Abstract
Background.
To the best of our knowledge, no studies have evaluated the effects of inspiratory muscle training (IMT) on recovered COVID-19 patients after weaning from mechanical ventilation. Therefore, this study assessed the efficacy of IMT on recovered COVID-19 patients following mechanical ventilation.
Methods.
Forty-two recovered COVID-19 patients (33 men and 9 women) weaned from mechanical ventilation with a mean age of 48.05 ± 8.85 years were enrolled in this pilot control clinical study. Twenty-one patients were equipped to 2-week IMT (IMT group) and 21 matched peers were recruited as a control (control group). Forced vital capacity (FVC%), forced expiratory volume in 1 second (FEV1%), dyspnea severity index (DSI), quality of life (QOL), and six-minute walk test (6-MWT) were assessed initially before starting the study intervention and immediately after intervention.
Results.
Significant interaction effects were observed in the IMT when compared to control group, FVC% (F = 5.31, P = .041, ηP2 = 0.13), FEV1% (F = 4.91, P = .043, ηP2 = 0.12), DSI (F = 4.56, P = .032, ηP2 = 0.15), QOL (F = 6.14, P = .021, ηP2 = 0.17), and 6-MWT (F = 9.34, P = .028, ηP2 = 0.16). Within-group analysis showed a significant improvement in the IMT group (FVC%, P = .047, FEV1%, P = .039, DSI, P = .001, QOL, P < .001, and 6-MWT, P < .001), whereas the control group displayed nonsignificant changes (P > .05).
Conclusions.
A 2-week IMT improves pulmonary functions, dyspnea, functional performance, and QOL in recovered intensive care unit (ICU) COVID-19 patients after consecutive weaning from mechanical ventilation. IMT program should be encouraged in the COVID-19 management protocol, specifically with ICU patients.
Keywords: COVID-19, dyspnea, functional capacity, inspiratory muscle training, mechanical ventilation, quality of life
1. Introduction
Recently, in December 2019, a novel pandemic viral disease is known as a coronavirus (COVID-19), which has been occurred in China[1] and widespread worldwide to become a global pandemic disease.[2] Generally, COVID-19 is identified as an acute discovered disorder and could be fatal. The onset of severe diseases may lead to death because of substantial damage to lung alveoli and a massive failure of the respiratory system to conduct its functional gas exchange.[3]
Xu et al,[4] 2020 have reported the pathological characters of a dead patient of severe respiratory failure and severe infection through carrying out biopsy postmortem with aiming to find safe and efficient clinical approaches to prevent the progression of this disease. They have documented that the dead patient was suffering from fever, cough, chills, severe inflammation with a progressive infiltrating and diffusing grid shadows in the lungs followed by severe hypoxia, shortness of breathing, and cardiac arrest ending by death. Regrettably, this case continually repudiated to conduct a ventilator tool in the intensive care unit (ICU) as he experienced claustrophobia.[4]
COVID-19 patients are suffering from a severe pneumonia, developed ARDS, and markedly require admission to ICU and oxygen support. A very short time was observed between hospitalization and ARDS.[5]
Mechanical ventilation is considered the commonest modality of saving lives in the ICU and is the cornerstone of the traditional intervention of respiratory failure. Although it provides many benefits, it could lead to several respiratory and systemic complications[6] that cause prolonged hospitalization, increased morbidity, and mortality rates.[7]
Respiratory muscle dysfunction is an important issue following a long duration of mechanical ventilation. Respiratory muscle weakness is approximately 2-fold of extremity muscles weakness following 1 day of mechanical ventilation.[8] In addition, myotrauma could occur subsequent to under assistance mode of mechanical ventilation,[9] whereas respiratory muscle weakness could occur subsequent to over assistance mode of mechanical ventilation.[10] Consequently, weakness of inspiratory muscles occurs following mechanical ventilation, irrespective to the provided mechanical ventilation mode. In addition, sepsis, muscle immobilization, and steroids contribute to ICU acquired weakness.[11]
Several previous studies explained that inspiratory muscle training (IMT) is a feasible and safe modality in ICU patients.[12,13] In addition, a recent study approved that IMT may enhance respiratory muscle strength and improves aerobic capacity among patients with respiratory muscle weakness.[14] In addition, previous systematic reviews documented that IMT may improve inspiratory muscle strength and endurance, and diminishes dyspnea.[15–17]
Rare studies evaluated the effects of IMT on COVID-19 subsequent to mechanically ventilated patients. Therefore, our present study aims to assess the efficacy of IMT on COVID-19 following mechanical ventilation hypothesizing that IMT could improve pulmonary functions, dyspnea index, and quality of life (QOL) in COVID patients after mechanical ventilation.
2. Materials and methods
2.1. Study design and ethics
This study was a prospective pilot clinical study. It was ethically approved by the local Ethics Committee of the Department of Health and Rehabilitation Sciences at Prince Sattam bin Abdulaziz University (RHPT/020/050) consistent with ethical guidelines and standards of the Helsinki Declaration 1964.
2.2. Subjects
Forty-two recovered COVID-19 patients (33 men and 9 women) weaned from mechanical ventilation with a mean age of 48.05 ± 8.85 years were enrolled in the study. They were recruited in the ICU at King Khalid Hospital, Al-Kharj, Saudi Arabia. Twenty-one patients were equipped to 2-week IMT (study group) and 21 age-matched peers were recruited as a control (control group) as demonstrated in the flow diagram of the study (Fig. 1). The inclusion criteria of the study were negative COVID, hemodynamically stable, respiratory rate <25 breath/min, negative inspiratory force <−25 cm H2O, minute ventilation <10 L/min, oxygen tension (PO2)/fraction of inspired oxygen (FIO2) >200.[18] The patients were excluded from the study if they have neurological, neuromuscular, and musculoskeletal limitations, cognitive dysfunction, end-stage of chronic diseases, and body mass index (BMI) >35 kg/m2. All patients were instructed to sign a written informed consent after explanation about the details of the study procedures.
Figure 1.
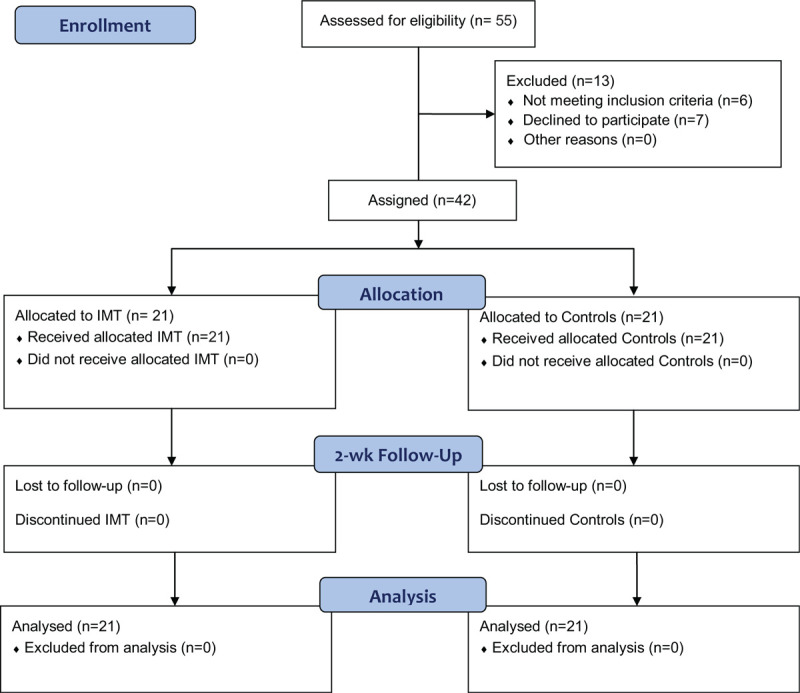
The CONSORT flow diagram of the study.
2.3. Intervention
Both IMT and control groups have conducted an incentive spirometer exercise. After weaning from MV, each patient was instructed to perform incentive breathing exercise in a relaxed sitting position 2 times daily for 2 following weeks.
The IMT group has received a program of IMT using a threshold inspiratory muscle trainer (Respironics, Cedar Grove, NJ), 2 sessions daily, 5 days a week for 2 consecutive weeks. Each session has consisted of 6 inspiratory cycles; each cycle has remained around 5 min of resisted inspiration, followed by 60-second rest time intending to improve inspiratory muscle strength. At the fifth and sixth cycle, each patient was instructed to breath regularly as much as possible in tending to improve inspiratory muscle fitness. The inspiratory threshold was controlled by a device loading valve that provided a threshold load with 50% of the maximal inspiratory pressure (MIP).
2.4. Outcome measures
Pulmonary function test (PFT), dyspnea severity index (DSI), QOL questionnaire, and functional performance were assessed initially before starting the study intervention and immediately after 2-week IMT by a blinded experienced examiner who was not included in the study intervention.
2.5. Assessment pulmonary function test
PFT was conducted by a blind well-trained technician for assessing the predicted values of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) using A Portable Digital Spirometer (Contec Medical Systems Co., Ltd., China). Each patient has conducted the PFT in a standing position and has been instructed to tightly close their lips on the mouthpiece, use a malleable nose caliper, and normally breathe in and out a number of times. Subsequently, each patient has been instructed to slowly breathe-in as deep as possible and followed by vigorous breathe out. The PFT has been repeated 3 times and the highest predicted value was recorded.[19]
2.6. Dyspnea severity index
A validated and reliable Arabic 12-item DSI questionnaire was used to assess dyspnea. Each item is scored 0 to 3; 0 = none and3 = severe and total score ranged from 0 to 36; 0 means no breathlessness and 36 means maximum severity.[20,21]
2.7. Quality of life
QOL was assessed utilizing a valid and reliable EuroQuality-5Dimensions-3Levels (EQ-5D-3L) questionnaire.[22] It is a valid instrument to assess different QOL domains involving self-care, mobility, anxiety/depression, pain/discomfort, and usual activity. Each domain represents 3-level scores; no problems, mild/moderate problems, and severe problems. The EQ-5D-3L responses were represented as a score ranged from 0 to 100, 0 represents the worst intelligible health status and 100 represent the best intelligible health status. Each patient was instructed to provide the score based on the health status during the assessment.[23]
2.8. Assessment of functional capacity
The functional capacity was assessed by a six-minute walk test (6-MWT). It is a valid and simple tool that objectively used to assess the physical functional capacity in patients experiencing respiratory disorders.[24]
2.9. Statistical analysis
All data were analyzed using Stata V. 15.1 software (Stata Corp., College Station, TX) in form of mean ± standard deviation (SD). The data were checked for normal distribution by the Shapiro-Wilk test. Data were analyzed based on a 2-way repeated analysis of variance (ANOVA) “F-test” analysis method to determine the differences between the 2 groups with group × time interactions. The partial eta-squared () effect size was calculated to assess the differences between and within IMT and control groups. The pre-post changes within groups were calculated using paired t-test where ANOVA was significant. The significance level was set at P < .05 for all outcome measures.
3. Results
Between July and October 2020, of 55 recovered COVID-19 patients after weaning from mechanical ventilation, 42 have completed the study program. Six patients did not meet the inclusion criteria and 7 patients refused to participate in the study. This pilot control clinical study has included 2 groups of recovered COVID-19 patients; the IMT group (n = 21), comprised 17 men and 4 women, and same number of age-matched peers regarding sex, BMI, mechanical ventilation duration, severity of the disease, and medications as a control group (n = 21, 16 men and 5 women). As displayed in Table 1, nonsignificant differences were detected between the 2 groups regarding the initial characteristics (sex, P = .707, age, P = .856, BMI, P = .634, duration of mechanical ventilation, P = .872, acute physiology and chronic health evaluation II score, P = .614, oxygen tension [PO2]/fraction of inspired oxygen [FIO2] ratio, P = .553, and medications, P > .05).
Table 1.
Initial characteristics of the participants.
| Characteristics | IMT group (n = 21) | Control group (n = 21) | P |
| Sex, M/F | 17 (80.9)/4 (19.1) | 16 (76.2)/5 (23.8) | .707 |
| Age, yr | 48.3 ± 8.5 | 47.8 ± 9.2 | .856 |
| BMI, kg/m2 | 28.5 ± 3.8 | 27.9 ± 4.3 | .634 |
| Respiratory rate breaths/min | 22.2 ± 2.3 | 21.7 ± 1.9 | .447 |
| Duration of MV, d | 13.3 ± 7.6 | 12.9 ± 8.4 | .872 |
| APACHE II score at ICU admission | 8.2 ± 2.4 | 8.6 ± 2.7 | .614 |
| P/F ratio n (%) before MV | |||
| Mild | 0 (0) | 0 (0) | .553 |
| Moderate | 10 (47.6) | 8 (38.1) | |
| Severe | 11 (52.4) | 13 (61.9) | |
| Medications n (%) | |||
| Corticosteroids | 14 (66.6) | 16 (76.2) | .494 |
| Muscle relaxant | 21 (100) | 21 (100) | 1.000 |
| Sedatives | 21 (100) | 21 (100) | 1.000 |
Continuous variables are presented as mean ± standard deviation; categorical variables are presented as frequency (percentage).
APACHE = acute physiology and chronic health evaluation, BMI = body mass index, F = females, ICU = intensive care unit, IMT = inspiratory muscle training, M = males, MV = Mechanical ventilation, P/F = oxygen tension (PO2)/ fraction of inspired oxygen (FIO2).
As displayed in Table 2, the mixed ANOVA test showed significant “group × time” interactions regarding predicted values of PFT in favor of the IMT group, FVC% (F = 5.31, P = .041, ηP2 = 0.13) and FEV1% (F = 4.91, P = .043, ηP2 = 0.12). Analyzing the PFT within each group, the IMT group displayed significant improvements in FVC% and FEV1% (P = .047, P = .039, respectively); however, the control group showed nonsignificant changes (FVC%, P = .754 and FEV1%, P = .871). Regarding the DSI, there was a significant “group × time” interaction supporting the favorable improvement in the IMT group (F = 4.56, P = .032, ηP2 = 0.15), whereas, analysis within groups demonstrated a significant reduction after intervention in the IMT group (P = .001) and nonsignificant changes in the controls (P = .621). Analysis of the total score of the EQ-5D-3L showed a significant “group × time” interaction with more advantageous to the IMT group (F = 6.14, P = .021, ηP2 = 0.17), whereas within-group analysis showed a significant improvement in the IMT group (P < .001) and statistical nonsignificant changes in the controls (P = .173). For the 6-MWT, a statistical analysis detected a significant group × time interaction (F = 9.34, P = .028, ηP2 = 0.16) in favor of the IMT group. Analyzing within-group changes, the IMT group showed a significant increase after the intervention (P < .001), whereas controls showed nonsignificant changes (P = .624).
Table 2.
Before and after-intervention changes within and between groups.
| Group × time interaction | ||||
| Measures | IMT group (n = 21) | Control group (n = 21) | P | ηP2 |
| FVC, % predicted | ||||
| Before | 78.7 ± 13.5 | 77.2 ± 12.6 | .041 | 0.13 |
| After | 84.2 ± 10.3 | 76.8 ± 11.7 | ||
| P | .047 | .754 | ||
| FEV1, % predicted | ||||
| Before | 76.2 ± 12.7 | 75.4 ± 12.2 | .043 | 0.12 |
| After | 83.7 ± 10.5 | 75.1 ± 12.4 | ||
| P | .039 | .871 | ||
| DSI | ||||
| Before | 18.5 ± 4.3 | 17.8 ± 5.1 | .032 | 0.15 |
| After | 14.2 ± 3.5 | 17.1 ± 4.8 | ||
| P | .001 | .621 | ||
| Eq-5D-3L, total score | ||||
| Before | 38.6 ± 5.8 | 40.7 ± 6.2 | .021 | 0.17 |
| After | 59.4 ± 8.3 | 43.3 ± 6.5 | ||
| P | <.001 | .173 | ||
| 6-MWT, min | ||||
| Before | 332.6 ± 34.5 | 329.7 ± 37.8 | .028 | 0.16 |
| After | 376.5 ± 39.4 | 334.8 ± 38.2 | ||
| P | <.001 | .624 | ||
6-MWT = six-minute walk test, DSI = dyspnea severity index, EQ-5D-3L = EuroQuality–5-dimensions-3-levels, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, IMT = inspiratory muscle training. ηP2 = 0.01 considered “small,” 0.09 “medium,” and 0.25 “large” effect-sized differences between groups.
4. Discussion
The main objective of the current study was to examine the effects of IMT on COVID-19 after mechanical ventilation hypothesizing that IMT could improve pulmonary functions, dyspnea, and QOL in COVID patients following the weaning from mechanical ventilation. The main findings of the study showed that FVC, FEV1, DSI, and QOL have improved significantly following 2-week IMT that confirm our hypothesis.
During mechanical ventilation, diaphragm muscle is being limited in mobility that leads to prompt onset of impaired functions of respiratory muscles[10] and could be aggravated by other elements including the patient's age, controlled mode of the ventilator, prolonged mechanical ventilation, malnutrition, and some medical interventions such as muscle relaxants and corticosteroids.[25] Therefore, new conservative strategies are required to promote and restore respiratory muscle functions.
Clinically, IMT has displayed valuable and significant improvements in impaired inspiratory muscle functions in patients suffering from asthma, chronic obstructive pulmonary disease (COPD),[26] and impaired diaphragmatic function.[27] The current study results showed significant improvements in the assessed variables following a 2-week IMT following the weaning from mechanical ventilation.
This is consistent with prior researches on the IMT in patients during mechanical ventilation and reported that IMT improved an MIP and reduced the time of weaning.[28,29] It is likely that some of the seeming improvements might be associated with learning influences assumed likeness of the IMT program and maximum inspiratory pressure Assessment maneuver. Patients admitted in ICU are liable to brisk skeletal and respiratory muscle wasting after short period and improved within few days following a proper IMT protocol despite few patients responds more to the intervention than others.[29]
Like our current study results previous study reported that pulmonary functions have improved following IMT exercise. Patients examined in this document have been admitted to ICU. Similar medications to this prior study, COVID-19 patients have received corticosteroid in their management.[30] In addition, El-Deen et al,[31] 2018 found that IMT is a beneficial interventional modality to improve FVC% and FEV1% in hemodialytic patients. Furthermore, Leelarungrayub et al,[32] 2017 documented that IMT improves FVC%, FEV1%, QOL, and dyspnea score in patients with COPD. Moreover, a recent systematic review by Wang et al,[33] has demonstrated that IMT may enhance pulmonary functions, respiratory muscle performance in patients with spinal cord injuries.
EQ-5D-3L has significantly improved following 2-week IMT in recovered COVID-19 patients after weaning from mechanical ventilation compared with control group. It is a valuable clinical outcome in those patients who fights with a low score of QOL after weaning. The Euro-QOL has been accepted to assess the QOL in ICU patients.[34] Similarly, Bisset et al,[12] 2016 concluded that IMT after consecutive weaning from mechanical ventilation improves QOL using a validated and reliable EQ-5D-3L tool.
It was observed that 6-MWT and DSI have significantly improved following IMT. These results were supported by a prior review which explained that IMT could improve respiratory muscle function, functional performance, dyspnea, and QOL in patients experiencing COPD.[35]
This pilot current study provides important strength points. It is the first study to assess the therapeutic value of 2-week IMT after weaning mechanical ventilated COVID-19 patients. We observed that the IMT group was strongly satisfied to complete IMT sessions regularly as they were feeling better status from session to session. This study has assessed various clinical measures including pulmonary functions, DSI, QOL, and 6-MWT in recovered ICU COVID-19 patients after consecutive weaning from mechanical ventilation.
In contrast, there are some limitations have been included in the study. This study selected the study patients depending on their availability rather than the calculation of the sample size and power analysis. Also, the 2-week IMT was a short period to provide complete results; however, the given outcomes indicate the safe and valuable effects of the IMT in recovered COVID-19 patients. One more limitation, the study is not a randomized controlled group for ethical considerations. Finally, the study has limited follow-up observation. Randomized controlled studies are needed with a larger number of patients and longer duration with follow-up observation. In addition, IMT should be started early during mechanical ventilation to avoid the complication of interventional medications and mechanical ventilation.
5. Conclusions
Along with the outcomes of the stud, 2-week IMT improves pulmonary functions, dyspnea, functional performance, and QOL in recovered ICU COVID-19 patients after consecutive weaning from mechanical ventilation. IMT program should be encouraged to be included in the COVID-19 management protocol, specifically with ICU patients.
Acknowledgments
This project was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University under the research project No. 2020/03/17300. The authors would like to acknowledge all patients who included in the study.
Author contributions
Conceptualization: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Elsayed A. Awad, Ibrahim E. Elalfy, Hosni A. Salem, Shereen H. Elsayed.
Data curation: Ahmed M. Abodonya, Elsayed A. Awad, Ibrahim E. Elalfy, Hosni A. Salem.
Formal analysis: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Elsayed A. Awad, Ibrahim E. Elalfy, Hosni A. Salem, Shereen H. Elsayed.
Funding acquisition: Ahmed M. Abodonya.
Investigation: Ahmed M. Abodonya, Elsayed A. Awad, Shereen H. Elsayed.
Methodology: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Elsayed A. Awad, Ibrahim E. Elalfy, Shereen H. Elsayed.
Project administration: Ahmed M. Abodonya, Walid Kamal Abdelbasset.
Resources: Ibrahim E. Elalfy, Hosni A. Salem.
Software: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Elsayed A. Awad, Hosni A. Salem.
Supervision: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Hosni A. Salem.
Validation: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Hosni A. Salem.
Visualization: Ahmed M. Abodonya, Ibrahim E. Elalfy, Hosni A. Salem.
Writing – original draft: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Elsayed A. Awad, Ibrahim E. Elalfy, Hosni A. Salem, Shereen H. Elsayed.
Writing – review & editing: Ahmed M. Abodonya, Walid Kamal Abdelbasset, Elsayed A. Awad, Ibrahim E. Elalfy, Hosni A. Salem, Shereen H. Elsayed.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, DSI = dyspnea severity index, EQ-5D-3L = EuroQuality-5-dimensions-3-levels, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, ICU = intensive care unit, IMT = inspiratory muscle training, MIP = maximal inspiratory pressure, 6-MWT = six-minute walk test, QOL = quality of life.
How to cite this article: Abodonya AM, Abdelbasset WK, Awad EA, Elalfy IE, Salem HA, Elsayed SH. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine. 2021;100:13(e25339).
Data availability: The data used that support the study findings are not available because of the medicolegal guidelines of the hospital.
This project was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University under the research project No. 2020/03/17300.
The authors declare no competing interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. World Health Organization (WHO), 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed April 2020. [Google Scholar]
- [3].Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis 2020;221:1757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cui J, Li F, Shi Z. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2018;17:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee WL, Slutsky AS. Ventilator-induced lung injury and recommendations for mechanical ventilation of patients with ARDS. Semin Respir Crit Care Med 2001;22:269–80. [DOI] [PubMed] [Google Scholar]
- [7].Herridge MS. Legacy of intensive care unit-acquired weakness. Crit Care Med 2009;37:S457–61. [DOI] [PubMed] [Google Scholar]
- [8].Dres M, Dube BP, Mayaux J, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med 2017;195:57–66. [DOI] [PubMed] [Google Scholar]
- [9].Goligher EC, Brochard LJ, Reid WD, et al. Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med 2019;7:90–8. [DOI] [PubMed] [Google Scholar]
- [10].Al-Bassam W, Dade F, Bailey M, et al. Likely overassistance” during invasive pressure support ventilation in patients in the intensive care unit: a multicentre prospective observational study. Crit Care Resusc 2019;21:18–24. [PubMed] [Google Scholar]
- [11].Friedrich O, Reid MB, Van den Berghe G, et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev 2015;95:1025–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bissett BM, Leditschke IA, Neeman T, et al. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax 2016;71:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bissett B, Gosselink R, Van Haren FMP. Respiratory muscle rehabilitation in patients with prolonged mechanical ventilation: a targeted approach. Crit Care 2020;24:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moawd SA, Azab AR, Alrawaili SM, et al. Inspiratory muscle training in obstructive sleep apnea associating diabetic peripheral neuropathy: a randomized control study. Biomed Res Int 2020;2020:5036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moodie L, Reeve J, Elkins M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: a systematic review. J Physiother 2011;57:213–21. [DOI] [PubMed] [Google Scholar]
- [16].O’Brien K, Geddes EL, Reid WD, et al. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. J Cardiopulm Rehabil Prev 2008;28:128–41. [DOI] [PubMed] [Google Scholar]
- [17].Lötters F, van Tol B, Kwakkel G, et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J 2002;20:570–6. [DOI] [PubMed] [Google Scholar]
- [18].Tobin M, Manthous C. Mechanical ventilation. Am J Respir Crit Care Med 2017;196:3–4. [DOI] [PubMed] [Google Scholar]
- [19].Culver BH, Graham BL, Coates AL, et al. ATS Committee on Proficiency Standards for Pulmonary Function Laboratories. Recommendations for a standardized pulmonary function report. An official American Thoracic Society technical statement. Am J Respir Crit Care Med 2017;196:1463–72. [DOI] [PubMed] [Google Scholar]
- [20].Yorke J, Moosavi SH, Shuldham C, et al. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax 2010;65:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Al-Gamal E, Yorke J, Al-Shwaiyat Mohammed KEA. Dyspnea-12-Arabic: testing of an instrument to measure breathlessness in Arabic patients with chronic obstructive pulmonary disease. Heart Lung 2014;43:244–8. [DOI] [PubMed] [Google Scholar]
- [22].Ringbaek T, Brøndum E, Martinez G, et al. EuroQoL in assessment of the effect of pulmonary rehabilitation COPD patients. Respir Med 2008;102:1563–7. [DOI] [PubMed] [Google Scholar]
- [23].Van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708–15. [DOI] [PubMed] [Google Scholar]
- [24].Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:382–6. [DOI] [PubMed] [Google Scholar]
- [25].Carlucci A, Ceriana P, Prinianakis G, et al. Determinants of weaning success in patients with prolonged mechanical ventilation. Crit Care 2009;13:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beaumont M, Forget P, Couturaud F, et al. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clin Respir J 2018;12:2178–88. [DOI] [PubMed] [Google Scholar]
- [27].Caleffi Pereira M, Dacha S, Testelmans D, et al. Assessing the effects of inspiratory muscle training in a patient with unilateral diaphragm dysfunction. Breathe (Sheff) 2019;15:e90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cader SA, Vale RG, Castro JC, et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. J Physiother 2010;56:171–7. [DOI] [PubMed] [Google Scholar]
- [29].Martin AD, Davenport PD, Franceschi AC, et al. Use of inspiratory muscle strength training to facilitate ventilator weaning: a series of 10 consecutive patients. Chest 2002;122:192–6. [DOI] [PubMed] [Google Scholar]
- [30].Ahmad H, Justine M, Othman Z, et al. The outcomes of short term inspiratory muscle training (IMT) combined with chest physiotherapy in hospitalized COPD patients. Bangladesh J Med Sci 2013;12:398–404. [Google Scholar]
- [31].El-Deen HAB, Alanazi FS, Ahmed KT. Effects of inspiratory muscle training on pulmonary functions and muscle strength in sedentary hemodialysis patients. J PhysTherSci 2018;30:424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Leelarungrayub J, Pinkaew D, Puntumetakul R, et al. Effects of a simple prototype respiratory muscle trainer on respiratory muscle strength, quality of life and dyspnea, and oxidative stress in COPD patients: a preliminary study. Int J Chron Obstruct Pulmon Dis 2017;12:1415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang X, Zhang N, Xu Y. Effects of respiratory muscle training on pulmonary function in individuals with spinal cord injury: an updated meta-analysis. Biomed Res Int 2020;2020:7530498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Granja C, Morujao E, Costa-Pereira A. Quality of life in acute respiratory distress syndrome survivors may be no worst than in other ICU survivors. Intensive Care Med 2003;29:1744–50. [DOI] [PubMed] [Google Scholar]
- [35].Gosselink R, De Vos J, Van den Heuvel SP, et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011;37:416–25. [DOI] [PubMed] [Google Scholar]


