Abstract
Purpose
Limited evidence is available on whether the white blood cell (WBC) count is a predictor of type 2 diabetes mellitus (T2DM) in non-obese individuals. This study aimed to determine whether WBC count could be used as an indicator for the prediction of incident T2DM among non-obese individuals using a large, community-based Korean cohort that was observed over 10 years.
Patients and methods
A total of 4211 non-obese adults without diabetes aged 40–69 years were selected from the Korean Genome and Epidemiology Study. The participants were divided into four groups according to WBC count quartiles. We prospectively assessed the hazard ratios (HRs) with 95% confidence intervals (CIs) for incident T2DM, based on the American Diabetes Association criteria, using multivariate Cox proportional hazards regression models over 10 years after the baseline survey.
Results
During the follow-up period, 592 (14.1%) participants had newly developed T2DM. The higher quartile of WBC count groups showed significantly higher cumulative T2DM incidence over 10 years after the baseline survey (log-rank test, P < 0.001). Compared with the HRs for individuals in the referent lowest quartile, the HR (95% CI) for incident T2DM in individuals in the highest quartile was 1.55 (1.10–2.18) after adjusting for confounding variables.
Conclusion
A higher WBC count predicts future incident T2DM among community-dwelling non-obese Korean adults. This study suggests that WBC count could facilitate the prediction of non-obese individuals susceptible to T2DM.
Keywords: white blood cell count, type 2 diabetes mellitus, chronic low-grade inflammation, non-obese adults
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by relative insulin deficiency caused by insulin resistance in target organs and pancreatic β-cell dysfunction.1 Worldwide, it is a challenging public health problem because of its high prevalence and concomitant risks of various complications, and it is a major leading cause of disability and death in adults globally.2,3 In addition, individuals with T2DM, on an average, have approximately 2.3 times higher medical expenditures than those without T2DM.4 The global prevalence of T2DM increased from 4.7% in 1980 to 9.3% in 2019, and is estimated to rise to 10.2% (578 million) by 2030.5,6 Similarly, in South Korea, the prevalence of T2DM has rapidly increased during the past several decades from 8.6% in 2001 to 14.4% in 2016.7,8 Furthermore, T2DM was the sixth leading cause of death in South Korea in 2018.9
Although obesity has long been considered a major risk factor for T2DM, susceptibility to T2DM has been reported to have heterogeneous features according to ethnicity and cultural subgroups.10 Specifically, Asians develop T2DM at a lower body-mass index (BMI) when compared with Caucasians, and the risk of T2DM starts at a lower BMI for Asians than for the Western populations.11,12 Indeed, the proportion of non-obese individuals among all T2DM patients was 52.6% for men and 47.9% for women in 2013 in Korea.8 Interestingly, the prevalence of obesity among Korean women has decreased gradually from around 2000,13,14 whereas the incidence of T2DM has increased steadily over the last decade.15 Thus, early identification of individuals at high risk of T2DM in non-obese adults is considered important from a public health perspective.
Insulin resistance and T2DM are closely related to chronic low-grade inflammation.16,17 Inflammation markers, such as the white blood cell (WBC) count, high-sensitivity C-reactive protein (hsCRP), and interleukin-6, are known to be associated with metabolic syndrome and T2DM.18–20 Furthermore, several studies have revealed that WBC count, which is commonly measured in routine laboratory examination panels, is related to insulin resistance and predicts the development of T2DM.21–24 In particular, it was reported that WBC count could be used as an indicator for the prediction of T2DM incidence among obese individuals.25 However, few prospective cohort studies have examined the relationship between WBC count and incident T2DM among non-obese individuals. Thus, this study aimed to determine whether WBC count could be used as an indicator for the prediction of incident T2DM among non-obese individuals using a large, community-based Korean cohort that was observed over 10 years.
Patients and Methods
Study Overview and Study Participants
Participants were recruited from the Korea Association Resource study, which is a part of the Korean Genome and Epidemiology Study (KoGES), a population-based prospective cohort study conducted by the Korean Centers for Disease Control and Prevention. Detailed information about the study participants and methodology of KoGES has been described in a previous study.26 In the KoGES study, adults aged 40–69 years in Ansung (a rural area) and Ansan (an urban area) were enrolled to investigate the prevalence of and risk factors for chronic diseases in Korea.
A total of 8840 adults aged 40–69 years were included in the baseline study conducted from 2001 to 2002. Baseline examinations were conducted in 2001–2002, and follow-up examinations continued biennially until 2012. Among the 8840 participants in the baseline survey, we excluded 3062 obese participants. We defined obese participants as those individuals with a BMI ≥ 25 kg/m2 based on the Asia-Pacific regional guidelines of the World Health Organization and International Obesity Task Force.27 We also excluded 613 participants because they either were previously diagnosed with T2DM or met the diagnostic criteria for T2DM in the baseline survey. Of the remaining participants, we also excluded 464 participants with a WBC count <4000 cells/mL or >10,000 cells/μL to rule out the possibility of an inflammatory disorder, infection, or bone marrow suppressive illness. During the 10-year follow-up period, 490 participants were further excluded due to loss to follow-up and incomplete follow-up data. After these exclusions, 4211 participants were included in the final analysis. All individuals voluntarily participated in the study, and informed consent was obtained from all participants. This study was approved by the Institutional Review Board of Yonsei University Yongin Severance Hospital (Institutional Review Board number 9–2016-0013). In addition, this study complied with the ethical principles underlined in the Declaration of Helsinki.
Clinical and Biochemical Measurement
The study data included the medical history and sociodemographic information recorded by a self-administered questionnaire, anthropometric measurements, and laboratory biochemical measurements. All study participants were also requested to answer a health-related behavior questionnaire that included topics related to smoking and alcohol consumption. In the present study, current smokers were individuals who smoked ≥ 100 cigarettes in their lifetime and currently smoked at the time of the study, and an alcohol drinker was defined as an individual who consumed alcohol more than twice a week.
Participants’ body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with participants wearing light indoor clothing and no shoes. The BMI was calculated as weight (kg) divided by the square of the height (m2). The waist circumference was measured to the nearest 0.1 cm at the midpoint between the lower border of the rib cage and the iliac crest at the end stage of normal expiration. Systolic and diastolic blood pressures were measured twice using a standard mercury sphygmomanometer (Baumanometer Standby; W.A. Baum, New York, NY, USA).
Blood samples were obtained in the morning after an overnight fast. WBC count was determined using a hematological analyzer (ADVIA 120; Siemens, Tarrytown, NY, USA). Glucose, total cholesterol, triglycerides, and high-density lipoprotein cholesterol levels were measured using an automatic analyzer (ADVIA 1650; Siemens, Tarrytown, NY, USA). Glycated hemoglobin A1c (HbA1c) levels were measured using high-performance liquid chromatography (Variant II; BioRad Laboratories, Hercules, CA, USA). Insulin levels were determined using a radioimmunoassay kit (LINCO Research, St. Charles, MO, USA) with intra-assay and inter-assay coefficients of variation ranging from 2.1% to 8.3%. The Denka Seiken (Tokyo, Japan) assay, which has been validated against the Dade Behring method, was used to measure hsCRP level. The homeostatic model assessment of insulin resistance (HOMA-IR) was determined using the following equation: fasting glucose (mg/dL) × fasting insulin (μIU/mL)/405. T2DM was defined as the presence of one or more of the following conditions: fasting glucose level ≥ 126 mg/dL, HbA1c ≥ 6.5%, and 2-hour plasma glucose level of oral glucose tolerance test ≥ 200 mg/dL, based on the American Diabetes Association,28 or ongoing treatment with oral antidiabetic medications or insulin therapy. Prediabetes was defined as one or more of the following conditions: fasting glucose level between 100–125 mg/dL, HbA1c level between 5.7–6.4%, and 2-hour plasma glucose level between 140–199 mg/dL in the oral glucose tolerance test.
Statistical Analyses
WBC count quartiles were categorized as follows: Q1, ≤ 5200; Q2, 5300–6100; Q3, 6200–7300; and Q4, ≥ 7400 cells/μL. The baseline characteristics of the study population according to WBC count quartiles were compared using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables according to the normality of distributions. The chi-squared test was used to compare the categorical variables. Continuous data are presented as means ± standard deviation or medians (interquartile range). Categorical data are presented as frequencies.
The hazard ratios (HRs) with 95% confidence intervals (CIs) for incident T2DM were calculated using multivariate Cox proportional hazards regression models after adjusting for potentially confounding variables. The lowest quartile, Q1, was set as the reference group for the WBC count. The cumulative incidence of T2DM was determined using the Kaplan-Meier curve. Log-rank tests were conducted to determine the differences in the cumulative incidence of T2DM among the groups. The Contal and O’Quigley method was used to determine the optimal cut-off point for the WBC count with maximized log-rank statistics.
All analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA). A p-value < 0.05 was considered as significant.
Results
Table 1 presents the baseline characteristics of 4211 participants without T2DM at baseline according to their WBC count quartiles. The mean values of a few cardiometabolic variables, such as blood pressure, triglycerides, and hsCRP levels, tended to increase proportionally with the WBC count quartiles. However, there was no significant difference in the median values of insulin and HOMA-IR among the WBC count quartiles.
Table 1.
Baseline Characteristics of the Study Population According to WBC Count Quartiles
| Total (n = 4211) | WBC Count Quartiles | |||||
|---|---|---|---|---|---|---|
| Q1 (≤ 5200) (n = 1006) | Q2 (5300–6100) (n = 1067) | Q3 (6200–7300) (n = 1115) | Q4 (≥ 7400) (n = 1023) | p-value | ||
| Age (years) | 52.2 ± 9.0 | 51.8 ± 9.0 | 52.3 ± 8.9 | 52.3 ± 9.3 | 52.2 ± 8.8 | 0.550 |
| Sex (%) | 47.8 | 36.6 | 45.5 | 50.4 | 58.4 | <0.001 |
| BMI (kg/m2) | 22.4 ± 1.8 | 22.4 ± 1.8 | 22.5 ± 1.7 | 22.5 ± 1.8 | 22.5 ± 1.8 | 0.360 |
| Waist circumference (cm) | 79.4 ± 8.0 | 78.6 ± 8.1 | 79.3 ± 7.8 | 79.6 ± 7.9 | 80.2 ± 8.1 | <0.001 |
| Systolic blood pressure (mmHg) | 119.6 ± 18.3 | 117.9 ± 17.4 | 119.4 ± 18.1 | 119.8 ± 18.7 | 121.5 ± 18.9 | <0.001 |
| Diastolic blood pressure (mmHg) | 77.8 ± 10.7 | 77.8 ± 10.7 | 78.7 ± 11.3 | 79.4 ± 11.5 | 79.8 ± 11.5 | <0.001 |
| Fasting plasma glucose (mg/dL) | 82.5 ± 8.7 | 82.2 ± 8.5 | 82.5 ± 8.8 | 82.7 ± 8.4 | 82.5 ± 9.2 | 0.545 |
| Insulin (μIU/mL) | 6.5 (4.9–8.8) | 6.4 (4.8–8.4) | 6.6 (4.9–8.7) | 6.6 (5.0–8.8) | 6.6 (4.8–9.1) | 0.359 |
| HOMA-IR | 1.32 (0.98–1.80) | 1.29 (0.97–1.74) | 1.32 (0.98–1.80) | 1.32 (1.00–1.82) | 1.32 (0.96–1.85) | 0.197 |
| Total cholesterol (mg/dL) | 187.5 ± 33.7 | 184.7 ± 32.5 | 187.3 ± 33.8 | 186.7 ± 33.2 | 191.3 ± 34.9 | <0.001 |
| Triglyceride (mg/dL) | 124.0 (94.0–169.0) | 111.0 (86.0–147.0) | 122.0 (93.0–164.0) | 127.0 (98.0–173.0) | 138.0 (101.0–192.0) | <0.001 |
| HDL cholesterol (mg/dL) | 46.1 ± 10.3 | 47.1 ± 10.3 | 46.1 ± 9.9 | 45.5 ± 10.2 | 45.7 ± 10.9 | 0.002 |
| hsCRP (mg/L) | 0.12 (0.05–0.22) | 0.10 (0.04–0.17) | 0.12 (0.05–0.19) | 0.13 (0.05–0.23) | 0.15 (0.07–0.28) | <0.001 |
| Current smoker (%) | 26.2 | 14.9 | 20.9 | 27.0 | 41.8 | <0.001 |
| Alcohol drinker (%) | 22.8 | 17.5 | 20.7 | 24.7 | 28.0 | <0.001 |
| Family history of diabetes (%) | 9.9 | 9.7 | 10.1 | 10.1 | 9.7 | 0.976 |
| Prediabetes (%) | 35.9 | 30.1 | 34.1 | 37.4 | 41.9 | <0.001 |
Notes: Data are expressed as the mean ± standard deviation, median (interquartile range), or percentage; p-values were calculated using ANOVA, the Kruskal-Wallis test, or chi-squared test.
Abbreviations: WBC, white blood cell; BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein.
Table 2 shows the incidence of T2DM during the 10-year follow-up period, during which the incidence rates were calculated biennially. A total of 592 individuals (14.1%, 592 of 4211) developed T2DM during the 10-year follow-up period. The incidence rate per 2 years ranged from 1.2–5.9.
Table 2.
Incidence of Type 2 Diabetes During the Follow-Up Study
| Year Range | Follow-Up | n | Incidence of Cases (n) | Incidence Rate per 2 Years |
|---|---|---|---|---|
| 2001–2002 | Baseline | 4211 | ||
| 2003–2004 | 2 years | 3973 | 47 | 1.2 |
| 2005–2006 | 4 years | 3559 | 83 | 2.3 |
| 2007–2008 | 6 years | 3161 | 109 | 3.5 |
| 2009–2010 | 8 years | 3188 | 188 | 5.9 |
| 2011–2012 | 10 years | 2994 | 165 | 5.5 |
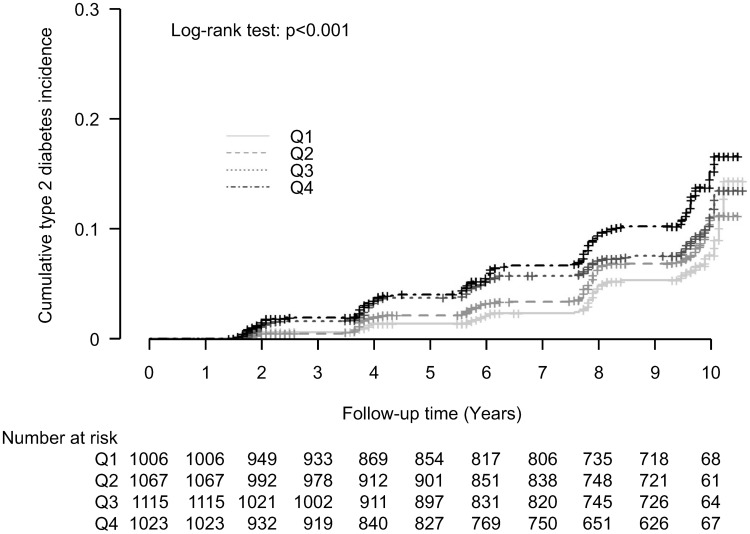
The cumulative incidence of T2DM according to WBC count quartiles is illustrated in Figure 1 as a Kaplan–Meier curve. The higher quartile of WBC count groups showed significantly higher cumulative T2DM incidence over 10 years after the baseline survey (log-rank test, P < 0.001).
Figure 1.
Cumulative incidence of type 2 diabetes according to WBC count quartiles in the non-obese population.
Table 3 shows the results of the multivariate Cox proportional hazards regression analysis for predicting T2DM according to WBC count quartiles. In Model 1, we calculated the hazard ratios after adjusting for age and sex. In model 2, we adjusted for additional potential confounding variables, including smoking, alcohol consumption, waist circumference, systolic blood pressure, fasting plasma glucose, and family history of T2DM. In model 3, we investigated the association between WBC count and incident T2DM by additional adjustment for HOMA-IR, hsCRP levels, and prediabetes. Compared to the reference first quartile, the HRs (95% CIs) of the incidence of T2DM in the second, third, and fourth quartiles increased in a dose–response manner among all models. Compared to the lowest quartiles, the HRs (95% CIs) of the incidence of T2DM in the highest WBC count quartile were 2.04 (1.48–2.81) in the unadjusted model, 1.96 (1.42–2.70) in model 1, 1.71 (1.22–2.39) in model 2, and 1.55 (1.10–2.18) in model 3.
Table 3.
Hazard Ratios and 95% Confidence Intervals for Incident Type 2 Diabetes According to WBC Count Quartiles
| WBC Count Quartiles | ||||
|---|---|---|---|---|
| Q1 (≤ 5200) (n = 1006) | Q2 (5300–6100) (n = 1067) | Q3 (6200–7300) (n = 1115) | Q4 (≥ 7400) (n = 1023) | |
| New cases of diabetes, n | 58 | 75 | 87 | 108 |
| Mean follow-up, years | 8.41 | 8.25 | 7.99 | 7.92 |
| Person-years of follow-up | 8463 | 8807 | 8905 | 8098 |
| Unadjusted | 1.00 | 1.28 (0.91–1.80) | 1.48 (1.06–2.06) | 2.04 (1.48–2.81) |
| Model 1 | 1.00 | 1.23 (0.87–1.73) | 1.42 (1.02–1.98) | 1.96 (1.42–2.70) |
| Model 2 | 1.00 | 1.27 (0.90–1.80) | 1.45 (1.03–2.03) | 1.71 (1.22–2.39) |
| Model 3 | 1.00 | 1.22 (0.96–1.72) | 1.39 (0.99–1.95) | 1.55 (1.10–2.18) |
Notes: Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, smoking, alcohol consumption, waist circumference, systolic blood pressure, fasting plasma glucose, and family history of type 2 diabetes. Model 3: adjusted for age, sex, smoking, alcohol consumption, waist circumference, systolic blood pressure, fasting plasma glucose, family history of type 2 diabetes, HOMA-IR, hsCRP, and prediabetes.
Abbreviations: WBC, white blood cell; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein.
Supplementary Figure S1 suggests the potential of a clinically meaningful WBC count threshold at ≥ 6600 cells/μL.
Discussion
In this large-scale prospective community-based Korean cohort that was observed over 10 years, we found that a higher WBC count was positively and independently associated with incident T2DM among community-dwelling non-obese Korean adults. This association between WBC count and incidence of T2DM was independent of insulin resistance, even after adjusting for HOMA-IR. Our findings are consistent with the results of previous studies showing that an increased WBC count is positively associated with incident T2DM.21–24 Moreover, our results suggest that WBC count could be a useful marker for non-obese adults susceptible to T2DM, independent of insulin resistance. Few previous studies have examined the relationship between WBC count and the incidence of T2DM among non-obese adults. We believe that this is the first large population-based cohort study to reveal a positive relationship between WBC count and the incidence of T2DM regardless of baseline insulin resistance in a non-obese population. Thus, our results expand upon earlier findings regarding the association between WBC count and incident T2DM. Smoking, waist circumference, and hsCRP levels tended to increase with WBC count quartiles, and they could influence the relationship between WBC count and incident T2DM. We included these factors as confounding variables in Cox regression analyses to control for potential confounding. An interaction test was also performed, and it identified that the influence of the WBC count is not dependent on each variable. Further studies are warranted to elucidate the relationship between WBC count and incident T2DM among non-obese adults by matching these factors, or subgroup analysis, in more participants.
Chronic low-grade inflammation is known to be involved in the pathogenesis of obesity-related T2DM.16,29 Adipose tissue is generally considered as the major and earliest trigger of chronic low-grade inflammation in obesity-related T2DM, with prominent infiltration of lymphocytes, macrophages, and other immune cells into this tissue.30–33 These cells are essential for the production of pro-inflammatory cytokines, which function in an autocrine and paracrine fashion to induce insulin resistance by inhibiting insulin signaling in peripheral tissues.34–36
Non-obese individuals with T2DM were also identified by an increase in pro-inflammatory cytokines, such as interleukin-8 and interleukin-18.37 Unlike obesity-related T2DM, dysfunctional adipocytes could not be considered as the major determinants of chronic low-grade inflammation, and over-nutrition could not be regarded as the only trigger of chronic low-grade inflammation in the case of non-obese individuals with T2DM. Other factors, such as pancreatic β-cell failure and alterations in gut microbiota composition, are also possible mechanisms of inflammation that are involved and can also be found in non-obese T2DM. It has been suggested that the “stressed” pancreatic β-cell may trigger local inflammation and alter the balance between pancreatic β-cell mass and function in Langerhans islets in individuals with a genetic predisposition, which leads to a decrease in both pancreatic β-cell number and function.38–41 Macrophages, interleukin-1β, and other immune cells are known to be involved in islet inflammation in T2DM.42–44 In addition, altered gut microbiota can directly influence immune cells in the gut and indirectly influence immune cells through microbial products, such as short-chain fatty acids, lipopolysaccharides, and other metabolites, all of which can have an effect on insulin resistance.45–48 Short-chain fatty acids are believed to regulate gene expression of human monocytes and decrease chemokine production and inflammatory cytokine, whereas lipopolysaccharides can lead to subclinical inflammation mediated by the induction of pro-inflammatory cytokines by immune cells.45,49
This study has several limitations that should be considered. First, only one measurement of WBC count was included in the analysis, and thus, it was not possible to determine whether an acute and brief episode of infection affected the findings observed in this study. In an attempt to minimize this limitation, participants with a WBC count ≥ 10,000 cells/mL were excluded. Second, this study did not show the effect of sequential changes in the WBC count, since we only considered the baseline measurements of the WBC count. Third, the KoGES did not duplicate the measurement of blood glucose levels, and T2DM diagnosis could not be confirmed by repeat testing. Fourth, this study did not exclude the presence of potential autoimmune or inflammatory disorders. Therefore, further studies that consider latent autoimmune diabetes in adults, autoimmune diseases, and inflammatory diseases are needed. Fifth, despite the large population size, this study was performed in a Korean population. Therefore, our results may not be generalizable to other racial/ethnic populations. Lastly, the study population may not represent the general Korean population, since the participants resided in a rural area and an urban area. Therefore, this study may have been subject to a selection bias. Despite these limitations, we believe that our findings would have clinical implications with regard to preventive public health strategies among non-obese individuals who may be at a high risk of developing T2DM.
Conclusion
In conclusion, a higher WBC count predicts future incident T2DM that is independent of other associated variables, including HOMA-IR, among community-dwelling non-obese Korean adults. In clinical practice, WBC count, which is inexpensive and commonly measured in routine laboratory examination panels, could facilitate the prediction of non-obese individuals susceptible to T2DM.
Acknowledgments
This work was supported by the Technology Innovation Program (20002781, A Platform for Prediction and Management of Health Risk Based on Personal Big Data and Lifelogging) funded by the Ministry of Trade, Industry & Energy (MOTIE, South Korea).
Abbreviations
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; T2DM, type 2 diabetes mellitus; WBC, white blood cell.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/s0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 2.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global report on diabetes [homepage on the internet]. Geneva: World Health Organization; 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng;jsessionid=BF5106442C6C33727D424A2C64F767AA?sequence=1. Accessed January6, 2021. [Google Scholar]
- 6.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 7.Kim BY, Won JC, Lee JH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019;43(4):487–494. doi: 10.4093/dmj.2019.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha KH, Kim DJ. Trends in the diabetes epidemic in Korea. Endocrinol Metab (Seoul). 2015;30(2):142–146. doi: 10.3803/EnM.2015.30.2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vital Statistics Division, Statistics Korea, Shin H-Y, Kim J, et al. Cause-of-death statistics in 2018 in the Republic of Korea. J Korean Med Assoc. 2020;63(5):286–297. doi: 10.5124/jkma.2020.63.5.286 [DOI] [Google Scholar]
- 10.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64–91. doi: 10.1111/nyas.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(Suppl 1):53–61. doi: 10.1111/j.1467-789X.2007.00439.x [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408–418. doi: 10.1016/s0140-6736(09)60937-5 [DOI] [PubMed] [Google Scholar]
- 13.Kang HT, Shim JY, Lee HR, Park BJ, Linton JA, Lee YJ. Trends in prevalence of overweight and obesity in Korean adults, 1998–2009: the Korean National Health and Nutrition Examination Survey. J Epidemiol. 2014;24(2):109–116. doi: 10.2188/jea.je20130017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Subramanian SV, Oh J, Razak F. Trends in the distribution of body mass index and waist circumference among South Korean adults, 1998–2014. Eur J Clin Nutr. 2018;72(2):198–206. doi: 10.1038/s41430-017-0024-7 [DOI] [PubMed] [Google Scholar]
- 15.Bae JC. Trends of diabetes epidemic in Korea. Diabetes Metab J. 2018;42(5):377–379. doi: 10.4093/dmj.2018.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. doi: 10.1016/j.diabres.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 17.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38(3):183–191. doi: 10.1016/j.diabet.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Park JM, Lee DC, Lee YJ. Relationship between high white blood cell count and insulin resistance (HOMA-IR) in Korean children and adolescents: Korean National Health and Nutrition Examination Survey 2008–2010. Nutr Metab Cardiovasc Dis. 2017;27(5):456–461. doi: 10.1016/j.numecd.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Mirza S, Hossain M, Mathews C, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012;57(1):136–142. doi: 10.1016/j.cyto.2011.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elimam H, Abdulla AM, Taha IM. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(1):800–804. doi: 10.1016/j.dsx.2018.11.061 [DOI] [PubMed] [Google Scholar]
- 21.Twig G, Afek A, Shamiss A, et al. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care. 2013;36(2):276–282. doi: 10.2337/dc11-2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Yang Z, Zhang W, et al. White blood cell subtypes and risk of type 2 diabetes. J Diabetes Complications. 2017;31(1):31–37. doi: 10.1016/j.jdiacomp.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 23.Vatcheva KP, Fisher-Hoch SP, Rahbar MH, Lee M, Olvera RL, McCormick JB. Association of total and differential white blood cells to development of type 2 diabetes in Mexican Americans in Cameron County hispanic cohort. Diabetes Res. 2015;1(4):103–112. doi: 10.17140/droj-1-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi N, Yoshida H, Matsuo Y, Suzuki K, Tatara K. White blood-cell count and the risk of impaired fasting glucose or Type II diabetes in middle-aged Japanese men. Diabetologia. 2002;45(1):42–48. doi: 10.1007/s125-002-8243-1 [DOI] [PubMed] [Google Scholar]
- 25.Gu Y, Hu K, Huang Y, et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes Res Clin Pract. 2018;141:140–147. doi: 10.1016/j.diabres.2018.04.041 [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Han BG, KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol. 2017;46(2):e20. doi: 10.1093/ije/dyv316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Regional office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment [homepage on the internet]. Sydney: Health Communications Australia; 2000. Available from: https://apps.who.int/iris/handle/10665/206936. Accessed January6, 2021. [Google Scholar]
- 28.American Diabetes Association. Standards of medical care in diabetes—2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14–37. doi: 10.2337/cd17-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71(4):174–187. [PubMed] [Google Scholar]
- 30.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu CJ, Benoist C, Mathis D. The immune system’s involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24(6):436–442. doi: 10.1016/j.smim.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci (Lond). 2012;122(5):203–214. doi: 10.1042/cs20110357 [DOI] [PubMed] [Google Scholar]
- 34.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/jci29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/jci19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposito K, Nappo F, Giugliano F, et al. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2003;78(6):1135–1140. doi: 10.1093/ajcn/78.6.1135 [DOI] [PubMed] [Google Scholar]
- 38.Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50–59. doi: 10.15420/ecr.2018.33.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halban PA, Polonsky KS, Bowden DW, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J Clin Endocrinol Metab. 2014;99(6):1983–1992. doi: 10.1210/jc.2014-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks-Worrell B, Palmer JP. Immunology in the clinic review series; focus on metabolic diseases: development of islet autoimmune disease in type 2 diabetes patients: potential sequelae of chronic inflammation. Clin Exp Immunol. 2012;167(1):40–46. doi: 10.1111/j.1365-2249.2011.04501.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57(2):241–254. doi: 10.1373/clinchem.2010.157016 [DOI] [PubMed] [Google Scholar]
- 42.Kamata K, Mizukami H, Inaba W, et al. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid. 2014;21(3):191–201. doi: 10.3109/13506129.2014.937857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavelti-Weder C, Babians-Brunner A, Keller C, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35(8):1654–1662. doi: 10.2337/dc11-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57(3):491–501. doi: 10.1007/s00125-013-3116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr. 2017;147(7):1468s–1475s. doi: 10.3945/jn.116.240754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheithauer TPM, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5(9):759–770. doi: 10.1016/j.molmet.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burcelin R. Gut microbiota and immune crosstalk in metabolic disease. Mol Metab. 2016;5(9):771–781. doi: 10.1016/j.molmet.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez-Curto E, Milligan G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 49.Arora P, Moll JM, Andersen D, et al. Body fluid from the parasitic worm Ascaris suum inhibits broad-acting pro-inflammatory programs in dendritic cells. Immunology. 2020;159(3):322–334. doi: 10.1111/imm.13151 [DOI] [PMC free article] [PubMed] [Google Scholar]