Abstract
Autism spectrum disorder (ASD) is characterized by impaired social communication and poor adaptation to change; thus, the onset of puberty may be a pivotal transition. This cross-sectional study measured pubertal timing to examine hypothesized differences for sex (female vs. male) and group (ASD vs. Typical development (TD)). Participants included 239 children (137 ASD, 102 TD) between 10-to-13-years. The ASD group included 35 females and 102 males; the TDs included 44 females and 58 males. Pubertal onset measured by genital or pubic stage was investigated with linear regression using main effects of sex and age-by-sex interactions in TD and ASD groups and main effects of diagnosis and diagnosis-by-age interactions in males and females, controlling for body mass index, SES, and race. In TD, examination of main effects for genital (penis/breast) stage showed no difference for male and female children (t=1.33, p=0.187, rdf=92); however, there were significant differences in ASD (t=2.70, p=0.008, rdf=121). For diagnosis modelled separately by sex, there was significantly earlier pubertal development in females with ASD (t=1.97, p=0.053, rdf=70, but not males (t=1.329, p=0.186, rdf=143). Additionally, analysis of menses revealed females with ASD had significantly earlier onset than TD (t=−2.56, p=0.018, rdf=21). Examination of pubic stage revealed expected sex differences for TD (t=2,674, p=0.009, rdf=91) and ASD (t=3.482, p=0.001, rdf=121). Females with ASD evidence advanced pubertal onset relative to ASD males and TD females. Findings underscore the need for enhanced understanding of pubertal development in ASD, as differences may have significant psychological, social, physiological and developmental consequences.
Keywords: autism, puberty, adolescence, female, development
Lay Summary
Children with autism spectrum disorder (ASD) have difficulty with social communication and respond poorly to change, which may include the onset and course of puberty. The study measured the timing of puberty in 239 children (137 ASD and 102 TD) between 10-to-13-years based on pubertal stage of genital (breast/penis) and pubic hair development. Females with ASD evidence advanced pubertal onset relative to ASD males and TD females. Findings underscore the need for an enhanced understanding of pubertal development in ASD.
Introduction
Adolescence, the progression from childhood to adulthood, is a time of remarkable physiological, psychological, and social changes. It refers to the developmental transition of juvenile social and cognitive processes to their adult forms and is closely associated with chronological age and experience (Spear, 2000; Steinberg, 2005). Alternatively, puberty refers to biological maturation contributing to significant changes in morphology, cognition, emotion regulation and physiological stress (e.g., (Chrousos, Torpy, & Gold, 1998; Spear, 2000; Steinberg, 2005)). Maturational changes are particularly present in sexual systems resulting in significant physical and psychological changes for both sexes. The emergence of secondary sexual characteristics signals the onset of puberty, involving breast development in females and pubic hair in both sexes.
The biological changes related to puberty are initiated by the activation of hypothalamic-pituitary-gonadal (HPG) axis resulting in interrelated neuroendocrine processes: gonadarche (e.g., estrogen, testosterone), adrenarche (e.g., dehydroepiandrosterone; DHEA) and rapid physical growth (e.g., growth hormone) (Buck Louis et al., 2008; Dahl, 2004). The cascade of gonadotrophins leads to an increase of estrogen in females (e.g., menstruation and breast development) and testosterone in males (e.g., phallic growth, voice changes). In addition, pubertal hormones facilitate the motivation and development of social and sexual relationships with peers (Forbes & Dahl, 2010; Sisk & Foster, 2004).
The dynamic reorganization of neural networks in adolescence is initiated by sex steroid hormonal signaling and results in sexual dimorphic gray (e.g., decrease) and white matter (e.g., increase) volume differences in trajectory and shape over pubertal development (Lenroot et al., 2007; Sisk & Foster, 2004). Neural changes during this dynamic period are prominent in the amygdala and limbic circuitry, which influence changes in social-affective behavior (Scherf, Behrmann, & Dahl, 2012). There are also changes in age-and-sex-related cortical thickness in lateral temporal lobes and thinning in frontal and parietal cortices (Bramen et al., 2012). Adolescence is shaped by profound experience-dependent brain reorganization associated with planning, decision-making, risk-taking and reward value (Andersen, 2003) making this a period of enhanced responsivity and vulnerability.
The age of pubertal onset is influenced by demographic (e.g., ethnic/racial) (Mendle, Beltz, Carter, & Dorn, 2019) and biobehavioral (e.g., genetic, endocrine and environmental) factors resulting in normative, precocious or delayed pubertal onset. Earlier research pointed to racial and ethnic factors as contributing to pubertal timing, such that in the United States the mean age of menarche was 12.88 years for Caucasians and 12.16 for African-Americans (Herman-Giddens et al., 1997). However, socioeconomic status (SES) rather than race, which include relevant factors such as education, nutrition and family composition, can play an important role in pubertal timing (Ellis & Garber, 2000; Mendle, Turkheimer, & Emery, 2007; Obeidallah, Brennan, Brooks-Gunn, Kindlon, & Earls, 2000). For example, paternal absence at age 14, rather than maternal absence or presence of a stepfather, may contribute to differences in pubertal onset (Bogaert, 2005). Furthermore, differential impact of ethnicity and race may intersect with paternal absence resulting in a complex picture. Deardorff and colleagues (Deardorff et al., 2011) conducted a study with a relatively large diverse sample of females (N = 444, 6–8 years at baseline) and reported that in addition to main effects for body mass index (BMI), the absence of the father predicted earlier breast development in higher income households, and earlier pubic hair development only in higher income African American families.
In addition to the broad biological, psychosocial and selection factors that contribute to pubertal timing (Mendle et al., 2007), there is strong evidence from the Pediatric Research in Office Settings (PROS) studies indicating the timing of pubertal development is decreasing in the United States for females (Herman-Giddens et al., 1997) such that African-American females enter puberty 1-to-1.5-years earlier than white females, and white females enter puberty 6-months-to-1-year earlier than previous normative studies (Herman-Giddens et al., 1997). A recent meta-analysis supports a persistent worldwide downward trajectory of breast gland development (thelarche) revealing a decrease of 0.24 years per decade between 1977 and 2013 (Eckert-Lind et al., 2020). Similar to the secular trend in females, the PROS network found that males showed lower mean ages of pubertal onset, approximately 6-months-to-2-years earlier than previous reports (Herman-Giddens et al., 2012).
Assessment of the onset of puberty is important for establishing developmental milestones and recalibrating normative standards. The manner in which pubertal development is documented is important. Recently, significant discrepancies in pubertal staging were demonstrated when comparing physician exam to parental- and self-report favouring the exam (Corbett, Muscatello, Tanguturi, McGinn, & Ioannou, 2019). However, physical exam remains a proxy for pubertal development as hormone-based changes often occur before physical changes are evident (e.g., (Biro, Lucky, Huster, & Morrison, 1995; Blakemore, Burnett, & Dahl, 2010; Spear, 2000). Another important determinant that can influence pubertal timing is the level of body fat measured by body mass index (BMI); obesity can contribute to accelerated pubertal onset (Karlberg, 2002) whereas low body fat (Roemmich, Richmond, & Rogol, 2001) can result in delayed pubertal onset.
In concert, there are psychological and social changes associated with the transformation in body image. Such perceptions can have negative psychological effects, which may be intensified when pubertal timing is atypical. Early pubertal onset can confer higher risk for mental health problems (Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Kaltiala-Heino, Marttunen, Rantanen, & Rimpela, 2003; Waylen & Wolke, 2004). The earlier onset can extend the adolescent period thereby lengthening the divergence between the social environment, neural development and hormonally responsive motivational and affective behaviors (Casey, Duhoux, & Malter Cohen, 2010). Thus, the relative emotional reactivity, cognitive imbalance and incongruence with physical maturation can contribute to enhanced risk for mental health problems (Graber et al., 1997; Kaltiala-Heino et al., 2003; Waylen & Wolke, 2004).
The timing of the release of pubertal hormones contributes to individual differences in sex-biased psychopathological conditions (Sisk & Zehr, 2005). Females who experience early puberty are at an increased risk for depression (Conley & Rudolph, 2009; Ge, Conger, & Elder, 2001; Llewellyn, Rudolph, & Roisman, 2012). For males, the relationship is mixed with some studies showing that early puberty may contribute to increased symptoms of depression (Ge et al., 2001; Mendle, Harden, Brooks-Gunn, & Graber, 2010) whereas others show no effect (e.g., (Conley & Rudolph, 2009; Mendle, Harden, Brooks-Gunn, & Graber, 2012). Late puberty can also confer risk especially in males as late onset has been associated with lower cognitive skills, educational attainment, and earning outcomes in adulthood (Koerselman & Pekkarinen, 2018).
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder impacting an estimated 2% of children between 6-to-17 years of age in the United States (CDC, 2013). The sex-based ratio of males-to-females is reported to be 4:1 (APA, 2013). However, more recent research suggests a ratio closer to 3:1 (Loomes, Hull, & Mandy, 2017). Females may be better able to camouflage their autistic symptoms than males with ASD contributing to a diagnostic gender bias (Dean, Harwood, & Kasari, 2017; Ratto et al., 2018). The primary domains of impairment include reciprocal social communication and a repertoire of restricted, repetitive and stereotyped thought and behaviour (APA, 2013). As a result, individuals with ASD often respond poorly to novelty and changes in life patterns. Due to the dynamic challenges in physical, cognitive, social and emotional development, the onset and course of puberty may be a pivotal transition for adolescents with ASD. Indeed, many individuals with ASD show poor adaptation to developmental transitions such as from adolescence to adulthood (Taylor, Adams, & Bishop, 2017; Taylor & Seltzer, 2010).
Adolescence has been characterized as a time marked by significant changes in social-affective engagement and goal flexibility (Crone & Dahl, 2012), both of which are inherently challenging for individuals with ASD. Relatively little research has focused on the adolescent transition and the role of pubertal development is understudied (Picci & Scherf, 2015). On the positive side, some research on the transition from childhood to adolescence in ASD has reported improvement in core symptomology (Seltzer, Shattuck, Abbeduto, & Greenberg, 2004), social cognition (Anderson et al., 2007; Anderson, Oti, Lord, & Welch, 2009) and hyperactivity and irritability (Anderson, Maye, & Lord, 2011; Brown, 1969; Eisenberg, 1956; Gillberg & Schaumann, 1981; Rutter, 1970). Conversely, social withdrawal often intensifies during puberty (Anderson et al., 2011) and approximately a third of individuals with ASD experience significant-to-profound difficulty in psychosocial outcome (e.g., independent living, peer relations, employment) (Billstedt, Gillberg, & Gillberg, 2005; Gillberg & Steffenburg, 1987).
Peer rejection in adolescence is a major contributor to anxiety (Ladd, 2006) and impairments in social skills inherent to autism become increasingly more apparent due to the enhanced complexity and demand for social competence (Tantam, 2003). Youth with ASD often develop significant anxiety directly related to their social impairments (Bellini, 2006). Moreover, enhanced insight of their challenges may contribute to growing anxiety (Tantam, 2003) and stress (Corbett & Simon, 2013). Thus, the risk of psychopathologies such as depression and anxiety may arise or worsen in adolescents with ASD (Gotham, Unruh, & Lord, 2015; Kuusikko et al., 2008; Weisbrot, Gadow, DeVincent, & Pomeroy, 2005; White & Roberson-Nay, 2009).
Despite the importance of this vital developmental window, research on pubertal timing in autism has been limited and largely mixed. An uncontrolled clinically-referred case study from Denmark reported precocious puberty in a female with ASD (Mouridsen, 1989; Pohl, Cassidy, Auyeung, & Baron-Cohen, 2014). An early case report from the US described a female with severe autism and intellectual disability with possible pubertal regression resulting in loss of therapeutic gains following onset of menses (Ayres & Mailloux, 1983), which was consistent with another study reporting deterioration of functioning in five cases from Sweden, three of whom were females (Gillberg & Schaumann, 1981). A more recent online, retrospective, self-referred community sample from the United Kingdom, also reported early puberty and growth spurt with no difference in menarche (Pohl et al., 2014). Meanwhile, others have reported delayed pubertal maturation to include an uncontrolled clinically-referred sample from New South Wales (Harper & Collins, 1979), a retrospective self-report from the United Kingdom (Knickmeyer, Wheelwright, Hoekstra, & Baron-Cohen, 2006) and two other studies from Turkey and Australia, respectively, suggested later age of menarche that was associated with symptoms of autism (Herguner & Herguner, 2016; Whitehouse, Maybery, Hickey, & Sloboda, 2011). More recently, secondary analysis from an Australian population sample did not find any differences in pubertal timing (May, Pang, O’Connell, & Williams, 2017) between ASD vs. TD participants based on parent-report and self-report. The one small (N = 12) uncontrolled clinically-referred sample of males with ASD from France reported precocious puberty (Tordjman, Ferrari, Sulmont, Duyme, & Roubertoux, 1997). Collectively, prior research examining pubertal timing has been marked by limited scientific rigor in the assessment of pubertal maturation (e.g., online reporting, based strictly on menarche), and considerable differences in approach (e.g., retrospective self-report, online survey, clinical vs. community referred), and characterization of the samples (e.g., case studies, clinic-referred, community-referred, parent-reported diagnosis). While the extant research is limited, it suggests that adolescence and the pubertal transition may be met with a combination of improvement and decline. Therefore, careful consideration of pubertal timing is warranted.
The purpose of the current study was to examine pubertal timing in a large sample of early adolescents for possible sex (female vs. male) and group (ASD vs. TD) differences. The aim was to cross-sectionally compare the timing of pubertal onset between and within diagnostic groups based on sex. Although previous research has been rather mixed, there is a preponderance of early pubertal development in four studies in females with ASD (Ayres & Mailloux, 1983; Gillberg & Schaumann, 1981; Mouridsen, 1989; Pohl et al., 2014) and the only study in males (Tordjman et al., 1997). Therefore, we hypothesized that: 1) females with ASD would evidence earlier pubertal onset compared to TD females, and 2) males with ASD would evidence earlier pubertal onset compared to TD males.
Methods
The research was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Vanderbilt Institutional Review Board approved the study. Informed written consent and assent was obtained from all parents and study participants, respectively, prior to inclusion in the study.
Participants
Data were collected as part of the SENSE longitudinal study on pubertal development and stress (Corbett, 2017). The current study includes data from Year-1 enrollment when the children were between 10-years-0-months to 13-years-11-months of age.
The total sample included 239 youth, of which 234 completed the physical exam described below. At enrollment, adolescents were between 10-years-0-months and 13-years-11-months, which equated to 137 ASD (mean age = 11.43) and 102 TD (mean age = 11.72) participants. The ASD group included 35 females and 102 males and the TD group included 44 females and 58 males. Five participants (3ASD males, 2TD males) who did not complete the physical exam were removed from the analyses and an additional TD participant (female) was missing the pubic hair stage variable due to not wanting to complete that portion of the exam.
The racial and ethnic characterization of the sample included 7.9% African-Americans, 83.6% Caucasians, and 8.2% Mixed. Participants were recruited from a broad community sample in the southern United States covering a 200-mile radius that targeted medical and health-related services, clinics, research registries, regional autism/disability organizations, schools, and social media platforms. Inclusion required an intelligence quotient (IQ) score ≥ 70 due to task demands in the source longitudinal study (Corbett, 2017). Exclusion criteria included current use of medications known to alter the Hypothalamic-Pituitary-Adrenal (HPA) axis (e.g., corticosteroids; see (Granger, Hibel, Fortunato, & Kapelewski, 2009)) or HPG axis (e.g., growth hormone), or medical condition known to impact pubertal development (e.g., Cushing’s Disease). At screening, there were 24 participants not enrolled who were classified as ineligible (i.e., lower IQ, insufficient language level, extreme aggression, or for TD children, having a sibling with an ASD diagnosis). Demographic information for each group is presented in Table 1.
Table 1.
Demographics and Diagnostic Variables.
| ASD (n = 137) |
TD (n = 102) |
||||||
|---|---|---|---|---|---|---|---|
| N | Proportion | Proportion | 𝜒 2 | p | |||
| Sex: F | 239 | 0.26 (35/137) | 0.43 (44/102) | 8.182 | <0.01 | ||
| Race | 239 | 9.542 | 0.01 | ||||
| Caucasian | 0.87 (89/102) | 0.81 (111/137) | |||||
| Black | 0.02 (2/102) | 0.12 (17/137) | |||||
| Mixed Race | 0.11 (11/102) | 0.07 (9/137) | |||||
| N | M | SD | M | SD | F | p | |
| IQ | 237 | 100.91 | 20.77 | 117.37 | 13.83 | 43.433 | <0.01 |
| SCQ | 236 | 17.36 | 8.34 | 2.51 | 2.68 | 287.513 | <0.01 |
| ADOS Total | 137 | 12.57 | 4.58 | -- | -- | -- | -- |
| BMI (Percentile) | 235 | 65.36 | 31.19 | 55.43 | 31.29 | 6.493 | 0.01 |
| SES Education | 230 | 3.73 | 1.37 | 4.64 | 1.31 | 25.893 | <0.01 |
N is the number of non-missing value.
Kruskal-Wallis.
Pearson.
Wilcoxon.
Note. ASD, Autism spectrum disorder; TD, Typical development; F, Female; IQ, Intelligence Quotient; SCQ, Social Communication Questionnaire; ADOS, Autism Diagnostic Observation Schedule; BMI, Body Mass Index; SES, Socioeconomic Status. BMI Percentile defined as age- and sex-adjusted percentile according to CDC guidelines (https://www.cdc.gov/healthyweight/bmi/calculator.html).
Diagnostic Procedures
The diagnosis of ASD was based on the Diagnostic and Statistical Manual-5 (APA, 2013) and confirmed by: (1) a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with autism expertise; (2) current clinical judgment, and (3) corroborated by the Autism Diagnostic Observation Schedule (ADOS-2; (Lord et al., 2012), administered by research-reliable personnel.
Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; (Lord et al., 2012) is a semi-structured play and interview-based instrument used to support the diagnosis of ASD.
Social Communication Questionnaire (SCQ; (Rutter, Bailey, & Lord, 2003) is a screening instrument for symptoms of ASD. A score of 15 is suggestive of ASD. Recent research reported lower sensitivity and specificity (Barnard-Brak, Brewer, Chesnut, Richman, & Schaeffer, 2016); therefore, TD children with a score ≥ 10 were excluded.
Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II, (Wechsler, 2011) is a measure of cognitive ability used to obtain an estimate of the child’s intellectual functioning (IQ ≥ 70 required).
Dependent Measures
To rigorously and comprehensively measure pubertal development, the source study employed three different approaches: a physical exam, a parent-report measure, and self-report based on visual representation of Tanner stages (Marshal & Tanner, 1970; Marshall & Tanner, 1969). The primary dependent variable for pubertal development used in the current study was the physical exam. Recent research with the sample comparing exam to parent- and self-report demonstrated that physical exam is the optimal approach for accurate pubertal measurement (Corbett et al., 2019). Findings showed significant discrepancies between self-report and parent-report, which were far less accurate than physical exam for precisely estimating pubertal development (Corbett et al., 2019); therefore, these measures are not included in the current study. Importantly, while Tanner staging is preferred, it may not precisely coincide with hormone-dependent, brain-based pubertal progression (Spear, 2000).
Physical Examination (PE).
The PE was completed to reliably identify pubertal development and assign Tanner stage (Marshal & Tanner, 1970; Marshall & Tanner, 1969). The exam ascertained two measures with 5 stages for Genitals (G1-G5 for males) and Breasts (B1-B5 for females) (GB stage) and Pubic hair (P1-P5 for both genders) (PH stage). The exam consisted of visual inspection and categorization of pubertal and genital maturation. To be consistent with the original Tanner staging and to maximize participation, palpation of breasts or measurement of testes was not conducted.
Pubertal assessment consisted of a brief, standardized physical exam conducted by trained, licensed study physicians. A male physician conducted the majority of the exams, but a female physician was provided same-gender exams as requested. Research indicates the sex of the physician is usually not as important as the participant’s comfort level and the competence of the physician (Dorn, Dahl, Woodward, & Biro, 2006). Study physicians spent approximately 5-minutes to establish rapport, explain the rationale for the exam and address any questions or concerns, which helped to normalize the experience. During the exam, the adolescent was requested to loosen clothing to fully expose breast and lower genital region, rather than disrobing, which aided in the level of comfort for the participants. A companion (e.g., parent or same-gender research member) was offered to accompany the participant during the exam, which was conducted in a clinic exam room.
Physicians were blinded to parental- and self-reports. Inter-rater reliability was established between study physicians on 10 randomly selected participants. Intraclass correlations were calculated between study physicians to assess the degree to which raters were able to identify Tanner stages for GB and PH markers. Inter-rater reliability for markers ranged from 0.62 to 0.75 (all p <0.001). Kappa was calculated to assess the extent to which physicians were able to reliably and independently identify when participants had initiated pubertal maturation (Stage 2) for each marker. Kappa ranged from 0.62 to 1.00 (good to very good).
Body Mass Index (BMI):
BMI was calculated using the standard formula (lb./in2) x 703 for use with the CDC growth charts for children and adolescents (2 through 19 years; https://www.cdc.gov/healthyweight/bmi/calculator.html). Percentiles were calculated based on sex and age.
Statistical Analysis
The Tanner genital and pubic stage reflect two different endocrine processes and combining into one score may not be optimal (Dorn, 2006; Dorn & Biro, 2011). Thus, pubertal onset measured by GB or PH was modeled with linear regression using 4 models; first we investigated sex differences in TD and ASD separately and then investigated diagnosis differences separately for each sex. We used this more intuitive way to present the results than making these comparisons using linear contrasts within a single model as we were not explicitly interested in testing the diagnosis by sex interaction. For models in TD and ASD groups, we also tested an age-by-sex interaction in order to see whether there was a difference in the change in Tanner stage between males and females in our developmental time period. Similarly, for the models in males and females we used diagnosis-by-age interaction models. All models controlled for a linear effect of BMI since it can impact pubertal timing (Karlberg, 2002; Roemmich et al., 2001). In addition, we included parental education as a proxy for SES, and race, both of which may be related to pubertal timing as well (Mendle et al., 2019). Parental education was modeled continuously (1 = less than high school, 2 = high school or equivalent, 3 = associates, 4 = bachelors, 5 = masters, 6 = doctorate, 7 = professional). These variables are included as covariates in the primary analyses within the sex/diagnosis strata. In each model, we investigated main effects and interactions using type 2 sums of squares, so that main effects were tested without the interaction terms in the model. Robust standard errors were used to compute all test statistics (Mackinnon & White, 1985).
Results
Sample demographics are presented in Table 1. There were no differences based on age. However, there were significant differences in IQ.
GB stage outcome
In TD, there was no evidence for differences in development-related changes in GB stage (age by sex interaction, F=0.078, p=0.783; df=1, rdf=92) through our samples’ age range and no difference in mean stage between male and female children, controlling for age. BMI, race and SES, (t=1.33, p=0.187, rdf=92; Figure 1). There was also no difference in development-related changes in genital stage for ASD (age by sex interaction, F=0.459, p=0.499; df=1, rdf=121). However, there were significant differences in mean genital stage between males and females with ASD (t=2.70, p=0.008, rdf=121), while controlling for the aforementioned covariates (Supplementary tables S1 and S2). These mean differences in our age range (10–14 years) provide evidence for an earlier onset of pubertal development in ASD females relative to ASD males.
Figure 1. Scatterplot of sex difference in genital/breast (GB) and pubic hair (PH) Tanner stage for typically developing (TD) individuals.
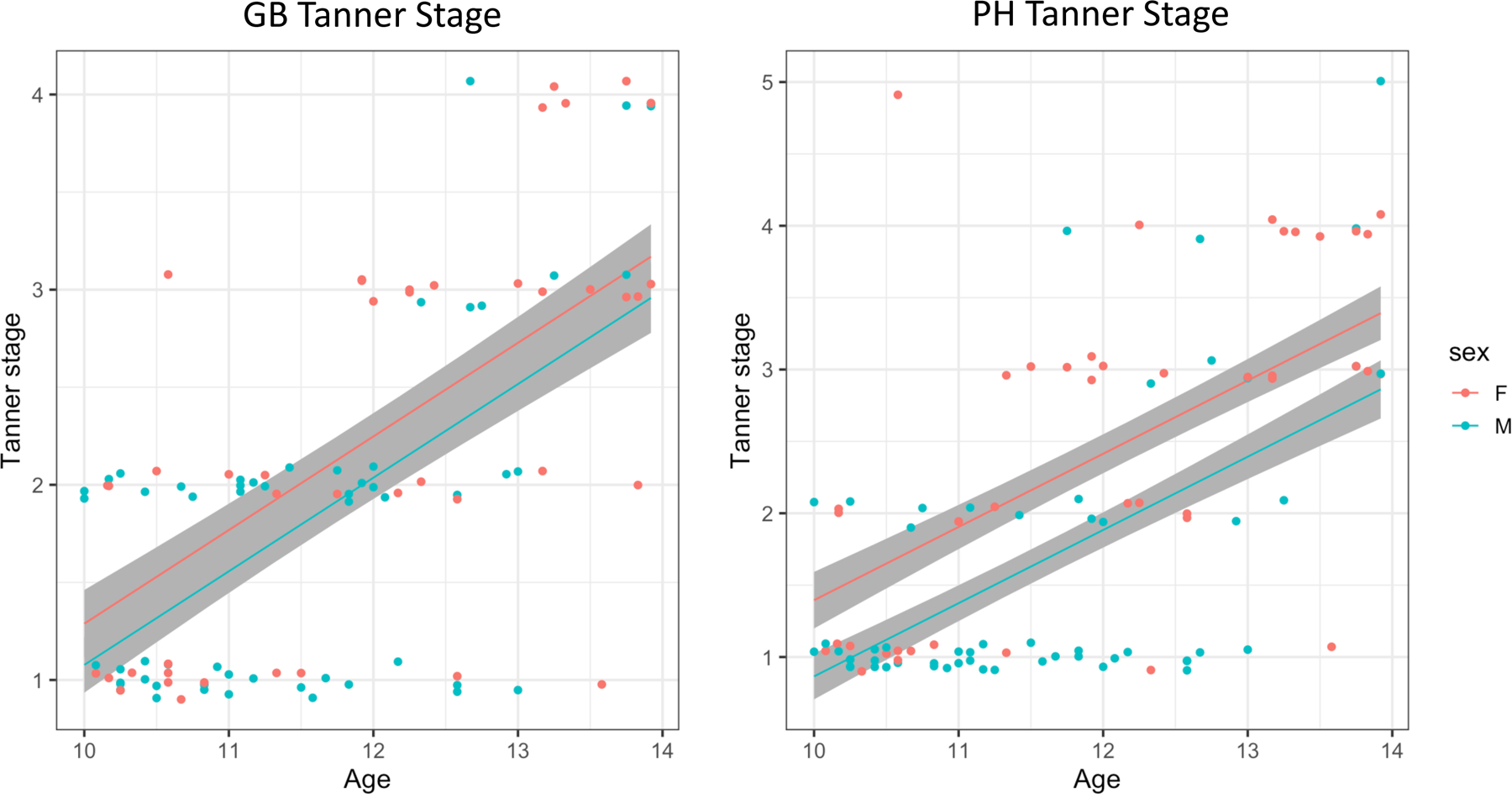
No sex differences in development-related changes in GB or PH stage were detected in our sample’s age range (no difference in slopes). Sex differences (mean shift) were detected in the PH stage, but not in the GB stage. Jitter of 0.1 was added to the y-axis to avoid overplotting.
We fit separate linear models for GB stage in males and females to separately assess differences related to ASD diagnosis while controlling for age, BMI, race and SES. There was no significant age by diagnosis interaction in males (F=0.008, p=0.929, df=1, rdf=143) or females (F= 0.257, p=0.614, df=1, rdf=70), suggesting that the shape of the trajectories may be similar in this range. However, mean differences were evident in females (t=1.97, p=0.053, rdf=70; Figure 2C, Tables S3 and S4, suggesting earlier pubertal development in ASD relative to TD, but not in males with ASD (t=1.329, p=0.186, rdf=143; Figure 2A).
Figure 2. Scatterplot of diagnosis differences in genital/breast (GB) and pubic hair (PH) Tanner stage for males and females.
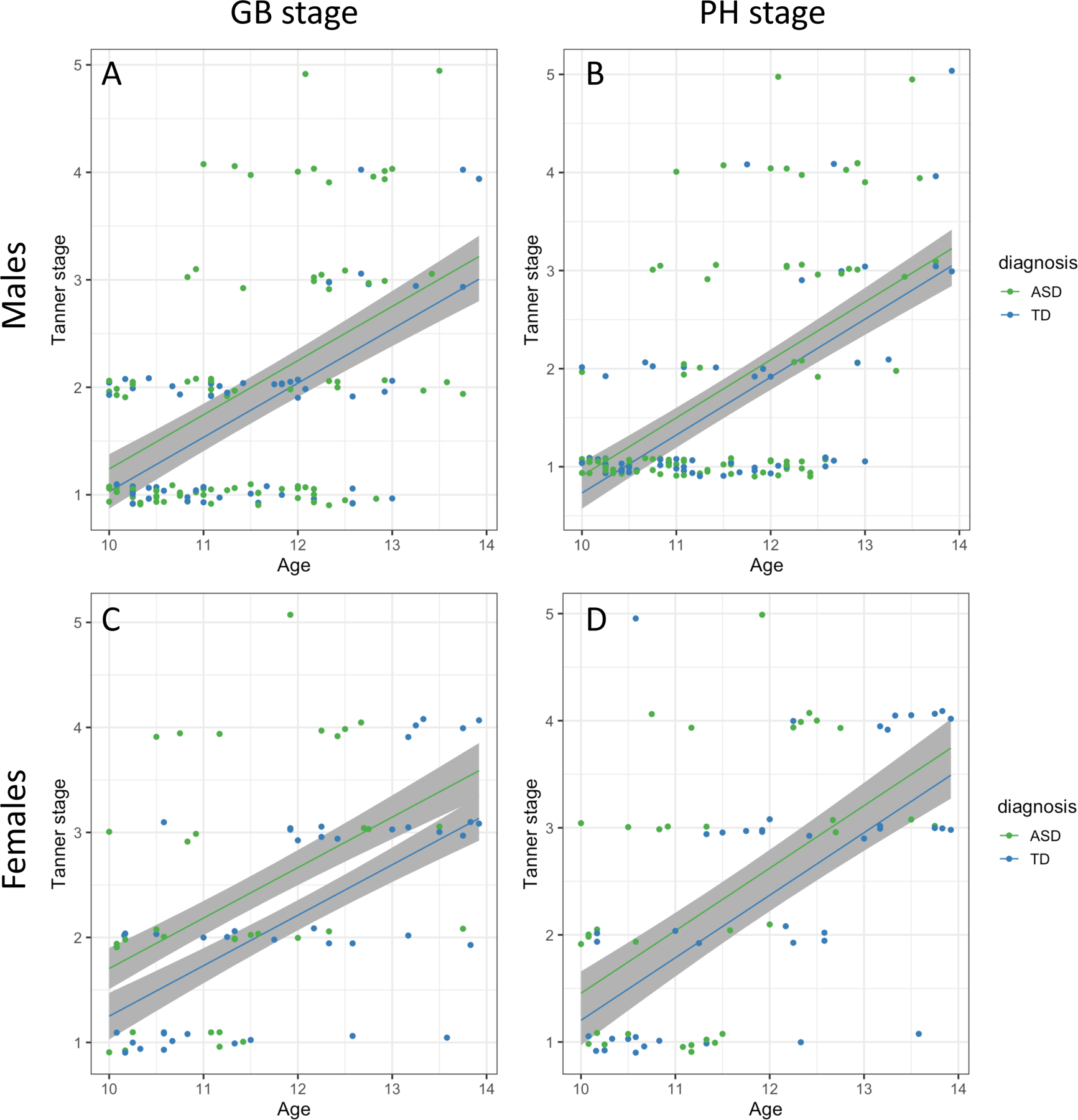
No differences in development-related changes in GB or PH stage were detected between diagnoses in our sample’s age range for males or females (no difference in slopes), but ASD was associated with a later GB stage in females throughout the age range (mean shift in panel C). Jitter of 0.1 was added to the y-axis to avoid overplotting.
There was a substantial effect of BMI in all models of GB stage, except the model in males; greater BMI was associated with higher Tanner stage (Supplementary Tables 1-4).
PH stage outcome
We performed parallel analyses using PH staging as the outcome (see Supplementary Tables S5-S8). As above, there were no significant age by sex interactions in either diagnostic group (TD, F=0.793, p=0.375, df=1, rdf=91; ASD, F=0.385, p=0.536, df=1, rdf=121) suggesting similar development-related changes between males and females in this age range for both groups. However, examination of mean differences in PH stage revealed expected sex differences for TD (t=2,674, p=0.009, rdf=91; Figure 1) and ASD (t=3.482, p=0.001, rdf=121) groups.
We fit separate linear models for PH stage in males and females to assess differences related to ASD diagnosis. There was no significant age by diagnosis interaction in males F=1.4, p=0.238, df=1, rdf=143) or females (F=0.004, p=0.949, df=1, rdf=69), suggesting that the shape of the PH stage trajectories may be similar for ASD and TD in this range. There was not significant evidence for a mean difference in PH stage between diagnostic groups for males (t=1.067 p=0.288, rdf=143; Figure 2B) or females (t=1.06, p=0.294, rdf=69; Figure 2D).
There was a significant effect of BMI in all models of PH stage (Supplementary Tables S5-S8) where BMI was associated with higher tanner stage.
In order to further assess the effect of diagnosis on puberty in females, we fit a model of age of menarche in a subset of 27 females, controlling for BMI, race and SES percentile. There was a significant difference between the groups (t=−2.56, p=0.018, rdf=21) indicating an earlier onset in females with ASD. While roughly a third of the sample (11ASD, 16TD) had begun menstruating, the onset of menarche in ASD females was 9.5 months earlier than TD females.
Discussion
Puberty is a time of considerable plasticity when various physiological changes coincide with the dynamic neural, social and behavioural transition of adolescence. While it is a pivotal and rapid developmental period, relatively little is known about the timing and consequences of pubertal onset in individuals with ASD. Therefore, the purpose of the study was to carefully examine pubertal timing between and within diagnostic groups (ASD vs. TD) and across sexes (female vs. male).
A series of linear models were conducted to examine the effects of age, diagnosis and sex on pubertal timing, while controlling for age and BMI. Pubertal level was determined based on physical exam using Tanner staging for genital/breast (GB) and pubic hair (PH) stage. In the TD group, there were no differences in development-related changes in either GB or PH stage in the sample age range evidenced by comparable slopes (Figure 1). There were no mean sex differences in GB pubertal stage for male and female children; however, PH stage showed expected developmental sex differences between males and females.
Difference in pubertal timing of GB and PH were examined separately for the diagnostic groups. There were no differences in development-related changes in GB or PH between diagnoses in the sample age range for males or females. However, females with ASD showed earlier pubertal onset than males with ASD. When GB stage was modeled separately by sex to examine differences associated with ASD diagnosis and age, there was a similar shape in the developmental trajectories during this age range. However, there was a significant GB stage difference in females with ASD compared to TD females indicating earlier breast development confirming our initial hypothesis. Importantly, the early onset is within the context of already declining age of breast development in the US (Biro et al., 2010) and around the world (Aksglaede, Olsen, Juul, & Sorensen, 2009). In addition to GB differences, females with ASD had significantly earlier onset of menses than TD females. In a large multisite, longitudinal study, Biro and colleagues reported that menses is positively correlated with breast development albeit negatively correlated with BMI (Biro et al., 2018). Therefore, it is not surprising that early breast development would coincide with early menses in females with ASD.
Examination of PH stage revealed expected sex differences for TD and ASD groups. Moreover, pubic development was comparable for females with and without ASD. Since puberty is comprised of different hormonally-driven neuroendocrine processes (e.g., gonadarche, adrenarche (e.g., (Havelock, Auchus, & Rainey, 2004; Lucky, Biro, Simbartl, Morrison, & Sorg, 1997)), the differences observed between breast/menses and pubic development may reflect distinct maturational endocrine processes such as the influence of estrogen vs. DHEA, respectively.
The findings showing earlier pubertal onset for ASD females is consistent with some reports (Mouridsen, 1989; Pohl et al., 2014; Yoshirmura, Naiki, Horikawa, & Tanaka, 2005). In two early small case reports, early pubertal timing was met with loss of therapeutic gains (Ayres & Mailloux, 1983) and deterioration of functioning (Gillberg & Schaumann, 1981). Similarly, an online, retrospective, self-referred community sample, also reported early puberty although no differences were noted in timing of menarche (Pohl et al., 2014).
In contrast, some studies have reported delayed pubertal onset (Harper & Collins, 1979; Herguner & Herguner, 2016; Knickmeyer et al., 2006; Whitehouse et al., 2011). More recently, a population sample did not find any differences in pubertal timing (May et al., 2017) between a sample of ASD vs. TD participants although with only 21 females with ASD, it may have been underpowered to find effects. Importantly, pubertal status in May et al. (May et al., 2017) was estimated based on parent-report and self-report. Recent results from the study team demonstrated the limitations of using parent and self-report, which lack precision in estimating pubertal stage especially when compared to physician exam (Corbett et al., 2019).
The current prospective study indicated females with ASD experience earlier onset of physical maturation of secondary sexual characteristics. While the consequences for the timing are not yet known, for TD females, early or precocious pubertal development may contribute to increased risk for peer victimization (e.g., relational, reputational or sexual) (Compian, Gowen, & Hayward, 2009; Nadeem & Graham, 2005; Petersen & Hyde, 2009). Also, adolescent females tend to experience increased emotional reactivity and intensity (DeRose & Brooks-Gunn, 2008; Silk et al., 2009). Similarly, females with ASD may find themselves at risk for relational, reputational or sexual victimization and heightened emotional dysregulation.
The PROS study reported a downward trend in pubertal onset in females (Herman-Giddens et al., 1997) suggesting that standards need to be redefined in order to provide appropriate education and care by professionals and parents alike. Historically, precocious puberty (onset < 8 years in females and 9 years in males) or early puberty (onset between 8 and 9 years in females and between 9 and 10.5 years in males) can be considered a normal variant (Winter, Durand, & Brauner, 2019). A portion of the females with ASD would likely meet criteria for precocious puberty and an even larger proportion would meet criteria for early puberty. Due to the observed higher percentage of early onset in ASD compared to TD females, the findings would be hard to dismiss as a normal variant.
For males, there were no differences across the groups in pubertal timing to include genital or pubic stage thereby not supporting the second hypothesis. The results for males with ASD are in contrast to the one small study in 12 clinically-referred males that presented with precocious puberty (Tordjman et al., 1997). Importantly, the current sample was large (N=118) derived from the community and not clinic-based.
Although not the focus of the study, the amount of body fat can influence pubertal timing such that obesity can accelerate pubertal onset (Karlberg, 2002) and low body fat can contribute to delayed pubertal onset (Roemmich et al., 2001). In the current study, BMI was significant across all of the models except genital stage in males. Due to the notable rise in obesity in children and youth, findings serve as a reminder to monitor this important health index as it relates to many aspects of development (e.g., pubertal, medical, physical, psychological, and social).
The aforementioned findings have important clinical implications especially for females with ASD. Menarche is an important developmental milestone for females. For those in the current sample who were menstruating, the age of menarche in the ASD group occurred 9.5 months earlier than TD females. The onset of menses and ongoing menstruation is often accompanied by notable challenges with mood and emotion regulation (Burke, Kalpakian, Smith, & Quint, 2010) (Hamilton, Marshal, & Murray, 2011; Obaydi & Puri, 2008). Females with ASD often experience intensification of symptoms such as heightened sensory experiences (Hamilton et al., 2011; Steward, Crane, Roy, Remington, & Pellicano, 2018). While the challenges and long-term consequences are understudied, a recent study of autistic females, highlighted that young women would benefit from more education pertaining to menstruation before and during menses to assist with understanding factors related to health, duration, pain, hygiene and changes in mood status (Steward et al., 2018). Additionally, psychological and sexual education training such as the Tackling Teenage program (Dekker et al., 2015; Visser et al., 2017) may be beneficial.
The unique profile of females with ASD may complicate the identification of the mental health challenges they face. For example, typically developing females who experience early puberty are at an increased risk for depression (Conley & Rudolph, 2009; Ge et al., 2001; Llewellyn et al., 2012), which may be masked in ASD. It has been shown that females with ASD are better at camouflaging their symptoms thereby appearing more competent than they may be (Burns & Tierney, 2016; Dean et al., 2017; Dworzynski, Ronald, Bolton, & Happe, 2012; Gould & Ashton-Smith, 2011; Rynkiewicz et al., 2016; Tierney, Burns, & Kilbey, 2016). As a result, the diagnosis of ASD in females is often delayed, questioned or non-existent (Kopp & Gillberg, 1992; Ratto et al., 2018), and efforts to conceal their challenges are associated with increased mental health costs (Cage & Troxell-Whitman, 2019). Therefore, females with ASD may be hiding – and struggling - in plain sight. Amidst enhancing our ability to diagnose and characterize females with ASD, future studies with careful attention to adolescence and mental health are needed.
Despite the strengths of the study (e.g., comprehensive examination, well-characterized large sample), there are limitations. The ratio of males-to-females is often reported to be 4:1 (APA, 2013). We actively recruited and enrolled female participants with ASD resulting in a 2.9:1 ratio. Even so, there are fewer females with ASD which significantly limits the power to conduct some analyses and limits the strength of the findings. Also, we enrolled children beginning at 10-years of age; therefore, if pubertal onset occurred before this age at entry, which appears the case especially for some females, this could result in an upward bias and earlier onset than would be captured in the current cohort. It is presumed that the early onset for females with ASD is even earlier than represented here. The cross-sectional design of the current study is unable to determine the tempo of puberty or how rapid or slow development progresses through pubertal stages. The longitudinal study, which follows the sample will be better positioned to examine pubertal tempo (Corbett, 2017). The study also did not have access to some factors that have been shown to impact pubertal timing, such as paternal absence (e.g., (Deardorff et al., 2011) and adverse childhood experiences (e.g., (Zhang, Zhang, & Sun, 2019). Moreover, due to the size of the sample and focus on autism, we are unable to covary all possible factors identified and controlled for in large-scale, national and world-wide studies. Future studies are needed to comprehensively address the various factors that may impact pubertal onset in youth with and without ASD. Finally, pubertal examination was based on standardized physical exams by a study physician using the original Tanner Staging visual inspection (Marshal & Tanner, 1970; Marshall & Tanner, 1969); it did not include palpation of the breast or testes which may be more rigorous but would have likely resulted in reduced participation. Nevertheless, the study team has shown that physical pubertal exam as described here, is superior to either parent- or self-report (Corbett et al., 2019).
In conclusion, pubertal onset, which marks the physiological beginning of adolescence - the vital and sometimes precarious psychological, social, and physical transition - may amplify an already steep climb to adulthood. Indeed, the findings underscore the need to continue the pursuit to elucidate pubertal development in ASD as even minor differences in the timing may have developmental consequences (Picci & Scherf, 2015). By definition, pubertal onset sets into motion a cascade of events which may magnify and further complicate an already vulnerable trajectory, especially in females. While risks may be plentiful, it will also be essential to identify characteristics and environments that may smoothen the transition to adulthood and make children with ASD more resilient to the multitude of psychological, physical and social changes that define adolescence.
Supplementary Material
Acknowledgment:
This study was funded by the National Institute of Mental Health (MH111599 PI: Corbett) with core support from the National Center for Advancing Translational Sciences (CTSA UL1 TR000445). Funders had no role in the conduct of the research or preparation of the article.
Footnotes
Conflict of Interest: The authors declare no conflict of interests.
References
- Aksglaede L, Olsen LW, Juul A, & Sorensen TIA (2009). Age at pubertal growth spurt in girls and boys in relation to body mass index during the emerging obesity epidemic. Hormone Research, 72, 122–122. [Google Scholar]
- Andersen SL (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev, 27(1–2), 3–18. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, . . . Pickles A (2007). Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol, 75(4), 594–604. doi: 10.1037/0022-006X.75.4.594 [DOI] [PubMed] [Google Scholar]
- Anderson DK, Maye MP, & Lord C (2011). Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. Am J Intellect Dev Disabil, 116(5), 381–397. doi: 10.1352/1944-7558-116.5.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Oti RS, Lord C, & Welch K (2009). Patterns of growth in adaptive social abilities among children with autism spectrum disorders. J Abnorm Child Psychol, 37(7), 1019–1034. doi: 10.1007/s10802-009-9326-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders, Fifth Edition (DSM-5) Washinton, D.C.: American Psychiatric Association. [Google Scholar]
- Ayres AJ, & Mailloux ZK (1983). Possible pubertal effect on therapeutic gains in an autistic girl. Am J Occup Ther, 37(8), 535–540. doi: 10.5014/ajot.37.8.535 [DOI] [PubMed] [Google Scholar]
- Barnard-Brak L, Brewer A, Chesnut S, Richman D, & Schaeffer AM (2016). The sensitivity and specificity of the social communication questionnaire for autism spectrum with respect to age. Autism Res, 9(8), 838–845. doi: 10.1002/aur.1584 [DOI] [PubMed] [Google Scholar]
- Bellini S (2006). The development of social anxiety in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 21(3), 138–145. [Google Scholar]
- Billstedt E, Gillberg IC, & Gillberg C (2005). Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord, 35(3), 351–360. [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, . . . Wolff MS (2010). Pubertal Assessment Method and Baseline Characteristics in a Mixed Longitudinal Study of Girls. Pediatrics, 126(3), E583–E590. doi: 10.1542/peds.2009-3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Lucky AW, Huster GA, & Morrison JA (1995). Pubertal staging in boys. J Pediatr, 127(1), 100–102. doi: 10.1016/s0022-3476(95)70265-2 [DOI] [PubMed] [Google Scholar]
- Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, . . . Teitelbaum SL (2018). Age of Menarche in a Longitudinal US Cohort. J Pediatr Adolesc Gynecol, 31(4), 339–345. doi: 10.1016/j.jpag.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Hum Brain Mapp, 31(6), 926–933. doi: 10.1002/hbm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert AF (2005). Age at puberty and father absence in a national probability sample. J Adolesc, 28(4), 541–546. doi: 10.1016/j.adolescence.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, . . . Sowell ER (2012). Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One, 7(3), e33850. doi: 10.1371/journal.pone.0033850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL (1969). Adolescent Development of Children with Infantile Psychosis. Seminars in Psychiatry, 1(1), 79–&. [Google Scholar]
- Buck Louis GM, Gray LE Jr., Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, . . . Euling SY (2008). Environmental factors and puberty timing: expert panel research needs. Pediatrics, 121 Suppl 3, S192–207. doi: 10.1542/peds.1813E [DOI] [PubMed] [Google Scholar]
- Burke LM, Kalpakian CZ, Smith YR, & Quint EH (2010). Gynecologic issues of adolescents with Down syndrome, autism, and cerebral Palsy. Journal of Pediatric Adolescent Gynecology, 23(1), 11–15. [DOI] [PubMed] [Google Scholar]
- Burns J, & Tierney S (2016). The imitation game: Being an adolescent girl with autism spectrum disorder. Journal of Intellectual Disability Research, 60(7–8), 774–774. [Google Scholar]
- Cage E, & Troxell-Whitman Z (2019). Understanding the Reasons, Contexts and Costs of Camouflaging for Autistic Adults. Journal of Autism and Developmental Disorders, 49(5), 1899–1911. doi: 10.1007/s10803-018-03878-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, & Malter Cohen M (2010). Adolescence: what do transmission, transition, and translation have to do with it? Neuron, 67(5), 749–760. doi: 10.1016/j.neuron.2010.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2013). Changes in prevalence of parent-reported Autism Spectrum Disorder in school-aged U.S. Children: 2007 to 2011–2012. Health Statistics Reports, 65. [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, & Gold PW (1998). Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med, 129(3), 229–240. [DOI] [PubMed] [Google Scholar]
- Compian LJ, Gowen LK, & Hayward C (2009). The Interactive Effects of Puberty and Peer Victimization on Weight Concerns and Depression Symptoms Among Early Adolescent Girls. Journal of Early Adolescence, 29(3), 357–375. doi: 10.1177/0272431608323656 [DOI] [Google Scholar]
- Conley CS, & Rudolph KD (2009). The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Dev Psychopathol, 21(2), 593–620. doi: 10.1017/S0954579409000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA (2017). Examining stress and arousal across pubertal development in ASD In: National Institute of Mental Health. [Google Scholar]
- Corbett BA, Muscatello RA, Tanguturi Y, McGinn E, & Ioannou S (2019). Pubertal Development Measurement in Children With and Without Autism Spectrum Disorder: A Comparison Between Physical Exam, Parent- and Self-Report. J Autism Dev Disord doi: 10.1007/s10803-019-04192-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, & Simon D (2013). Adolescence, stress and cortisol in autism spectrum disorders. OA Autism, 1(1), 2. [PMC free article] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci, 13(9), 636–650. doi: 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Dahl RE (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci, 1021, 1–22. doi: 10.1196/annals.1308.001 [DOI] [PubMed] [Google Scholar]
- Dean M, Harwood R, & Kasari C (2017). The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism, 21(6), 678–689. doi: 10.1177/1362361316671845 [DOI] [PubMed] [Google Scholar]
- Deardorff J, Ekwaru JP, Kushi LH, Ellis BJ, Greenspan LC, Mirabedi A, . . . Hiatt RA (2011). Father absence, body mass index, and pubertal timing in girls: differential effects by family income and ethnicity. J Adolesc Health, 48(5), 441–447. doi: 10.1016/j.jadohealth.2010.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker LP, van der Vegt EJ, Visser K, Tick N, Boudesteijn F, Verhulst FC, . . . Greaves-Lord K (2015). Improving psychosexual knowledge in adolescents with autism spectrum disorder: pilot of the tackling teenage training program. J Autism Dev Disord, 45(6), 1532–1540. doi: 10.1007/s10803-014-2301-9 [DOI] [PubMed] [Google Scholar]
- DeRose LM, & Brooks-Gunn J (2008). Pubertal development in early adolescence: implications for affective processes. Adolescent Emotional Development and the Emergence of Depressive Disorders, 56–73. doi:Doi 10.1017/Cbo9780511551963.004 [DOI] [Google Scholar]
- Dorn LD (2006). Measuring puberty. J Adolesc Health, 39(5), 625–626. doi: 10.1016/j.jadohealth.2006.05.014 [DOI] [PubMed] [Google Scholar]
- Dorn LD, & Biro FM (2011). Puberty and Its Measurement: A Decade in Review. Journal of Research on Adolescence, 21(1), 180–195. doi: 10.1111/j.1532-7795.2010.00722.x [DOI] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, & Biro F (2006). Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10(1), 30–56. [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happe F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry, 51(8), 788–797. doi: 10.1016/j.jaac.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Brauner EV, & Juul A (2020). Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr, e195881. doi: 10.1001/jamapediatrics.2019.5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg L (1956). The Autistic-Child in Adolescence. American Journal of Psychiatry, 112(8), 607–612. doi:DOI 10.1176/ajp.112.8.607 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Garber J (2000). Psychosocial antecedents of variation in girls’ pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Dev, 71(2), 485–501. doi: 10.1111/1467-8624.00159 [DOI] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2010). Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cognition, 71(1), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, & Elder GH Jr. (2001). Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol, 37(3), 404–417. [DOI] [PubMed] [Google Scholar]
- Gillberg C, & Schaumann H (1981). Infantile autism and puberty. J Autism Dev Disord, 11(4), 365–371. [DOI] [PubMed] [Google Scholar]
- Gillberg C, & Steffenburg S (1987). Outcome and prognostic factors in infantile autism and similar conditions: a population-based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders, 2, 273–287. [DOI] [PubMed] [Google Scholar]
- Gotham K, Unruh K, & Lord C (2015). Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism, 19(4), 491–504. doi: 10.1177/1362361314536625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J, & Ashton-Smith J (2011). Missed diagnosis or misdiagnosis: Girls and women on the autism spectrum. Good autism practice, 12(1), 34–41. [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, & Brooks-Gunn J (1997). Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry, 36(12), 1768–1776. doi: 10.1097/00004583-199712000-00026 [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. doi: 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Hamilton A, Marshal MP, & Murray PJ (2011). Autism spectrum disorders and menstruation. J Adolesc Health, 49(4), 443–445. doi: 10.1016/j.jadohealth.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Harper JF, & Collins JK (1979). Physical growth and development in a sample of autistic girls from New South Wales. Aust Paediatr J, 15(2), 110–112. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, & Rainey WE (2004). The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med, 22(4), 337–347. doi: 10.1055/s-2004-861550 [DOI] [PubMed] [Google Scholar]
- Herguner A, & Herguner S (2016). Association Between Age at Menarche and Autistic Traits in Turkish University Students. American Journal of Human Biology, 28(1), 44–47. doi: 10.1002/ajhb.22739 [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, & Hasemeier CM (1997). Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics, 99(4), 505–512. doi: 10.1542/peds.99.4.505 [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, . . . Reiter EO (2012). Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics, 130(5), e1058–1068. doi: 10.1542/peds.2011-3291 [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, & Rimpela M (2003). Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med, 57(6), 1055–1064. [DOI] [PubMed] [Google Scholar]
- Karlberg J (2002). Secular trends in pubertal development. Horm Res, 57 Suppl 2, 19–30. doi: 10.1159/000058096 [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Wheelwright S, Hoekstra R, & Baron-Cohen S (2006). Age of menarche in females with autism spectrum conditions. Dev Med Child Neurol, 48(12), 1007–1008. doi: 10.1017/S0012162206222229 [DOI] [PubMed] [Google Scholar]
- Koerselman K, & Pekkarinen T (2018). Cognitive consequences of the timing of puberty. Labour Economics, 54, 1–13. doi: 10.1016/j.labeco.2018.05.001 [DOI] [Google Scholar]
- Kopp S, & Gillberg C (1992). Girls with social deficits and learning problems: Autism, atypical Asperger syndrome or a variant of these conditions. Eur Child Adolesc Psychiatry, 1(2), 89–99. doi: 10.1007/BF02091791 [DOI] [PubMed] [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, Ebeling H, . . . Moilanen I (2008). Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. J Autism Dev Disord, 38(9), 1697–1709. doi: 10.1007/s10803-008-0555-9 [DOI] [PubMed] [Google Scholar]
- Ladd GW (2006). Peer rejection, aggressive or withdrawn behavior, and psychological maladjustment from ages 5 to 12: an examination of four predictive models. Child Dev, 77(4), 822–846. doi:CDEV905 [pii]10.1111/j.1467–8624.2006.00905.x [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, . . . Giedd JN (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage, 36(4), 1065–1073. doi: 10.1016/j.neuroimage.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn N, Rudolph KD, & Roisman GI (2012). Other-Sex Relationship Stress and Sex Differences in the Contribution of Puberty to Depression. J Early Adolesc, 32(6), 824–850. doi: 10.1177/0272431611429945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry, 56(6), 466–474. doi: 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule (ADOS-2) (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Lucky AW, Biro FM, Simbartl LA, Morrison JA, & Sorg NW (1997). Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr, 130(1), 30–39. doi: 10.1016/s0022-3476(97)70307-x [DOI] [PubMed] [Google Scholar]
- Mackinnon JG, & White H (1985). Some Heteroskedasticity-Consistent Covariance-Matrix Estimators with Improved Finite-Sample Properties. Journal of Econometrics, 29(3), 305–325. doi:Doi 10.1016/0304-4076(85)90158-7 [DOI] [Google Scholar]
- Marshal WA, & Tanner JM (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1969). Variations in pattern of puberal development in girls. Archives of Disease in Childhood, 44(235), 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, Pang KC, O’Connell MA, & Williams K (2017). Typical Pubertal Timing in an Australian Population of Girls and Boys with Autism Spectrum Disorder. J Autism Dev Disord, 47(12), 3983–3993. doi: 10.1007/s10803-017-3281-3 [DOI] [PubMed] [Google Scholar]
- Mendle J, Beltz AM, Carter R, & Dorn LD (2019). Understanding Puberty and Its Measurement: Ideas for Research in a New Generation. J Res Adolesc, 29(1), 82–95. doi: 10.1111/jora.12371 [DOI] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, & Graber JA (2010). Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev Psychol, 46(5), 1341–1353. doi: 10.1037/a0020205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, & Graber JA (2012). Peer relationships and depressive symptomatology in boys at puberty. Dev Psychol, 48(2), 429–435. doi: 10.1037/a0026425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, & Emery RE (2007). Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Dev Rev, 27(2), 151–171. doi: 10.1016/j.dr.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen SE (1989). Pervasive developmental disorder and idiopathic precocious puberty in a 5-year-old girl. J Autism Dev Disord, 19(2), 351–353. [DOI] [PubMed] [Google Scholar]
- Nadeem E, & Graham S (2005). Early puberty, peer victimization, and internalizing symptoms in ethnic minority adolescents. Journal of Early Adolescence, 25(2), 197–222. doi: 10.1177/0272431604274177 [DOI] [Google Scholar]
- Obaydi H, & Puri BK (2008). Prevalence of premenstrual syndrome in autism: a prospective observer-rated study. J Int Med Res, 36(2), 268–272. doi: 10.1177/147323000803600208 [DOI] [PubMed] [Google Scholar]
- Obeidallah DA, Brennan RT, Brooks-Gunn J, Kindlon D, & Earls F (2000). Socioeconomic status, race, and girls’ pubertal maturation: results from the Project on Human Development in Chicago neighborhoods. Journal of Research on Adolescence, 10, 443–464. [Google Scholar]
- Petersen JL, & Hyde JS (2009). A longitudinal investigation of peer sexual harassment victimization in adolescence. J Adolesc, 32(5), 1173–1188. doi: 10.1016/j.adolescence.2009.01.011 [DOI] [PubMed] [Google Scholar]
- Picci G, & Scherf KS (2015). A Two-Hit Model of Autism: Adolescence as the Second Hit. Clin Psychol Sci, 3(3), 349–371. doi: 10.1177/2167702614540646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl A, Cassidy S, Auyeung B, & Baron-Cohen S (2014). Uncovering steroidopathy in women with autism: a latent class analysis. Molecular Autism, 5. doi:Artn 27 10.1186/2040–2392-5–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto AB, Kenworthy L, Yerys BE, Bascom J, Wieckowski AT, White SW, . . . Anthony LG (2018). What About the Girls? Sex-Based Differences in Autistic Traits and Adaptive Skills. J Autism Dev Disord, 48(5), 1698–1711. doi: 10.1007/s10803-017-3413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich JN, Richmond RJ, & Rogol AD (2001). Consequences of sport training during puberty. J Endocrinol Invest, 24(9), 708–715. [DOI] [PubMed] [Google Scholar]
- Rutter M (1970). Autistic Children - Infancy to Adulthood. Seminars in Psychiatry, 2(4), 435-&. [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The Social Communication Questionnaire Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, & Baron-Cohen S (2016). An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol Autism, 7, 10. doi: 10.1186/s13229-016-0073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, & Dahl RE (2012). Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev Cogn Neurosci, 2(2), 199–219. doi: 10.1016/j.dcn.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, & Greenberg JS (2004). Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev, 10(4), 234–247. doi: 10.1002/mrdd.20038 [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, & Dahl RE (2009). Pubertal changes in emotional information processing: pupillary, behavioral, and subjective evidence during emotional word identification. Dev Psychopathol, 21(1), 7–26. doi: 10.1017/S0954579409000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, & Foster DL (2004). The neural basis of puberty and adolescence. Nat Neurosci, 7(10), 1040–1047. doi: 10.1038/nn1326 nn1326 [pii] [DOI] [PubMed] [Google Scholar]
- Sisk CL, & Zehr JL (2005). Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol, 26(3–4), 163–174. doi: 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev, 24(4), 417–463. doi:S0149-7634(00)00014-2 [pii] [DOI] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9(2), 69–74. doi: 10.1016/j.tics.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Steward R, Crane L, Roy EM, Remington A, & Pellicano E (2018). “Life is Much More Difficult to Manage During Periods”: Autistic Experiences of Menstruation. Journal of Autism and Developmental Disorders, 48(12), 4287–4292. doi: 10.1007/s10803-018-3664-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantam D (2003). The challenge of adolescents and adults with Asperger syndrome. Child Adolesc Psychiatr Clin N Am, 12(1), 143–163, vii-viii. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Adams RE, & Bishop SL (2017). Social participation and its relation to internalizing symptoms among youth with autism spectrum disorder as they transition from high school. Autism Research, 10(4), 663–672. doi: 10.1002/aur.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, & Seltzer MM (2010). Changes in the autism behavioral phenotype during the transition to adulthood. J Autism Dev Disord, 40(12), 1431–1446. doi: 10.1007/s10803-010-1005-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney S, Burns J, & Kilbey E (2016). Looking behind the mask: Social coping strategies of girls on the autistic spectrum. Research in Autism Spectrum Disorders, 23, 73–83. doi: 10.1016/j.rasd.2015.11.013 [DOI] [Google Scholar]
- Tordjman S, Ferrari P, Sulmont V, Duyme M, & Roubertoux P (1997). Androgenic activity in autism. Am J Psychiatry, 154(11), 1626–1627. [DOI] [PubMed] [Google Scholar]
- Visser K, Greaves-Lord K, Tick NT, Verhulst FC, Maras A, & van der Vegt EJM (2017). A randomized controlled trial to examine the effects of the Tackling Teenage psychosexual training program for adolescents with autism spectrum disorder. J Child Psychol Psychiatry, 58(7), 840–850. doi: 10.1111/jcpp.12709 [DOI] [PubMed] [Google Scholar]
- Waylen A, & Wolke D (2004). Sex ‘n’ drugs ‘n’ rock ‘n’ roll: the meaning and social consequences of pubertal timing. Eur J Endocrinol, 151 Suppl 3, U151–159. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence II (Vol. Second Edition). San Antonio, TX: PsychCorp. [Google Scholar]
- Weisbrot DM, Gadow KD, DeVincent CJ, & Pomeroy J (2005). The presentation of anxiety in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol, 15(3), 477–496. doi: 10.1089/cap.2005.15.477 [DOI] [PubMed] [Google Scholar]
- White SW, & Roberson-Nay R (2009). Anxiety, social deficits, and loneliness in youth with autism spectrum disorders. J Autism Dev Disord, 39(7), 1006–1013. doi: 10.1007/s10803-009-0713-8 [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Maybery MT, Hickey M, & Sloboda DM (2011). Brief report: autistic-like traits in childhood predict later age at menarche in girls. J Autism Dev Disord, 41(8), 1125–1130. doi: 10.1007/s10803-010-1129-1 [DOI] [PubMed] [Google Scholar]
- Winter S, Durand A, & Brauner R (2019). Precocious and Early Central Puberty in Children With Pre-existing Medical Conditions: A Single Center Study. Front Pediatr, 7. doi:ARTN 35 10.3389/fped.2019.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshirmura K, Naiki Y, Horikawa R, & Tanaka T (2005). Three patients with autism and central precocious puberty. Clinical Pediatrics Endocrinology, 14(Suppl 24), 55–57. [Google Scholar]
- Zhang L, Zhang D, & Sun Y (2019). Adverse Childhood Experiences and Early Pubertal Timing Among Girls: A Meta-Analysis. Int J Environ Res Public Health, 16(16). doi: 10.3390/ijerph16162887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


