Abstract
The architecture of the myocardial fibers defines the different components of left ventricular (LV) contraction. Subendocardial layers are primarily responsible for longitudinal LV deformation and subepicardial layers for circumferential LV deformation. The specific analysis of the different components of LV deformation by echocardiography might offer new diagnostic options in patients with acute myocarditis (?IM). This case report focuses on specific pathological findings of regional LV deformation in a patient with IM and preserved LV systolic function.
Keywords: Cardiac magnetic resonance, echocardiography, layered-specific strain, myocardial work, myocarditis, rotation rate
INTRODUCTION
The architecture of the myocardial fibers defines the different components of left ventricular (LV) contraction. Subendocardial layers are primarily responsible for longitudinal and subepicardial layers for circumferential LV deformation. Myocardial edema, hyperemia, and failure of structural integrity of the myocardial cells are often documented by cardiac magnetic resonance imaging (cMRI) within the outer LV layers in viral acute myocarditis (IM).[1,2,3] Thus, it can be expected that alterations of circumferential and rotational LV deformation could be detected by speckle tracking in IM, especially if global LV function is preserved. The specific analysis of the different components of LV deformation by echocardiography might offer new diagnostic options in patients with IM. This case report focuses on specific pathological findings of regional LV deformation in a patient with IM and preserved LV systolic function.
CASE REPORT
History
Admission to emergency care unit of a 60-year-old female patient with chest pain, dyspnea, and flu-like symptoms (cough, head and limb pain, and palpitations). Medical history: stable Crohn's disease, stable Hashimoto's thyroiditis, and no significant cardiovascular risk factors.
Diagnostic/findings (baseline)
Electrocardiogram (ECG), cardiac and inflammatory markers (leukocytes and C-reactive protein), and thyroid-stimulating hormone were normal. Echocardiography showed preserved left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) of -21% (normal ranges in female patients: 17.8%–28.2%) and global radial strain (GRS) of 48% (apical) and 40% (basal) (normal ranges: 21.5%–54.8%).[4] In contrast, lower global circumferential strain (GCS) of - 20% (apical) and - 8% (basal) (normal ranges in female patients: -23.6–-40.7%), especially subepicardial layer strain were observed in the basal inferior, posterior, lateral, and anterior as well as the apical lateral LV segment.[4] Line graphs of apical and basal rotation and LV twist and untwisting showed specific pathological findings [Figure 1].
Figure 1.
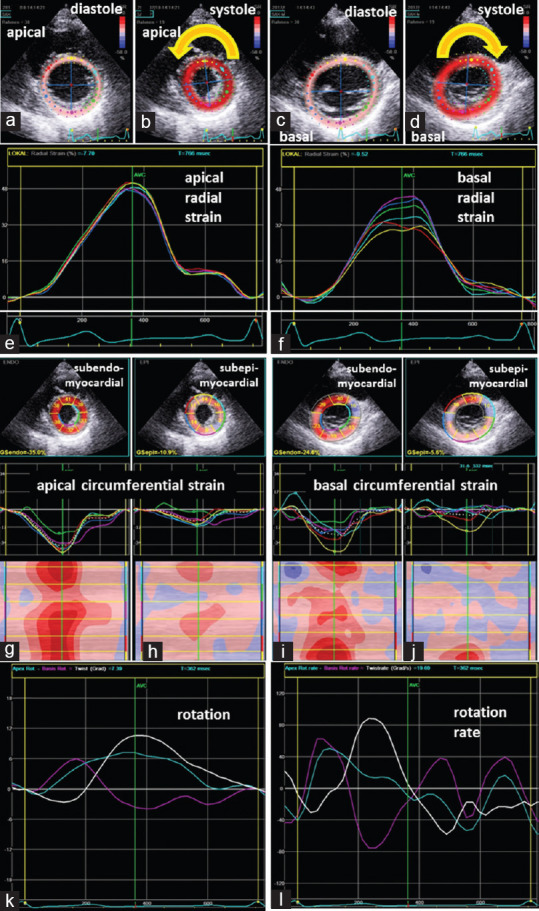
Documentation of echocardiographic findings: Illustration ofthe tracking area of apical (a and b) and basal (c and d) left ventricular segments in parasternal short-axis views during diastole (a and c) and systole (b and d) documenting the counterclockwise rotation of the apex (b) and the clockwise rotation of the base (d). Line graphs of normal regional radial deformation of the apical and basal left ventricular segments (e and f). Two-dimensional parasternal short-axis views representing segmental subendo- and subepicardial circumferential deformation (g-j) including segmental strain graphs and color-M-Modes; Conspicuous deformation was observed in the basal inferior, posterior, lateral and anterior as well as the apical lateral left ventricular segment (j); line graphs of apical (blue) and basal rotation (magenta) and left ventricular twist (white) (k) as well as corresponding line graphs of rotation rate (l) showing conspicuous left ventricular twist and untwisting
IM was confirmed by positive Lake Louise criteria documented by myocardial edema in the inferoseptal, inferior, and posterior LV segments by T2-short Tau inversion recovery (STIR) sequences, by hyperemia in the basal septal and the apical LV segments by T1-black blood fast spin (echo-early enhancement sequences, and by cellular damage in the posterior and lateral basal LV segments and predominantly subepicardial in the apical lateral LV by late enhancement by phase-sensitive inversion recovery (PSIR) sequences using cMRI [Figure 2].
Figure 2.
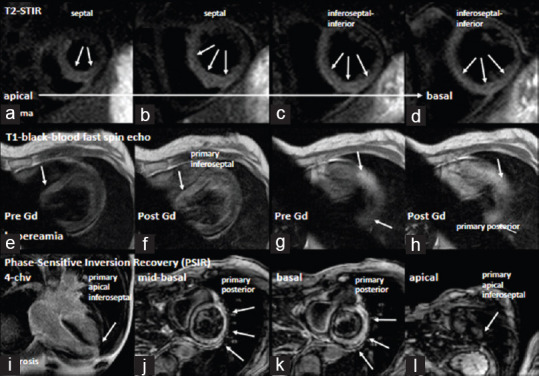
Documentation of cardiac magnetic resonance imaging findings: Documentation of a predominant edema in the septal and inferior apical to basal left ventricular segments (a-d). Arrows label regions of increased T2 ratio in in T2-STIR (short tau inversion recovery) sequences. Documentation of hyperemia by T1-BBS sequences (black blood fast spin) by early gadolinium enhancement (e-h). Arrows label regions of increased early enhancement (e, g-prior to contrast, f, h-after contrast). Documentation of fibrosis by late gadolinium enhancement in phase sensitive inversion recovery (PSIR) sequences (i-4-chamber view, j-l-short-axis views). Arrows label regions of transmural and subepicardial delayed enhancement
Follow-up (after 6 months)
Normal GLS (-19%) and higher GRS (52% (apical); 42% (basal)) were documented in the follow-up after 6 months [Figure 3]. GCS improved (-24% (apical); -10% (basal)), whereas subepicardial circumferential strain values were still lower in the basal and apical lateral LV segments. Rotation line graph showed normal apical rotation and biphasic basal rotation as well as a “chaotic” rotation rate-pattern during diastole [Figure 3]. Normal rotation and rotation rate patterns of a healthy subject are shown in Figure 4.
Figure 3.
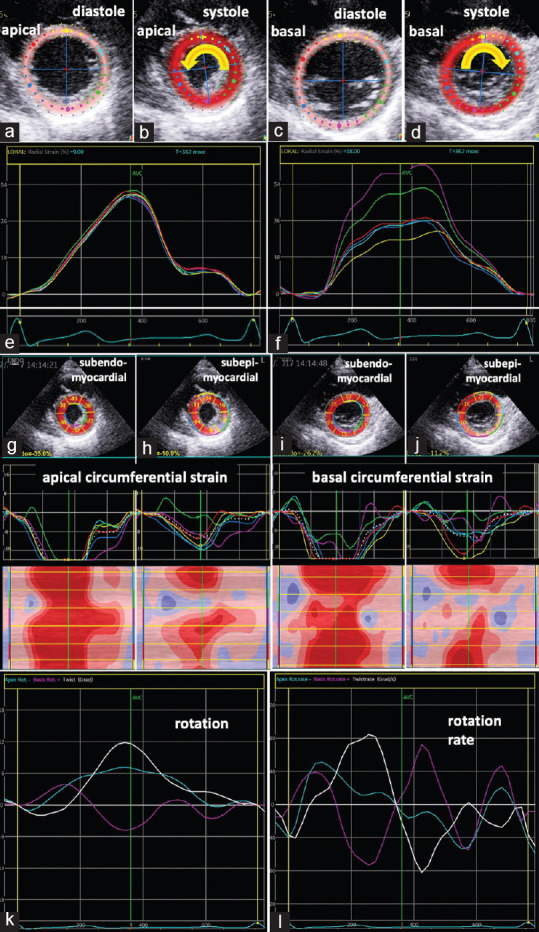
Documentation of echocardiographic findings at follow-up after 6 months: Illustration of the tracking area of apical (a and b) and basal (c and d) left ventricular segments in parasternal short-axis views during diastole (a and c) and systole (b and d) documenting the counterclockwise rotation of the apex (b) and the clockwise rotation of the base (d). Line graphs of increased regional radial deformation of the apical and basal left ventricular segments (e and f). Two-dimensional parasternal short-axis views representing segmental subendo- and subepicardial circumferential deformation (g-j) including segmental strain graphs and color-M-Modes; Conspicuous circumferential strain was only observed subepicardial in the basal and apical lateral left ventricular segments (h and j); line graphs of apical (blue) and basal rotation (magenta) and left ventricular twist (white) (k) as well as corresponding line graphs of rotation rate (l) showed normal apical rotation and biphasic basal rotation as well as still a “chaotic” rotation rate-pattern during diastole (l)
Figure 4.
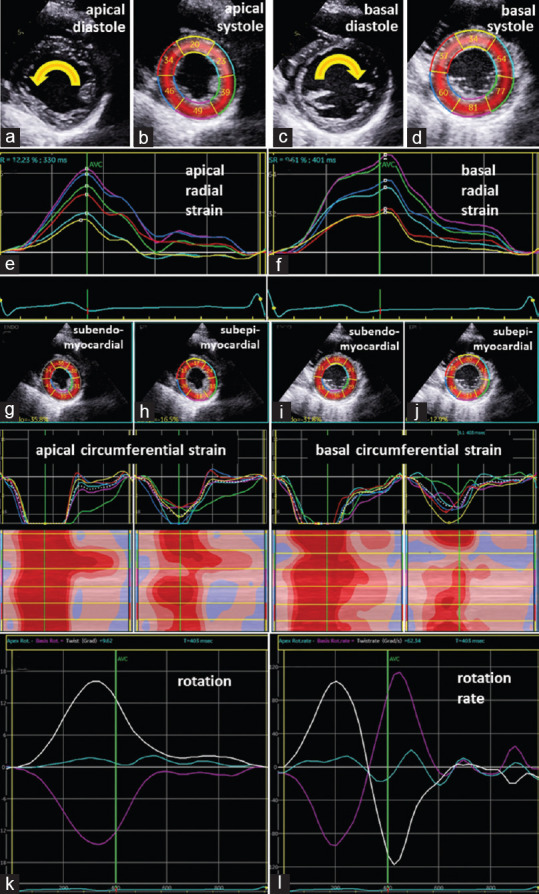
Documentation of rotational deformation patterns in a healthy volunteer: Illustration of conventional two-dimensional images (a and c) and the tracking area (b and d) of apical (a and b) and basal (c and d) left ventricular segments in parasternal short-axis views during diastole (a and c) and systole (b and d) documenting the counterclockwise rotation of the apex (a) and the clockwise rotation of the base (c). Line graphs of normal regional radial deformation of the apical and basal left ventricular segments (e and f). Two-dimensional parasternal short-axis views representing segmental subendo- and subepicardial circumferential deformation (g-j) including segmental strain graphs and color-M-Modes; normal monophasic deformation is documented in all subendo- and subepicardial left ventricular segments; line graphs of apical (blue) and basal rotation (magenta) and left ventricular twist (white) (k) as well as corresponding line graphs of rotation rate (l) showed normal left ventricular twist and untwisting
Whereas no significant alterations of regional longitudinal strain could be documented between baseline and follow-up, the analysis of global and regional longitudinal myocardial work index (MWI) showed specific pathological findings. Higher MWI was observed in the apical LV segments (2908 mmHg%) and in the basal posterior LV segment (2758 mmHg%) (normal ranges of MWI in female patients: 1310–2538 mmHg%), indicating elevated constructive work.[5] After 6 months, global MWI was still in normal ranges (1996 mmHg% vs. 1718 mmHg%), whereas only slightly increased MWI was still observed apical inferior (2521 mmHg%) [Figure 5].
Figure 5.
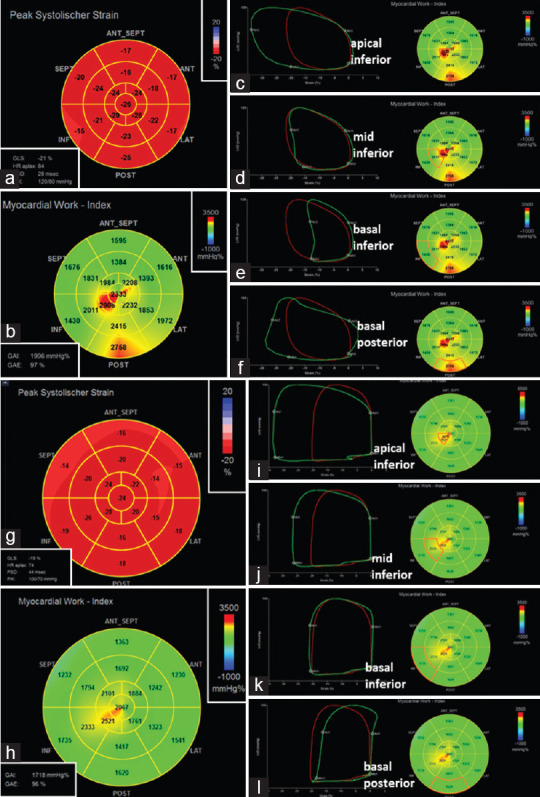
Documentation of longitudinal strain pattern (a) and regional myocardial work index (b) in acute myocarditis (baseline): In c-f regional pressure–strain loops are presented for the apical (c), mid (d) and basal inferior (e) as well as basal posterior left ventricular segments documenting increased myocardial work index mainly in the mid-apical inferior and basal posterior left ventricular segments. Documentation of the corresponding patterns at follow-up after 6 months (g-l). The longitudinal strain pattern (g) did not significantly change, the myocardial work index has improved-except in the apical inferior left ventricular segment (h and i)
DISCUSSION
To our knowledge, dynamics of strain and rotation patterns as well as of regional MWI are described for the first time. In the presence of normal ECG and preserved LVEF, it is challenging to diagnose IM by echocardiography, because in clinical practice, conventional echocardiography usually focuses on the assessment of global and regional LV systolic function by LVEF and GLS. As documented in this case, GLS is not necessarily impaired in patients with IM and preserved LVEF. Nevertheless, making the diagnosis of IM is obviously important in patients with preserved LV function because these patients are at considerably high risk of cardiovascular events, e.g., life-threatening arrhythmias due to myocardial inflammation.[6]
As demonstrated in this case, IM induces primarily alterations of subepicardial circumferential strain, LV rotation, and rotation rate underlining the predominant involvement of the subepicardial LV layers by viral inflammation. In contrast to the impairment of regional rotational deformation, an increase of regional MWI was observed in regions adjacent to LV segments with impaired circumferential deformation. This finding can be interpreted as a compensatory mechanism counteracting reduced circumferential strain of the outer LV layers by increased longitudinal contractility indicated by the regional elevation of MWI. The higher regional MWI at the acute stage of IM might also explain the preserved global LV systolic function in the presence of impaired LV twist and untwisting [Figure 5]. In general, LV performance can be estimated noninvasively by pressure–strain loop areas measuring LV pressure by brachial artery cuff pressure and longitudinal strain by speckle-tracking echocardiography. The pressure–strain loop areas can serve as the regional MWI. Global work index represents the mean pressure–strain loop area of all segmental pressure–strain loop areas. Constructive work describes the total work during shortening in systole and negative work during lengthening at isovolumetric relaxation. Wasted work can be described by negative work during lengthening in systole and positive work during shortening at isovolumetric relaxation. Constructive and wasted work can be calculated from global and regional LV pressure–strain loop areas.
A comparable scenario about the components of LV deformation has been described as a potential compensation mechanism after initiation of trastuzumab therapy by an improvement of GCS in contrast to an impairment of GLS at early stages of cardiotoxicity.[7] Thus, the assessment of GRS, circumferential layer strain, LV twist and untwisting (rotational deformation), as well as MWI might presumably provide an easy and noninvasive tool to detect subepicardial involvement in patients with IM.[8,9,10] Pathological findings of circumferential and rotational deformation might initiate further diagnostic procedures, e.g., cMRI or myocardial biopsy, to confirm the diagnosis of IM.
CONCLUSION
This case highlights the noninvasive approach to detect IM by significant alterations of LV deformation using speckle tracking echocardiography, especially in patients with normal ECG and normal global LV systolic function and GLS. The hypothesis about the compensatory mechanism of increased regional MWI due to impaired circumferential strain sets the stage for further trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Torrent-Guasp F, Ballester M, Buckberg GD, Carreras F, Flotats A, Carrió I, et al. Spatial orientation of the ventricular muscle band: physiologic contribution and surgical implications. J Thorac Cardiovasc Surg. 2001;122:389–92. doi: 10.1067/mtc.2001.113745. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833–9. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto T, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18:833–40. doi: 10.1093/ehjci/jex140. [DOI] [PubMed] [Google Scholar]
- 5.Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2019;20:582–90. doi: 10.1093/ehjci/jey188. [DOI] [PubMed] [Google Scholar]
- 6.Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy. Am J Cardiol. 1993;72:952–7. doi: 10.1016/0002-9149(93)91113-v. [DOI] [PubMed] [Google Scholar]
- 7.Cadeddu C, Piras A, Dessì M, Madeddu C, Mantovani G, Scartozzi M, et al. Timing of the negative effects of trastuzumab on cardiac mechanics after anthracycline chemotherapy. Int J Cardiovasc Imaging. 2017;33:197–207. doi: 10.1007/s10554-016-0987-9. [DOI] [PubMed] [Google Scholar]
- 8.Ünlü S, Mirea O, Duchenne J, Pagourelias ED, Bézy S, Thomas JD, et al. Comparison of Feasibility, Accuracy, and Reproducibility of Layer-Specific Global Longitudinal Strain Measurements Among Five Different Vendors: A Report from the EACVI-ASE Strain Standardization Task Force. J Am Soc Echocardiogr. 2018;31:374–800. doi: 10.1016/j.echo.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 9.White B, Voigt JU, Thomas JD. Sifting through the layers of myocardial deformation imaging. J Am Soc Echocardiogr. 2019;32:102–4. doi: 10.1016/j.echo.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur Heart J. 2012;33:724–33. doi: 10.1093/eurheartj/ehs016. [DOI] [PMC free article] [PubMed] [Google Scholar]


