Abstract
Background:
Despite the well-recognized effect of vitamin D in metabolism and homeostasis, there is now growing interest in its probable association with pneumonia. This study aims to supply vitamin D3 (Cholecalciferol) (100,000 IU) to pneumonic children to minimize the duration of illness and improve their outcome.
Methods:
A double-blinded, randomized, placebo-controlled trial was conducted in a Pediatric Cairo University affiliated hospital. An intervention arm (93 children) and a control arm (98 children), who had pneumonia with an insufficient or deficient level of vitamin D and whose parental permission was obtained, were enrolled in the trial. All children were treated with antibiotics according to WHO guidelines. Children were given a single injection of 1 mL of 100,000 IU of vitamin D3 or placebo. Clinical data were recorded every eight hours for all children. Outcomes were assessed 7 days after vitamin D injection.
The primary outcome variable was the change in serum level of 25(OH)D, while the secondary outcomes were the medical state of the assigned cases (improvement or death) and duration between enrollment and hospital discharge for improved cases.
Results:
In the supplementation group, the percentage of patients who suffered either deficient (38.7%) or insufficient levels (61.3%) of 25 (OH)D at day one had significantly decreased in the seventh day to (11.8%) and (52.7%), respectively. Kaplan--Meier plots highlighted that the median time to recover of the placebo group was significantly longer than that of the supplementation group (Log Rank P value < .001).
Conclusion:
VDD was detected in pediatric critical care children. In pneumonic children with high VDD, it is illustrated that Vitamin D supplementation is accompanied by lowered mortality risk and pSOFA scores, reduced time to recover, and improved PaO2/FiO2.
Trial registration:
Trial Identifier number: NCT04244474. Registered on 27 January 2020- Retrospectively registered at ClinicalTrials.gov https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S0009JXO&selectaction=Edit&uid=U0004UO8&ts=152&cx=9cceq6
Keywords: cholecalciferol, duration of stay, outcome, PICU, pneumonia
1. Introduction
Globally, pneumonia is considered a leading prominent factor of children’ illness, particularly the developing countries, causing 29% of the under-Five mortality.[1] World Health Organization estimates 66% of deaths due to pneumonia occur in infancy, where 90% occur in developing countries.[2]
In Egypt, according to UNICEF 2018, acute respiratory tract infections (ARTIs) were estimated to account for 11% and 19% of the under-five and post-neonatal mortalities respectively.[3]
Despite the well-recognized effect of vitamin D in metabolism and homeostasis in all populations, there is now growing interest in its probable association with pneumonia.[4]
Twenty-five -hydroxyl vitamin D (25(OH) D) and1,25-dihydroxy vitamin D (1,25(OH)2 D) are surrogate markers of the active form of vitamin D in the body. Recently, it has been demonstrated that 1,25(OH)2D has an important role in host defenses, inflammation, and immunity. Furthermore, 1,25(OH)2D promotes the expression of B-defensin (antimicrobial peptides) and cathelecidin that are broadly released in the human body and play a vital role in immunity as a result of their toxin neutralization and chemotactic mechanism.[5]
1,25(OH)2D is proposed to provide a more valid measure for better detection of the vitamin D role in the prognosis, reducing the severity and improving the treatment outcome of patients with ARTIs due to its short half-life (12–36 hours) compared with 25(OH)D (3 weeks).[6]
Globally, about 30% to 90% of under-five children experience vitamin D deficiency (VDD). This could vary among children according to the socioeconomic, environmental, and behavioral circumstances.[6]
Studies evaluating the relation between1,25 (OH)2D deficiency and the prognosis of respiratory tract infection are rare and showed controversial findings.[7,8]
However, a systematic review that used the results of 12 selected studies including 2279 participants, highlighted the significant correlation between VDD and occurrence and severity of acute lower respiratory infections (ALRIs).[9]
In Yemen (2009), a prospective cohort study was conducted to examine the ability of deficient levels of vitamin D to predict outcomes of severe pneumonia. The study documented the significant association of VDD with neutropenia and hypoxia in patients with severe pneumonia, thus, predicting poor prognosis.[10]
In Egypt (2010), children aged less than 5 years were subjected to a case--control study to examine the impact of VDD on the susceptibility of pneumonia. The study illustrated that higher incidence and more severe pneumonia is associated with VDD.[11]
Hashemian et al,[12] advocated providing children (particularly suffering from pneumonia) with adequate amounts of vitamin D supplements. Nevertheless, little research has been conducted to measure the impact of supplementation of vitamin D on the outcome of pneumonic children.
In this regard, we were urged to conduct a randomized controlled trial (RCT) in Abou El-Reesh Tertiary Pediatric hospital to evaluate the effects of vitamin D3 supplementation on children with pneumonia. Our team postulated that supplementation of 100,000 IU of vitamin D3 (Cholecalciferol) will minimize the illness duration and improve the outcome of those children.
2. Materials and methods
2.1. Study setting and design
The study is a randomized, double-blinded, placebo-controlled trial that was conducted in Cairo University Abou El-Reesh Children hospital (a university-affiliated teaching hospital in Egypt) to evaluate the effect of vitamin D3 supplementation on the outcome of treatment of pneumonic children. Pneumonia cases were recruited from 2 of the hospital general pediatric departments (chosen randomly out of a total of 6 hospital departments) and the 4 pediatric intensive care units (PICUs).
2.2. Study population and sampling
Eighty-six children per group (172 total children) were the minimum number to be recruited to verify a 20% difference in the mean pneumonia duration between the vitamin D3 arm and control arm [5 vs 6 days (SD 2)] achieving Type I error of 5% and Type II error of 10%.[13] According to the prevalence of VDD in eastern India at a pediatric hospital,[14] and after allowing 10% for nonresponse or loss of follow-up and 25% for the exclusion of children (diagnosed with sufficient or toxic level of vitamin D after vitamin D testing) (Fig. 1); 233 was the minimum number to start with.[15,16]
Figure 1.

Levels of vitamin D.
Mild and moderate pneumonia cases were recruited from 2 of the hospital general pediatric departments, while severe pneumonia cases were recruited from the four PICUs of the hospital.
All children (259 children) between 1 month and 12 years of age admitted to the selected departments during the period from 9 September 2019 to 15 February 2020 and who were diagnosed clinically as pneumonia according to World Health Organization criteria of severity (Fig. 2),[17] were screened for the inclusion criteria. Children who had rickets (2 children), marked illnesses (severe malnutrition, measles, heart or renal disorders, meningitis, endocrine dysfunction, hypercalcemia, hyperthyroidism, and suspected tuberculosis) (4 children), or were known in the past 3 months to have been treated with a high dose of vitamin D (3 children) were all excluded from the study. Children with sufficient (52 children) or toxic (7 children) levels of vitamin D were also excluded.
Figure 2.

Criteria for diagnosis of pneumonia.
Thus, 191 children (93 in the intervention arm and 98 in the control arm) who had pneumonia with an insufficient or deficient level of vitamin D and whose parents approved their participation were enrolled in the trial. The baseline and outcome assessments were completed and the daily clinical follow-up was continued until the discharge from the hospital (improvement and recovery or death). (Fig. 3).
Figure 3.
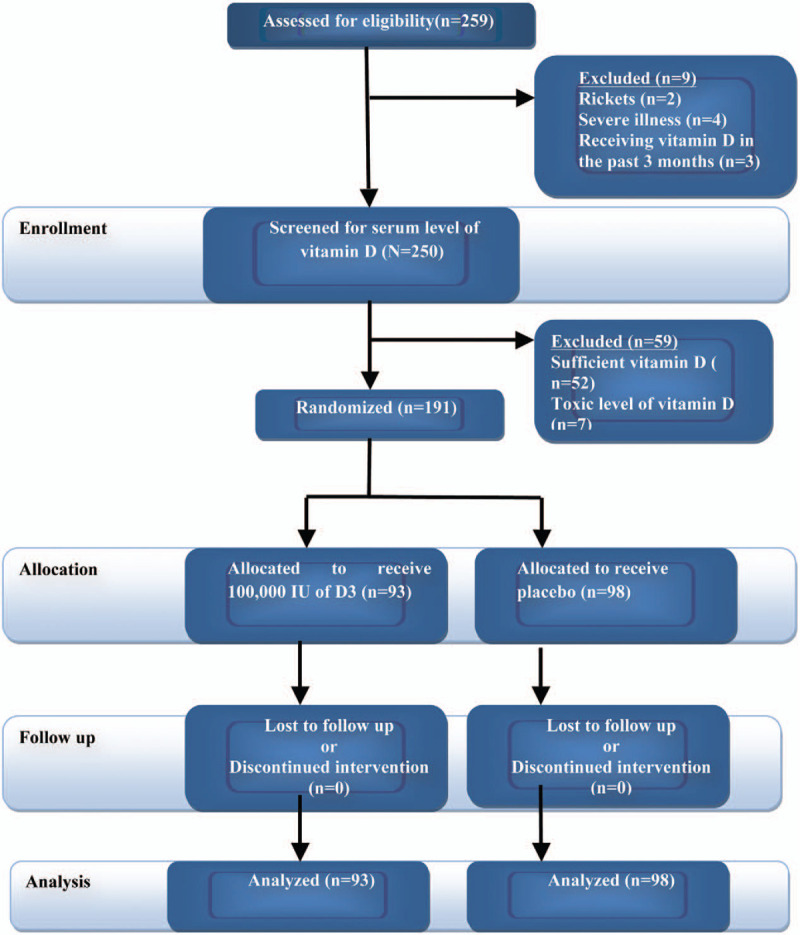
Clinical trial flowchart.
2.3. Vitamin D testing
About 2 mL of venous blood was taken, left to clot, and then centrifuged at 3000 rpm for 5 minutes. The serum samples were stored at -20°C till the time of assay and used for detection of concentrations of serum 25(OH)D and total (25OH Vitamin D2 and D3) by enzyme-linked immunosorbent assay (ELISA) using (DIA source 25OH Vitamin D Total ELISA Kit, Catalogue No. KAP 1971) supplied by DIA source Immunoassays S.A. (Rue Du Bosquet, 2 B-1348 Louvain-la Neuve, Belgium). According to the factory instructions and based on the principle of solid-phase, ELISA was performed on microliter plates. The size of substrate change has been assessed colorimetrically by measuring the absorbance, which is inversely proportional to the total (25OH Vitamin D2 and D3) concentration. The total (25OH Vitamin D2 and D3) concentrations of the samples have been measured by dose interpolation from the calibration curve.
2.4. Random allocation
By using an Excel spreadsheet, a random number sequence was elicited. The children were randomized into an intervention or control group. The allocation was further hidden by using closed dark envelopes. A biostatistician and a secretary who were not members of the research group did independently the randomization, repackaging, sequencing, and allocation concealment. None of the research team and participants’ parents were aware of the supplementation or placebo being given. Only at the time of final data analysis, the codes were revealed.
The children were followed clinically on a daily basis by the 3 study pediatricians to evaluate the resolution or deterioration of symptoms and signs of pneumonia till being discharged from the hospital.
2.5. Baseline assessment
Data were collected for each participant in terms of demographic variables (e.g., feeding practices, age, sex), presenting symptoms degree and duration. Vital signs for all children (oxygen saturation, blood pressure, respiratory rate, heart rate, and temperature), nasal flaring, cyanosis, pallor, grunt, and mental status, were evaluated. Partial oxygen/fractional oxygen (PaO2/FiO2) was recorded and serum creatinine, C-reactive protein, platelet count, and serum bilirubin laboratory tests were done. Pediatric Sequential Organ Failure Assessment (pSOFA) score was assessed for severe pneumonia cases.
2.6. Intervention
According to WHO guidelines for the treatment of childhood pneumonia at health facilities (2012), all children were treated with antibiotic[17,18] at enrollment after obtaining consent from parents and completing the baseline assessment. Children were given a single injection of one ml of 100,000 IU of vitamin D3 (Cholecalciferol). Vitamin D3 obtained from 2 mL vials containing 200,000 IU each (Devarol- S- 200,000 IU produced by Memphis. for Pharmaceutical and Chemical Industries) and stored in a dry and cool environment for 1 to 16 weeks (based on recruitment date) according to the manufacturers’ recommended conditions. They were alternatively given placebo, which is 1 mL saline injection. Syringes were labeled with a unique ID number and given by the blinded researchers selecting the next syringe with a randomization number (only office secretary was aware of randomization codes). The routine treatment of inpatient children is free of charge in AbouEl-Reesh Children hospital, while cost of vitamin D3 was paid by the researchers to be given free of charge to the children in the intervention arm during the study period.
2.7. Follow-up
Data were monitored and recorded for all children every 8 hours for auscultation findings, fever, respiratory rate, cyanosis, chest indrawing, oxygen saturation, feeding, and mental status. In cases where fast breathing and fever were absent for 2 consecutive days, the child was discharged.
2.8. Outcomes assessment
Outcomes were assessed 7 days after vitamin D supplementation, particularly, the first day where vitamin D reaches its maximum level in the blood, to guarantee the assessment of all participants before their discharge.[19] The primary outcome variables were the change in serum level of 25(OH)D, PaO2/FiO2, serum creatinine, C reactive protein, and serum bilirubin levels and platelet count, in addition to the pSOFA scores for severe cases.
The secondary outcomes were the medical state of the assigned cases (improvement or death), the duration between enrollment and hospital discharge or death, and the time to recover from the episode of pneumonia.
2.9. Statistical analysis
To quantify the degrees of change before and after vitamin D3 (Cholecalciferol) administration, patients’ percentage change of Vitamin D (25 (OH)2D) was calculated through the equation:
Pediatric, Blood Vitamin D(BVD) level % change. [(BVD after – BVD before) ÷ BVD before] × 100
Statistical Package for Social Science Software (SPSS) program (version 21.0 IBM) was utilized for the analysis of collected data. Data are not normally distributed as disclosed by tests of normality (such as Shapiro–Wilk test). Univariable comparisons to quantify continuous variables associations were carried out using Mann–Whitney and Kruskal–Wallis tests (nonparametric tests). The median and interquartile ranges were applied for summarizing quantitative variables. Statistically, significance was heeded when P values were below .05. The Cox regression model was conducted to find out the independent influence of vitamin D3 supplementation on the overall survival of pneumonic children.
There were no stopping or interim analyses due to the one single intervention available and the limited period of the study follow-up.
A comparison of the meantime to recovery for the episode of pneumonia at recruitment was done for the vitamin D and placebo groups. To compare the time to recover from the episode of pneumonia between the vitamin D and placebo groups, Kaplan–Meier plots and log-rank tests were done.
2.10. Ethics approval and consent to participate
The study protocol and the consent form have been reviewed and approved in July 2019 before study implementation by the departments of public health and pediatrics at KasrAl-Aini faculty of medicine. The research protocol was also agreed upon by Cairo University Research Ethical committee (REC) under number D-12-2019.
The trial was registered at ClinicalTrials.gov with the Trial Identifier number: NCT04244474. https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S0009JXO&selectaction=Edit&uid=U0004UO8&ts=152&cx=9cceq6
If the child met the required study standards after clear explanation of the study objectives, the nature of the intervention, risk and benefits from participating in the study, and alternatives in case of participation refusal, written consent was obtained from one of the child's care givers or parents.
3. Results
Out of the 191 children who had pneumonia with an insufficient or deficient level of vitamin D, 93 were assigned to receive vitamin D3 supplementation and 98 received the placebo. Basic sociodemographic and clinical characteristics were fairly shared between both groups. Both of them did not show statistically significant differences regarding gender, age, feeding patterns, exposure to sun, maternal age, and education. Cases with severe pneumonia accounted for (68.8%) of the supplementation group compared with (61.2%) of the placebo group. Besides, a statistically significant difference was highlighted between the 2 groups concerning the median duration of hospital stay; 11 (IQR 10–14) days and 10 (IQR 8–13) days in the supplementation and placebo group, respectively (P < .001) (Table 1).
Table 1.
Baseline demographic factors, comorbidities, and outcome among the randomly assigned supplementation and placebo groups.
| Variable | D3 Supplementation group (n = 93) | Placebo group (n = 98) | P |
| Age, mo | 24 (18--48) | 24 (10--48) | .613∗ |
| Sex | |||
| Male | 65 (69.9) | 71 (72.4) | .697† |
| Female | 28 (30.1) | 27 (27.6) | |
| Ever breast fed | 71 (76.3) | 78 (79.6) | .588† |
| Bottle fed | |||
| Cow milk | 43 (46.2) | 49 (50) | .603† |
| Artificial milk | 50 (53.8) | 49 (50) | |
| Age of weaning, mo | 8 (6–12) | 7 (6 - 9) | .348∗ |
| Cow milk intake after weaning | |||
| < 250 mL/day | 47 (50.5) | 50 (51) | .931† |
| 250–500 mL/day | 38 (40.9) | 41 (41.8) | |
| > 500 mL/day | 8 (8.6) | 7 (7.1) | |
| No. of children in house | 3 (2–3) | 3 (2–3) | .428∗ |
| Maternal age, yr | 32 (30–35) | 32 (30–35) | .892∗ |
| Maternal education | |||
| Not educated | 9 (9.7) | 16 (16.3) | .315† |
| Primary education | 43 (46.2) | 46 (46.9) | |
| Secondary education | 34 (36.6) | 33 (33.7) | |
| High education | 7 (7.5) | 3 (3.1) | |
| Exposure to sun | |||
| Less frequent | 25 (26.9) | 28 (28.6) | .794† |
| More frequent (most of days) | 68 (73.1) | 70 (71.4) | |
| Comorbidities | 48 (51.6) | 42 (42.9) | .226† |
| Severe pneumonia | 64 (68.8) | 60 (61.2) | .272† |
| Duration of hospital stay, d | 11 (10--14) | 10 (8 - 13) | .001∗ |
| Total duration of stay | |||
| < 2 wks | 67 (72) | 75 (76.5) | .478† |
| 2 wks or more | 26 (28) | 23 (23.5) | |
| Mortality | 23 (24.7) | 32 (32.7) | .227† |
Qualitative variables described as number (percentage). Quantitative variables described as median (interquartile range).
Bold values represent statistically significant P values were below .05.
Mann--Whitney test.
Chi-square test.
Although 28% of the children in the supplementation group stayed longer for 2 weeks or more compared with the 23.5% in the placebo group, the mortality rate was higher in the placebo group (32.7%) compared with (24.7%) the supplementation group (Table 1).
No statistically significant difference was reported between both groups concerning baseline (day one) clinical findings, signs, respiratory rates, temperature, laboratory measures, 25(OH)D concentration, and severity scores (Table 2).
Table 2.
Clinical findings, signs, laboratory measures, and severity scores among the randomly assigned supplementation and placebo groups.
| Variable | D3 Supplementation group (n = 93) | Placebo group (n = 98) | P |
| Temperature | 38 (38–39) | 38 (38–39) | .184∗ |
| Respiratory rate | 45 (40–50) | 40 (40–50) | .603∗ |
| Mechanical ventilation | 23 (24.7) | 34 (34.7) | .133† |
| D1-serum Ca | 7.9 (7.4–8.9) | 8.2 (7.2–9) | .392∗ |
| D1-serum creatinine | 0.2 (0.2–0.2) | 0.2 (0.1–0.2) | .079∗ |
| D1-serum albumin | 3.5 (2.9–4) | 3.2 (2.9–3.7) | .096∗ |
| D1-Bilirubin | 0.3 (0.3–0.3) | 0.3 (0.3–0.4) | .502∗ |
| D1-platelet count | 249 (170–370) | 227 (170–300) | .663∗ |
| D1-CRP | 55 (35–70) | 57.5 (45–70) | .138∗ |
| D1-pSOFA score | 2 (2–3) | 2.5 (2–4) | .344∗ |
| D7-serum Ca | 8.9 (8.2–9) | 9 (8–9.2) | .663∗ |
| D7-serum creatinine | 0.2 (0.2–0.2) | 0.2 (0.1–0.2) | .220∗ |
| D7-serum albumin | 3.7 (3.5–4) | 3.7 (3.1–4) | .066∗ |
| D7-Bilirubin | 0.3 (0.3–0.3) | 0.3 (0.2–0.3) | .070∗ |
| D7-platelet count | 300 (250–350) | 250 (250–300) | .017∗ |
| D7-CRP | 6 (6–6) | 6 (5–35) | .370∗ |
| D7-pSOFA score | 1 (0–2) | 1 (0 –2) | .991∗ |
| D1–25 (OH)D concentration | 15.8 (7–18.2) | 16.7 (8.9–21.7) | .119∗ |
| D1Vitamin 25 (OH)D | |||
| Deficient | 36 (38.7) | 33 (33.7) | .469† |
| Insufficient | 57 (61.3) | 65 (66.3) | |
| D7–25 (OH)D concentration | 67.7 (58.8–84.5) | 14.3 (8.9–20.6) | .000∗ |
| D7Vitamin 25 (OH)D | |||
| Deficient | 11 (11.8) | 33 (33.7) | .000† |
| Insufficient | 49 (52.7) | 65 (66.3) | |
| Sufficient | 33 (35.5) | 0 (0) | |
| Vitamin D 25 (OH)D % change | 403.1 (261.4–868.2) | -5 (-11.6 to 0) | .000∗ |
| D1-PaO2/FIO2 | 300 (290–360) | 340 (230–400) | .824∗ |
| D7-PaO2/FIO2 | 360 (330–400) | 345 (290–400) | .005∗ |
| D1-PaO2/FIO2 | |||
| Normal | 62 (66.7) | 54 (55.1) | .194† |
| Acute lung disease | 25 (26.9) | 32 (32.7) | |
| Acute respiratory distress syndrome | 6 (6.5) | 12 (12.2) | |
| D7-PaO2/FIO2 | |||
| Normal | 87 (93.5) | 68 (69.4) | .000† |
| Acute lung disease | 3 (3.2) | 21 (21.4) | |
| Acute respiratory distress syndrome | 3 (3.2) | 9 (9.2) |
D1:Day1, D7:Day7.
Qualitative variables described as number (percentage). Quantitative variables described as median (interquartile range).
Bold values represent statistically significant P values were below .05.
Mann–Whitney test.
Chi-square test.
On the seventh day of the intervention, the supplementation group showed significantly higher levels of 25(OH)D concentration and higher percentage change than in the placebo group. In the supplementation group, the percentage of patients who suffered either deficient (38.7%) or insufficient levels (61.3%) of 25 (OH)D at day one had significantly decreased in the seventh day to (11.8%) and (52.7%), respectively. Also, 93.5% of the supplementation group showed significantly normal PaO2/FiO2 score values (P < .001) (Table 2).
Kaplan–Meier's plots highlighted that the median time to recover of the placebo group was significantly longer than the Vitamin D supplementation group (Log Rank P value < .001) (Fig. 4).
Figure 4.
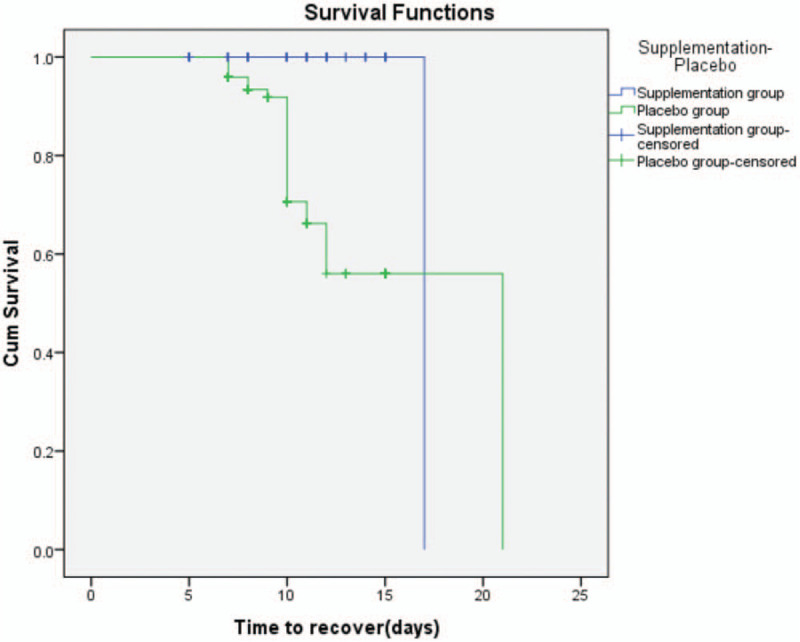
Kaplan--Meier analysis for D3 Supplementation group and Placebo group.
The Cox regression model revealed that vitamin D3 supplementation significantly decreases the risk of mortality of pneumonic children by nearly 50% while fixing the effect of age, CRP, pSOFA score, and PaO2/FiO2 at day one (Table 3).
Table 3.
Cox regression analysis to estimate the independent contribution of Vitamin D supplementation on the overall survival of ICU patients.
| Variable | P | HR | 95.0% CI for HR |
| Vitamin D3 Supplementation | .013 | 0.492 | 0.281–0.863 |
| Age, mo | .528 | 1.003 | 0.993–1.013 |
| D1-CRP | .120 | 1.008 | 0.998–1.018 |
| D1-pSOFA score | .972 | 0.997 | 0.841–1.182 |
| D1-PaO2/FIO2 | .547 | 1.001 | 0.997–1.005 |
D1 = Day1, CI = Confidence interval, HR = hazard ratio.
4. Discussion
The present study is a double-blinded trial randomized, well-managed RCT evaluating the effect of vitamin D3 supplementation on the prognosis of vitamin D insufficient children with pneumonia.
The study findings were in harmony with those of an Egyptian study conducted at PICU of Cairo University Pediatric Hospital among critically ill children where moderate and severe VDD prevalence accounted for 44% and 34%, respectively. Also, VDD was stated to be related to multiple organ dysfunctions and rapid clinical deterioration.[20]
Correspondingly, a Turkish study revealed that more than half (58.5%) of critically ill children suffered VDD at admission.[21]
Meanwhile, recent Indian studies disclosed that the prevalence of VDD was 74% among children with sepsis.[22]
Information as well from a multicenter prospective observational research extremely indicates that most critically ill Canadian children are vitamin D deficient at their admission to PICU. The prevalence of VDD was 69%; with an extra 23% are at risk of deficiency or having insufficient vitamin D levels.[23]
Factors as varying cut-off values, dissimilar ways of measuring 25(OH)D, genotype divergence, and its proteins plunged in metabolism, functioning, and transportation of vitamin D, vitamin D supplementation, dietary intake, differing populations studied, sunlight exposure, and weather, all might contribute to the different range of variations in the occurrence of VDD in various researches.[24,25]
There is a wealth of evidence in favor of the preventive role of vitamin D supplementation to pneumonic children. Such evidence includes enhancing the immune function through the human innate immune system. Accelerated formation of antimicrobial proteins (AMPs) such as cathelicidin and stimulation of toll-like receptors (TLRs) in charge of identifying organisms in epithelial cells, monocytes, and macrophages represent eminent innate immunity host defense mechanisms against infections.[26] The present trial demonstrated significantly elevated levels of 25(OH)D concentration and increased percent change in the supplementation compared with the placebo group. This goes hand in hand with previous researches, which evinced that critically ill children in their acute status suffer from changed metabolism, fluid administration, and transcapillary leak, resulting in reduced levels of serum 25(OH)D. After supplementation, vitamin D body reserve showed rapid restoration due to a significant increase in 25(OH)D levels.[27,28]
In the same context, on the seventh day of the intervention, the supplementation group showed significant elevated levels of 25(OH)D concentration, accompanied by improved laboratory parameters and lower pSOFA score. This could indicate an improvement in the children's condition and decrease in the disease severity.[29]
The current study revealed achieving normal PaO2/FiO2 scores by the majority of the Vit D group (7 days after the intervention) indicating retraction and subsidence of lung injury severity among children on mechanical ventilation. This goes in agreement with previous studies wthathich highlighted the intense need for mechanical ventilation for children experiencing VDD on admission, attaining higher PaO2/FiO2 scores and prolonged duration on mechanical ventilation.[22,30]
In contrast, other studies have shown no direct relation between plasma 25(OH)D levels and illness severity scoring, such as higher PaO2/FiO2 scores. In other words, hypovitaminosis D did not show higher prediction of risk scores for mortality.[31,32]
The range of hospital stay duration was slightly longer in the supplementation group, yet the mortality was lower among those children. The length of hospital stay is an important clinical outcome proved to be significantly associated with VDD. During hospital stay, the addition of catecholamines, mechanical ventilation, or fluid administration represent multifactorial mechanisms, which may affect the association between the length of stay and VDD.[22,33]
Longer disease course in the supplementation children and therefore prolonged hospital stay duration could be attributable to diversities in children patient's population such as age, underlying nutrition status (degree of malnutrition), genetic heterogeneity, status on admission (grade of pneumonia), and presence of other comorbidities (medical or surgical conditions).[34]
Previous studies evinced that factors coupled with elongated hospital stay included lower grades of mothers’ education and lack of exclusive breastfeeding. Education helps mothers to identify an illness in the early stages and seek early treatment.[35]
Moreover, the survival curve analysis showed a significantly longer time to recover among placebo group in comparison to the vitamin D supplementation group. This matches a recent study showing that therapeutic elevation in levels of 25(OH)D was accompanied by improved recovery time in patients with cystic fibrosis and improvement in lung function for adult patients.[36] Also, a study by Braun et al[37] assessing preadmission 25(OH)D observed lower concentrations as an independent predictor of survival at PICU.
Concerning the regression model in the present study, a 50% reduction in the mortality risk among the intervention group was demonstrated. This conforms to a study conducted in Kabul (2010) aiming at determining the effect of supplementation of oral 100,000 IU of cholecalciferol (vitamin D3) together with antibiotics on the range of duration of illness among pneumonic children. The stated study showed that children in the vitamin D3 group survived more without suffering a recurrent episode [72 vs 59 days; P = .02; hazard ratio (HR) 0.71; 95% confidence interval (95% CI) 0.53–0.95].[38]
Furthermore, in agreement with this, 2 recent meta-analysis pediatric studies comprising only observational studies presented circulating VDD among critically ill children, specifically children suffering from sepsis and reported an increased risk of mortality in those critically ill patients due to VDD.[24,39]
These findings also correspond to Amrein et al,[40] which reported the significant reduction of in-hospital mortality following cholecalciferol supplementation in critically ill patients.
4.1. Limitations
Although 1,25(OH)2D is proposed to provide a more valid measure for better detection of the vitamin D role in the prognosis and the treatment outcome of ARTIs patients, yet 25(OH)D was the one we measured owing to the unavailability of 1,25(OH)2D and high expenses to obtain theses kits in Egypt. Regarding the calculation of the sample size, it is focused on adequately powering the trial to evaluate whether supplementation of 100,000 IU of vitamin D3 (Cholecalciferol) will minimize the disease duration in pneumonic children and improve their treatment outcome. This study is not powered to evaluate these or other important outcomes as PICU length of stay, as the duration range of PICU stay was slightly longer among the supplementation group. This corresponds to a previous randomized control trial among ill adults involving vitamin D3 supplementation showing no significant variations in length of PICU stay or other clinical outcomes after vitamin D3 supplementation to the placebo group.[40]
5. Conclusion
VDD was detected in pediatric critical care children. In pneumonic children with high VDD, it is illustrated that Vitamin D supplementation is accompanied by lowered mortality risk, reduced time to recover, improved PaO2/FiO2, and lower pSOFA scores.
However, globally, these disclosures need to be documented in different trials and places. Also, the impact of supplementation upon less risky children and other critical diseases needs to be studied to evaluate the whole advantage of this intervention in regards to improving child health.
Additional research is needed to adjudge if larger doses of vitamin D are needed, and where possible, a multicenter trial would provide the inevitable statistical power to assert the inscribed beneficial effects over the measured foreshowed outcomes.
The strengths of the present study are the use of valid criteria for the assessment of pneumonia severity and its outcome.
Acknowledgments
The authors especially thank all the nursing staff at Cairo University AbouEl-Reesh Children hospital for their support and help while conducting this study.
Author contributions
Conceptualization: John Rene Labib, Sally Kamal Ibrahem, Mohamed M. Ismail, Hadeel Mohammad El-Hanafi, Mai Hamed Kamel.
Data curation: Sally Kamal Ibrahem, Hadeel Mohammad El-Hanafi, Mai Hamed Kamel.
Formal analysis: Shaimaa A.M. Abd El Fatah.
Funding acquisition: Sally Kamal Ibrahem, Mohamed M. Ismail, Shaimaa A.M. Abd El Fatah, Amal Samir Sedrak, Mona Adel Soliman Attia, Hadeel Mohammad El-Hanafi, Mai Hamed Kamel.
Investigation: Hadeel Mohammad El-Hanafi, Mai Hamed Kamel.
Methodology: John Rene Labib, Shaimaa A.M. Abd El Fatah, Amal Samir Sedrak, Mona Adel Soliman Attia, Hadeel Mohammad El-Hanafi, Mai Hamed Kamel.
Project administration: John Rene Labib, Sally Kamal Ibrahem, Mohamed M. Ismail.
Software: Shaimaa A.M. Abd El Fatah.
Validation: Shaimaa A.M. Abd El Fatah, Amal Samir Sedrak, Mona Adel Soliman Attia.
Writing – original draft: John Rene Labib, Sally Kamal Ibrahem, Mohamed M. Ismail, Shaimaa A.M. Abd El Fatah, Amal Samir Sedrak, Mona Adel Soliman Attia, Hadeel Mohammad El-Hanafi, Mai Hamed Kamel.
Writing – review & editing: John Rene Labib, Shaimaa A.M. Abd El Fatah, Amal Samir Sedrak, Mona Adel Soliman Attia.
Footnotes
Abbreviations: 1,25(OH)2 D = 1,25-dihydroxy vitamin D, 25(OH) D = 25-hydroxy vitamin D, ALRIs = acute lower respiratory infections, ARTIs = acute respiratory tract infections, BVD = blood Vitamin D, ELISA = enzyme-linked immunosorbent assay, PaO2/FiO2 = partial oxygen/fractional oxygen, PICU = pediatric intensive care unit, PO = per oral, PSOFA = Pediatric Sequential Organ Failure Assessment, RCT = randomized controlled trial, REC = Research Ethical Committee, SD = standard deviation, SPSS = Statistical Package for Social Science, VDD = vitamin D deficiency.
How to cite this article: Labib JR, Ibrahem SK, Ismail MM, Fatah SA, Sedrak AS, Attia MA, El-Hanafi HM, Kamel MH. Vitamin D supplementation and improvement of pneumonic children at a tertiary pediatric hospital in Egypt: a randomized controlled trial. Medicine. 2021;100:13(e25011).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Elsevier, Kliegman RM, Geme JSt. Nelson Textbook of Pediatrics. 21th ed.2019;2088-94. [Google Scholar]
- [2].Tiewsoh K, Lodha R, Pandey RM, et al. Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatric 2009;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].UNICEF. Levels & Trends in Child Mortality. Estimates developed by the UN Inter-agency Group for Child Mortality Estimation. Report 2019. Available at: https://childmortality.org/wpcontent/uploads/2019/10/UN-IGME-Child-Mortality-Report-2019.pdf. Accessed March, 2019. [Google Scholar]
- [4].Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008;88:491S–9S. [DOI] [PubMed] [Google Scholar]
- [5].Underwood MA, Bevins CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics 2010;125:1237–47. [DOI] [PubMed] [Google Scholar]
- [6].Tavahen N, Pourmoghaddas Z, Esteki B, et al. Active form and reservoir form of Vitamin D in children with acute lower respiratory infections and its association with severity of the infection. Arch Pediatr Infect Dis 2019;7:e83431. [Google Scholar]
- [7].Larkin A, Lassetter J. Vitamin D deficiency and acute lower respiratory infections in children younger than 5 years: identification and treatment. J Pediatr Health Care 2014;28:572–82. quiz 583-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bergman P, Lindh AU, Bjorkhem-Bergman L, et al. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2013;8:e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].RamJat K. Vitamin D deficiency and lower respiratory tract infections in children: a systematic review and meta-analysis of observational studies. Trop Doct 2017;47:77–84. [DOI] [PubMed] [Google Scholar]
- [10].Banajeh SM. Nutritional rickets and vitamin D deficiency--association with the outcomes of childhood very severe pneumonia: a prospective cohort study. Pediatr Pulmonol 2009;44:1207–15. [DOI] [PubMed] [Google Scholar]
- [11].Albanna EAM, Ali YF, Elkashnia RAM. Vitamin D and LL-37 in children with pneumonia. Egypt J Pediatr Allergy Immunol 2010;8:81–6. [Google Scholar]
- [12].Hashemian H, Heidarzadeh A. Role of Vitamin D [25(OH) D] deficiency in development of pneumonia in children. Arch Pediatr Infect Dis 2017;5:e57276. [Google Scholar]
- [13].MedCalc Statistical Software version 18.2.1. MedCalc Software bvba. Ostend, Belgium; 2018. Available at: http://www.medcalc.org. [Google Scholar]
- [14].Basu S, Gupta R, Mitra M, et al. Prevalence of Vitamin D deficiency in a pediatric hospital of Eastern India. Indian J Clin Biochem 2015;30:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].World Health Organization. Technical Bases for the WHO Recommendations on the Management of Pneumonia in Children at First-Level Health Facilities.(1995) Report No. WHO/ARI /91.20. Geneva: WHO. Available at: https://www.who.int/maternal_child_adolescent/documents/ari_91_20/en/ [Google Scholar]
- [16].Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr 2012;142:498–507. [DOI] [PubMed] [Google Scholar]
- [17].World Health Organization. Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities. Evidence summaries, 2014. Available at: https://apps.who.int/iris/bitstream/handle/10665/137332/WHO_FWC_MCA_14.9_eng.pdf [PubMed] [Google Scholar]
- [18].Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Disease Society of America. Clin Infect Dis 2011;53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Han JE, Jones JL, Tangpricha V, et al. High dose Vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized control trial. J Clin Transl Endocrinol 2016;4:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Badawia NES, Algebalyb HF, ElSayed R, et al. Vitamin D deficiency in critically ill children. Kasr Al Ainy Med J 2017;23:6–11. [Google Scholar]
- [21].Aşilioğlu N, Çiğdem H, Paksu MS. Serum Vitamin D status and outcome in critically ill children. Indian J Crit Care Med 2017;21:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sankar J, Lotha W, Ismail J, et al. Vitamin D deficiency and length of pediatric intensive care unit stay: a prospective observational study. Ann Intensive Care 2016;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McNally JD, Menon K, Chakraborty P, et al. The association of vitamin D status with pediatric critical illness. Pediatrics 2012;130:429–36. [DOI] [PubMed] [Google Scholar]
- [24].McNally JD, Nama N, O’Hearn K, et al. Vitamin D deficiency in critically ill children: a systematic review and meta-analysis. Crit Care 2017;21:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cariolou M, Cupp MA, Evangelou E, et al. Importance of vitamin D in acute and critically ill children with subgroup analyses of sepsis and respiratory tract infections: a systematic review and meta-analysis. BMJ Open 2019;9:e027666.doi: 10.1136/bmjopen-2018-027666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Quraishi SA, De Pascale G, Needleman JS, et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med 2015;43:1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 2012;95:1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gottschlich MM, Mayes T, Khoury J, et al. Clinical trial of Vitamin D2vs D3 supplementation in critically ill pediatric burn patients. J Parent Enteral Nutr 2017;41:412–21. [DOI] [PubMed] [Google Scholar]
- [29].McNally JD, Amrein K, O’Hearn K, et al. Study protocol for a phase II dose evaluation randomized controlled trial of cholecalciferol in critically ill children with vitamin D deficiency (VITdAL-PICU study). Pilot Feasibility Stud 2017;3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ebenezer K, Job V, Antonisamy B, et al. Serum Vitamin D status and outcome among critically ill children admitted to the pediatric intensive care unit in South India. Indian J Pediatr 2016;83:120–5. [DOI] [PubMed] [Google Scholar]
- [31].Khorasani NR, Moazzami B, Tajrishi FZ, et al. The association between low levels of Vitamin D and clinical outcomes in critically-ill children: a systematic review and meta-analysis. Fetal Pediatr Pathol 2019;11:1–5. [DOI] [PubMed] [Google Scholar]
- [32].McNally JD, Amrein K. Vitamin D deficiency in pediatric critical care. J Pediatr Intensive Care 2016;5:142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shah SK, Lodha R. Implications of Vitamin D deficiency in critically ill children. Indian J Pediatr 2015;82:977–9. [DOI] [PubMed] [Google Scholar]
- [34].Han JE, Jones JL, Tangpricha V, et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol 2016;4:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tiewsoh K, Lodha R, Pandey RM, et al. Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatrics 2009;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grossmann RE, Zughaier SM, Kumari M, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol 2012;4:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med 2012;40:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Manaseki-Holland S, Qader G, Masher MI, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 2010;15:1148–55. [DOI] [PubMed] [Google Scholar]
- [39].Cariolou M, Cupp MA, Evangelou E, et al. Importance of vitamin D in acute and critically ill children with subgroup analyses of sepsis and respiratory tract infections: a systematic review and meta-analysis. BMJ Open 2019;9:e027666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Amrein K, Schnedl C, Holl A, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 2014;312:1520–30. [DOI] [PubMed] [Google Scholar]


