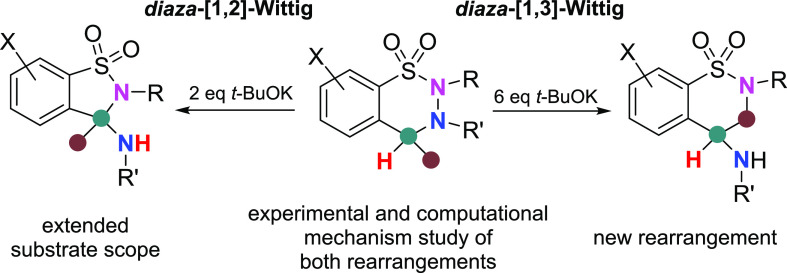
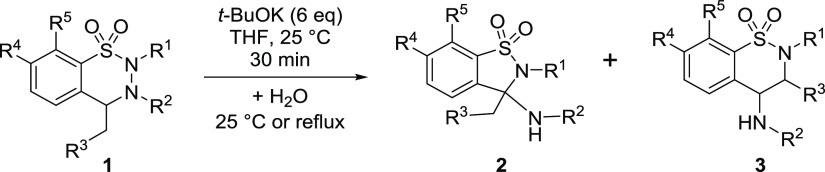
Abstract
The base-induced (t-BuOK) rearrangement reactions of 3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxides result in a ring opening along the N–N bond, followed by ring closure with the formation of new C–N bonds. The position of the newly formed C–N bond can selectively be tuned by the amount of the base, providing access to new, pharmacologically interesting ring systems with high yield. While with 2 equiv of t-BuOK 1,2-benzisothiazoles can be obtained in a diaza-[1,2]-Wittig reaction, with 6 equiv of the base 1,2-benzothiazine 1,1-dioxides can be prepared in most cases as the main product, in a diaza-[1,3]-Wittig reaction. DFT calculations and detailed NMR studies clarified the mechanism, with a mono- or dianionic key intermediate, depending on the amount of the reactant base. Also, the role of an enamide intermediate formed during the workup of the highly basic (6 equiv of base) reaction was clarified. The substrate scope of the reaction was also explored in detail.
Introduction
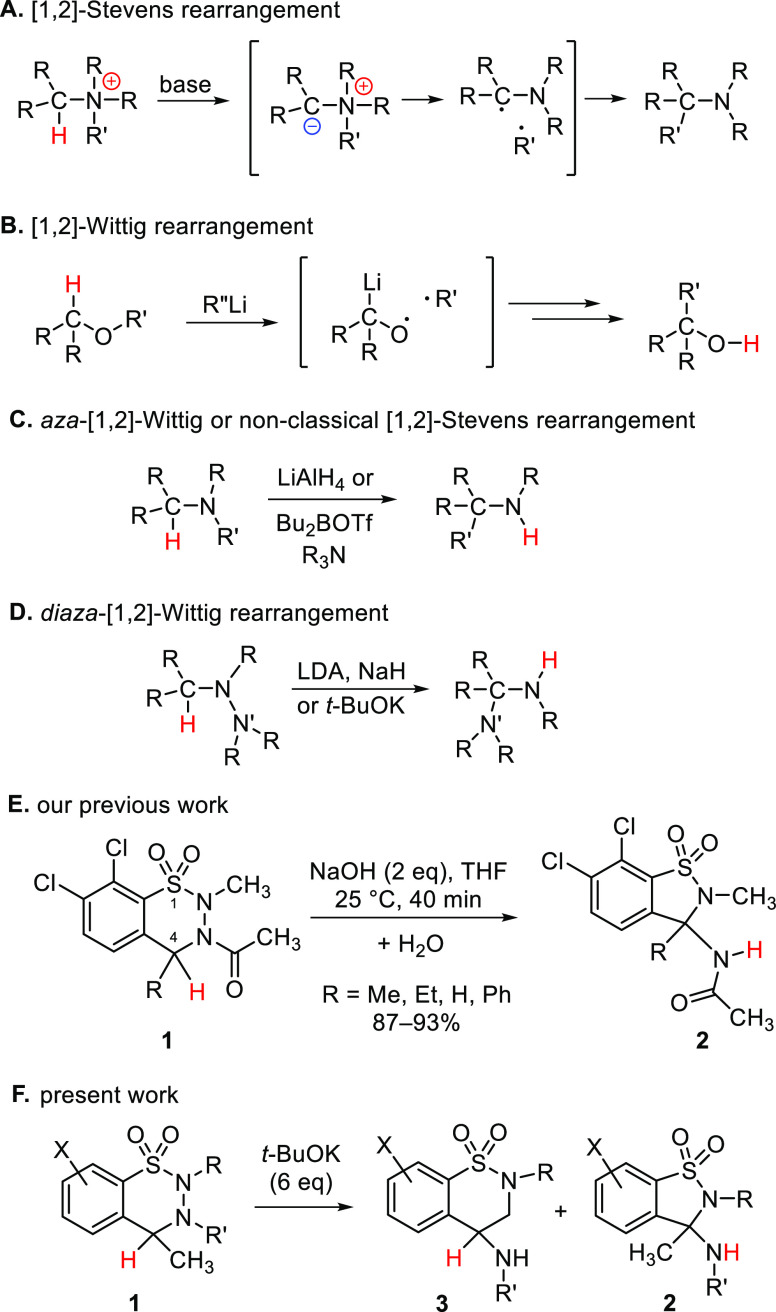
Carbon, nitrogen, and oxygen atoms are the main constituents of organic frameworks, thus the selective formation of their bonds belongs to the most important organic transformations. [1,2]-Stevens reaction (Scheme 1A) is a well investigated example,1 where the ylide (formed after a base-induced deprotonation of one of the α-carbon atoms of a quaternary ammonium salt) takes part in a rearrangement via a [1,2]-shift of a nitrogen substituent. During this reaction step, a tight radical pair is usually formed after the cleavage of the C–N bond, but with certain substituents a concerted mechanism is proposed.2,3 [1,2]-Wittig rearrangements (Scheme 1B)4 with oxygen as the heteroatom and aza-[1,2]-Wittig rearrangements (Scheme 1C) are related transformations. This latter reaction can be considered as a nonclassical Stevens rearrangement, since here the reactant is a neutral species. While Wittig reactions including their variants5 are well investigated, likewise the Stevens reaction, the aza-[1,2]-Wittig transformation is rare6−8 and is often a side reaction, competing with aza-[2,3]-Wittig rearrangement.9,10 In the case of tetrasubstituted hydrazines, the N–N bond is breaking and the terminal amino unit is shifting, resulting in a diaza-[1,2]-Wittig rearrangement (Scheme 1D).11−16 Exposure of these derivatives to bases can also result in imines and amines as possible intermediates,16,17 indicating that with the breaking of the N–N bond during the reaction, the formation of new N–H bonds becomes also feasible. Diaza-1,4-Wittig rearrangement is also precedented;18 nonetheless, the diaza-[1,3]-Wittig reaction is a missing link.
Scheme 1. Stevens, Wittig, and Related Rearrangements; Our Previous Work, All Involving α-Deprotonation and [1,2]-Migration Steps; and the Present Work.
Recently, we have reported a facile and (at the 4-position) substituent tolerant diaza-[1,2]-Wittig rearrangement of 3-acetyl-7,8-dichloro-2-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxides (1, Scheme 1E) to the corresponding 3-acetamido substituted 2,3-dihydro-1,2-benzisothiazole 1,1-dioxides (2), representatives of a compound family of biological relevance,19−25 in the presence of a suspension of 2 equiv of NaOH (or t-BuOK) in THF.26 While in our previous work we tentatively proposed an ionic pathway for this diaza-[1,2]-Wittig reaction yielding the benzisothiazole dioxides (2, Scheme 1E),26 the mechanism was not investigated in detail. During these investigations we now surprisingly found that, in the presence of a larger amount of base, the reaction gave also rise to the formation of an unexpected benzothiazine derivative (3, Scheme 1F). These new results are reported below.
Results and Discussion
When seeking mechanistic information on the reaction starting from compound 1a, we realized that depending on the basicity of the system (i.e., on the amount of t-BuOK applied), the reaction could result either in the previously described benzisothiazole 2a(26) or in an unexpected benzothiazine derivative 3a (Scheme 2), the latter being again a molecular framework in the focus of recent pharmacological interest.27−35 Hereby we explore the mechanism of this highly complex reaction and demonstrate that it is possible to selectively influence the outcome of the reaction toward any of the two different products. The corresponding experimental and computational studies are described below, with further details disclosed in the Supporting Information (SI).
Scheme 2. Discovery of the Basicity-Dependent Rearrangement Reactions.
Computational Examination of the Deprotonation of Benzothiadiazine Dioxide 1a, As the Initial Step of the Rearrangement
Having observed the diaza-[1,2]-Wittig reaction leading to 1,2-benzisothiazole 2a(26) and considering the lack of mechanistic studies on this transformation, first we aimed to investigate the mechanism of the ring contraction of 4-methyl derivative 1a. Since the presumable first elementary step of the rearrangement is the deprotonation of 1a, we studied the effect of various bases (2 equiv) on the reaction shown in Scheme 2 (left). If the basicity was sufficient for deprotonation (t-BuOK, NaOH, NaOMe, and DBU), 2a could be isolated in excellent yields (Table S1 in the SI), otherwise (DIPEA, NaOAc) 1a was recovered. The possible positions for gas-phase proton abstraction were considered computationally (Scheme 3). As expected, the far most favored deprotonation site is the C(4) atom yielding anion I–, which is the likely starting point of the [1,2]-rearrangement. (For gas-phase acidity of some other systems at the same level of theory, see Table S2 in the SI). For further investigations, we decided to use t-BuOK as the deprotonating agent, since 2 equiv of this base gave 1,2-benzisothiazole dioxide 2a selectively and with excellent isolated yield (Scheme 2).26
Scheme 3. Evaluation of the Gas-Phase Acidity of 1a at Different Positions and the Anion (I–) Obtained after Deprotonation.
Deprotonation Gibbs free energies are given in kcal/mol.
NMR Study of the Effect of the Amount of t-BuOK on the Reaction Mechanism
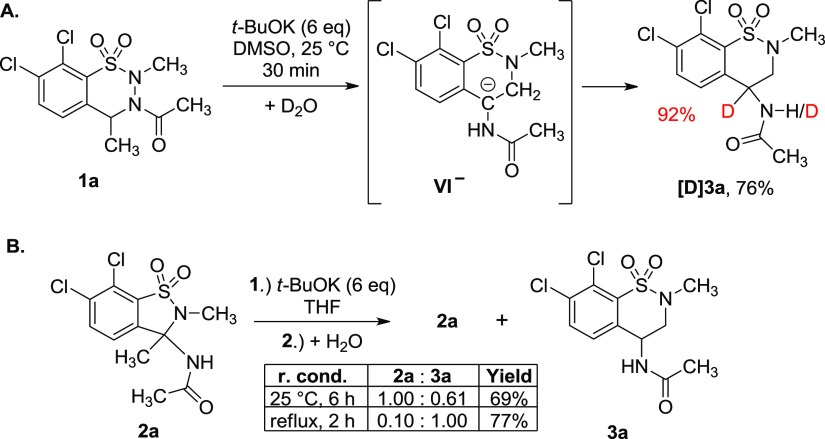
Aiming to obtain information on the reaction mechanism, the 1H NMR spectrum of the reaction mixture of 1a and 2 equiv of t-BuOK was investigated in [D6]DMSO, immediately after mixing the reactants (Figure 1b). The detected signals corresponded to two compounds present in comparable amounts (ca. 1.1:1.0 molar ratio). When decreasing the amount of t-BuOK to 1 equiv, the signals marked by red (Figure 1a) become dominant. On the basis of the appearance of the characteristic 2.58/77.9 three-bond HMBC cross-peak (see the SI, page S26) caused by the vicinal 3J(NCH3/C-4) coupling, the compound can unequivocally be assigned to structural formula II–. Thus, the presence of III–, which would be indistinguishable by simple 1H NMR, can be excluded. The presence of intermediate II– featuring the 1,2-benzisothiazole ring is also in accordance with the formation of 2a upon protonation (i.e., quenching by water) and subsequent crystallization. Furthermore, it has to be noted that the formation of acylimine 4a (the apparent protonation product of III–) was neither here, nor in our further experiments (see below) observed.
Figure 1.
1H NMR analysis of reaction mixtures measured immediately after mixing 1a (20 mg, 6.2 mmol) and 1 equiv (a, red), 2 equiv (b, green), or 6 equiv (c, blue) of t-BuOK (6.2, 12.4, or 37.1 mmol, respectively) in [D6]DMSO (800 μL) at 22 °C; and the corresponding neutral products obtained after quenching with water (d).
In the presence of 6 equiv t-BuOK, however, the peaks marked by blue (Figure 1/c) including the characteristic signals of a methylidene H2C= group (δ 4.56/3.94 ppm) become dominant. This dependence on the concentration of the base indicates that a dianionic compound, which was assigned as enamide dianion IV2–, was formed in the reaction mixture after removal of a second proton by the large excess of base. (For a detailed discussion of the 1H NMR and LC-MS spectra of the reaction mixture containing IV2–, see page S3 in the SI.) After quenching this reaction mixture with water and immediate extraction with DCM, not only product 3a (47%) and the minor component 2a (8%) could be identified by their 1H NMR spectra, but also enamide (5a, Figure 1/d, 45%) could be detected (Figure S3 in the SI). However, after our usual workup (evaporation of THF from the quenched mixture in vacuo) no 5a was present in the precipitated product, which was obtained in 84% overall isolated yield, consisting mainly (92%) 1,2-benzothiazine 1,1-dioxide 3a, with 2a as the minor product (8%). The structure of compound 3a was confirmed by detailed NMR studies and by single-crystal X-ray measurement (as a monohydrate). Structural details of compounds 1a(26) and 3a·H2O are given in the SI (Figures S11 and S12 and Tables S4 and S5).
Effect of the Reaction Conditions on the Selectivity of the Rearrangements
It became obvious that the formation of the rearranged products 2a and/or 3a largely depended on the amount and strength of the base applied, providing a simple and convenient way to selectively tune the outcome of the reaction. As the next step, the conversion of compound 1a has been investigated under various basic conditions (Table 1). The use of up to 2 equiv of t-BuOK in THF gave 2a selectively in high yields (Table 1, entries 1 and 2). Upon increasing the amount of t-BuOK gradually to 8 equiv (entries 3–6), mixtures of 2a and 3a were isolated, and the selectivity could be shifted to 3a (best with 6 equiv, entry 5). Change of the counterion to Na+ (entry 7) gave similar results. Use of 6 equiv of other bases in THF led to the formation of 2a in high yields (entries 8–10). When applying 6 equiv of t-BuOK in other ether-type solvents or DMF, 3a was obtained again as the major product (entries 11–14). Most importantly, from DMSO pure 3a could be crystallized in 80% yield (entry 15), thereby making this reaction variant a practical synthesis of benzothiazine dioxide 3a. On the contrary, using the protic t-BuOH, where the formation of the dianion intermediate is unlikely, 2a (entry 16) was the sole product obtained.
Table 1. Effect of the Reaction Conditions on the Formation of Rearranged Products 2a and 3a.
| entrya | base | amount of base (eq) | solvent | 2a:3a ratiob | yield (%) |
|---|---|---|---|---|---|
| 1 | t-BuOK | 1 | THF | 1:0 | 83 (2a) |
| 2 | t-BuOK | 2 | THF | 1:0 | 90 (2a) |
| 3 | t-BuOK | 3 | THF | 1.00:0.88 | 87 (2a+3a) |
| 4 | t-BuOK | 4 | THF | 0.31:1.00 | 80 (2a+3a) |
| 5 | t-BuOK | 6 | THF | 0.09:1.00 | 84 (2a+3a) |
| 6 | t-BuOK | 8 | THF | 0.13:1.00 | 88 (2a+3a) |
| 7 | t-BuONa | 6 | THF | 0.09:1.00 | 85 (2a+3a) |
| 8 | KOH | 6 | THF | 1:0 | 89 (2a) |
| 9 | NaOMe | 6 | THF | 1:0 | 76 (2a) |
| 10 | NaNH2 | 6 | THF | 1:0 | 74 (2a) |
| 11 | t-BuOK | 6 | 2-Me-THF | 0.14:1.00 | 88 (2a+3a) |
| 12 | t-BuOK | 6 | 1,4-dioxane | 0.10:1.00 | 87 (2a+3a) |
| 13 | t-BuOK | 6 | DME | 0.14:1.00 | 79 (2a+3a) |
| 14 | t-BuOK | 6 | DMFc | 0.12:1.00 | 93 (2a+3a) |
| 15 | t-BuOK | 6 | DMSOd | 0:1 | 84 (3a) |
| 16 | t-BuOK | 6 | t-BuOH | 1:0 | 88 (2a) |
Reagents and reaction conditions: 1a (0.6 mmol), base, solvent (3 mL), 25 °C, 30 min, then quenching with H2O, evaporation and crystallization.
The product ratio of the isolated mixtures was determined by 1H NMR.
H2O was added after evaporation.
Without evaporation.
DFT Calculations on the Ring Transformations
In order to provide a reasonable mechanism for the rearrangement of 1a to 2a and 3a, density functional theory (DFT) calculations have been carried out (Scheme 4, for the energy profile diagram see Figure S5 in the SI). Based on the investigation of the Kohn-Sahm molecular orbitals of I–, the concerted mechanism furnishing 1,2-benzisothiazole anion II– in one step can be excluded. Though the HOMO is localized at C(4) (see Figure S4 in the SI), N(2) has no significant contribution to the LUMO. In accordance with this, we were not able to find any transition structure for a concerted pathway of the [1,2]-shift yielding II– in one step. On the contrary, we could locate the transition state TS1 corresponding to the N–N bond cleavage with a barrier of 18.8 kcal/mol leading to the formation of acylimine anion III–, which is significantly (by 15.3 kcal/mol) more stable than I–. The closed shell wave function turned out to be stable for both III– and the corresponding transition structure. This is remarkable since the analogous step was claimed to have a biradical character in some cases of related Stevens rearrangements involving ylides,2 but it is in agreement with the observed lack of any electron spin resonance (ESR) signal or chemically induced dynamic nuclear polarization (CIDNP) signal intensity enhancement in the NMR during our experiments. In III– the HOMO is at the N(2) atom, and the LUMO has a significant contribution at the C=N double bond (see Figure S4 in the SI), indicating a subsequent facile ring closure to form II–. Indeed, this step (TS2) has only a 3.6 kcal/mol activation barrier facilitating the rapid conversion (note that no NMR signal was observed for III–) to the thermodynamically more (by 13.8 kcal/mol) stable II–. It should be mentioned that the alternative pathway, i.e., the direct tautomerization of acylimine anion III– to enamide anion V–, has a high reaction barrier furthermore V– is by 12.6 kcal/mol less stable than II–. Thus, even if the formation of V– from III– might be possible by a base-catalyzed reversible deprotonation/protonation procedure via IV2–, the thermodynamic sink at the monoanionic level is II–, in accordance with the NMR results (see above).
Scheme 4. DFT Study of the Reaction Mechanism at M06-2X/6-31+G* (smd: THF) Level of Theory.

Reaction Gibbs free energy values are presented in kcal/mol.
Altogether, the selective formation of 2a after reprotonation of II– (during the workup with water) from the reaction of 1a with 1–2 equiv of t-BuOK via the monoanionic pathway is fully justified on thermodynamic as well as on kinetic grounds.
When applying an excess of base, the formation of dianion IV2– can easily be achieved by two alternative ways. First is by deprotonation of III– with an excess of t-BuOK at the CH3 group via a barrier of 4.1 kcal/mol, calculated with respect to an incoming t-BuO– and III– as shown in Scheme 4. This barrier is comparable to that leading to the formation of II– from III– (3.6 kcal/mol). Alternatively, we can consider the deprotonation of the experimentally detected II– at the C(3)-methyl group. Since any attempted optimization of the dianion derived by deprotonation of II– resulted in a barrierless ring opening and finally in the formation of IV2–, and this pathway is also viable apart from the direct deprotonation of III– discussed above.
Upon quenching the reaction mixture with excess of H2O, the protonation of IV2– yields V– thermodynamically somewhat more favorably than III–. Nevertheless, the energy difference between III– and V– is small (1.2 kcal/mol), in agreement with the presence of a small amount of 2a (that can be derived from III– as discussed above) in the isolated product. From V–, enamide 5a can easily be obtained by further protonation, and this product was indeed observed after an immediate extraction of the reaction mixture (carried out with 6 equiv t-BuOK) with DCM, as discussed above. The neutral form of acylimine (4a, Scheme 4) is less stable than 5a, and indeed, it remained experimentally unobserved. Since after quenching the solution is still highly basic, enamide 5a can rearranged in a base-catalyzed reaction sequence, where the first step is a deprotonation. Thus, we should consider the reactivity of anion V–, which can cyclize in a thermodynamically downhill process via a reasonable 23.4 kcal/mol barrier to carbanion VI–,37 which might lead, after a proton exchange, to 1,2-benzothiazine anion VII–. Either VI– or VII– can be transformed to 3a by protonation. Product 3a could in principle be obtained directly from dianion VIII2– as well, but the direct ring closure of IV2– to dianion VIII2– is unlikely, since this transformation has a high (32.9 kcal/mol) activation barrier (TS5). Indeed, no other major 1H NMR signals than those of IV2– could be seen in the presence of 6 equiv of base (Figure 1).
Experimental Studies on the Formation of Benzothiazine Dioxide Derivative 3a
When the reaction of 1a was carried out with 6 equiv of t-BuOK in DMSO, but quenched with D2O instead of water, [D]3a was obtained in 76% yield with a 92% deuteration ratio at position 4 (Scheme 5A and Figure S6 in the SI). This finding supports the suggested mechanism involving the formation of intermediate VI–. Clearly, in the CH2 unit of the thiazine ring no deuterium exchange was observed, showing that this methylene unit remains intact during the series of transformations, in accordance with our proposed mechanism starting from IV2–.
Scheme 5. Experimental Studies on the Formation of the Benzothiazine Ring: (A) Partial Deuteration Observed after Quenching the Reaction Mixture with D2O and (B) Ring Expansion of Benzisothiazole 2a to Benzothiazine 3a.
The question arose whether 1,2-benzisothiazole 2a could be rearranged to 1,2-benzothiazine 3a in a sufficiently basic medium. Treatment of 1,2-benzisothiazole 2a with 6 equiv of t-BuOK at 25 °C for 6 h and subsequent quenching with water and crystallization afforded a mixture of 1,2-benzisothiazole 2a and 1,2-benzothiazine 3a (in 1.00:0.61 ratio), proving the occurrence of the 2a → 3a rearrangement (Scheme 5B). Furthermore, 2 h reflux and subsequent workup provided the “pseudoequilibrial” product ratio (0.10:1.00, Scheme 5B), in accordance with that obtained from the reaction of t-BuOK (6 equiv) and 1a (Table 1, entry 5). It is noteworthy that this product ratio corresponds to the Boltzmann population of III– and V– as derived from their calculated 1.2 kcal/mol energy difference (Scheme 4).
While the reaction of 1a with 6 equiv t-BuOK with the standard aqueous workup procedure led to 3a as the main product (Table 1, entry 5), it is noteworthy that, with acidic workup, 1,2-benzisothiazole 2a was obtained in excellent (92%) yield (see pages S13–14 in the SI). Thus, in the selective tuning of the outcome of the reaction, not only the amount of the base, but also the conditions of the workup are of high importance. Clearly, the thermodynamic sink is the formation of 1,2-benzisothiazole 2a (see Scheme 4).
Extension of the Substrate Scope of the Rearrangement of Benzothiadiazine 1,1-Dioxides (1) Leading to Benzisothiazole 1,1-Dioxides (2) Using 2 equiv of t-BuOK
With the useful information in hand on how to control the outcome of the rearrangements in the case of 3-acetyl-7,8-dichloro-2,4-dimethyl substituted derivative, we turned our attention to the experimental evaluation of the substituent effects. Therefore, variously substituted benzothiadiazine 1,1-dioxides 1 were synthesized as starting materials (for synthetic methods, see the SI) using the procedures described earlier.26,38 The ring contraction of derivatives 1 to 1,2-benzisothiazoles 2 was conducted in the presence of 2 equiv of t-BuOK in THF (Scheme 6), and in our standard procedure, the reaction mixture was quenched with water. Under these conditions, compounds 1a–j (i.e., substrates bearing various 2-alkyl substituents, various acyl or alkyl groups at position 3, and chlorine atoms both at positions 7 and 8) were transformed smoothly and effectively to 2a–j (in case of 1g, the hydrolysis of the trifluoroacetylamino group lowered the yield). The synthesis of 2a was successfully scaled up to 1.2 mmol of starting material 1a with an unchanged yield (90%). Modifications in the aromatic substitution pattern (1k–o), however, have significantly reduced the formation of the precipitated 1,2-benzisothiazole (2k–o) in our usual workup procedure, and the corresponding enamides (5k–o) became the main products. For example, when the reaction mixture of 1o was quenched with water and extracted with DCM, the isolated mixture contained the corresponding enamide (5o) predominantly, according to 1H NMR (Figures S7 and S8 in the SI), while benzisothiazole dioxide 2o was only a minor product (12%). In case of 7-chloro-8-unsubstituted analogue 1k, cyclization to 2k did not take place under these conditions, it could only be forced at elevated temperature. For derivatives 1l–o, cyclization with good to excellent yield could be fostered also at room temperature, by quenching the reaction mixture with aqueous (1 w/w%) hydrochloric acid instead of water. Benzothiazines 3 were not present in these experiments.
Scheme 6. Study on the Substituent Effect on the Preparation of 1,2-Benzisothiazoles 2, Carried out with 2 equiv of t-BuOK.
In the presence of the acid catalyst, the transformation of enamides 5l–o to 2l–o is easily understandable, considering that after protonation at the most basic site, i.e., at the methylidene group of 5, carbocation I+ forms, which can be attacked by the nitrogen lone pair of the sulfonamide moiety to give II+, losing finally a proton from this nitrogen atom to result in neutral products 2l–o (Scheme 7). Our DFT calculations on enamide 5o fully supported the above mechanism, as shown in Figure S9 in the SI. The calculated proton affinity of the neutral 5o is as high as 270.2 kcal/mol, and the resulting cation Io+ undergoes a ring closure via a tiny (2.3 kcal/mol) barrier (for more details see Figure S9 in the SI) to the thermodynamically more stable (by 8.9 kcal/mol) IIo+. In case of 5a, similar proton affinity (261.8 kcal/mol) and reaction energy (−12.9 kcal/mol) were obtained, and scan calculations indicated a barrierless ring closure (Figure S10 in the SI). Altogether the high proton affinity and the small barrier indicate that the proton-catalyzed ring closure is a robust and generally applicable route for the formation of 2 for a wide range of substituents, in accordance with the above experimental observations.
Scheme 7. Proposed Mechanism for the Formation of 1,2-Benzisothiazoles 2l–o with Acidic Work-Up.
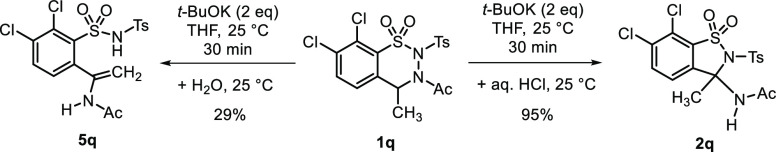
When starting from compound 1p bearing a 2-mesyl group, a hydrolysis occurred under the usual reaction conditions, and no product (2p) was isolated (Scheme 6). In the case of 2-tosyl derivative 1q, our standard procedure resulted in the precipitation of enamide 5q (Scheme 8), isolated in 29% yield. If the same reaction mixture was treated with aqueous (1 w/w%) hydrochloric acid, again the thermodynamic sink, i.e., benzisothiazole 2q, was obtained in excellent yield via the acid-catalyzed mechanism.
Scheme 8. Dependence of the Product on the Workup Conditions in the Reaction of 3-Acetyl-2-tosyl Derivative 1q.
Elaboration of the Targeted Synthesis of Benzothiazine 1,1-Dioxides (3) Using 6 equiv of t-BuOK
When studying the effect of the substituents on the pathway leading to benzothiazine 3a, the effects stabilizing the key intermediates (dianion IV2– and anion V–) are of importance, clearly benefiting from the delocalization along the C=C—N—C=O unit. Accordingly, when reacting 6 equiv of t-BuOK with compounds 1a–f (all having an acyl moiety at position 3), a mixture of products 2 and 3 could be isolated in high overall yields (Table 2). A good selectivity was observed toward benzothiazine dioxides 3a–f (entries 1–5), the only exception being 4-ethyl derivative 1b, where benzothiazine 3b was obtained as a mixture of diastereomers, in a ratio of 1.00:0.16 (entry 2), in accord with the expected effect of the 3-acyl group. In contrast, 2,3-dimethyl compound 1i underwent even with 6 equiv t-BuOK a ring contraction to give 2i in a virtually identical yield (85%, entry 6) as with 2 equiv of t-BuOK (see Scheme 6). The reactions of 3-acetyl-2-methyl derivatives bearing various substitution patterns (other than 7,8-dichloro) on the aromatic ring, likewise with 2 equiv of base, stopped at the enamide level. These reactions could be forced at reflux temperature to cyclization, however, in these cases again 2 was formed instead of 3 (entries 7–9).
Table 2. Study on the Substituent Effect in the Reaction of Compounds 1 Carried out with 6 equiv of t-BuOK.
| entrya | 1–3 | R1 | R2 | R3 | R4 | R5 | 2:3 ratiob | yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | a | Me | C(O)Me | H | Cl | Cl | 0.09:1.00 | 84c (2a+3a) |
| 2 | b | Me | C(O)Me | Me | Cl | Cl | 1.00:0.88d | 54c (2b+3b) |
| 3 | c | Et | C(O)Me | H | Cl | Cl | 0.13:1.00 | 90c (2c+3c) |
| 4 | d | Bn | C(O)Me | H | Cl | Cl | 0.25:1.00 | 62c (2d+3d) |
| 5 | f | Me | C(O)Pr | H | Cl | Cl | 0.18:1.00 | 86c (2f+3f) |
| 6 | i | Me | Me | H | Cl | Cl | 1:0 | 85c (2i) |
| 7 | l | Me | C(O)Me | H | OMe | Cl | 1:0 | 74e (2l) |
| 8 | n | Me | C(O)Me | H | H | OMe | 1:0 | 35e (2n) |
| 9 | o | Me | C(O)Me | H | H | H | 1:0 | 59e (2o) |
Reagents and reaction conditions: substrate (0.6 mmol), t-BuOK (3.6 mmol), THF (3 mL), 30 min, then quenching with H2O.
The product ratio of the isolated mixtures was determined by 1H NMR.
The reaction was carried out at 25 °C.
dr = 1.00:0.16.
The reaction was carried out at reflux temperature.
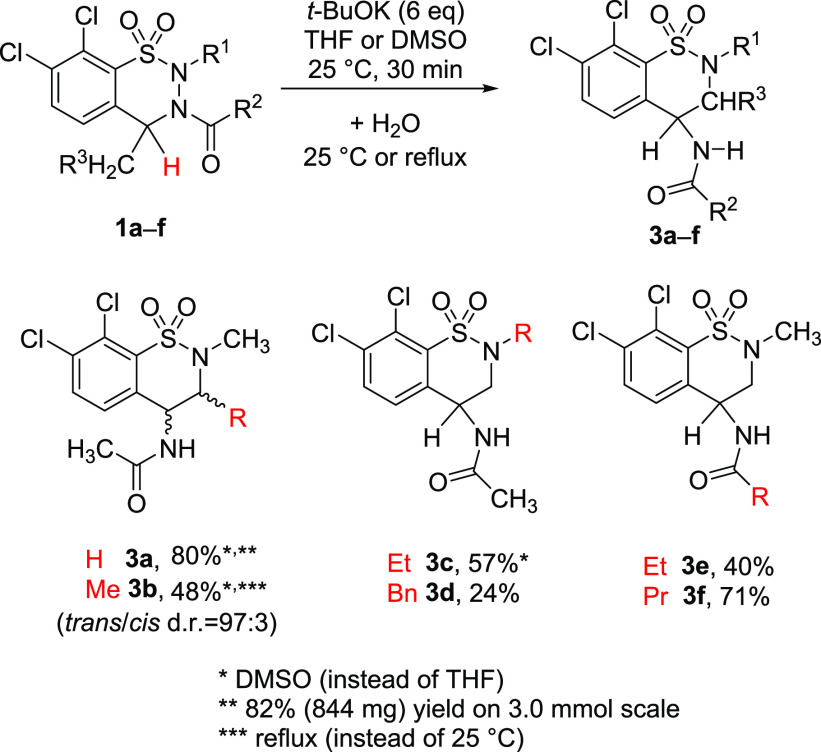
The formation of compounds 3 is particularly noteworthy since [1,3]-rearrangements involving a N–N bond cleavage and inclusion of a C2 unit are very rare in the literature and occur with a different mechanism.39−41 The targeted synthesis of 1,2-benzothiazine 1,1-dioxides 3a–f was finally conducted with 6 equiv of t-BuOK (Scheme 9), by crystallization either in DMSO (3a–c) or in THF (3d–f), yielding the products in a pure form even without chromatographic purification. Contrary to the other reactions taking place at room temperature, the quenched reaction mixture of 4-ethyl derivative 1b had to be heated to reflux to cyclize to 3b (trans–cis diastereomeric ratio = 97:3).
Scheme 9. Targeted Synthesis of Variously Substituted 1,2-Benzothiazine 1,1-Dioxides 3.
Computations
DFT calculations were carried out with the Gaussian 09 software package.42 The level of theory was validated (Table S3), and the M06-2X43 functional was used in conjunction with the 6-31+G* basis set for conformational analysis on all reactants, transition states, and intermediates to identify the most plausible conformers (unless otherwise stated). For frequency calculations, ultrafine grid was used, and free energies are reported in kcal/mol at 1 atm and 25 °C. Free energies for gas-phase acidity were calculated with fine grid and G(H+(gas)) = −6.28 kcal/mol44 was used. Normal mode analysis has been performed, as well as intrinsic reaction coordinate (IRC) calculations to verify the transition state geometries. The possible pathways under study were modeled using the solvation model based on density (SMD)45 in THF. Further calculations on I– were carried out using the B3LYP46 and the ωB97X-D47 functionals giving similar results as shown in the SI (Table S3). Stability of the wave functions was checked and the energies of anions and the corresponding radicals were compared to evade unnoticed electron loss. The Avogadro software48 was used for the visualization of PMOs and CYLview49 for geometries.
Conclusion
In this paper we presented new, switchable base-catalyzed C–N bond forming reactions proceeding through a sequence of rearrangements. The ring contraction of variously substituted 3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxides (1) to 1,2-benzisothiazole 1,1-dioxides (2) could be achieved in a diaza-[1,2]-Wittig reaction using 2 equiv of t-BuOK, followed by quenching with water and subsequent crystallization. With certain substitution patterns, the formation of enamide intermediates 5 was observed; however, when heating or using a 1% HCl solution for quenching, the selective formation of the thermodynamically most stable compounds 2 could be forced. The mechanism of the facile proton-catalyzed transformation of enamides 5 to benzisothiazoles 2 was explored by DFT calculations. When applying a larger excess (6 equiv) of t-BuOK in the reaction of compounds 1, a hitherto unknown diaza-[1,3]-Wittig rearrangement providing 1,2-benzothiazines (3) was identified, and in a noteworthy way this reaction also turned out to be a practical synthetic method (80% isolated yield for 3a). The mechanism of these reactions was explored in a combined experimental and theoretical study. NMR studies proved that when starting from 1a with 2 equiv of t-BuOK base, the monoanionic cyclic intermediate II– (the deprotonated form of 2a) formed predominantly. DFT calculations clearly described the thermodynamics and the kinetics for the formation of II– via a closed-shell ionic pathway with the involvement of the ring-opened short-lived intermediate III–. The formation of the thermodynamically stable 2 from its deprotonated anion II– upon reaction with water is apparent. With a large excess (6 equiv) of the base, enamide dianion IV2– could be obtained according to 1H NMR studies, and again DFT studies supported this pathway. When quenching the reaction mixture with water, 3a and enamide 5a were predominantly formed, together with some 2a. Under the basic conditions present, 5a transformed during the workup to 3a, in accordance with the DFT calculations on a base-catalyzed mechanism, which was also supported by deuterium labeling. In a noteworthy way, the final 3a:2a ratio represents the Boltzmann population of V– to III– in agreement with the calculations. In addition, the substituent effects of the base-induced (6 equiv) rearrangement of 1,2-benzothiadiazine dioxides (1) leading to 1,2-benzisothiazole dioxides (2) and to 1,2-benzothiazine dioxides (3) was explored, as well. The investigation of the substituent tolerance of these reactions revealed that while the formation of derivatives 2 could be achieved with most substitution patterns, the reaction leading to the formation of compounds 3 is more sensitive. For example, replacement of the N(3)-acyl group to N(3)-alkyl in the starting materials 1 destabilizes the enamide dianion IV2–, preventing the formation of 3.
Experimental Section
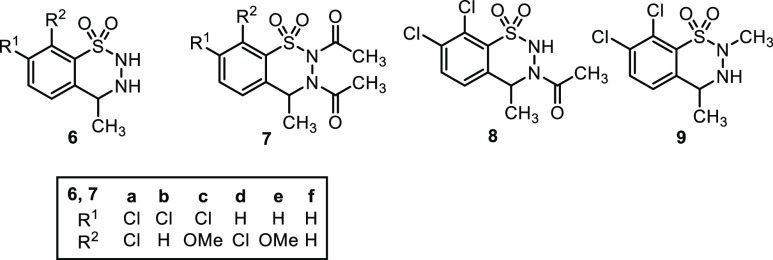
Melting points were determined using either a Leica Galen III melting point apparatus; IR spectra were obtained on a Bruker ALPHA FT-IR spectrometer in KBr pellet, and ν̃ was reported in cm–1. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance III 400 (400/100 MHz) or a Bruker Avance III HD 600 (600/150 MHz) spectrometer equipped with a Prodigy cryo-probehead. CDCl3 or [D6]DMSO was used as the solvent and tetramethylsilane (TMS) as the internal standard, and δ was reported in ppm. Structural assignments were made with additional information from gHSQC and gHMBC experiments. A Waters Acquity UPLC equipment coupled with a Thermo Scientific LTQ XL iontrap MS was used to obtain mass spectroscopic data. High resolution mass spectra were recorded on a micromass GCT or on a Bruker O-TOF MAXIS Impact mass spectrometer coupled with a Dionex Ultimate 3000 RS system with a diode array detector. Single crystal X-ray diffraction measurements were carried out on a Rigaku R-AXIS SPIDER diffractometer using image plate detection and monochromated Cu Kα radiation. The reactions were followed by analytical thin layer chromatography on silica gel 60 F254 and HPLC-MS on a Shimadzu LC-20 HPLC utilizing a SPD-M20A diode array detector and a LCMS-2020 spectrometer. Flask heating blocks were used for reactions that required heating. All unspecified reagents were purchased from commercial sources. Compounds 1a,b,261i,j,396b–f,397a,268,26 and 9(39) (Figure 2) were obtained as described previously.
Figure 2.
Further starting materials and intermediates used in this study.
Synthesis of Starting Materials 7
2,3-Diacetyl-7-chloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (7b)
A mixture of 7-chloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (6b, 3.69 g, 15.9 mmol) and acetic anhydride (28 mL) was refluxed at 140 °C under stirring for 19 h. Then it was poured onto ice water (70 g). The precipitated product was filtered and washed with H2O (2 × 10 mL), cold EtOH (2 × 5 mL), and hexane (2 × 10 mL). Yield 4.49 g (89%); colorless crystals; mp 152.5–153.5 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.87 (d, J = 2.1 Hz, 1H; 8-H), 7.57 (dd, J = 8.4, 2.1 Hz, 1H; 6-H), 7.26 (d, J = 8.4 Hz, 1H; 5-H), 6.02 (q, J = 7.0 Hz, 1H; 4-H), 2.63 (s, 3H; O=C–CH3), 2.13 (s, 3H; O=C–CH3), 1.42 (d, J = 7.0 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 172.0 (O=C), 167.2 (O=C), 137.2, 136.5, 134.3, 133.6, 128.9, 123.9, 49.3 (C4), 24.3, 20.6, 19.6; IR (KBr): ν̃ = 1741 (s; C=O), 1687 (vs; C=O), 1352 (vs; SO2), 1151 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H14ClN2O4S 317.0357; Found 317.0362.
2,3-Diacetyl-8-chloro-7-methoxy-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (7c)
A mixture of 8-chloro-7-methoxy-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (6c, 800 mg, 3.0 mmol) and acetic anhydride (4.5 mL) was refluxed at 140 °C under stirring for 24 h. Then it was poured onto ice water (50 g). The precipitated product was filtered and washed with H2O (2 × 10 mL) and hexane (2 × 10 mL). Yield 925 mg (88%); colorless crystals; mp 201–204 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.17 (d, J = 8.5 Hz, 1H; Ar–H), 7.14 (d, J = 8.5 Hz, 1H; Ar–H), 5.93 (q, J = 6.9 Hz, 1H; 4-H), 3.95 (s, 3H; OCH3), 2.67 (s, 3H; O=C–CH3), 2.13 (s, 3H; O=C–CH3), 1.40 (d, J = 6.9 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 171.8 (O=C), 167.6 (O=C), 134.9 (C), 132.6 (C), 126.6 (CH), 120.0 (C), 115.9 (CH), 56.8 (OCH3), 49.6 (C4), 24.8 (O=C–CH3), 20.3 (4-CH3), 19.5 (O=C-CH3); IR (KBr): ν̃ = 1731 (s; C=O), 1689 (vs; C=O), 1377 (vs; SO2), 1157 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C13H16ClN2O5S 347.0463; Found 347.0464.
2,3-Diacetyl-8-chloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (7d)
A mixture of 8-chloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (6d, 6.37 g, 27.4 mmol) and acetic anhydride (41 mL) was refluxed at 140 °C under stirring for 17 h. Then it was poured onto ice water (50 g). The precipitated product was filtered and washed with H2O (3 × 40 mL). Yield 7.76 g (90%); colorless crystals; mp 171–173 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.52–7.46 (m, 2H; Ar–H), 7.22 (dd, J = 7.3, 1.7 Hz, 1H; Ar–H), 6.00 (q, J = 7.1 Hz, 1H; 4-H), 2.67 (s, 3H; O=C–CH3), 2.13 (s, 3H; O=C–CH3), 1.44 (d, J = 7.1 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 171.8 (C=O), 167.5 (C=O), 142.1 (C), 133.9 (C),133.3 (CH), 131.4 (C), 130.7 (CH), 126.0 (CH), 50.1 (C4), 24.8 (O=C–CH3), 20.1 (4-CH3), 19.5 (O=C-CH3); IR (KBr): ν̃ = 1741 (s; C=O), 1686 (vs; C=O), 1354 (vs; SO2), 1158 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H14ClN2O4S: 317.0357; Found 317.0360.
2,3-Diacetyl-8-methoxy-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (7e)
A mixture of 8-methoxy-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (6e, 3.00 g, 13.1 mmol) and acetic anhydride (19 mL) was refluxed at 140 °C under stirring for 19 h. Then it was poured onto ice water (50 g). The precipitated product was filtered and washed with H2O (2 × 20 mL) and hexane (10 mL). Yield 3.80 g (93%); colorless crystals; mp 167–169 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.51 (t, J = 8.0 Hz, 1H; 6-H), 6.92 (d, J = 8.0 Hz, 1H; Ar–H), 6.85 (d, J = 8.0 Hz, 1H; Ar–H), 5.94 (q, J = 7.0 Hz, 1H; 4-H), 4.01 (s, 3H; OCH3), 2.66 (s, 3H; O=C–CH3), 2.13 (s, 3H; O=C–CH3), 1.41 (d, J = 7.0 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 171.9 (C=O), 167.7 (C=O), 157.1 (C), 141.5 (C), 134.1 (CH), 124.2 (C), 119.1 (CH), 111.0 (CH), 56.8 (OCH3), 49.6 (C4), 24.8 (O=C–CH3), 19.9 (4-CH3), 19.5 (O=C-CH3); IR (KBr): ν̃ = 1736 (s; C=O), 1681 (s; C=O), 1347 (s; SO2), 1161 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C13H17N2O5S 313.0853; Found 313.0858.
2,3-Diacetyl-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (7f)
A mixture of 4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (6f, 1.86 g, 9.4 mmol) and acetic anhydride (15 mL) was refluxed at 140 °C under stirring for 24 h. Then it was quenched with EtOH (20 mL), evaporated in vacuo, crystallized from hexane (15 mL), filtered and washed with H2O (10 mL) and EtOH (5 mL). Yield 2.32 g (87%); colorless crystals; mp 155–157 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.89 (d, J = 7.7 Hz, 1H; Ar–H), 7.61 (td, J = 7.7, 1.3 Hz, 1H; Ar–H), 7.49 (t, J = 7.7 Hz, 1H; Ar–H), 7.31 (d, J = 7.7 Hz, 1H; Ar–H), 6.04 (q, J = 7.1 Hz, 1H; 4-H), 2.64 (s, 3H; O=C–CH3), 2.14 (s, 3H; O=C–CH3), 1.44 (d, J = 7.1 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 172.1 (C=O), 167.4 (C=O), 138.9 (C), 135.3 (C), 133.3 (CH), 128.1 (CH), 127.5 (CH), 123.9 (CH), 49.6 (C4), 24.4 (O=C–CH3), 20.6 (4-CH3), 19.7 (O=C–CH3); IR (KBr): ν̃ = 1745 (s; C=O), 1690 (vs; C=O), 1347 (s; SO2); HRMS (ESI): m/z calcd for C12H15N2O4S 283.0747; Found 283.0752.
Synthesis of 2,3-Disubstituted Benzothiadiazine Dioxides (1)
3-Acetyl-7,8-dichloro-2-ethyl-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1c)
To a suspension of t-BuOK (449 mg, 4.0 mmol) in DMF (4 mL) was added a solution of 2,3-diacetyl-7,8-dichloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (7a, 702 mg, 2.0 mmol) in DMF (8 mL) at room temperature. After 30 min, EtI (485 μL, 936 mg, 6.0 mmol) was added dropwise. The mixture was stirred for 4 h. It was poured into ice water (60 g). The precipitated product was filtered. Yield 620 mg (92%); colorless crystals; mp 146–147 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.00 (d, J = 8.7 Hz, 1H; 6-H), 7.69 (d, J = 8.7 Hz, 1H; 5-H), 5.69 (q, J = 7.0 Hz, 1H; 4-H), 3.84 (dq, 1H, J = 13.9, 7.1 Hz; N–CH), 3.22 (dq, 1H, J = 13.9, 7.1 Hz; N–CH), 2.22 (s, 3H; O=C–CH3), 1.67 (d, J = 7.0 Hz, 4-CH3), 1.28 (t, J = 7.1 Hz, 3H, CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 172.8 (C=O), 139.5 (C4a), 134.3 (C6), 132.8 (C8a*), 132.7(C7*), 128.8 (C5), 128.6 (C8), 48.2 (N–CH2), 46.8 (C4), 21.5 (4-CH3), 20.6 (O=C-CH3), 12.5 (CH2–CH3); IR (KBr): ν̃ = 1672 (vs; C=O), 1366 (vs; SO2), 1187 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S 337.0175; Found 337.0179.
3-Acetyl-2-benzyl-7,8-dichloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1d)
To a suspension of t-BuOK (449 mg, 4.0 mmol) in DMF (4 mL) was added a solution of 7a (702 mg, 2.0 mmol) in DMF (8 mL) at room temperature. After 30 min, BnBr (713 μL, 1026 mg, 6.0 mmol) was added dropwise. The mixture was stirred for 4 h. It was poured into ice water (60 g). The precipitated product was filtered, washed with hexane (3 × 5 mL). Yield 722 mg (90%); colorless crystals; mp 187–189 °C (iPrOH); 1H NMR (400 MHz, [D6]DMSO): δ = 8.04 (d, J = 8.6 Hz, 1H; 6-H); 7.73 (d, J = 8.6 Hz, 1H; 5-H); 7.47 (d, J = 7.5 Hz, 2H; o-H); 7.43 (t, J = 7.5 Hz, 2H; m-H); 7.39 (t, J = 7.5 Hz, 1H; p-H); 5.72 (q, J = 6.9 Hz, 1H; 4-H); 5.00 (d, J = 14.5 Hz, 1H; N–CH); 4.42 (d, J = 14.5 Hz, 1H; N–CH); 1.82 (d, J = 6.9 Hz, 3H; 4-CH3); 1.80 (s, 3H; O=C–CH3); 13C{1H} NMR (100 MHz, [D6]DMSO): δ = 172.7 (C=O), 139.7 (C), 134.8 (C), 134.4 (CH), 133.4 (C), 132.8 (C), 129.9 (CH), 128.91 (CH), 128.88 (CH), 128.6 (CH), 128.5 (C), 57.0 (2-CH2), 47.5 (C4), 22.3 (4-CH3), 20.2 (O=C-CH3); IR (KBr): ν̃ = 1685 (vs; C=O), 1365 (s; SO2), 1178 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C17H17Cl2N2O3S 399.0331; Found 399.0332.
7,8-Dichloro-2,4-dimethyl-3-propionyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1e)
A mixture of 7,8-dichloro-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (9, 700 mg, 2.5 mmol) and propionic anhydride (16 mL) was heated at 130 °C under stirring for 16 h. Then it was quenched with H2O (20 mL), extracted with EtOAc (20 mL), the organic layer was washed with H2O (20 mL), aq NaHCO3 (saturated, 20 mL) and brine (10 mL), dried over MgSO4 and evaporated. The residue was treated with H2O (15 mL), decanted, stirred in hexane–diethyl ether (1:1, 10 mL) under cooling, filtered, and washed with hexane. Yield 498 mg (59%); light yellow crystals; mp 135–137 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.00 (d, J = 8.7 Hz, 1H; 6-H), 7.69 (d, J = 8.7 Hz, 1H; 5-H), 5.67 (q, J = 7.0 Hz, 1H; 4-H), 3.26 (s, 3H; NCH3), 2.69 (dq, J = 17.4, 7.4, 1H; O=C–CH), 2.56 (dq, J = 17.4, 7.4, 1H; O=C–CH), 1.67 (d, J = 7.0 Hz, 3H; 4-CH3), 1.04 (t, J = 7.4 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 175.0 (C=O), 139.8 (C4a), 134.2 (C6), 132.8 (C7), 132.5(C8a), 128.9 (C5), 128.6 (C8), 47.0 (C4), 41.2 (NCH3), 24.8 (4-CH3), 22.1 (O=C-CH2), 8.7 (CH2–CH3); IR (KBr): ν̃ = 1678 (vs; C=O), 1358 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S 337.0175; Found 337.0179.
3-Butyryl-7,8-dichloro-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1f)
A mixture of 9 (703 mg, 2.5 mmol) and butyric anhydride (20 mL) was heated at 130 °C under stirring for 15 h. Then it was quenched with H2O (20 mL), extracted with EtOAc (20 mL), the organic layer was washed with H2O (20 mL), aq NaHCO3 (saturated, 20 mL) and brine (10 mL), dried over MgSO4, and evaporated. The residue was treated and washed with diethyl ether (4, then 2 × 2 mL). Yield 545 mg (62%); light yellow crystals; mp 141–143 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.00 (d, J = 8.6 Hz, 1H; Ar–H), 7.69 (dd, J = 8.8, 0.8 Hz, 1H; Ar–H), 5.70–5.65 (m, 1H; 4-H), 3.26 (s, 3H; 2-CH3), 2.64 (dt, J = 16.7, 7.5, 1H; O=C–CH), 2.54 (dt, J = 16.7, 7.5, 1H; O=C–CH), 1.67 (d, J = 7.1 Hz, 3H; 4-CH3), 1.61–1.53 (m, 2H; CH2-CH3), 0.92 (t, J = 7.3 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 174.2 (C=O), 139.8, 134.2, 132.8, 132.5, 128.9, 128.6, 47.0, 41.3, 33.2, 22.1, 17.5, 13.8; IR (KBr): ν̃ = 1680 (vs; C=O), 1359 (vs; SO2), 1183 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C13H17Cl2N2O3S 351.0331; Found 351.0336.
7,8-Dichloro-3-trifluoroacetyl-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1g)
A suspension of 9 (703 mg, 2.5 mmol) and trifluoroacetic anhydride (20 mL) was stirred at room temperature for 14 h. The precipitated product was filtered and washed with H2O. Yield 743 mg (79%); colorless crystals; mp 151–152 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.08 (d, J = 8.7 Hz, 1H; Ar–H), 7.73 (d, J = 8.7 Hz, 1H; Ar–H), 5.68 (q, J = 6.9 Hz, 1H; 4-H), 3.27 (s, 3H; 2-CH3), 1.80 (d, J = 6.9 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 156.7 (q, J = 36.9 Hz; C=O), 137.7, 134.7, 133.4, 131.2, 129.0, 128.8, 115.9 (q, J = 288.8 Hz; CF3); 49.1 (4C), 42.1 (2-CH3), 21.4 (4-CH3); IR (KBr): ν̃ = 1721 (s; C=O), 1373 (vs; SO2), 1187 (vs; SO2); HRMS (ESI) m/z: [M + NH4]+ Calcd for C11H13F3Cl2N3O3S 394.0001; Found 394.0001.
7,8-Dichloro-3-difluoroacetyl-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1h)
To 9 (463 mg, 1.65 mmol) was added difluoroacetic anhydride (3 mL) under ice water cooling, and then it was stirred at room temperature for 2.5 h. The mixture was poured into ice water (40 g), the precipitated product was filtered and washed with water. Yield 564 mg (95%); colorless crystals; mp 162–163 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.73 (d, J = 8.6 Hz, 1H; 6-H), 7.29 (d, J = 8.6 Hz, 1H; 5-H), 6.65 (dd, J = 54.8, 51.6 Hz, 1H; CHF2), 5.62 (q, J = 7.2 Hz, 1H; 4-H), 3.28 (s, 3H; 2-CH3), 1.82 (d, J = 7.2 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 163.2 (dd, J = 30.1, 24.2 Hz; C=O), 136.8 (C4a), 135.1 (C7), 134.1 (C6), 132.6 (C8a), 131.4 (C8), 126.4 (C5), 105.8 (dd, J = 244.2, 246.9 Hz; CHF2); 47.9 (4C), 41.7 (2-CH3), 22.2 (4-CH3); IR (KBr): ν̃ = 1716 (s; C=O), 1368 (s; SO2), 1065 (m; SO2); HRMS (ESI) m/z: [M + Na] C11H10F2Cl2N2O3S 380.8649; Found 380.8652.
3-Acetyl-7-chloro-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1k)
To a mixture of 7b (1.58 g, 5.0 mmol) and DMF (10 mL) was added t-BuOK (1.12 g, 10.0 mmol) and DMF (20 mL) at room temperature. After 10 min, MeI (934 μL, 2.13 g, 15.0 mmol) was added dropwise. The mixture was stirred for 4.5 h. It was poured into ice water (160 g). The precipitated product was filtered. Yield 697 mg (48%); beige crystals; mp 156–157 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 7.98 (d, J = 2.2 Hz, 1H; H-8), 7.84 (dd, J = 8.6, 2.2 Hz, 1H; H-6), 7.72 (d, J = 8.6 Hz, 1H; H-5), 5.61 (q, J = 7.0 Hz, 1H; 4-H), 3.13 (s, 3H; NCH3), 2.21 (s, 3H; O=C–CH3), 1.63 (d, J = 7.0 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 172.4 (C=O), 136.6, 133.6, 133.0, 132.6, 130.7, 124.0, 46.1 (C4), 40.4 (NCH3), 22.4 (4-CH3), 20.9 (O=C-CH3); IR (KBr): ν̃ = 1686 (vs; C=O), 1343 (vs; SO2), 1164 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H14ClN2O3S 289.0414; Found 289.0413.
3-Acetyl-8-chloro-7-methoxy-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1l)
To a suspension of t-BuOK (324 mg, 2.9 mmol) in DMF (3 mL) was added a solution of 7c (500 mg, 1.4 mmol) in DMF (6.5 mL) at room temperature. After 30 min, MeI (269 μL, 614 mg, 4.3 mmol) was added dropwise. The mixture was stirred for 2.5 h. It was poured into ice water (50 g). The precipitated product was filtered. Yield 415 mg (90%); colorless crystals; mp 176–177 °C; 1H NMR (400 MHz, [D6]DMSO): δ = 7.62 (d, J = 8.7 Hz, 1H; Ar–H), 7.52 (d, J = 8.7 Hz, 1H; Ar–H), 5.56 (q, J = 7.0 Hz, 1H; 4-H), 3.94 (s, 3H; OCH3), 3.23 (s, 3H; NCH3), 2.21 (s, 3H; O=C–CH3), 1.65 (d, J = 7.0 Hz, 3H; 4-CH3); 13C{1H} NMR (100 MHz, [D6]DMSO): δ = 172.1 (C=O), 154.6, 131.4, 131.2, 128.3, 118.4, 117.2, 57.1 (OCH3), 46.5 (C4), 41.0 (NCH3), 22.4 (4-CH3), 20.6 (O=C-CH3); IR (KBr): ν̃ = 1678 (s; C=O), 1349 (vs; SO2), 1292; HRMS (ESI) m/z: [M + H]+ Calcd for C12H16ClN2O4S 319.0514; Found 319.0518.
3-Acetyl-8-chloro-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1m)
To a suspension of t-BuOK (1.06 g, 9.5 mmol) in DMF (10 mL) was added a solution of 7d (1.50 g, 4.7 mmol) in DMF (20 mL) at room temperature. After 10 min, MeI (884 μL, 2.02 g, 14.2 mmol) was added dropwise. The mixture was stirred for 85 min. It was poured into ice water (150 g). The precipitated product was filtered. Yield 1.21 g (89%); colorless crystals; mp 133–134 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 7.70 (t, J = 7.9 Hz, 1H; 6-H), 7.67–7.63 (m, 2H; Ar–H), 5.65 (q, J = 7.0 Hz, 1H; 4-H), 3.26 (s, 3H; NCH3), 2.22 (s, 3H; O=C–CH3), 1.68 (d, J = 7.0 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 172.2 (C=O), 141.0 (C), 133.9 (C), 130.6 (CH), 130.5 (C), 127.5 (CH), 46.8 (C4), 40.9 (NCH3), 22.3 (4-CH3), 20.6 (O=C–CH3) (one quaternary C signal not resolved); IR (KBr): ν̃ = 1686 (vs; C=O), 1351 (vs; SO2), 1166 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H14ClN2O3S 289.0408; Found 289.0412.
3-Acetyl-8-methoxy-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1n)
To a suspension of t-BuOK (1.80 g, 1.60 mmol) in DMF (1.5 mL) was added a solution of 7e (200 mg, 0.64 mmol) in DMF (2.5 mL) at room temperature. After 30 min, MeI (140 μL, 318 mg, 2.24 mmol) was added dropwise. The mixture was stirred for 2 h. It was poured into ice water (20 g). The precipitated product was filtered. Yield 140 mg (77%); colorless crystals; mp 189–190 °C (EtOH); 1H NMR (400 MHz, [D6]DMSO): δ = 7.89–7.47 (m, 1H; Ar–H), 7.47–6.88 (m, 2H; Ar–H), 5.82–5.27 (m, 1H; 4-H), 3.90 (s, 3H; OCH3), 3.17 (s, 3H; NCH3), 2.19 (s, 3H; O=C–CH3), 1.88–1.31 (m, 3H; 4-CH3) (broad signals); 13C{1H} NMR (100 MHz, [D6]DMSO): δ = 172.2 (C=O), 157.2 (C), 139.8 (C), 134.2 (CH), 120.8 (C), 119.6 (CH), 111.7 (CH), 56.8 (OCH3), 46.2 (C4), 40.8 (NCH3), 22.3 (4-CH3), 20.7 (O=C-CH3); IR (KBr): ν̃ = 1688 (vs; C=O), 1345 (vs; SO2), 1159 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H17N2O4S 285.0904; Found 285.0904.
3-Acetyl-2,4-dimethyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1o)
To a suspension of t-BuOK (898 mg, 8.0 mmol) in DMF (7 mL) was added a solution of 7f (1129 mg, 4.0 mmol) in DMF (10 mL) at room temperature. After 30 min, MeI (747 μL, 1703 mg, 12.0 mmol) was added dropwise. The mixture was stirred for 2 h. It was poured into ice water (100 g). The precipitated product was filtered. Yield 861 mg (85%); colorless crystals; mp 115–117 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 7.88 (dd, 1H, J = 7.8, 1.1 Hz; Ar–H), 7.75 (td, 1H, J = 7.8, 1.1 Hz; Ar–H), 7.68 (d, 1H, J = 7.8 Hz; Ar–H) 7.59 (t, 1H, J = 7.8 Hz; Ar–H), 5.60 (q, J = 7.0 Hz, 1H; 4-H), 3.11 (s, 3H; NCH3), 2.22 (s, 3H; O=C–CH3), 1.65 (d, J = 7.0 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 172.4 (C=O), 137.6, 133.6, 130.9, 128.6, 128.3, 124.4, 46.2 (C4), 40.4 (NCH3), 22.5 (4-CH3), 20.9 (O=C-CH3); IR (KBr): ν̃ = 1677 (vs; C=O), 1342 (vs; SO2), 1183 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H15N2O3S 255.0798; Found 255.0802.
3-Acetyl-7,8-dichloro-2-(methylsulfonyl)-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1p)
To a stirred mixture of 8 (500 mg, 1.6 mmol) and TEA (0.5 mL, 3.6 mmol) in DCM (6.5 mL) was added MsCl in two portions (250 μL, 3.2 mmol and after 2 h 125 μL, 1.6 mmol) at room temperature. After 4 h stirring, it was poured into ice water (20 g), and the precipitated product was filtered. Yield 425 mg (68%); colorless crystals; mp 226–227 °C (EtOH); 1H NMR (600 MHz, CDCl3): δ = 7.69 (d, J = 8.5 Hz, 1H; Ar–H), 7.24 (dd, J = 8.5, 0.7 Hz, 1H; Ar–H), 5.88 (∼qd, J = 7.1, 0.7 Hz, 1H; 4-H), 3.66 (s, 3H; SO2CH3), 3.11 (s, 3H; O=C–CH3), 1.72 (d, J = 7.1 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 172.3 (C=O), 138.6 (C), 134.8 (C), 134.33 (C), 134.31 (CH), 130.0 (C), 127.1 (CH), 49.6 (C4), 45.1 (CH3), 20.7 (CH3), 19.9 (CH3); IR (KBr): ν̃ = 1705 (s; C=O), 1372 (vs; SO2), 1158 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H13Cl2N2O5S2 386.9637; Found 386.9636.
3-Acetyl-7,8-dichloro-4-methyl-2-(4-toluenesulfonyl)-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-Dioxide (1q)
To a stirred mixture of 3-acetyl-7,8-dichloro-4-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine 1,1-dioxide (8, 500 mg, 1.6 mmol) and TEA (0.5 mL, 3.6 mmol) in DCM (8.5 mL) was added TsCl (617 mg, 3.2 mmol) at room temperature. After 1.5 h stirring, it was poured into ice water (30 g), and the precipitated product was filtered. Yield 490 mg (65%); colorless crystals; mp 243–244 °C (EtOAc); 1H NMR (600 MHz, CDCl3): δ = 7.96 (d, J = 8.3 Hz, 2H; o-H), 7.67 (d, J = 8.6 Hz, 1H; 6-H), 7.42 (d, J = 8.3 Hz, 2H; m-H), 7.25 (d, J = 8.6 Hz, 1H; 5-H), 5.92 (q, J = 7.1 Hz, 1H; 4-H), 2.50 (s, 3H; p-CH3), 2.36 (s, 3H; O=C–CH3), 1.85 (d, J = 7.1 Hz, 3H; 4-CH3); 13C{1H} NMR (150 MHz, CDCl3): δ = 172.9 (C=O), 147.1 (p-C), 138.7 (C4a), 135.3 (C8a), 134.4 (C7), 134.1 (ipso-C), 134.0 (C6), 130.0 (4C; o-C and m-C), 129.9 (C8), 126.9 (C5), 49.6 (C4), 21.9 (p-CH3), 20.8 (4-CH3), 20.2 (O=C-CH3); IR (KBr): ν̃ = 1698 (vs; C=O), 1376 (vs; SO2), 1169 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C17H17Cl2N2O5S2 462.9950; Found 462.9956.
Preparation of 1,2-Benzisothiazole 1,1-Dioxides (2)
General Procedure for the Synthesis of Compounds 2a–f,i,j
Method A. To the mixture of 1a–d,f,i (0.60 mmol) in THF (3 mL) was added t-BuOK (1.2 mmol, 135 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (10 mL), and THF was evaporated. After stirring and cooling with ice water bath for 1 h, the precipitated product was filtered and washed with water to give 2a–d,f,i. Method B. To the solution of 1e,j (0.30 mmol) in THF (1.5 mL) was added t-BuOK (0.60 mmol, 67 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (5 mL), and THF was evaporated. After stirring and cooling with an ice water bath for 1 h, the precipitated product was filtered and washed with water to give 2e,j.
N-(6,7-Dichloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2a)
Using 1a (194 mg). Yield 174 mg (90%). Identical with our previous report;26 mp 212 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.79 (s, 1H; NH), 7.94 (d, J = 8.4 Hz, 1H; 5-H), 7.57 (d, J = 8.4 Hz; 4-H), 2.67 (s, 3H; NCH3), 1.82 (s, 3H; O=C–CH3), 1.56 (s, 3H; 3-CH3); 13C{1H} NMR (600 MHz, [D6]DMSO): δ = 168.6 (O=C), 143.4 (C3a), 135.0 (C5), 132.8 (C7a), 132.5 (C6), 124.7 (C7), 123.3 (C4), 71.5 (C3), 25.2 (3-CH3), 23.0 (O=C-CH3), 22.7 (NCH3). Scaled-up experiment with modified workup: To the mixture of 1a (1.2 mmol, 389 mg) in THF (6 mL) was added t-BuOK (2.4 mmol, 269 mg) at 25 °C and stirred for 30 min. Then, THF was evaporated and aq HCl (5 w/w%, 10 mL) was added to the residue. Upon cooling with ice water, the precipitated product was filtered and washed with water to give 2a (347 mg, 90%).
N-(6,7-Dichloro-3-ethyl-2-methyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2b)
Using 1b (202 mg). Yield 187 mg (92%). Identical with our previous report;26 mp 209–210 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.67 (s, 1H; NH), 7.94 (d, J = 8.3 Hz, 1H; 5-H), 7.52 (d J = 8.3 Hz, 1H; 4-H), 2.63 (s, 3H; NCH3), 2.13–1.94 (m, 2H; 3-CH2), 1.81 (s, 3H; O=C–CH3), 0.48 (t, J = 7.2 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.8 (O=C), 141.0 (C3a), 134.9 (C5), 134.2 (C7a), 132.5 (C6), 124.7 (C7), 123.2 (C4), 75.0 (C3), 29.5 (CH2), 22.9 (O=C-CH3), 22.4 (NCH3), 6.9 (CH2–CH3).
N-(6,7-Dichloro-2-ethyl-3-methyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2c)
Using 1c (202 mg). Yield 172 mg (85%); colorless crystals; mp 221–222 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.83 (s, 1H; NH), 7.92 (d, J = 8.4 Hz, 1H; 5-H), 7.54 (d, J = 8.4 Hz, 1H; 4-H), 3.31–3.15 (m, 2H; 2-CH2), 1.82 (s, 3H; O=C–CH3), 1.59 (s, 3H; 3-CH3), 1.26 (t, J = 7.2 Hz, 3H; CH2–CH3); DEPTQ (150 MHz, [D6]DMSO): δ = 168.1 (C=O), 143.4 (C3a), 134.9 (C5), 133.0 (C7a), 132.4 (C6), 124.6 (C7), 123.2 (C4), 71.9 (C3), 33.6 (2-CH2), 26.6 (3-CH3), 23.0 (O=C–CH3), 15.3 (CH2–CH3); IR (KBr): ν̃ = 3265 (m; NH), 1672 (s; C=O), 1305 (vs; SO2), 1191 (vs; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S 337.0175; Found 337.0175.
N-(2-Benzyl-6,7-dichloro-3-methyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2d)
Using 1d (240 mg). Yield 226 mg (94%); colorless crystals; mp 225–227 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.71 (s, 1H; NH), 7.94 (d, J = 8.3 Hz, 1H; 5-H), 7.52 (d, J = 8.4 Hz, 1H; 4-H), 7.43 (d, J = 7.3 Hz, 2H; o-H), 7.33 (t, J = 7.3 Hz, 2H; m-H), 7.27 (t, J = 7.3 Hz, 1H; p-H), 4.39 (s, 2H; 2-CH2), 1.54 (s, 3H; 3-CH3), 1.53 (s, 3H; O=C–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.8 (O=C), 143.5 (C3a), 137.1 (ipso-C), 135.1 (C5), 132.6 (C7a*), 132.5 (C6*), 128.3 (2C; o-C), 128.2 (2C; m-C), 127.4 (p-C), 124.8 (C7), 123.2 (C4), 72.1 (C3), 41.6 (2-CH2), 26.6 (3-CH3), 22.6 (O=C-CH3); IR (KBr): ν̃ = 3268 (m; NH), 3067 (m; C = C–H), 1673 (s; C=O), 1300 (vs; SO2), 1174 (vs; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C17H17Cl2N2O3S: 339.0331; Found 339.0332.
N-(6,7-Dichloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)propionamide (2e)
Using 1e (101 mg). Yield 86 mg (85%); colorless crystals; mp 224–225 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.71 (s, 1H; NH), 7.94 (d, J = 8.3 Hz, 1H; 5-H), 7.55 (d, J = 8.3 Hz, 1H; 4-H), 2.66 (s, 3H; 2-CH3), 2.19–2.06 (m, 2H; CH2-CH3), 1.56 (s, 3H), 0.89 (t, J = 7.5 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 172.5 (C=O), 143.5 (C3a), 135.0 (C5), 132.8 (C7a), 132.5 (C6), 124.7 (C7), 123.2 (C4), 71.5 (C3), 28.4 (O=C-CH2), 25.2 (3-CH3), 22.7 (2-CH3), 9.5 (CH2–CH3); IR (KBr): ν̃ = 3262 (w; NH), 1669 (m; C=O), 1306 (s; SO2), 1155 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S 337.0175; Found 337.0174.
N-(6,7-Dichloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)butyramide (2f)
Using 1f (211 mg). Yield 200 mg (95%); colorless crystals; mp 258–260 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.73 (s, 1H; NH), 7.95 (d, J = 8.3 Hz, 1H; 5-H), 7.54 (d, J = 8.3 Hz, 1H; 4-H), 2.66 (s, 3H; 2-CH3), 2.14–2.03 (m, 2H; O=C–CH2), 1.56 (3-CH3), 1.46–1.37 (m, 2H; CH2-CH3), 0.80 (t, J = 7.4 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 171.6 (C=O), 143.5 (C3a), 135.0 (C5), 132.9 (C7a), 132.5 (C6), 124.7 (C7), 123.2 (C4), 71.5 (C3), 37.1 (O=C-CH2), 25.2 (3-CH3), 22.7 (2-CH3), 18.4 (CH2–CH2-CH3), 13.7 (CH2–CH3); IR (KBr): ν̃ = 3258 (m; NH), 3205 (m; NH), 3067 (C=C–H), 1663 (s; C=O), 1301 (s; SO2), 1159 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C13H17Cl2N2O3S 351.0332; Found 351.0331.
N-(6,7-Dichloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)trifluoroacetamide (2g)
To the solution of 1g (226 mg) in THF (3 mL) was added t-BuOK (1.2 mmol, 135 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (10 mL), and THF was evaporated. After stirring and cooling with ice water bath, the precipitated side product was filtered. The filtrate was extracted with DCM (3 × 15 mL), the combined organic layer was dried over MgSO4 and evaporated. Yield 83 mg (37%); colorless crystals; mp 198–199 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 10.26 (s, 1H; NH), 8.03 (d, J = 8.3 Hz, 1H; 5-H), 7.73 (d, J = 8.3 Hz, 1H; 4-H), 2.73 (s, 3H; 2-CH3), 1.72 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 155.4 (q, J = 37.5 Hz; O=C), 141.1 (C4a), 135.7 (C5), 133.5 (C7a*), 132.8 (C6*), 125.1 (C7), 123.5 (C4), 115.2 (q, J = 289.5 Hz; CF3), 72.1 (C3), 24.5 (3-CH3), 22.9 (2-CH3); IR (KBr): ν̃ = 3354 (w; NH), 1727 (m; C=O), 1550 (m, O=C-NH), 1304 (m; SO2), 1167 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H10Cl2F3N2O3S 376.9736; Found 376.9735.
N-(6,7-Dichloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)difluoroacetamide (2h)
To the solution of 1h (108 mg) in THF (1.5 mL) was added t-BuOK (0.60 mmol, 67 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (5 mL), and THF was evaporated. After stirring and cooling with ice water bath, the precipitated byproduct was filtered. The filtrate was extracted with DCM (3 × 15 mL), the combined organic layer was dried over MgSO4 and evaporated. Yield 71 mg (67%); colorless crystals; mp 90–91 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 9.67 (s, 1H; NH), 8.02 (d, J = 8.3 Hz, 1H; 5-H), 7.64 (d, J = 8.3 Hz, 1H; 4-H), 6.19 (t, J = 53.5 Hz, 1H; CHF2), 2.77 (s, 3H; 2-CH3), 1.67 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 161.4 (t, J = 26.0 Hz; O=C), 141.8 (C3a), 135.4 (C5), 133.2 (C7a*), 132.8 (C6), 124.9 (C7), 123.5 (C4), 107.9 (t, J = 247.3 Hz; CHF2), 71.6 (C3), 24.7 (3-CH3), 22.8 (2-CH3); IR (KBr): ν̃ = 3330 (w; NH), 1714 (m; C=O), 1548 (w, O=C-NH), 1303 (m; SO2), 1162 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H11Cl2F2N2O3S 358.9830; Found 358.9830.
6,7-Dichloro-2,3-dimethyl-3-(methylamino)-2,3-dihydro-1,2-benzisothiazole 1,1-Dioxide (2i)
Using 1i (177 mg). Yield 148 mg (84%); colorless crystals; mp 187–189 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.03 (d, J = 8.3 Hz, 1H; 5-H), 7.62 (d, J = 8.3 Hz, 1H; 4-H), 3.64 (q, J = 5.7 Hz, 1H; NH), 2.68 (s, 3H; 2-CH3), 1.77 (d, J = 5.7 Hz, 3H; HN-CH3), 1.46 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 142.9 (C3a), 135.4 (C5), 134.4 (C7a*), 133.3 (C6*), 124.9 (C6), 124.5 (C4), 79.1 (C3), 28.1 (HN–CH3), 25.3 (3-CH3), 21.5 (2-CH3); IR (KBr): ν̃ = 3350 (m; NH), 1287 (vs; SO2), 1160 (vs; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C10H13Cl2N2O2S 295.0069; Found 295.0068.
6,7-Dichloro-3-(ethylamino)-2,3-dimethyl-2,3-dihydro-1,2-benzisothiazole 1,1-Dioxide (2j)
Using 1j (93 mg). Yield 74 mg (80%); colorless crystals; mp 112–113 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.02 (d, J = 8.2 Hz, 1H; 5-H), 7.64 (d, J = 8.2 Hz, 1H; 4-H), 3.58 (q, J = 4.2 Hz, 1H; NH), 2.69 (s, 3H; 2-CH3), 2.25–2.15 (m, 1H; N–CH), 1.83–1.72 (m, 1H; N–CH), 1.47 (s, 3H; 3-CH3), 0.91 (t, J = 7.1 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 143.5 (C3a), 135.4 (C5), 134.2 (C7a*), 133.2 (C6*), 124.9 (C7), 124.4 (C4), 78.6 (C3), 36.2 (N–CH2), 25.4 (3-CH3), 21.6 (2-CH3), 15.0 (CH2–CH3); IR (KBr): ν̃ = 3348 (m; NH), 1291 (s; SO2), 1156 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H15Cl2N2O2S 309.0226; Found 309.0227.
N-(6-Chloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2k)
To the solution of 1k (87 mg, 0.30 mmol) in THF (1.5 mL) was added t-BuOK (0.60 mmol, 67 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (5 mL), and THF was evaporated. Then, the aqueous mixture was refluxed for 1 h. After stirring and cooling with ice water bath for 1 h, the precipitated product was filtered and washed with water. Yield 56 mg (65%); colorless crystals; mp 277–278 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.71 (s, 1H; NH), 8.05 (d, J = 1.8 Hz, 1H; 7-H), 7.73 (dd, J = 8.5, 1.8 Hz, 1H; 5-H), 7.57 (d, J = 8.5 Hz, 1H; 4-H), 2.65 (s, 3H; 2-CH3), 1.82 (s, 3H; O=C–CH3), 1.55 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.7 (O=C), 140.7 (C3a), 135.1 (C6*), 134.0 (C7a*), 133.4 (C5), 125.1 (C4), 120.8 (C7), 72.6 (C3), 25.2 (3-CH3), 23.1 (O=C-CH3), 22.6 (2-CH3); IR (KBr): ν̃=, 3265 (m; NH), 1669 (m; O=C), 1298 (m; SO2), 1157 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H14ClN2O3S: 289.0408; Found 289.0408.
General Procedure for the Synthesis of Compounds 2l–o,q
To the mixture of 1l–o, 1q (0.3 mmol) in THF (1.5 mL) was added t-BuOK (0.60 mmol, 67 mg) at 25 °C and stirred for 30 min. Then, it was quenched with aq HCl (1 w/w%, 2.5 mL), and THF was evaporated. Upon cooling with ice water, the precipitated product was filtered and washed with water to give compounds 2l–o,q.
N-(7-Chloro-6-methoxy-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2l)
Using 1l (96 mg). Yield 89 mg (93%); colorless crystals; mp 251–252 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.63 (s, 1H; NH), 7.48 (d, J = 8.5 Hz, 1H; 4-H), 7.45 (d, J = 8.5 Hz, 1H; 5-H), 3.94 (s, 3H; OCH3), 2.65 (s, 3H; 2-CH3), 1.81 (s, 3H; O=C–CH3), 1.55 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.8 (O=C), 155.2 (C6), 135.4 (C3a), 132.2 (C7), 122.7 (C4), 117.6 (C5), 114.0 (C7a), 71.5 (C3), 57.3 (OCH3), 25.6 (3-CH3), 23.2 (O=C-CH3), 22.7 (2-CH3); IR (KBr): ν̃ = 3452 (m; NH), 3264 (w; NH), 1649 (m; O=C), 1282 (w; SO2), 1151 (s; SO2); HRMS (ESI) m/z: [M + NH4]+ Calcd for C12H19ClN3O4S 336.0779; Found 336.0784.
N-(7-Chloro-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2m)
Using 1m (87 mg). Yield 72 mg (83%); colorless crystals; mp 257–258 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.73 (s, 1H; NH), 7.68 (t, J = 7.9 Hz, 1H; 5-H), 7.63 (d, J = 7.9 Hz, 1H; 6-H), 7.51 (d, J = 7.9 Hz, 1H; 4-H), 2.66 (s, 3H; 2-CH3), 1.82 (s, 3H; O=C–CH3), 1.55 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.7 (O=C), 144.7 (C3a), 134.8 (C5), 131.0 (C7a), 130.0 (C6), 126.4 (C7), 122.0 (C4), 71.9 (C3), 25.4 (3-CH3), 23.0 (O=C-CH3), 22.5 (2-CH3); IR (KBr): ν̃ = 3267 (m; NH), 1671 (s; O=C), 1294 (vs; SO2), 1158 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H14ClN2O3S 289.0408; Found 289.0408.
N-(7-Methoxy-2,3-dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2n)
Using 1n (85 mg). Yield 44 mg (52%); colorless crystals; mp 189–190 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.58 (s, 1H; NH), 7.60 (t, J = 8.1 Hz, 1H; 5-H), 7.14 (d, J = 8.1 Hz, 1H; 6-H), 7.04 (d, J = 7.9 Hz, 1H; 4-H), 3.93 (s, 3H; OCH3), 2.60 (s, 3H; 2-CH3), 1.81 (s, 3H; O=C–CH3), 1.51 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.5 (O=C), 153.9 (C7), 144.2 (C3a), 135.1 (C5), 120.9 (C7a), 114.4 (C4), 111.5 (C6), 72.1 (C3), 56.3 (OCH3), 25.5 (3-CH3), 23.2 (O=C-CH3), 22.5 (2-CH3); IR (KBr): ν̃=, 3306 (w; NH), 1673 (m; O=C), 1291 (s; SO2), 1155 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H17N2O4S 285.0904; Found 285.0903.
N-(2,3-Dimethyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2o)
Using 1o (76 mg). Yield 57 mg (75%); colorless crystals; mp 193–194 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.64 (s, 1H; NH), 7.82 (d, J = 7.7 Hz, 1H; 7-H), 7.68 (t, J = 7.7 Hz, 1H; 5-H), 7.58 (t, J = 7.7 Hz, 1H; 6-H), 7.54 (d, J = 7.7 Hz, 1H; 4-H), 2.65 (s, 3H; 2-CH3), 1.82 (s, 3H; O=C–CH3), 1.56 (s, 3H; 3-CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.6 (O=C), 141.8 (C3a), 133.4 (C7a), 133.2 (C7), 129.4 (C4), 123.1 (C6), 120.7 (C5), 72.9 (C3), 25.4 (3-CH3), 23.2 (O=C–CH3), 22.5 (2-CH3); IR (KBr): ν̃ = 3370 (w; NH), 1670 (m; O=C), 1284 (m; SO2), 1159 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H15N2O3S 255.0797; Found 255.0799.
N-(6,7-Dichloro-3-methyl-2-tosyl-1,1-dioxo-2,3-dihydro-1,2-benzisothiazol-3-yl)acetamide (2q)
Using 1q (139 mg). Yield 132 mg (95%); colorless crystals; mp 199.5–200.5 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 9.30 (s, 1H; NH), 7.98 (d, J = 8.5 Hz, 1H; 5-H), 7.88 (d, J = 7.9 Hz, 2H; o-H), 7.50 (d, J = 8.5 Hz, 1H; 4-H), 7.43 (d, J = 7.9 Hz, 2H; m-H), 2.39 (s, 3H; p-CH3), 1.95 (s, 3H; 3-CH3), 1.21 (s, 3H; O=C–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 168.6 (O=C), 145.2 (p-C), 141.4 (C3a), 136.1 (C5), 135.9 (ipso-C), 133.1 (C6), 131.6, (C7a), 129.7 (2C; m-CH), 128.3 (2C; o-CH), 124.9 (C7), 122.9 (C4), 75.7 (C3), 29.8 (3-CH3), 22.2 (O=C-CH3), 21.3 (p-CH3); IR (KBr): ν̃ = 3250 (w; NH), 3200 (w; NH), 1672 (m; O=C), 1370 (m; SO2), 1196 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C17H17Cl2N2O5S2 462.9950; Found 462.9950.
Synthesis of N-{1-[3,4-dichloro-2-(N-tosylsulfamoyl)phenyl]vinyl}acetamide (5q)
To the suspension of 1q (0.60 mmol) in THF (3 mL) was added t-BuOK (1.2 mmol, 135 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (10 mL), and THF was evaporated. After stirring and cooling with ice water bath for 1 h, the precipitated product was filtered and washed with water. Yield 80 mg (29%); colorless crystals, mp 264–265 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 9.11 (s, 1H; NH), 7.67 (d, J = 8.3 Hz, 1H), 7.48 (d, J = 7.9 Hz, 2H), 7.26–7.08 (m, 3H), 5.75 (s, 1H; = CH), 4.44 (s, 1H; = CH), 2.33 (s, 3H), 1.88 (s, 3H), (sulfonimide NH is under the noise level); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 167.8, 143.4, 142.5, 142.4, 140.7, 138.3, 132.3, 131.7, 131.0, 128.7 (2C), 125.8 (2C), 101.0 (=CH2), 24.2, 21.1; IR (KBr): ν̃ = 3328 (m; NH), 1668 (s; O=C), 1526 (s; O=C-NH), 1311 (s; SO2), 1089 (vs; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C17H17Cl2N2O5S2 462.9950; Found 462.9959.
Synthesis of 1,2-Benzothiazine 1,1-Dioxides (3)
N-(7,8-Dichloro-2-methyl-1,1-dioxo-3,4-dihydro-2H-1,2-benzothiazin-4-yl)acetamide Monohydrate (3a·H2O)
To the solution of 1a (0.60 mmol, 194 mg) in DMSO (3 mL) was added t-BuOK (3.6 mmol, 404 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (15 mL), and after intensive stirring for 1 h, the precipitated product was filtered and washed with water to give 3a·H2O (163 mg, 80%); colorless crystals; mp 186–187 °C (EtOH); 1H NMR (600 MHz, [D6]DMSO): δ = 8.53 (d, J = 8.5 Hz, 1H; NH), 7.90 (d, J = 8.5 Hz, 1H; 6-H), 7.38 (dd, J = 8.7, 0.7 Hz, 1H; 5-H), 5.38 (ddd, J = 10.2, 8.5, 5.4 Hz, 1H; 4-H), 3.88 (dd, J = 14.7, 10.2 Hz, 1H; 3-Hax), 3.66 (dd, J = 14.7, 5.4 Hz, 1H; 3-Heq), 2.98 (s, 3H; 2-CH3), 1.92 (s, 3H; O=C–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 170.0 (O=C), 139.3 (C4a), 135.7 (C8a), 133.7 (C6), 133.0 (C7), 129.8 (C5), 128.4 (C8), 50.5 (C3), 40.7 (C4), 36.2 (2-CH3), 22.9 (O=C-CH3); IR (KBr): ν̃ = 3451 (m; NH), 3258 (m; NH), 1644 (vs; O=C), 1329 (vs; SO2), 1172 (s; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C11H13Cl2N2O3S 323.0018; Found 323.0017; Elemental analysis Calcd (%) for C11H12Cl2N2O3S·H2O C 38.72, H 4.14, Cl 20.78, N 8.21, S 9.40; Found C 38.53, H 4.20, Cl 21.27, N 8.14, S 9.55. Scaled-up experiment: To the solution of 1a (3.00 mmol, 970 mg) in DMSO (15 mL) was added t-BuOK (18.0 mmol, 2020 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (75 mL), the precipitated product was filtered and washed with water to give 3a·H2O (844 mg, 82%).
N-(7,8-Dichloro-2,3-dimethyl-1,1-dioxo-3,4-dihydro-2H-1,2-benzothiazin-4-yl)acetamide (3b)
To the solution of 1b (0.60 mmol, 202 mg) in DMSO (3 mL) was added t-BuOK (3.6 mmol, 404 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (15 mL), and refluxed at 98 °C for 2 h. After cooling to room temperature and intensive stirring, the product precipitated, then it was filtered and washed with water. Yield 97 mg (48%); trans–cis mixture, d.r. 97:3; gray crystals. Another reaction with sequential crystallization gave an analytical sample of pure trans racemate; colorless crystals; mp 217–218 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.53 (d, J = 8.9 Hz, 1H; NH), 7.88 (d, J = 8.7 Hz, 1H; 6-H), 7.33 (d, J = 8.7 Hz, 1H; 5-H), 5.15 (t, J = 10.0 Hz, 1H; 4-Hax), 4.29 (dq, J = 10.0, 6.7 Hz, 1H; 3-Hax), 2.80 (s, 3H; 2-CH3), 1.94 (s, 3H; O=C–CH3); 1.29 (t, J = 6.7 Hz, 3H; 3-CH3); DEPTQ (150 MHz, [D6]DMSO): δ = 170.3 (O=C), 139.7 (C4a), 135.2 (C8a), 133.8 (C6), 132.9 (C7), 129.6 (C5), 128.3 (C8), 54.6 (C3), 46.1 (C4), 29.8 (2-CH3), 22.9 (O=C-CH3), 15.8 (3-CH3); IR (KBr): ν̃ 3249 (w; NH), 1661 (m; O=C), 1553 (m; O=C-NH), 1344 (m; SO2), 1153 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S 337.0175; Found 337.0176.
N-(7,8-Dichloro-2-ethyl-1,1-dioxo-3,4-dihydro-2H-1,2-benzothiazin-4-yl)acetamide monohydrate (3c)
To the solution of 1c (0.30 mmol, 101 mg) in DMSO (1.5 mL) was added t-BuOK (1.8 mmol, 202 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (7.5 mL), and after intensive stirring for 1 h, the precipitated product was filtered and washed with water to give 3c (61 mg, 57%); colorless crystals; mp 85–86 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.54 (d, J = 8.5 Hz, 1H; NH), 7.89 (d, J = 8.6 Hz, 1H; 6-H), 7.38 (d, J = 8.6 Hz, 1H; 5-H), 5.29 (ddd, J = 9.9, 8.5, 5.4 Hz, 1H; 4-H), 3.83 (dd, J = 14.8, 9.9 Hz, 1H; 3-Hax), 3.70 (dd, J = 14.8, 5.4 Hz, 1H; 3-Heq), 3.45 (dq, J = 13.7, 7.1 Hz, 1H; CH-CH3), 3.26 (dq, J = 13.7, 7.1 Hz, 1H; CH-CH3), 1.91 (s, 3H; O=C–CH3), 1.19 (t, J = 7.1 Hz, 1H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 169.9 (O=C), 139.3 (C4a), 136.8 (C8a), 133.6 (C6), 133.0 (C7), 129.7 (C5), 128.1 (C8), 47.4 (C3), 43.2 (2-CH2), 41.7 (C4), 22.8 (O=C-CH3), 14.4 (CH2–CH3); IR (KBr): ν̃ = 3457 (m; NH), 3265 (m; NH), 1646 (vs; O=C), 1318 (s; SO2), 1169 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S: 337.0175; Found 337.0175; Elemental analysis Calcd (%) for C12H14Cl2N2O3S·H2O C 40.57, H 4.54, Cl 19.96, N 7.89, S 9.03; Found C 40.58, H 4.51, Cl 20.67, N 7.89, S 9.12.
N-(2-Benzyl-7,8-dichloro-1,1-dioxo-3,4-dihydro-2H-1,2-benzothiazin-4-yl)acetamide (3d)
To the suspension of 1d (0.45 mmol) in THF (2.25 mL) was added t-BuOK (2.7 mmol, 303 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (7.5 mL), and THF was evaporated. After stirring and cooling with ice water bath, then precipitate was filtered as a mixture of 2d and 3d (0.25:1, 110 mg, 62%). Upon dissolving in DMSO (4 mL), MeOH (20 mL) and water (38 mL) was added, and the precipitated product was filtered off to give pure 3d. Yield 43 mg (24%); colorless crystals; mp 178–180 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.52 (d, J = 8.3 Hz, 1H; NH), 7.91 (d, J = 8.5 Hz, 1H; 6-H), 7.46–7.37 (m, 5H), 7.37–7.29 (m, 1H), 5.33 (ddd, J = 9.9, 8.5, 5.4 Hz, 1H; 4-H), 4.61 (d, J = 14.7 Hz, 1H; 2-CH), 4.46 (d, J = 14.7 Hz, 1H; 2-CH), 3.77 (dd, J = 14.9, 9.6 Hz, 1H; 3-Hax), 3.47 (dd, J = 14.9, 5.2 Hz, 1H; 3-Heq), 1.86 (s, 3H; O=C–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 169.7 (O=C), 139.3 (C4a), 136.9 (C8a), 136.0 (ipso-C), 133.7 (C6), 133.0 (C7), 130.0 (C5), 128.9 (2C; m-C), 128.6 (2C; o-C), 128.1 (C8), 128.0 (p-C), 51.3 (2-CH2), 47.3 (C3), 41.9 (C4), 22.8 (O=C-CH3); IR (KBr): ν̃ = 3364 (m; NH), 1683 (s; O=C), 1296 (m; SO2), 1153 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C17H17Cl2N2O3S 399.0331; Found 339.0331.
N-(7,8-Dichloro-2-methyl-1,1-dioxo-3,4-dihydro-2H-1,2-benzothiazin-4-yl)propionamide (3e)
To the solution of 1e (202 mg, 0.60 mmol) in THF (3 mL) was added t-BuOK (3.6 mmol, 404 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (10 mL), and THF was evaporated. After stirring and cooling with ice water bath, precipitate was filtered as a mixture of 2e and 3e (160 mg, 79%). Upon dissolving in DMSO (5 mL), MeOH (25 mL) and water (47.5 mL) was added, and the precipitated product was filtered off to give pure 3e. Yield 80 mg (40%); colorless crystals; mp 200–201 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.45 (d, J = 8.6 Hz, 1H; NH), 7.90 (d, J = 8.5 Hz, 1H; 6-H), 7.35 (d, J = 8.5 Hz, 1H; 5-H), 5.38 (∼ddd, J = 10.5, 8.6, 5.5 Hz, 1H; 4-H), 3.90 (dd, J = 14.7, 10.5 Hz, 1H; 3-Hax), 3.65 (dd, J = 14.7, 5.5 Hz, 1H; 3-Heq), 2.98 (s, 3H; 2-CH3), 2.24–2.15 (m, 2H; O=C–CH2), 1.05 (t, J = 7.7 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 173.6 (O=C), 139.4 (C4a), 135.7 (C8a), 133.7 (C6), 133.0 (C7), 129.7 (C5), 128.4 (C8), 50.4 (C3), 40.5 (C4), 36.2 (2-CH3), 28.7 (O=C-CH2); 9.9 (CH2–CH3); IR (KBr): ν̃ = 3265 (w; NH), 1655 (m; O=C), 1349 (m; SO2), 1166 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C12H15Cl2N2O3S 377.0175; Found 377.0175.
N-(7,8-Dichloro-2-methyl-1,1-dioxo-3,4-dihydro-2H-1,2-benzothiazin-4-yl)butyramide (3f)
To the solution of 1f (211 mg, 0.60 mmol) in THF (3 mL) was added t-BuOK (3.6 mmol, 404 mg) at 25 °C and stirred for 30 min. Then, it was quenched with water (10 mL), and THF was evaporated. After stirring and cooling with ice water bath, the precipitate was filtered as a mixture of 2f and 3f (182 mg, 86%). Upon dissolving in DMSO (7.4 mL), MeOH (37 mL) and water (70 mL) was added, and the precipitated product was filtered off to give pure 3f. Yield 149 mg (71%); colorless crystals; mp 205–206 °C; 1H NMR (600 MHz, [D6]DMSO): δ = 8.48 (d, J = 8.7 Hz, 1H; NH), 7.91 (d, J = 8.7 Hz, 1H; 6-H), 7.34 (d, J = 8.7 Hz, 1H; 5-H), 5.38 (∼ddd, J = 10.4, 8.7, 5.5 Hz, 1H; 4-H), 3.90 (dd, J = 14.8, 10.4 Hz, 1H; 3-Hax), 3.65 (dd, J = 14.8, 5.5 Hz, 1H; 3-Heq), 2.98 (s, 3H; 2-CH3), 2.21–2.08 (m, 2H; O=C–CH2), 1.62–1.52 (m, 2H; CH2–CH2-CH3), 0.89 (t, J = 7.4 Hz, 3H; CH2–CH3); 13C{1H} NMR (150 MHz, [D6]DMSO): δ = 172.7 (O=C), 139.4 (C4a), 135.7 (C8a), 133.7 (C6), 133.0 (C7), 129.7 (C5), 128.4 (C8), 50.4 (C3), 40.6 (C4), 37.5 (O=C-CH2), 36.2 (2-CH3), 18.8 (CH2–CH2-CH3), 13.8 (CH2–CH3); IR (KBr): ν̃ = 3274 (w; NH), 1652 (m; O=C), 1347 (s; SO2), 1161 (m; SO2); HRMS (ESI) m/z: [M + H]+ Calcd for C13H17Cl2N2O3S 351.0331; Found 351.0331.
Acknowledgments
We are thankful to Mr. Péter Kővágó for the NMR and IR, to Dr. Éva Szabó and Mrs. Mónika Mezővári for the MS, to Dr. Mária Tóthné Lauritz, Ms. Dóra R. Németh and Dr. Péter Slégel for the HRMS, and to Dr. Nóra May for the ESR measurements. Z.K. is grateful for the general support of the Hungarian Academy of Science under the Premium Postdoctoral Research Program 2019. The financial support from NKFIH (OTKA K 120075) is also gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c02512.
Additional experimental and theoretical mechanism studies; detailed NMR structure eludication (including 1H and 13C NMR, DEPTQ, HSQC, HMBC, selective NOE, ROE, and TOCSY spectra) of new compounds; coordinates and energy values of the computed structures; conditions of X-ray diffraction measurements, cif files, and ORTEP diagrams (PDF)
Structure report files of 1a (CCDC 1995298) (PDF)
Structure report files of 3a·H2O (CCDC 1995299) (PDF)
Accession Codes
CCDC 1995298–1995299 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Bach R.; Harthong S.; Lacour J.. Nitrogen- and Sulfur-based Stevens and Related Rearrangements. Comprehensive Organic Synthesis II, 2nd ed.; Knochel P., Molander G. A., Eds.; Elsevier: Amsterdam, 2014; pp 992–1037. [Google Scholar]
- Ghigo G.; Cagnina S.; Maranzana A.; Tonachini G. The Mechanism of the Stevens and Sommelet-Hauser Rearrangements. A Theoretical Study. J. Org. Chem. 2010, 75, 3608–3617. 10.1021/jo100367z. [DOI] [PubMed] [Google Scholar]
- Biswas B.; Collins S. C.; Singleton D. Dynamics and a Unified Understanding of Competitive [2,3]- and [1,2]-Sigmatropic Rearrangements Based on a Study of Ammonium Ylides. J. Am. Chem. Soc. 2014, 136, 3740–3743. 10.1021/ja4128289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J. P.The Wittig Rearrangement. Comprehensive Organic Synthesis II, 2nd ed.; Knochel P., Molander G. A., Eds.; Elsevier: Amsterdam, 2014; pp 1038–1072. [Google Scholar]
- Rycek L.; Hudlicky T. Applications of the Wittig-Still Rearrangement in Organic Synthesis. Angew. Chem., Int. Ed. 2017, 56, 6022–6066. 10.1002/anie.201611329. [DOI] [PubMed] [Google Scholar]
- Dahn H.; Solms U. Rearrangement of Tertiary Amines by Lithium Aluminum Hydride. Helv. Chim. Acta 1951, 34, 907–915. 10.1002/hlca.19510340323. [DOI] [Google Scholar]
- Everett R. K.; Wolfe J. P. Aza-Wittig Rearrangements of N-Benzyl and N-Allyl Glycine Methyl Esters. Discovery of a Surprising Cascade Aza-Wittig Rearrangement/Hydroboration Reaction. J. Org. Chem. 2015, 80, 9041–9056. 10.1021/acs.joc.5b01286. [DOI] [PubMed] [Google Scholar]
- Everett R. K.; Wolfe J. P. Aza-[1,2]-Wittig Rearrangements of N-Benzyl Glycine Methyl Esters. A New Approach to the Synthesis of N-Aryl Phenylalanine Derivatives. Tetrahedron Lett. 2015, 56, 3393–3395. 10.1016/j.tetlet.2015.01.037. [DOI] [Google Scholar]
- Anderson J. C.; Flaherty A.; Swarbrick M. E. The Aza-[2,3]-Wittig Sigmatropic Rearrangement of Acyclic Amines: Scope and Limitations of Silicon Assistance. J. Org. Chem. 2000, 65, 9152–9156. 10.1021/jo0056343. [DOI] [PubMed] [Google Scholar]
- Motaleb A.; Rani S.; Das T.; Gonnade R. G.; Maity P. Phosphite-catalyzed C-H Allylation of Azaarenes via an Enantioselective [2,3]-Aza-Wittig Rearrangement. Angew. Chem., Int. Ed. 2019, 58, 14104–14109. 10.1002/anie.201906681. [DOI] [PubMed] [Google Scholar]
- Forrest A. K.; Schmidt R. R. De Novo Synthesis of Carbohydrates and Related Natural Products. 16. De Novo Synthesis of Carbohydrates - Preparation of 4-Amino-4-deoxy-D,L-ribose Derivatives. Tetrahedron Lett. 1984, 25, 1769–1772. 10.1016/S0040-4039(01)90037-1. [DOI] [Google Scholar]
- Baiocchi L.; Picccni G. The Isomerization of Dialkyl Indazolones to Dihydroquinazolinones. Tetrahedron Lett. 1986, 27, 5255–5256. 10.1016/S0040-4039(00)85183-7. [DOI] [Google Scholar]
- Taylor E. C.; Clemens R. J.; Davies H. M. L.; Haley N. F. Formation of Monocyclic and Bicyclic Aza-β-lactams and Other Novel Heterocycles from 1-(Diphenylmethylene)-3-oxo-1,2-diazetidinium Inner Salt. J. Am. Chem. Soc. 1981, 103, 7659–7660. 10.1021/ja00415a046. [DOI] [Google Scholar]
- Taylor E. C.; Davies H. M. L. Synthesis of Fused 1,2-Diazetidinones via an Intramolecular Horner-Emmons Reaction. J. Org. Chem. 1986, 51, 1537–1740. 10.1021/jo00359a029. [DOI] [Google Scholar]
- Gong Y.; Bausch M. J.; Wang L. Fluorenyl Participated Ring Transformation of Urazoles to Triazinanediones. Heterocycles 2001, 55, 163–170. 10.3987/COM-00-9087. [DOI] [Google Scholar]
- Gong Y.; Bausch M. J.; Wang L. Acylimine-mediated N-N Bond Cleavage of Pyrazolidinediones and Subsequent Conversion to Dihydropyrimidinediones and Malonamides. Tetrahedron Lett. 2001, 42, 1–4. 10.1016/S0040-4039(00)01865-7. [DOI] [Google Scholar]
- Magnus P.; Garizi N.; Seibert K. A.; Ornholt A. Synthesis of Carbamates from Diethoxycarbonyl Hydrazine Derivatives by E1cB Eliminative Cleavage of the N-N’-Bond Rather Than Reduction. Org. Lett. 2009, 11, 5646–5648. 10.1021/ol902313v. [DOI] [PubMed] [Google Scholar]
- Tayama E.; Kobayashi Y.; Toma Y. Diaza [1,4]-Wittig-type Rearrangement of N-Allylic-N-boc-hydrazines Into γ-Amino-N-boc-enamines. Chem. Commun. 2016, 52, 10570–10573. 10.1039/C6CC04626F. [DOI] [PubMed] [Google Scholar]
- For synthetic strategies, see:; a Zhang S.; Cha L.; Li L.; Hu Y.; Li Y.; Zha Z.; Wang Z. Asymmetric Formal Aza-Diels-Alder Reaction of Trifluoromethyl Hemiaminals with Enones Catalyzed by Primary Amines. J. Org. Chem. 2016, 81, 3177–3187. 10.1021/acs.joc.6b00087. [DOI] [PubMed] [Google Scholar]; b Mishra A.; Mukherjee U.; Vats T. K.; Deb I. Ir(III)/MPAA-catalyzed Mild and Selective C-H Amidation of N-Sulfonyl Ketimines: Access to Benzosultam-fused Quinazolines/Quinazolinones. J. Org. Chem. 2018, 83, 3756–3767. 10.1021/acs.joc.8b00125. [DOI] [PubMed] [Google Scholar]; c Dawsey A. C.; Potter C.; Knight J. D.; Kennedy Z. C.; Smith E. A.; Acevedo-Jake A. M.; Puciaty A. J.; Metz C. R.; Beam C. F.; Pennington W. T.; Van-Derveer D. G. Preparation of 2H-Spiro[benzo[d]isothiazole-3,3′-pyrazole]-1,1-dioxide-2′(4′H)-carboxylates from Dilithiated C(α),N-Carboalkoxyhydrazones and Methyl-2-(aminosulfonyl)benzoate. J. Heterocycl. Chem. 2009, 46, 231–236. 10.1002/jhet.50. [DOI] [Google Scholar]; d Ahn K. H.; Baek H.-H.; Lee S. J.; Cho C. W. Synthesis of Chiral Benzosultams: 3-Functionalized 1,2-Benzisothiazoline-1,1-dioxides. J. Org. Chem. 2000, 65, 7690–7696. 10.1021/jo000952n. [DOI] [PubMed] [Google Scholar]; e Hai Y.; Zou H.; Ye H.; You L. Three Switchable Orthogonal Dynamic Covalent Reactions and Complex Networks Based on the Control of Dual Reactivity. J. Org. Chem. 2018, 83, 9858–9869. 10.1021/acs.joc.8b01332. [DOI] [PubMed] [Google Scholar]; f Mahy J.-P.; Ciesielski J.; Dauban P. Catalytic C-H Amination: A Reaction Now Accessible to Engineered Natural Enzymes. Angew. Chem., Int. Ed. 2014, 53, 6862–6864. 10.1002/anie.201403654. [DOI] [PubMed] [Google Scholar]; g McIntosh J. A.; Coelho P. S.; Farwell C. C.; Wang Z. J.; Lewis J. C.; Brown T. R.; Arnold F. H. Enantioselective Intramolecular C-H Amination Catalyzed by Engineered Cytochrome P450 Enzymes In Vitro and In Vivo. Angew. Chem., Int. Ed. 2013, 52, 9309–9312. 10.1002/anie.201304401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For derivatives showing antidiabetic and antiobesity effect, see:; a Azevedo C. M. G.; Watterson K. R.; Wargent E. T.; Hansen S. V. F; Hudson B. D.; Kȩpczyńska M. A.; Dunlop J.; Shimpukade B.; Christiansen E.; Milligan G.; Stocker C. J.; Ulven T. Non-acidic Free Fatty Acid Receptor 4 Agonists with Antidiabetic Activity. J. Med. Chem. 2016, 59, 8868–8878. 10.1021/acs.jmedchem.6b00685. [DOI] [PubMed] [Google Scholar]; b Raimundo B.; Koltun E. S.; Griffin J.; Stangeland E.. Preparation of Heteroaryl Saccharins and Related Compounds Useful as Gpr120 Modulators for the Treatment of Diseases. Numerate Inc.; WO 2018049324.
- For derivatives with antimicrobial and antifungal effect, see:; a Singh R.; Jakhar K. Green Synthesis of Saccharin Substituted Urea and Thiourea Derivatives and Their Antimicrobial Evaluation. Der Pharma Chem. 2016, 8, 175–181. [Google Scholar]; b Taher E. S.; Ibrahim T. S.; Fares M.; Al-Mahmoudy A. M. M.; Radwan A. F.; Orabi K. Y.; El-Sabbagh O. I. Novel Benzenesulfonamide and 1,2-Benzisothiazol-3(2H)-one-1,1-dioxide Derivatives as Potential Selective COX-2 Inhibitors. Eur. J. Med. Chem. 2019, 171, 372–382. For anti-inflammatory activity, see: 10.1016/j.ejmech.2019.03.042. [DOI] [PubMed] [Google Scholar]
- For inhibitors of acetyl choline esterase, see:; Panek D.; Wiȩckowska A.; Wichur T.; Bajda M.; Godyn J.; Jonczyk J.; Mika K.; Janockova J.; Soukup O.; Knez D.; Korabecny J.; Gobec S.; Malawska B. Design, Synthesis and Biological Evaluation of New Phthalimide and Saccharin Derivatives with Alicyclic Amines Targeting Cholinesterases, Beta-secretase and Amyloid Beta Aggregation. Eur. J. Med. Chem. 2017, 125, 676–695. 10.1016/j.ejmech.2016.09.078. [DOI] [PubMed] [Google Scholar]
- For tyrosine kinase inhibitors, see:; Gençer N.; Demir D.; Sonmez F.; Kucukislamoglu M. New Saccharin Derivatives as Tyrosinase Inhibitors. Bioorg. Med. Chem. 2012, 20, 2811–2821. 10.1016/j.bmc.2012.03.033. [DOI] [PubMed] [Google Scholar]
- For derivatives with cancer-related carbonic anhydrase effect, see:; a Moeker J.; Peat T. S.; Bornaghi L. F.; Vullo D.; Supuran C. T.; Poulsen S.-A. Cyclic Secondary Sulfonamides: Unusually Good Inhibitors of Cancer-related Carbonic Anhydrase Enzymes. J. Med. Chem. 2014, 57, 3522–3531. 10.1021/jm500255y. [DOI] [PubMed] [Google Scholar]; b Carradori S.; Secci D.; De Monte C.; Mollica A.; Ceruso M.; Akdemir A.; Sobolev A. P.; Codispoti R.; De Cosmi F.; Guglielmi P.; Supuran C. T. A Novel Library of Saccharin and Acesulfame Derivatives as Potent and Selective Inhibitors of Carbonic Anhydrase IX and XII Isoforms. Bioorg. Med. Chem. 2016, 24, 1095–1105. 10.1016/j.bmc.2016.01.038. [DOI] [PubMed] [Google Scholar]; c D’Ascenzio M.; Carradori S.; De Monte C.; Secci D.; Ceruso M.; Supuran C. T. Design, Synthesis and Evaluation of N-Substituted Saccharin Derivatives as Selective Inhibitors of Tumor-associated Carbonic Anhydrase XII. Bioorg. Med. Chem. 2014, 22, 1821–1831. 10.1016/j.bmc.2014.01.056. [DOI] [PubMed] [Google Scholar]
- For 3-amino derivatives with insecticide activity, see: Pohlman M.; Von Deyn W.; Kaiser F.; Rack M.; Bastiaans H. M. M.; Anspaugh D. D.; Culbertson D. L.; Cotter H. V. T. (BASF Se.). Preparation of 3-Amino-1,2-benzisothiazole Derivatives as Acaricides, Insecticides and Nematocides. WO 2007057407.
- Porcs-Makkay M.; Gyűjtő I.; Simig G.; Volk B. Synthesis and Base-mediated Rearrangement of 3-Acetyl-2-methyl-3,4-dihydro-2H-1,2,3-benzothiadiazine-1,1-dioxides. Tetrahedron 2016, 72, 8463–8469. 10.1016/j.tet.2016.11.021. [DOI] [Google Scholar]
- a Hanchate V.; Muniraj N.; Prabhu K. R. Rh(III)-Catalyzed Oxidative Annulation of Sulfoximines with Arylalkynyl Silanes via Desilylation. J. Org. Chem. 2019, 84, 8248–8255. 10.1021/acs.joc.9b00743. [DOI] [PubMed] [Google Scholar]; b Cheng Y.; Bolm C. Regioselective Syntheses of 1,2-benzothiazines by Rhodium-catalyzed Annulation Reactions. Angew. Chem., Int. Ed. 2015, 54, 12349–12352. 10.1002/anie.201501583. [DOI] [PubMed] [Google Scholar]; c Shen B.; Wan B.; Li X. Enantiodivergent Desymmetrization in the Rhodium(III)-catalyzed Annulation of Sulfoximines with Diazo Compounds. Angew. Chem., Int. Ed. 2018, 57, 15534–15538. 10.1002/anie.201810472. [DOI] [PubMed] [Google Scholar]; d Sun Y.; Cramer N. Enantioselective Synthesis of Chiral-at-sulfur 1,2-benzothiazines by CpxRhIII-Catalyzed C-H Functionalization of Sulfoximines. Angew. Chem., Int. Ed. 2018, 57, 15539–15543. 10.1002/anie.201810887. [DOI] [PubMed] [Google Scholar]; e Xie H.; Lan J.; Gui J.; Chen F.; Jiang H.; Zeng W. Ru(II)-catalyzed Coupling-cyclization of Sulfoximines with alpha-Carbonyl Sulfoxonium Ylides as an Approach to 1,2-benzothiazines. Adv. Synth. Catal. 2018, 360, 3534–3543. 10.1002/adsc.201800753. [DOI] [Google Scholar]; f Li Y.; Liu X.-Y.; Xu Y.-J.; Dong L. Rhodium(III)-catalyzed Tandem Annulation Reaction to Build Polycyclic Benzothiazine Derivatives. Org. Chem. Front. 2019, 6, 2457–2461. 10.1039/C9QO00579J. [DOI] [Google Scholar]
- For reviews, see:; a Chattopadhyay S. K. Recent Advancement in the Synthesis of 1,2- and 2,1-Benzothiazines. Synth. Commun. 2018, 48, 3033–3078. 10.1080/00397911.2018.1527355. [DOI] [Google Scholar]; b Gouda M. A.; Hussein B. H. M.; El-Said Sherif Y. Synthesis and Medicinal Importance of Oxicams and Their Analogues. Synth. Commun. 2017, 47, 1709–1736. 10.1080/00397911.2017.1350983. [DOI] [Google Scholar]
- For nonsteroidal antiinflammatory activity (piroxicam, ampiroxicam), see:; Zia-Ur-Rehman M.; Choudary J. A.; Elsegood M. R. J.; Siddiqui H. L.; Khan K. M. A Facile Synthesis of Novel Biologically Active 4-Hydroxy-N’-(benzylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide-1,1-dioxides. Eur. J. Med. Chem. 2009, 44, 1311–1316. 10.1016/j.ejmech.2008.08.002. [DOI] [PubMed] [Google Scholar]
- For inhibitors of calpain I, see:; Bihovsky R.; Tao M.; Mallamo J. P.; Wells G. J. 1,2-benzothiazine 1,1-Dioxide α-Ketoamide Analogues as Potent Calpain I Inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 1035–1038. 10.1016/j.bmcl.2003.11.037. [DOI] [PubMed] [Google Scholar]
- For monoamine oxidase activity, see:; Saddique F. A.; Zaib S.; Jalil S.; Aslam S.; Ahmad M.; Sultan S.; Naz H.; Iqbal M.; Iqbal J. Synthesis, Monoamine Oxidase Inhibition Activity and Molecular Docking Studies of Novel 4-Hydroxy-N’-[benzylidene or 1-Phenylethylidene]-2-H/methyl/benzyl-1,2-benzothiazine-3-carbohydrazide-1,1-dioxide. Eur. J. Med. Chem. 2018, 143, 1373–1386. 10.1016/j.ejmech.2017.10.036. [DOI] [PubMed] [Google Scholar]
- For alkaline phosphatase activity, see:; Ashraf A.; Ejaz S. A.; Rahman S. U.; Siddiqui W. A.; Arshad M. N.; Lecka J.; Sévigny J.; Zayed M. E. M.; Asiri A. M.; Iqbal J.; Hartinger C. G.; Hanif M. Hybrid Compounds from Chalcone and 1,2-Benzothiazine Pharmacophores as Selective Inhibitors of Alkaline Phosphatase Isozymes. Eur. J. Med. Chem. 2018, 159, 282–291. 10.1016/j.ejmech.2018.09.063. [DOI] [PubMed] [Google Scholar]
- For aldose reductase activity for the treatment of diabetic complications, see:; Chen X.; Zhang S.; Yang Y.; Hussain S.; He M.; Gui D.; Ma B.; Jing C.; Qiao Z.; Zhu C.; Yu Q. 1,2-Benzothiazine-1,1-dioxide Carboxylate Derivatives as Novel Potent Inhibitors of Aldose Reductase. Bioorg. Med. Chem. 2011, 19, 7262–7269. 10.1016/j.bmc.2011.07.051. [DOI] [PubMed] [Google Scholar]
- For anti-inflammatory and anticancer activities, see:; Swaroop D. K.; Kumar N. R.; Ratnakarreddy K.; Raja G.; Srigiridhar K.; Poornachandra Y.; Kumar C. G.; Babu N. J.; Kumar G. S.; Narsaiah B. Novel 1,2,3-Triazole-functionalized 1,2-Benzothiazine-1,1-dioxide Derivatives: Regioselective Synthesis, Biological Evaluation and Docking Studies. Chemistry Select 2018, 3, 2398–2403. 10.1002/slct.201800072. [DOI] [Google Scholar]
- For antimicrobial and antioxidant effects, see:; Tamatam R.; Panga S. S.; Grigory Z.; Gundala S.; Nemallapudi B. R.; Vasikarla K. P.; Adivireddy P.; Venkatapuram P. Synthesis, Antimicrobial and Antioxidant Activities of Pyrimidinyl Benzothiazine Carboxamides. Chemistry Select 2019, 4, 6813–6820. 10.1002/slct.201900525. [DOI] [Google Scholar]
- Simple PMO analysis of V– also justifies this reversed reactivity (compared to enamides): the HOMO is localized at N(2), and the methylidene H2C= group has a significant contribution to the LUMO (SI, Figure S4). So far, only one example is present in the literature for the addition of an amine to the β-position of an enamide, but it occurs photocatalytically, see:; Musacchio A. J.; Lainhart B. C.; Zhang X.; Naguib S. G.; Sherwood T. C.; Knowles R. R. Catalytic Intermolecular Hydroaminations of Unactivated Olefins with Secondary Alkyl Amines. Science 2017, 355, 727–730. 10.1126/science.aal3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyűjtő I.; Porcs-Makkay M.; Lukács G.; Pusztai G.; Garádi Z.; Tóth G.; Nyulasi B.; Simig G.; Volk B. Synthesis of 4-Methyl-2H-1,2,3-benzothiadiazine-1,1-dioxides and Their Further Transformation via Alkylation and Reduction Steps. Synth. Commun. 2019, 49, 3475–3485. 10.1080/00397911.2019.1673777. [DOI] [Google Scholar]
- Peng J.; Chen C.; Wang Y.; Lou Z.; Li M.; Xi C.; Chen H. Direct Vicinal Disubstitution of Diaryliodonium Salts by Pyridine N-Oxides and N-Amidates by a [1,3]-Radical Rearrangement. Angew. Chem., Int. Ed. 2013, 52, 7574–7578. 10.1002/anie.201303347. [DOI] [PubMed] [Google Scholar]
- Barta-Szalai G.; Fetter J.; Lempert K.; Møller J.; Párkányi L. Electron Deficient Heteroaromatic Ammonioamidates. Part 24. N-(Quinazolin-3-io)amidates. Part 11. The Photochemistry of N-(6,7-Methylenedioxyquinazolin-3-io)amidates in Acetone. J. Chem. Soc., Perkin Trans. 1 1983, 2003–2009. 10.1039/P19830002003. [DOI] [Google Scholar]
- Riedl Z.; Kövér P.; Soós T.; Hajós G.; Egyed O.; Fábián L.; Messmer A. Unexpected Ring Transformation to Pyrrolo[3,2-b]pyridine Derivatives. Fused Azolium Salts. 22. J. Org. Chem. 2003, 68, 5652–5659. 10.1021/jo034231a. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; et al. Gaussian 09, revision A.02; Gaussian, Inc.: Wallingford, CT, 2016. [Google Scholar]
- Zhao Y.; Truhlar D. G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Tawa G. J.; Topol I. A.; Burt S. K.; Caldwell R. A.; Rashin A. A. Calculation of the Aqueous Solvation Free Energy of the Proton. J. Chem. Phys. 1998, 109, 4852–4863. 10.1063/1.477096. [DOI] [Google Scholar]
- Marenich A. V.; Cramer C. J.; Truhlar D. G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Chai J.-D.; Head-Gordon M. Long-range Corrected Hybrid Density Functionals with Damped Atom-atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- Hanwell M. D.; Curtis D. E.; Lonie D. C.; Vandermeersch T.; Zurek E.; Hutchison G. R. Avogadro: an Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminf. 2012, 4, 4. 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYLview , 1.0b; Legault C. Y.Université de Sherbrooke, 2009; http://www.cylview.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.