Abstract
OBJECTIVE
Ambrisentan, an endothelin receptor antagonist FDA-approved for the treatment of pulmonary arterial hypertension in adult patients, lacks an acceptable pediatric dosage form. The objective of this investigation was to determine the stability of an extemporaneously compounded ambrisentan suspension.
METHODS
Ambrisentan suspension was compounded to a concentration of 1 mg/mL using commercially available suspending agents. The suspension was then evenly split into 2 plastic amber prescription bottles. One bottle was stored at room temperature and under continuous fluorescent light while the other bottle was stored under refrigeration and protection from light. A fast and selective reversed-phase high-performance liquid chromatography (HPLC) method was developed and validated for the analysis of ambrisentan. HPLC analysis was performed on samples withdrawn from the stock bottles at predetermined time intervals, up to 90 days.
RESULTS
The developed HPLC method enabled the elution and detection of ambrisentan peak at 4.4 minutes. HPLC analysis revealed that all samples from both storage conditions retained >90% potency throughout the study timeframe. There were no signs of any ambrisentan breakdown products on HPLC analysis. Color and odor of the final product was also consistent throughout the 90-day storage period.
CONCLUSION
Ambrisentan suspension, compounded to 1 mg/mL, is stable at room temperature or under refrigeration for up to 90 days.
Keywords: ambrisentan; chromatography, high performance liquid; drug compounding; drug stability; pulmonary arterial hypertension
Introduction
Pediatric pulmonary arterial hypertension (PAH), characterized by elevation of pulmonary vascular resistance, can lead to right heart failure and early mortality if untreated. Therapeutic options include 3 classes of medications targeting altered pulmonary vascular endothelial function to promote pulmonary artery vasodilation. Over time, therapies have resulted in improved survival in pediatric patients with PAH.1,2
Ambrisentan, a selective endothelin A receptor antagonist, is currently approved by the FDA for the treatment of PAH in adult patients with improvement in outcome shown alone and in combination with tadalafil.3–5 Data in pediatric use are limited but suggest therapeutic benefit as well as tolerance.6 Despite limitations in administration to older patients given availability only in tablet form, ambrisentan continues to be used off-label in the treatment of pediatric PAH.7,8
Currently, the only FDA-approved medication for treatment of pediatric PAH is bosentan, which is now available in a dissolvable tablet for administration as a liquid to children >4 kg. Stability of a compounded suspension has been previously published.9 However, the need for monthly monitoring of liver function tests on bosentan given risk of hepatotoxicity makes ambrisentan a more attractive choice for children despite only being commercially available as a tablet in the United States.
Methods using reversed-phase high-performance liquid chromatography (HPLC) have been published for stability determination of solid tablet dosage form of ambrisentan.10–12 However, there are currently no reports for quality control of ambrisentan suspensions. The purpose of this study was to use HPLC to determine the length of stability of an oral suspension of ambrisentan extemporaneously compounded from commercial tablets and commercially available suspending agents under 2 sets of storage conditions.
Methods
Chemicals and Standards. Ambrisentan 5-mg tablets (Gilead Sciences, Inc, Foster City, CA) (LOT: 010976, expiration date: March 2021) were obtained from Children's Wisconsin. Reference standard ambrisentan and consumables for sample preparation and HPLC analysis were purchased from Fisher Scientific (Waltham, MA).
Instruments. HPLC analysis was performed on Prominence 2030 system (Shimadzu, Tokyo, Japan) equipped with Kinetix RP column (C18, 50 × 2.1 mm, 5 micron, Phenomenex, Torrance, CA), autosampler and ultraviolet-visible spectroscopy (UV-VIS) photodiode array detector. HPLC solvents were acetonitrile (solvent A) and 0.1% aqueous formic acid (solvent B). Gradient elution, at 25°C and a flow rate of 1 mL/min, was as follows: 5% (A in B) for 1 minute, 5% to 100% (A) for 6 minutes, 100% (A) for 1 minute, 5% (A) for 2 minutes. Samples were run in triplicates with an injection volume of 10 μL and detection at 260 nm.
Standard Stock Solutions. Stock 1 was used for generation of calibration curve and was prepared by dissolving 10 mg standard ambrisentan in 10 mL methanol (1000 mcg/mL). Stock 2 was used for validation of accuracy and precision and was prepared identical to stock 1.
Analytical Method Validation. The developed HPLC method was validated for specificity, accuracy, and precision according to FDA guidelines for quality control of human pharmaceuticals using standard ambrisentan solutions.13 A calibration curve was generated from stock 1 by preparing 7 concentrations of ambrisentan in methanol in the range of 20 to 200 mg/L. Linear regression was performed on data representing nominal concentration of each concentration and its corresponding peak area for ambrisentan. Specificity was confirmed by the lack of overlapping eluting peaks in forced degradation studies and compounded preparations. Ambrisentan peak purity was verified by overlaying ambrisentan UV spectra at peak start, middle, and end. Accuracy was validated by determining the concentration of stock 2 at 50, 100, and 150 mg/L (Table 1). Interday precision was validated by comparing the experimental concentration values of stock 2 at the 3 chosen levels for 3 consecutive days (Table 1). Injection volume was 10 μL and all samples were run in triplicates.
Table 1.
Method Accuracy and Precision at 3 Levels of Ambrisentan Concentrations
| Accuracy | Precision | |||||
|---|---|---|---|---|---|---|
| Target, mg/L | 50.0 | 100.0 | 150.0 | 50.0 | 100.0 | 150.0 |
| Percent,* mean ± SD (SE) | 50.3 ± 0.8 (1.7) | 98.3 ± 0.4 (0.4) | 147.6 ± 0.3 (0.2) | 49.3† ± 2.1 (4.2) | 97.9† ± 0.7 (0.7) | 148.8† + 1.8 (1.2) |
| Recovery, % | 100.5 | 98.3 | 98.4 | 98.5 | 97.9 | 99.2 |
* Mean of 3 replica for accuracy and intraday precision at each concentration.
† Mean of stock 2 over 3 consecutive days.
Forced Degradation Studies. Five samples were prepared by dissolving 100 μL stock 1 ambrisentan solution in 900 μL of each of the following: deionized water (×2), 0.5 N HCl, 0.5 N NaOH, and 10% H2O2 to obtain a nominal concentration of 100 mcg/mL. One of the 2 deionized water samples was subjected to 3 freeze-thaw cycles and then analyzed. The remaining samples were subjected to the conditions outlined in Table 2 and then analyzed via HPLC.
Table 2.
Summary of Forced Degradation Experiments of Standard Ambrisentan
| Stressor | Temperature, °C | Time, hr | Recovered Ambrisentan, % | Degradation Products |
|---|---|---|---|---|
| Acid hydrolysis (0.5 N HCl) | 60 | 3 | 1 | At least 1 |
| Base hydrolysis (0.5 N NaOH) | 60 | 8 | 100 | 0 |
| Aqueous hydrolysis | 60 | 8 | 100 | 0 |
| Oxidation (10% H2 O2) | 24 | 24 | 43 | At least 4 |
| Freeze/thaw | −20/24 | 18/6 (×3) | 100 | 0 |
Preparation of Ambrisentan Suspension. Ambrisentan suspension was compounded in a vertical flow hood with the compounding personnel wearing appropriate personal protective equipment for hazardous medications as defined by the National Institute for Occupational Safety and Health.14 Twelve 5-mg tablets of ambrisentan were used to prepare 60 mL of 1 mg/mL ambrisentan suspension. The 12 tablets were triturated in a porcelain mortar using a porcelain pestle. Approximately 5 mL of glycerin were added to the crushed tablets to aid in the production of a fine powder. After the tablets were sufficiently triturated and whetted with glycerin, approximately 10 mL of Ora-Plus (Humco, Austin, TX) were added while continuing to levigate the mixture with the pestle. The mixture was then transferred to a 60-mL light resistant prescription bottle. The mortar was rinsed several times with portions of the remaining Ora-Plus solution to ensure all ambrisentan was transferred to the prescription bottle. After a total of 30 mL of Ora-Plus had been added, the bottle was then brought to volume with approximately 25 mL of Ora-Sweet (Humco) and shaken well. The suspension, now containing the crushed ambrisentan tablets, 5 mL of glycerin, 30 mL of Ora-Plus, and approximately 25 mL of Ora-Sweet, was then divided evenly into 2 portions, both in light resistant plastic amber prescription bottles. Bottle A (SA) was stored at ambient room temperature (24 ± 3°C) under continuous florescent light exposure. Bottle B (SB) was stored in the dark and under refrigeration (4 ± 0.8°C). Sampling was done from both bottles at 7 time points (day 0, 5, 10, 14, 30, 60, and 90). Each bottle was vigorously shaken prior to each sampling. After shaking, three 1-mL samples were withdrawn from each bottle using a pipette. The collected samples were then labeled and stored at −20°C ± 0.4 until analysis.
Stability Sample Preparation for HPLC Analysis. Frozen samples were allowed to thaw to room temperature. Two hundred microliters were then transferred from each sample to a 10-mL volumetric flask, diluted to 10 mL with methanol-water (1:1) mixture, and ultrasonicated for 1 minute to generate a nominal ambrisentan concentration of 20 mcg/mL. An aliquot was passed through a 0.45-micron filter into an HPLC vial for analysis.
Data Acquisition and Management. LabSolution (Shimadzu) was used to control the HPLC instrument and to generate calibration curve and analytical chromatograms. Statistical analysis of data was performed in Excel (Microsoft Office 365, Seattle, WA) and Prism 5.0 (GraphPad Software, Inc, San Diego, CA). Comparisons of percent ambrisentan recovery at different time points were compared via Student t test. Statistical significance was set at an μ value of 0.05.
Results
The developed analytical method enabled the elution and detection of ambrisentan peak at 4.4 minutes (Figure 1A). No interfering peaks were observed in the HPLC chromatogram. Ambrisentan UV spectra overlapped at the beginning, middle, and end of each eluting peak, which verified peak purity. Two peaks, corresponding to methyl-p-hydroxybenzoate (methyl paraben) and benzoic acid eluted in all stability samples, were observed at 2.4 and 2.7 minutes, respectively (Figure 1B). Methyl paraben is a common ingredient in Ora-Plus and Ora-Sweet, and benzoic acid is one of its hydrolytic products. These observations demonstrate the specificity of the developed method.
Figure 1.
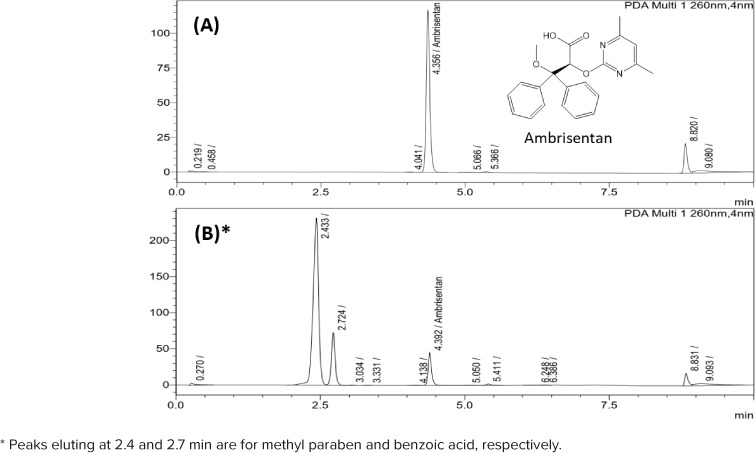
Chemical structure and reversed-phase high-performance liquid chromatography chromatograms of (A) ambrisentan reference standard (stock 1) and (B) compounded ambrisentan in Ora-Plus/Ora-Sweet mixture at day 0.
The 7-point calibration curve generated using stock 1 included the following concentrations: 20, 40, 60, 100, 120, 160, and 200 mcg/mL. The linear regression equation for the curve was calculated as y = 9176.4x + 32636 with a regression coefficient (R2) > 0.999.
Method accuracy and precision were also validated by repeated analysis of quality control samples prepared at 3 concentrations of stock 2 (50, 100, and 150 mcg/mL). Accuracy was 98.3% to 100.5% of target concentrations of quality control samples. Intraday and interday precision was also within acceptable range with standard error values for all analyses at less than 5%. A summary of method accuracy and precision is shown in Table 1. Forced degradation studies further supported method specificity due to lack of peak overlap between ambrisentan and degradation products obtained under different stress conditions as shown in Table 2 and Figure 2.10
Figure 2.
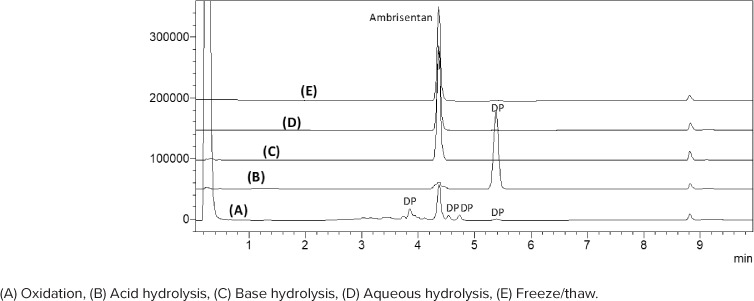
Reversed-phase high-performance liquid chromatography chromatograms of forced degradation experiments of standard ambrisentan showing degradation products (DP).
The ambrisentan formulation prepared from the ingredients and methods listed provided a pharmaceutically acceptable oral suspension with no signs of tablet clumping or other undesirable qualities. The color and odor did not change throughout the 90-day storage period for either bottle. Some settling of contents was observed after the bottles had been undisturbed for over 7 days but after shaking the suspension was quickly restored. HPLC chromatograms of all analyzed samples did not show major peaks indicative of significant decomposition products (Figure 3). Overall, ambrisentan potency in room temperature samples was comparable with that of the refrigerated samples with mean potency of 96.4 % and 97.5 %, respectively. There was no statistically significant difference between the potencies of the samples under different storage conditions (t(12) = 1.039, p = 0.319). Determined ambrisentan concentrations (as percent of target) in all tested samples are shown in Table 3.
Figure 3.
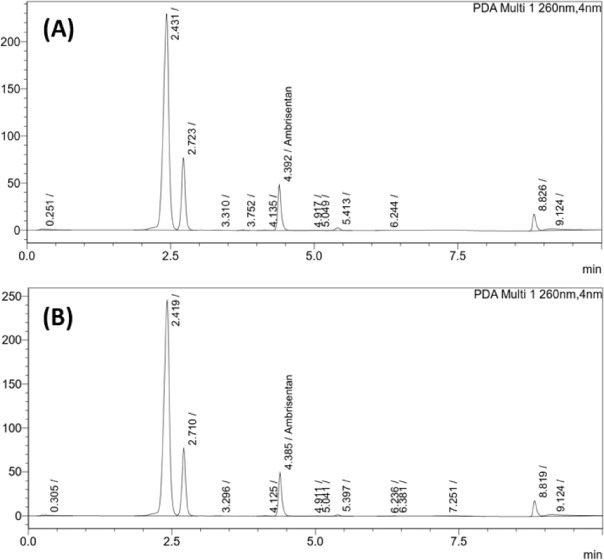
Reversed-phase high-performance liquid chromatography chromatograms of ambrisentan suspension after 90 days of storage at (A) room temperature and continuous light exposure; and (B) refrigeration in the dark at 4°C.
Table 3.
Percent of Target Concentration of Ambrisentan Samples Under 2 Sets of Storage Conditions
| Storage Conditions | Day | Cumulative Mean | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 14 | 30 | 60 | 90 | ||
| SA,* mean ± SD, % | 93.7 ± 0.1 | 94.7 ± 1.3 | 99.2 ± 2.4 | 96.5 ± 1.7 | 94.0 ± 7.7 | 100.1 ± 0.8 | 96.6 ± 5.3 | 96.4 ± 2.8 |
| SB,* mean ± SD, % | 97.8 ± 0.3 | 97.8 ± 8.5 | 97.2 ± 6.1 | 97.5 ± 4.5 | 99.1 ± 0.8 | 98.4 ± 4.7 | 94.8 ± 1.6 | 97.5 ± 3.8 |
SA, ambrisentan bottle A stored at room temperature of 24 ± 3°C; SB, ambrisentan bottle B stored in refrigerator at 4 ± 0.8°C
* n = 3.
Discussion
Ambrisentan, an endothelin receptor antagonist, is currently used off-label for the treatment of pediatric PAH. Availability only as a tablet limits use to older children or encourages splitting of tablets at home, which may be prone to dosage error as well as increase the risk of exposure given known embryo-fetal toxicity. To improve the ability to administer this therapy to younger children, this study assessed the stability of an extemporaneously prepared oral suspension of ambrisentan and determined retention of potency for up to 90 days. Currently, pediatric dosing is often based on simple fractions (10%, 25%, 50%, etc.) of the available commercial dosage forms. The chosen suspension concentration of 1 mg/mL allows for easily measurable liquid dosages of these fractions of the smallest tablet size (ambrisentan 5-mg tablet).
Previously, the reported stability of compounded bosentan along with trials in pediatric PAH patients demonstrating safety and tolerability prompted FDA approval of bosentan with dosage guidelines for patients ≥ 3 years of age and > 4 kg.9,15,16 Ambrisentan has several advantages over bosentan, such as avoiding drug-drug interactions with the phosphodiesterase type-5 inhibitors and potential synergistic effects when combined with tadalafil as shown in the Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension (AMBITION) trial.5 Additionally, the FDA-mandated bosentan risk evaluation and mitigation strategy program requires monthly liver function test monitoring for all patients, which proves difficult particularly in young infants and children. With the development of this stable compounded suspension of ambrisentan, a safe and effective alternate endothelin receptor antagonist therapy for pediatric patients is available.
A simple, specific, and fast HPLC method was developed, validated, and applied for quality control and stability testing of an ambrisentan suspension compounded from 5-mg tablets. This compounded ambrisentan suspension at 1 mg/mL will provide practitioners with the ability to safely and accurately prescribe patients small doses of ambrisentan that would have not otherwise been possible with the commercial dosage forms. Based on the described visual observation and analytical results, the suspension presented here is stable and devoid of any signs of potential breakdown products. It is compounded with a relatively simple formula that is familiar to pharmacists and with common ingredients. Finally, stability was shown at both room temperature and under refrigeration for up to 90 days, making transport and storage of the medication practical.
Conclusion
The developed HPLC method was validated for specificity, accuracy, and precision and used to determine the stability of ambrisentan suspensions. This is the first report on stability of an extemporaneously compounded ambrisentan suspension stored under 2 different conditions. With all samples retaining more than 90% of their potencies, compounded ambrisentan suspensions are acceptable for use within 90 days after preparation when stored in the refrigerator or at room temperature.
Acknowledgment
The authors thank Herma Heart Institute at Children's Wisconsin for providing ambrisentan tablets.
ABBREVIATIONS
- FDA
US Food and Drug Administration
- HPLC
reversed-phase high-performance liquid chromatography
- PAH
pulmonary arterial hypertension
- SA
ambrisentan bottle A
- SB
ambrisentan bottle B
Footnotes
Disclosure. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all data and take responsibility for the integrity and accuracy of the data analysis.
Ethical Approval and Informed Consent. Given the nature of this study, the institution board/ethics committee review was not required.
References
- 1.Ivy DD, Abman SH, Barst RJ et al. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62(suppl 25):D117–D126. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Ivy DD, Foreman AJ et al. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry) Am J Cardiol. 2014;113(1):147–155. doi: 10.1016/j.amjcard.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Olschewski H, Oudiz RJ et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hyper-tension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Am J Cardiol. 2014;113(1):147–155. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 4.Oudiz RJ, Galiè N, Olschewski H et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(21):1971–1981. doi: 10.1016/j.jacc.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Galie N, Barbera JA, Frost AE et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 6.Takatsuki S, Rosenzweig EB, Zuckerman W et al. Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr Pulmonol. 2013;48(1):27–34. doi: 10.1002/ppul.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abman SH, Hansmann G, Archer SL et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig EB, Abman SH, Adatia I et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53(1):1801916. doi: 10.1183/13993003.01916-2018. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik A, Gorman G, Coward L, Arnold JJ. Stability of an extemporaneously compounded oral suspension of bosentan. Hosp Pharm. 2016;51(5):389–395. doi: 10.1310/hpj5105-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramisetti NR, Kuntamukkala R. LC-MS/MS characterization of forced degradation products of ambrisentan: development and validation of a stability-indicating RPHPLC method. New J Chem. 2014;38(7):3050–3061. [Google Scholar]
- 11.Satheeshkumar N, Naveenkumar G. A stability-indicating reversed-phase high-performance liquid chromatography method for ambrisentan: an endothelin receptor antagonist. J Chromatogr Sci. 2014;52(8):894–898. doi: 10.1093/chromsci/bmt138. [DOI] [PubMed] [Google Scholar]
- 12.Narayana MB, Chandrasekhar KB, Rao BM. A validated specific stability-indicating RP-HPLC assay method for ambrisentan and its related substances. J Chromatogr Sci. 2014;52(8):818–825. doi: 10.1093/chromsci/bmt121. [DOI] [PubMed] [Google Scholar]
- 13.Leutzinger E, Frankewich R, Faustino P et al. Analytical procedures and method validation: highlights of the FDA's draft guidance. LCGC. 2001;19(1):74–79. [Google Scholar]
- 14.Connor TH, MacKenzie BA, DeBord DG, et al. National Institute for Occupational Safety and Health Cincinnati, OH: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2016. NIOSH list of antineoplastic and other hazardous drugs in health-care settings, 2016. DHHS (NIOSH) Publication Number 2016-161 (Supersedes 2014-138) [Google Scholar]
- 15.Berger RM, Haworth SG, Bonnet D et al. FUTURE-2: Results from an open-label, long-term safety and tolerability extension study using the pediatric formulation of bosentan in pulmonary arterial hypertension. Int J Cardiol. 2016;202:52–58. doi: 10.1016/j.ijcard.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 16.Berger RMF, Gehin M, Beghetti M et al. A bosentan pharmacokinetic study to investigate dosing regimens in paediatric patients with pulmonary arterial hypertension: FUTURE-3. Br J Clin Pharmacol. 2017;83(8):1734–1744. doi: 10.1111/bcp.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]


