Abstract
This observational, longitudinal retrospective, noncomparative study was designed to assess the persistence and effectiveness of golimumab as a second anti-tumor necrosis factor (TNF) drug in patients with spondyloarthritis requiring discontinuation from a first anti-TNF drug.
Data were collected retrospectively for all patients with axial spondyloarthritis or psoriatic arthritis from 20 rheumatology clinics in Spain who started golimumab as a second anti-TNF drug between January 2013 and December 2015. Golimumab persistence was assessed with Kaplan-Meier survival analysis, and associated factors were assessed with Cox regression analysis.
210 patients started golimumab as a second anti-TNF drug: 131 with axial spondyloarthritis and 79 with psoriatic arthritis. In axial spondyloarthritis patients, the mean (standard deviation) Bath Ankylosing Spondylitis Disease Activity Index score at baseline was 5.5 (2.1), decreasing to 3.9 (2.0) at month 3 and 3.5 (2.0) at year 1, and remaining stable thereafter. In psoriatic arthritis patients, mean (standard deviation) baseline Disease Activity Score was 4.0 (1.3), reducing to 2.5 (1.2) at month 3 and to 2.2 (1.3) at year 1. Corresponding improvements were recorded from baseline in C-reactive protein levels and erythrocyte sedimentation rates. The probability of persistence of treatment with golimumab was 80% at year 1, 70% at year 2 and 65% at years 3 and year 4, and was similar in those who had stopped the first anti-TNF due to loss of efficacy or other reasons. Cox regression analysis showed that the probability of survival with golimumab was higher in patients with higher erythrocyte sedimentation rate, in patients with axial spondyloarthritis than with psoriatic arthritis, and in those who had discontinued adalimumab as first anti-TNF. Seventy-two patients (34.3%) discontinued golimumab during follow-up, 50 of them due to lack of efficacy.
In patients with spondyloarthritis requiring discontinuation from a first anti-TNF drug, treatment with golimumab was effective and showed a high probability of persistence up to 4 years of treatment.
Keywords: ankylosing spondylitis, anti- tumor necrosis factor, golimumab, psoriatic arthritis, spondyloarthritis, tumor necrosis factor inhibitor
1. Introduction
Spondyloarthritis (SpA) comprises several rheumatic diseases, among which the most prevalent and studied are axial spondyloarthritis (axial SpA) and psoriatic arthritis (PsA). Both diseases impact patient quality of life at an early age and are associated with loss of productivity and sick leave.[1] Biological drugs – initially inhibitors of tumor necrosis factor (TNF) alpha and later drugs with other mechanisms of action – have changed the course of these diseases and improved patient quality of life. For patients with ankylosing spondylitis or nonradiographic axial SpA, notwithstanding the use of nonsteroidal antiinflammatory drugs, the American College of Rheumatology, Spondylitis Association of America, and Spondyloarthritis Research and Treatment Network, and the European League Against Rheumatism, recommend the use of anti-TNF drugs;[2,3] for patients with PsA, biological drugs are recommended when conventional therapy fails.[4] Whilst anti-TNF drugs are the most frequently used first biological drugs, the response declines over time in some patients, requiring a switch to another biological.[3,4]
Most information on the clinical efficacy of anti-TNF drugs is derived from controlled clinical trials or observational studies in patients who failed on conventional non-biological therapy and were naïve to anti-TNF drugs. There is less reported experience of the mid- and long-term effectiveness of biological drugs as second biologicals in patients who have discontinued a first anti-TNF drug.
Golimumab is a human anti-TNF monoclonal antibody with high affinity binding to soluble and transmembrane TNF.[5] It improves the signs and symptoms of disease in patients with active rheumatoid arthritis (RA), PsA and axial SpA [either non-radiographic SpA or ankylosing spondylitis (AS)], and in clinical trials golimumab showed a high retention rate in biological-naïve patients with RA, PsA or SpA, with around 70% of patients still on therapy after 5 years of treatment and with sustained efficacy.[6] However, except in RA – where the efficacy of golimumab after the failure of other anti-TNF drugs was studied in the prospective, controlled GO-AFTER study,[7] and a retrospective analysis of data from the LORHEN registry[8] – information on the effectiveness and persistence rate of golimumab as second-line treatment is scarce. In patients with axial SpA or PsA, information on golimumab retention rates in biological non-naïve patients (second or further biological drug) comes from subgroups of 2 studies in different lines of therapy.[9,10]
Given the scarce information available, we focused our research on the specific group of patients with spondyloarthropathies (axial SpA or PsA) treated with golimumab as a second biological agent. Thus, the main objective of this retrospective study was to assess the effectiveness of golimumab as a second anti-TNF drug in patients with SpA (either axial SpA or PsA) who discontinued a first anti-TNF drug. In particular, the study investigated the probability of persistence (retention rate of treatment with golimumab, or drug survival) over time and variables associated with higher persistence.
2. Patients and methods
2.1. Study design
The present study was an observational multicenter, longitudinal retrospective, and noncomparative study undertaken in 20 rheumatology clinics in Spain. Data were collected for all SpA (axial SpA or PsA) patients who had initiated golimumab as a second biological drug (after discontinuation of a first anti-TNF drug), between January 2013 and December 2015, according to standard prescribing information. The diagnosis of axial SpA or PsA was made according to clinical practice in Spain, where it is recommended to follow the Assessment of SpondyloArthritis International Society criteria for classification of axial SpA,[11,12] and the Classification Criteria for Psoriatic Arthritis criteria for PsA.[13] For patients presenting with mixed forms involving both axial and peripheral symptoms, investigators assigned them to the dominant group fulfilling criteria.
2.2. Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of the Hospital Universitari of Bellvitge, L’Hospitalet de Llobregat, Barcelona, Spain (ref EPA003/17) as reference center, and then by the participating centers. Signed informed consent forms were obtained from all available patients for whom data were used in the study. Where patients could not be located to obtain consent, their retrospective data were anonymized prior to inclusion. This was approved by the Clinical Research Ethics Committees.
2.3. Assessment criteria
The primary objective was to assess 1-, 2- and 3-year probability of persistence (survival on treatment, or retention rate) on golimumab and to explore possible variables associated with longer persistence on treatment with golimumab.
The following data were collected for stratified analyses: gender, smoking status, age above/below median, disease duration above/below median, axial SpA compared to PsA patients, reason for discontinuation of first anti-TNF drug (loss of efficacy versus other reasons), baseline C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), human leukocyte antigen (HLA) B27 status for axial SpA patients, presence or absence of radiographic erosions for PsA patients, prior anti-TNF drug used, presence or absence of concomitant immune disease and the nature of concurrent medications.
The effectiveness of golimumab was assessed at baseline and after 3 months and 1 year, using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axial SpA,[14,15] and Disease Activity Score (DAS28) for patients with PsA,[16,17] according to the available information contained in clinical records. For patients lacking DAS28 assessment, evolution of painful and swollen joints was recorded. In case patients presented with axial and peripheral symptoms, effectiveness was measured using the dominant disease assessment criterion. Due to the retrospective nature of the study, flexible windows of +/- 1 month for the 3-month assessment and of +/- 3 months for the 1-year assessment were permitted.
2.4. Patient selection
Patients aged ≥18 years who started golimumab for SpA as a second anti-TNF drug between January 2013 and December 2015 were included in the study if they had been treated successfully with a first anti-TNF drug for at least 3 months (i.e. the patient had obtained clinical benefit from a first anti-TNF drug) and discontinued it for 1 of the following reasons:
-
(1)
disease reactivation (loss of response or secondary failure after the patient had responded),
-
(2)
patient discomfort, poor tolerability or adverse event or
-
(3)
physician and patient agreement to substitute for patient convenience or preference, with no therapy failure.
Patients considered to have had a primary failure to a first anti-TNF drug (patient did not show clinical improvement on the first anti-TNF) were excluded, as were patients who, during the retrospective study period, participated in a clinical trial with antirheumatic drugs.
Patients were identified in the rheumatology clinic records or hospital pharmacy registry, and all patients fulfilling the criteria were included in the study. All patients still on golimumab signed informed consent before being enrolled into the study. Patients who had already discontinued golimumab were identified, localized and also invited to participate. For patients unable to attend the rheumatology clinic (e.g. due to death, change of address or other reason), anonymized data were recorded from their clinical records. This was done to avoid bias in the evaluation of the primary objective (probability of persistence on golimumab). For the same reason, it was mandatory that centers included all golimumab patients in the study, that is, patients still receiving golimumab and those who had discontinued golimumab. Centers that could not fulfil this commitment were excluded from participation to avoid selection bias.
General demographic data, disease-related data, previous anti-TNF drug use and reason for discontinuation, and baseline disease activity (prior to golimumab initiation) were collected from the clinical records. The first date of golimumab injection and last observation date with golimumab were recorded to assess the primary objective of the study. For patients who permanently discontinued golimumab, the reason for discontinuation was classified as:
-
(1)
primary failure (the patient never responded to golimumab),
-
(2)
disease reactivation (secondary failure, loss of response after golimumab was initially efficacious),
-
(3)
poor tolerability,
-
(4)
adverse event,
-
(5)
inactive disease or low disease activity or
-
(6)
other reason.
Patients who stopped golimumab temporarily (i.e. due to infection, programmed surgery or other reason), but resumed it successfully later on (grace period, 2 months), were considered as continuing on golimumab.
2.5. Statistical analysis
The sample size was calculated to assess the primary objective, and due to the lack of information on persistence (retention rate) of a second anti TNF alpha after discontinuation of a first anti TNF alpha in spondyloarthropathies at the time of the study design, a 50% retention rate was selected as the percentage requiring the highest sample size. With a confidence level (level 1-alpha) of 95% and 7% precision, the estimated sample size was 196 patients with SpA. The target sample size was increased by 15% (to 231 patients) to compensate for possible missing data. No stratification by background disease (axial SpA versus PsA) was done during recruitment since centers were obliged to recruit all patients, whether or not balanced.
Summary descriptive statistics are presented as means with standard deviations, or medians with percentiles and percentages when applicable. The probability of persistence (drug survival) was assessed by Kaplan-Meier analysis, for the overall population and each background disease. Patients who were still on golimumab at the study visit and those who had not discontinued golimumab, but for whom data were not available from a specific time point (due to death, emigration to other region or country, or end of data availability for other reasons), were right-censored. Comparisons of survival between different subgroups were evaluated with the log-rank test. Cox-regression analyses were performed to identify factors related to the probability of persistence on golimumab. R Software (version 3.4.3, R Core Team, 2017) and the RStudio interface (version 1.1.419) were used for statistical analyses.
3. Results
3.1. Patient demographics and baseline condition
Between January 2013 and December 2015, 210 patients from 20 hospital rheumatology clinics had initiated golimumab as a second anti-TNF drug for the treatment of axial SpA (n = 131; 28 with non-radiographic axial SpA and 103 with ankylosing spondylitis) or PsA (n = 79). Five patients from 1 center (which failed to include all golimumab patients) were excluded from all analyses.
Mean (standard deviation [SD]) age was 49.0 (12.1) years. There were 126 men (60.0%) and 84 women (40.0%). Median disease duration prior to golimumab initiation was 7 years (interquartile range: 3–13). Baseline characteristics of the population are displayed in Table 1. Previous anti-TNF therapy was etanercept (n = 53, 25.2%), adalimumab (n = 83, 39.5%), infliximab (n = 73, 34.8%) and certolizumab (n = 1, 0.48%) (Table 2). Reasons for discontinuation of the first anti-TNF drug were: loss of efficacy (n = 149, 71.0%), poor tolerability or adverse event (n = 24, 11.4%) and patient or physician preference (n = 37, 17.6%).
Table 1.
General characteristics of patients at golimumab initiation.
| Axial SpA (n = 131) | PsA (n = 79) | |
| Age | ||
| yr, mean (SD) | 48.0 (12.3) | 50.0 (11.7) |
| Gender | ||
| Male, n (%) | 83 (63.4) | 43 (54.4) |
| Female, n (%) | 48 (36.6) | 36 (45.6) |
| Duration of disease | ||
| Years, median (IQR) | 8.0 (4.0–16.0) | 6.0 (3.0–9.5) |
| Smoking habit | ||
| Never, n (%) | 47 (42.3) | 33 (55.9) |
| Current, n (%) | 40 (36.0) | 9 (15.3) |
| Past, n (%) | 24 (21.6) | 17 (28.8) |
| Not available, n | 20 | 20 |
| HLA B27 status | ||
| Positive, n (%) | 95 (76.7) | 8 (17.8) |
| Negative, n (%) | 29 (23.4) | 37 (82.2) |
| Not available, n | 7 | 34 |
| Concomitant immune-mediated diseases | ||
| Psoriasis, n (%) | 12 (9.2) | 38 (48.1) |
| Inflammatory Bowel Disease, n (%) | 6 (4.6) | 0 (0.0) |
| Uveitis, n (%) | 22 (16.8) | 1 (1.3) |
| Concomitant medication | ||
| NSAIDs | 101 (77.1) | 45 (57.0) |
| Steroids, n (%) | 13 (9.9) | 25 (31.7) |
| Non-biological DMARD | 41 (31.3) | 52 (65.8) |
| Methotrexate, n | 29 | 36 |
| Sulfasalazine, n | 11 | 6 |
| Leflunomide, n | 2 | 13 |
DMARD = disease modifying antirheumatic drug, HLA = human leukocyte antigen, IQR = interquartile range, NSAIDs = nonsteroidal antiinflammatory drugs, SD = standard deviation.
Table 2.
Characteristics of treatment with the first anti-TNF.
| Axial SpA (n = 131) | PsA (n = 79) | |
| First anti-TNF | ||
| Adalimumab, n (%) | 55 (42.0) | 28 (35.4) |
| Etanercept, n (%) | 26 (19.9) | 27 (34.2) |
| Infliximab, n (%) | 50 (38.1) | 23 (29.1) |
| Certolizumab, n (%) | – | 1 (1.3) |
| Duration of treatment with the first anti-TNF | ||
| Months, median (SD) | 35.0 (10.0–73.0) | 28.0 (10.5–59.0) |
| Reason for discontinuation | ||
| Loss of efficacy (disease reactivation), n (%) | 98 (74.8) | 51 (64.5) |
| Adverse event or intolerance, n (%) | 11 (8.4) | 13 (16.5) |
| Patients and/or physician preference, n (%) | 22 (16.8) | 15 (19.0) |
SD = standard deviation, TNF = tumor necrosis factor.
3.2. Effect of golimumab on disease activity
In axial SpA patients, mean (SD) BASDAI at baseline (n = 78) was 5.5 (2.1). It was rapidly reduced to a mean of 3.9 (2.0) at month 3 and 3.5 (2.0) at year 1, and remained stable thereafter. The percentage of patients with a BASDAI 50 response was 69.1% at month 3 and 58.5% at year 1; the percentages with BASDAI ≤4 were 53.2% and 60.4%, respectively. Baseline mean (SD) CRP (n = 121) was 0.9 (1.7) mg/dL and was reduced to 0.3 (0.6) mg/dL at month 3 and 0.5 (1.5) mg/dL at year 1. Similarly, ESR (n = 119) decreased from baseline 22.4 (24.8) mm/h to 13.0 (12.2) and 10.8 (9.8) mm/h at month 3 and year 1, respectively.
In PsA patients, mean (SD) DAS28 (n = 28) was 4.0 (1.3) at baseline and was reduced to 2.5 (1.2) at month 3 and to 2.2 (1.3) at year 1. The mean number of painful joints (n = 59) decreased from 4.2 (4.7) at baseline to 2.1 (2.2) and 1.1 (1.9) at month 3 and year 1, respectively, and the mean number of swollen joints from 2.4 (2.7) to 1.3 (2.3) and 0.7 (2.0), respectively. Baseline mean (SD) CRP (n = 68) was 1.0 (2.1) mg/dL and was reduced to 0.4 (0.6) at month 3 and 0.3 (0.5) mg/dL at year 1. ESR (n = 66) decreased from 18.9 (20.0) mm/h at baseline to 14.1 (17.7) and 12.9 (13.4) mm/h at month 3 and year 1, respectively.
3.3. Probability of persistence of treatment with golimumab
Patients were followed up for a mean (SD) period of 29.8 (16.5) months: axial SpA patients, 30.2 (17.1) months; PsA patients, 29.0 (16.5) months. During this period, 72 of 210 patients (34.3%) discontinued golimumab: 39 of 131 axial SpA patients (29.8%) and 33 of 79 PsA patients (41.8%). The probability of persistence of treatment with golimumab (survival of golimumab therapy) since initiation was 80% at year 1 (95% confidence interval [CI] 75–86), 70% at year 2 (95% CI 64–77), and 65% at year 3 and year 4 (95% CI 59–72) (Fig. 1). The probability of persistence was slightly but non-significantly higher in patients with axial SpA than in those with PsA (P = .121, Fig. 2A; Supplemental Digital Content Table 1). The probability curves were very similar in patients who discontinued their first anti-TNF due to loss of efficacy compared with those discontinuing for other reasons (P = .750, Fig. 2B; Supplemental Digital Content Table 2).
Figure 1.
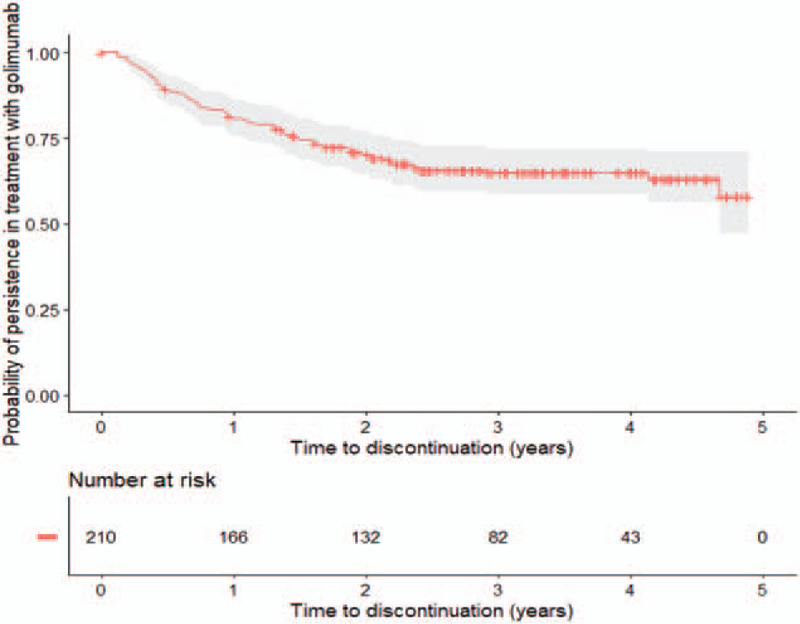
Probability of persistence with golimumab treatment in all patients.
Figure 2.
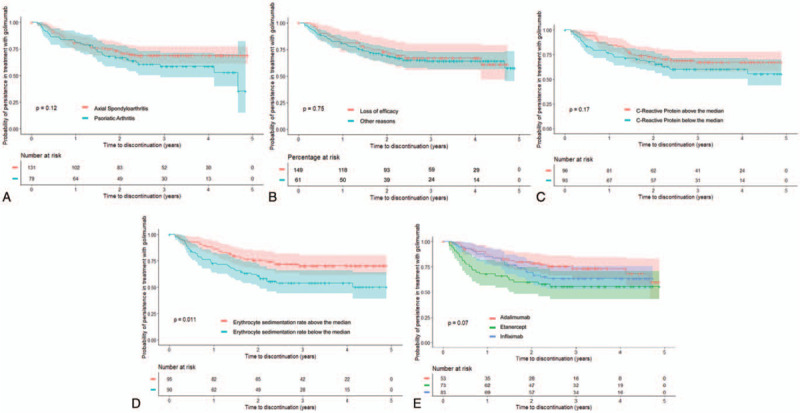
Probability of persistence with golimumab treatment in patients (A) diagnosed with axial SpA (red) or PsA (blue); (B) discontinuing the first anti-TNF drug for loss of efficacy (red) versus for other reasons (blue); (C) whose baseline C-reactive protein (CRP) level was over (red) or under (blue) the median value; (D) whose baseline erythrocyte sedimentation rate (ESR) level was over (red) or under (blue) the median value; (E) treated with adalimumab (red), etanercept (green) or infliximab (blue) as first anti-TNF drug.
There were no differences in the probability of persistence by gender (P = .313), age above/below the median (49 years, p = 1.000), smoking status (P = .381), or disease duration above/below the median (80 months, P = .985).
The probability of persistence with golimumab was slightly higher in patients with CRP values above the median (0.28 mg/dL) at the time of golimumab initiation compared to those with values below the median (P = .175, Fig. 2C; Supplemental Digital Content Table 3), as well as in those with ESR above the median when golimumab was started (16 mm/h) compared to those with values below the median (P = .011, Fig. 2D; Supplemental Digital Content Table 4).
There were no differences in the probability of persistence with regard to the HLA B27 status in patients with axial SpA, or with regard to the presence or not of radiographic erosions in PsA patients. The probability of persistence was higher in those previously treated with adalimumab as first anti-TNF (continuation rate at year 3: 73%) compared to in those who had stopped infliximab (63%) or etanercept (55%), but the difference was not statistically significant (P = .070, Figure 2e; Supplemental Digital Content Table 5).
Finally, the probability of persistence did not differ between patients with or without other concomitant immune disease (P = .731) and was also similar in those who were or were not treated with glucocorticoids, nonsteroidal anti-inflammatory drugs or non-biological disease-modifying antirheumatic drugs (P = .710, .490, and .657, respectively). Persistence was slightly, but non-significantly, higher (69% versus 63% at both year 3 and year 4) in those treated with methotrexate (P = .254; Supplemental Digital Content Table 6). In a post hoc analysis, persistence with/without methotrexate in patients with axial SpA or PsA was, in both cases, slightly but non-significantly higher in patients treated with methotrexate compared to those without methotrexate (P = .202 for axial SpA patients; P = .313 for PsA patients).
3.4. Multivariate analysis
Cox-regression analysis including CRP and ESR showed that patients with ESR above the median at golimumab initiation were less likely to discontinue golimumab than those below the median (hazard ratio [HR], 0.59, 95% CI 0.36–0.95; P = .031). CRP was not statistically related to discontinuation of golimumab in this model (Table 3).
Table 3.
Cox-regression analysis. Hazard ratios for risk of discontinuation of golimumab.
| Hazard ratio | 95% Confidence interval | P | |
| Model 1 | |||
| ESR (above vs below the median) | 0.59 | 0.36–0.95 | .031 |
| CRP (above vs below the median) | 0.74 | 0.48–1.19 | .217 |
| Model 2 | |||
| Gender (women vs men) | 0.58 | 0.25–1.34 | .206 |
| Age (above vs below the median) | 1.68 | 0.75–3.76 | .208 |
| Diagnosis (axial SpA vs PsA) | 0.44 | 0.18–1.05 | .063 |
| Previous anti-TNF (adalimumab vs etanercept) | 0.28 | 0.10–0.76 | .012 |
| Previous anti-TNF (infliximab vs etanercept) | 0.45 | 0.18–1.16 | .098 |
| Use of methotrexate (yes vs no) | 0.55 | 0.25–1.23 | .145 |
| ESR (above vs below the median) | 0.53 | 0.24–1.19 | .123 |
| CRP (above vs below the median) | 0.60 | 0.26–1.37 | .225 |
CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; PsA = psoriatic arthritis; SpA = spondyloarthritis; TNF = tumor necrosis factor.
A second model, including as predictive variables age, gender, methotrexate use and those variables with P-values ≤.2 in the bivariate analysis (diagnosis, previous anti-TNF drug, CRP and ESR when golimumab was started), showed that patients who had stopped treatment with adalimumab had a lower probability of discontinuation of golimumab therapy than those previously treated with etanercept (HR, 0.28, P = .012). Similarly, patients with axial SpA had a lower probability of discontinuation of golimumab therapy compared to PsA patients (HR, 0.44, P = .063) [Table 3, Model 2].
3.5. Reasons for discontinuation of golimumab
Overall, 72 of 210 patients (34.3%) discontinued golimumab during the follow-up. The reasons for discontinuation were primary failure (n = 21), disease reactivation or secondary failure (n = 29), poor tolerability (n = 4), adverse events (n = 10) and patient-physician agreement due to inactive disease or low disease activity (n = 8). Reasons for discontinuation did not differ between patients with axial SpA or those with PsA.
4. Discussion
Previous observational studies have described the golimumab retention rate in SpA patients previously treated with other biological drugs as subgroups of larger cohorts without stratifying non-naïve patients in second or further lines of therapy.[9,10] Our study assessed, specifically, the effectiveness and persistence (survival on therapy or retention rate) of golimumab as a second anti-TNF drug in patients with SpA (axial SpA or PsA) who had discontinued a first anti-TNF drug, with up to 4 years of follow-up. Golimumab was effective for the treatment of these patients in the short term, reducing disease activity (BASDAI and DAS28) and inflammatory markers (CRP and ESR). In the long term, there was a high rate of retention of golimumab therapy (65%) after 3–4 years. Golimumab showed similar short-term effectiveness in patients with axial SpA or PsA, but long-term persistence seemed better in patients with axial SpA.
The clinical development of golimumab comprised clinical trials in patients with RA, axial SpA and PsA who had failed conventional non-biological treatment, including disease-modifying antirheumatic drugs (methotrexate), in RA. For patients with RA (but not axial SpA or PsA), clinical trials also considered patients who had failed on previous biological medication. Thus, the current study provides specific information on the effectiveness of golimumab in a subset of patients who had not been included in clinical trials, and suggests that, in patients with spondyloarthropathies who need to discontinue using a first anti-TNF, switching to golimumab is a suitable option.
A review of English-language publications of clinical trials which reported on the efficacy of second-line biologicals concluded that, for PsA and AS patients failing a first anti-TNF drug, switching to a second is advisable.[18] Indeed, after the failure of a first anti-TNF, therapeutic guidelines describe the option of switching to another biological drug; either another anti-TNF or a drug with a different mechanism of action.[2–4] In particular, the recently updated 2019 American College of Rheumatology, Spondylitis Association of America and Spondyloarthritis Research and Treatment Network guidelines conditionally recommend a second anti-TNF after discontinuation of a first anti-TNF due to secondary nonresponse in axial spondyloarthritis, although with a very low level of evidence.[2] For patients with primary nonresponse, the guidelines recommend switching to a drug with another mechanism of action, also with a very low level of evidence.[2] Thus, data on the effectiveness of different biologicals after discontinuation of one anti-TNF drug are welcome. Recent research indicates that therapeutic drug monitoring (TDM) with serum level measurement and antidrug antibody detection could potentially be a useful tool for the individualization of biological therapy[19–21] and selection of a second or subsequent biological drug.
In the present study, probabilities of persistence with golimumab as a second anti-TNF drug in SpA patients were high and at least as good as data reported with other anti-TNF drugs, whether as first-line or subsequent lines of anti-TNF therapy. Among 990 anti-TNF treatment courses (with adalimumab, infliximab, etanercept or golimumab) as first, second or third anti-TNF in 765 patients with PsA treated in Finland between 2004 and 2014, the probability of persistence was 80% at 1 year and 72% at 2 years.[22] The probability of discontinuation of anti-TNF drug was similar whether it was the first or a subsequent drug.[22] Similarly, in a review of randomized controlled trials of anti-TNF drugs for axial SpA or AS (but mostly AS), across all anti-TNF drugs, drug survival was 70% to 80% after 1 year, 65% to 75% after 2 years and 55% at 5 years.[23] In AS patients, across all anti-TNF drugs, median drug survival was 3.1 years for a first anti-TNF drug, 1.6 years for a second and 1.8 years for a third; 2-year drug survival was 58%, 47%, and 49%, respectively (based on data from the Danish nationwide DANBIO registry[24]).[23] In contrast, in a longer-term analysis of patients with axial SpA receiving anti-TNF drugs at 2 specialist centers in the UK, median drug survival was 10.2 years for the first or index anti-TNF drug (n = 651) and 5.5 years for a second anti-TNF drug after switching from the index drug (n = 105). Drug survival was not influenced by the choice of anti-TNF drug.[25]
With regard to studies which have focused on golimumab, a retrospective, observational study in Greece involving 328 patients, reported drug survival rates with golimumab after 3 years of 76% in AS patients, 60% in RA patients, and 53% in PsA patients.[26] Regarding the use of golimumab as a second anti-TNF, a recent retrospective analysis of data from the LORHEN registry showed that the 2-year retention rate was higher with golimumab than with etanercept or adalimumab (53.4%, 39.8%, and 31.2%, respectively), and there was a lower discontinuation rate due to adverse events in patients treated with golimumab.[8] Iannone et al described 2-year persistence rates of 67.3% and 72.2% for the overall naïve and non-naïve arthritis population, with no differences for patients with axial SpA or PsA,[9] and recently Michelsen et al. reported a 4-year retention rate of around 30% in non-naïve golimumab patients,[10] but they did not stratify by second or further line of therapy. Finally, a retrospective analysis of the Spanish Registry of Biological Drugs in Rheumatic Diseases reported high continuation rates of golimumab as a second biological drug in rheumatic diseases up to 4 years of follow-up (up to 59.2% at year 4) including a small sample of patients with axial SpA (n = 52) or PsA (n = 33).[27]
Interestingly, in the current study, golimumab seemed to be similarly effective in patients who discontinued the first anti-TNF drug due to loss of efficacy compared with in those who discontinued for other reasons. Others have also reported no difference in survival analysis with a second anti-TNF drug according to reason for discontinuation of the first anti-TNF drug.[10] This finding supports the use of golimumab in these patients after failure or discontinuation of a first anti-TNF drug independently of the reason, except when discontinuation was due to primary nonresponse. Higher retention of golimumab was seen in patients with higher CRP or, significantly, higher ESR levels. These patients, with higher markers of inflammation at baseline, would seem better suited for obtaining clinical benefit from anti-TNF drugs. It has previously been shown that increased CRP levels and higher BASDAI scores are valuable predictors of clinical response to anti-TNF drugs.[28] In the GO-AHEAD study, the benefits of golimumab were not apparent in patients with normal CRP and no inflammatory signs by magnetic resonance imaging.[29] In the present study, mean CRP was relatively low because all patients had been previously treated with a biological drug and because up to 29% of patients were discontinued for reasons other than disease reactivation.
The higher probability of continuation with golimumab in patients who had received adalimumab compared with that in patients who had previously received other anti-TNF drugs requires further investigation with a larger sample of patients. The slightly better persistence seen with golimumab when used in combination with methotrexate is consistent with earlier reports.[30]
Possible explanations for these high retention rates with golimumab as a second anti-TNF could relate to the convenience of a once-monthly, self-administered medication,[22] and/or the fact that the drug was well tolerated (only 6.7% of patients discontinued golimumab for poor tolerability or adverse effects in the present study). From some studies it appears that, due to the molecular characteristics of golimumab, there is a lower frequency of anti-drug antibody formation,[21] and this may also contribute to the good retention rate of golimumab as a second biological drug. Because persistence with the same anti-TNF drug in second-line treatment decreases the overall disease-associated costs compared with non-persistence, this aspect also merits consideration by physicians and payers when choosing a biological drug.[31]
There were a number of limitations to this study. Because data were collected retrospectively, disease activity (BASDAI and DAS 28) was not reported adequately in the clinical records for all patients. Functional and motion activity indexes (Bath Ankylosing Spondylitis Functional Index, Bath Ankylosing Spondylitis Metrology Index), and other currently important activity indices like Ankylosing Spondylitis Disease Activity Score or Disease Activity for Psoriatic Arthritis, were not reported in the clinical records, so accurate information on disease evolution was limited. Similarly, data to calculate currently used indices, such as minimal disease activity in PsA, were also lacking, and body mass index data, an important variable, were not collected in many clinical records and, therefore, could not be analyzed. Previous studies have shown a relationship between overweight and obesity with poorer clinical outcomes and lower retention rate of biological drugs,[32,33] but unfortunately we could not analyze the impact of body mass index in our study. As real-world data are becoming indispensable for understanding the effectiveness of drugs in different populations and guiding the best therapeutic decisions, standardization of data collection among different clinics or regions/countries is needed to facilitate obtaining this important information more accurately. The absence of radiographic analysis meant that it was not possible to confirm whether the clinical effectiveness of golimumab in these patients was associated with reduced radiographic damage or progression. Also, whilst we considered some predictive factors, it would be interesting for future research to assess other aspects such as the type of arthritis in PsA (e.g. polyarthritis or oligoarthritis, enthesitis), psoriasis severity, extra-articular involvement such as uveitis or inflammatory bowel disease, and other comorbidities, for which a larger sample size of patients will be needed. In this case, the sample size was limited, precluding the identification of stronger associations. In particular, the influence of methotrexate on long-term effectiveness cannot be ruled out.
Changing from 1 biological drug to another has been reported to be associated with an increase in treatment costs.[31,34] Nonetheless, treatment switching is required (and recommended) where there is treatment failure or intolerance. Except for patients with primary nonresponse to anti-TNF drugs, starting a second anti TNF drug is supported by current recommendations.[2–4] Choosing a subsequent anti-TNF drug with a high survival or persistence rate is, therefore, an important factor in the economics of patient management for patients with rheumatic disease. Golimumab was effective and showed a high probability of persistence as a second anti-TNF drug in patients with SpA who need to discontinue the use of a first anti-TNF drug for reasons other than primary nonresponse.
Acknowledgments
Study implementation and statistical analysis were conducted by SERMES CRO, Spain. Medical writing assistance was provided by Jon Monk and David P. Figgitt PhD, ISMPP CMPP, Content Ed Net, with funding from Merck Sharp & Dohme Spain. Special thanks to the study coordinators, Beatriz Ramos, Sabela Fernández and Yvonne Mestre, Medical Affairs, Merck Sharp & Dohme Spain.
List of investigators: The authors want to acknowledge the participation of the following investigators in the study: María Martín (Hospital Universitario 12 de Octubre, Madrid), Miriam Retuerto, Antía Crespo and Carlota Íñiguez (Hospital Universitario de León), José Manuel Martín (Hospital Universitario Río Hortega, Valladolid), Noelia Álvarez and Tomás Ramón Vázquez (Hospital Universitario Lucus Agusti, Lugo), César Antonio Egües (Hospital Universitario de Donostia, San Sebastián), Belén Serrano (Hospital Universitario Gregorio Marañón, Madrid), Ángel Aragón and Ángel Jaime Zubieta (Hospital Universitario de Getafe), Ana Milena (Hospital de la Santa Creu i Sant Pau, Barcelona), Juan Víctor Tovar (Hospital General Universitario de Elche), Victoria Núñez and Ana Sendra (Hospital Universitario Doctor Pesset, Valencia), Enrique Raya and Cristina Cano (Hospital Universitario San Cecilio, Granada), Rosario Ibáñez and Ricardo Gutiérrez (Complejo Hospitalario de Navarra, Pamplona), Chesús Beltrán, Álvaro Lesta, Marta Medrano and Carlos Vázquez (Hospital Universitario Miguel Servet, Zaragoza), Jordi Pons (Hospital Royo Villanova, Zaragoza), Rosa Roselló and Blanca García (Hospital General San Jorge, Huesca), Emma Beltrán, Carolina Pérez, Jone Llorente, Tarek Carlos Salman-Monte, Luciano Polino and Jordi Monfort (Hospital del Mar, Barcelona), Sara García Carazo, Irene Monjo and Diana Peiteado (Hospital Universitario La Paz, Madrid).
Author contributions
Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González designed the study. Juan J Alegre-Sancho, Xavier Juanola, and Carlos M González interpreted the results. Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González drafted the manuscript. José M Rodríguez-Heredia, Javier Manero, Ignacio Villa-Blanco and Ana Laiz made substantial contributions to the draft manuscript. All authors reviewed and approved the final version of the manuscript. Authors from the sponsor (MSD Spain) participated in the study design, statistical analysis planning and in drafting the manuscript.
Conceptualization: Juan J Alegre-Sancho, Xavier Juanola, José M Rodríguez-Heredia, Javier Manero, Ignacio Villa-Blanco, Ana Laiz, María J Arteaga, Luis Cea-Calvo, Carlos M González.
Data curation: Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González.
Formal analysis: Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González.
Funding acquisition: María J Arteaga, Luis Cea-Calvo.
Investigation: Juan J Alegre-Sancho, Xavier Juanola, José M Rodríguez-Heredia, Javier Manero, Ignacio Villa-Blanco, Ana Laiz, María J Arteaga, Luis Cea-Calvo, Carlos M González.
Methodology: Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González.
Project administration: María J Arteaga, Luis Cea-Calvo, Carlos M González, Laura Perez (on behalf of the authors).
Resources: María J Arteaga, Luis Cea-Calvo.
Software: María J Arteaga, Luis Cea-Calvo.
Supervision: Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González.
Validation: Juan J Alegre-Sancho, José M Rodríguez-Heredia, Javier Manero, María J Arteaga, Luis Cea-Calvo, Carlos M González.
Visualization: Juan J Alegre-Sancho, Xavier Juanola, Ignacio Villa-Blanco, Ana Laiz, María J Arteaga, Luis Cea-Calvo, Carlos M González.
Writing – original draft: Juan J Alegre-Sancho, Xavier Juanola, María J Arteaga, Luis Cea-Calvo and Carlos M González.
Writing – review & editing: Juan J Alegre-Sancho, Xavier Juanola, José M Rodríguez-Heredia, Javier Manero, Ignacio Villa-Blanco, Ana Laiz, María J Arteaga, Luis Cea-Calvo, Carlos M González.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AS = ankylosing spondylitis, BASDAI = Bath Ankylosing Spondylitis Disease Activity Index, CI = confidence interval, CRP = C-reactive protein, DAS = disease activity score, ESR = erythrocyte sedimentation rate, PsA = psoriatic arthritis, RA = rheumatoid arthritis, SD = standard deviation, SpA = spondyloarthritis, TNF = tumor necrosis factor.
How to cite this article: Alegre-Sancho JJ, Juanola X, Rodríguez-Heredia JM, Manero J, Villa-Blanco I, Laiz A, Arteaga MJ, Cea-Calvo L, González CM. Effectiveness and persistence of golimumab as a second biological drug in patients with spondyloarthritis: a retrospective study. Medicine. 2021;100:13(e25223).
This study was funded by Merck Sharp & Dohme Spain, a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA.
Conflict of interest: Juan J Alegre-Sancho: speaker for MSD; Xavier Juanola: no conflicts of interest; José M Rodríguez-Heredia: board membership for Janssen, Sandoz and Sanofi, and speaker for BMS, UCB, Pfizer, Janssen, Novartis and Celgene; Javier Manero: no conflicts of interest; Ignacio Villa-Blanco: no conflicts of interest; Ana Laiz: board membership for Janssen, Novartis and Celgene, and speaker for Lilly, MSD, Janssen, Novartis and Celgene; María J Arteaga: full-time employee of Merck Sharp & Dohme Spain; Luis Cea-Calvo: full-time employee of Merck Sharp & Dohme Spain; Carlos M González: consultancy for MSD, Gilead, Pfizer, Roche, Novartis and Janssen, speaker for Roche, Novartis and Celgene, and travel and accommodation costs for meetings from Janssen, Pfizer and Roche.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Garrido-Cumbrera M, Hillmann O, Mahapatra R, et al. Improving the management of psoriatic arthritis and axial spondyloarthritis: roundtable discussions with healthcare professionals and patients. Rheumatol Ther 2017;4:219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken) 2019;71:1285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- [4].Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- [5].Zhou H, Jang H, Fleischmann RM, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol 2007;47:383–96. [DOI] [PubMed] [Google Scholar]
- [6].Tahir Z, Kavanaugh A. The role of golimumab in inflammatory arthritis. A review of the evidence. Ther Adv Musculoskelet Dis 2018;10:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor ( inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther 2015;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Favalli EG, Sinigaglia L, Becciolini A, et al. Two-year persistence of golimumab as second-line biologic agent in rheumatoid arthritis as compared to other subcutaneous tumor necrosis factor inhibitors: real-life data from the LORHEN registry. Int J Rheum Dis 2018;21:422–30. [DOI] [PubMed] [Google Scholar]
- [9].Iannone F, Santo L, Anelli MG, et al. Golimumab in real-life settings: 2 Years drug survival and predictors of clinical outcomes in rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum 2017;47:108–14. [DOI] [PubMed] [Google Scholar]
- [10].Michelsen B, Sexton J, Wierød A, et al. Four-year follow-up of inflammatory arthropathy patients treated with golimumab: data from the observational multicentre NOR-DMARD study. Semin Arthritis Rheum 2020;50:12–6. [DOI] [PubMed] [Google Scholar]
- [11].Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- [12].Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- [13].Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- [14].Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- [15].Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- [16].Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- [17].Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cantini F, Niccoli L, Nannini C, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum 2017;47:183–92. [DOI] [PubMed] [Google Scholar]
- [19].Balsa A, Sanmarti R, Rosas J, et al. Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor inhibitor therapies: the REASON study. Rheumatology (Oxford) 2018;57:688–93. [DOI] [PubMed] [Google Scholar]
- [20].Bornstein G, Lidar M, Langevitz P, et al. The prevalence and clinical effect of immunogenicity of TNF-(blockers in patients with axial spondyloarthritis. Clin Exp Rheumatol 2018;36:228–32. [PubMed] [Google Scholar]
- [21].Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs 2017;31:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aaltonen K, Heinonen A, Joensuu J, et al. Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin Arthritis Rheum 2017;46:732–9. [DOI] [PubMed] [Google Scholar]
- [23].Corbett M, Soares M, Jhuti G, et al. Tumour necrosis factor-(inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess 2016;20:1–334. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor ( inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 2013;72:1149–55. [DOI] [PubMed] [Google Scholar]
- [25].Yahya F, Gaffney K, Hamilton L, et al. Tumour necrosis factor inhibitor survival and predictors of response in axial spondyloarthritis-findings from a United Kingdom cohort. Rheumatology (Oxford) 2018;57:619–24. [DOI] [PubMed] [Google Scholar]
- [26].Thomas K, Flouri I, Repa A, et al. High 3-year golimumab survival in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: real world data from 328 patients. Clin Exp Rheumatol 2018;36:254–62. [PubMed] [Google Scholar]
- [27].Hernandez MV, Sanchez-Piedra C, Garcia-Magallon B, et al. Factors associated with long-term retention of treatment with golimumab in a real-world setting: an analysis of the Spanish BIOBADASER registry. Rheumatol Int 2019;39:509–15. [DOI] [PubMed] [Google Scholar]
- [28].Rudwaleit M, Listing J, Brandt J, et al. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 2004;63:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015;67:2702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Behrens F, Cañete JD, Olivieri I, et al. Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PsA: a systematic review of the literature. Rheumatology (Oxford) 2015;54:915–26. [DOI] [PubMed] [Google Scholar]
- [31].Dalén J, Svedbom A, Black CM, et al. Second-line treatment persistence and costs among patients with immune-mediated rheumatic diseases treated with subcutaneous TNF-alpha inhibitors. Rheumatol Int 2017;37:2049–58. [DOI] [PubMed] [Google Scholar]
- [32].Lopalco G, Venerito V, Cantarini L, et al. Different drug survival of first line tumour necrosis factor inhibitors in radiographic and non-radiographic axial spondyloarthritis: a multicentre retrospective survey. Clin Exp Rheumatol 2019;37:762–7. [PubMed] [Google Scholar]
- [33].di Minno MN, Peluso R, Iervolino S, et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res 2013;65:141–7. [DOI] [PubMed] [Google Scholar]
- [34].Gu T, Mutebi A, Stolshek BS, et al. Cost of biologic treatment persistence or switching in rheumatoid arthritis. Am J Manag Care 2018;24:S338–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


