montoro DT, Leung HM, Yang J, Shamseldin HE, Taylor MS, Dougherty GW, Zariwala MA, Carson J, Daniels MLA, Sears PR, Black KE, Hariri LP, Almogarri I, Frenkel EM, Vinarsky V, Omran H, Knowles MR, Tearney GJ, Alkuraya FS, Sabatini DM, Author Correction: A human ciliopathy reveals essential functions for NEK10 in airway mucociliary clearance., Nat. Med 26 (2020) 300. 10.1038/s41591-020-0773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


