Abstract
Background:
In response to the devastating effects of the coronavirus disease 2019 pandemic, several vaccine prototypes have been developed, with the Pfizer/BioNTech (BNT162b2) platform being the first to receive emergency use authorization. Although taken to market on an unprecedented timeline, the safety profile of the drug during clinical trials was shown to be favorable. Shortly after release, reports from the Centers for Disease Control and Prevention demonstrated a higher-than-average rate of anaphylaxis to the vaccine that has been the cause for concern for safety officials and the general public alike. Here, we present a unique case of protracted anaphylaxis in a recipient of the BNT162b2.
Case Summary:
The patient is a 55-year-old female with a history of multiple allergic reactions who presented with respiratory distress and hives after receiving the first dose of the BNT162b2, despite premedication with IV steroids and diphenhydramine. The refractory nature of her reaction was demonstrated by edema of her tongue (visualized on nasolaryngoscopy), requiring an epinephrine infusion for nearly 3 days. She was discharged from the hospital with instructions not to receive the second dose of the vaccine.
Conclusion:
Although the exact etiology of anaphylaxis secondary to this messenger RNA-based vaccine is not completely clear, our literature search and review of the patient’s course support either polyethylene glycol versus other excipient-related allergy as a possible cause. Based on the protracted nature to our patient’s anaphylaxis, critical care management for patients with a true anaphylactic reaction to BNT162b2 may require monitoring for an extended period of time.
Keywords: anaphylaxis; coronavirus disease 2019; messenger ribonucleic acid vaccine, Pfizer/BioNTech vaccine; polyethylene glycol allergy
The coronavirus disease 2019 (COVID-19) pandemic has had a devastating impact, infecting over 48 million people and resulting in over 1.2 million deaths across over 200 countries (1). In response to this growing outbreak, numerous vaccine prototypes have been developed, the first of which was tested in humans as early as March 13, 2020 (63 d after release of the virus’s genetic information). Forty-three of these vaccine prototypes entered clinical trials by September 2020. The Pfizer/BioNTech platform (BNT162b2) is a messenger RNA-based vaccine that acts to trigger rapid and immediate antigen expression without crossing over the nuclear membrane (2); it received emergency use authorization in the United States on December 11, 2020. As most vaccines take 12–15 years for production and testing, this compressed, under 1-year timeframe and reports of allergic reaction have been the cause of concern for the public (3). The overall safety profile was shown to be favorable with most reactions reported to be mild and transient with an prevalence of severe systemic event after the first dose at 0.9% or less (4). Here, we present the first case of protracted anaphylaxis to BNT162b2.
CASE PRESENTATION
The patient is a 55-year-old female with a history of significant allergies including prior anaphylactic reactions who presented to the emergency department (ED) 1 day after receiving the BNT162b2 with complaints of dyspnea. The aforementioned allergic reactions occurred with exposure to fresh fish, iodine solution, and the rabies vaccine. Her reaction to fresh fish was described as hives on her chest, dyspnea, and throat tightness that occurred within 30 minutes. Anaphylaxis to the rabies vaccine was most severe; within 40 minutes, she noted extreme dyspnea, wheezing, hives on her chest, and a sensation of throat closure, with an episode of syncope requiring bag-valve-mask resuscitation and nearly intubation. She denied allergic reactions to any other vaccines including pneumococcal and influenza vaccines and was undergoing evaluation and treatment with nightly cetirizine for possible mast cell activation disorder.
Despite premedication with IV diphenhydramine and dexamethasone 30 minutes prior to administration, she experienced hives on her chest, sensation of throat closure, dyspnea, and wheezing within 10 minutes of receipt. She administered her own intramuscular epinephrine and was evaluated in the ED where she received an additional dose of intramuscular epinephrine and IV fluids, and after significant improvement, she was discharged home. At that time, she denied gastrointestinal symptoms, pruritis, or chest pain and had no notable vital sign abnormalities.
Six hours later, she awoke with similar symptoms, readministered intramuscular epinephrine, and returned to the ED, where she was treated and admitted for prolonged anaphylaxis. Approximately 6 hours after admission, she again started having the same symptoms as prior, however this time had worsening dyspnea with tachycardia, tachypnea and notable tongue swelling, and angioedema, despite appropriate treatment with intramuscular epinephrine. An epinephrine infusion was initiated and the patient was upgraded to the ICU where she had a bedside nasolaryngoscopy, demonstrating nonobstructive edema at the base of her tongue nearly touching the epiglottis.
She remained on the epinephrine infusion for 24 hours and was gradually weaned off. After 9 hours of discontinuation, she developed the same symptoms as prior, which resolved with restarting the epinephrine infusion. She was transferred to our institution for further evaluation of protracted anaphylaxis. Ninety-six hours after vaccine administration, epinephrine was slowly titrated off and she was transitioned from IV to oral steroids without further recurrence of symptoms. In total, she received over 1 gram of methylprednisolone and over 73 hours of epinephrine infusion throughout her 5-day hospitalization (Fig. 1). She was discharged from the hospital with a prescription for a steroid taper and EpiPen, instructions to not receive the second dose of the COVID-19 vaccine and given a follow-up appointment for allergy skin testing. Informed consent was obtained by the patient for publication.
Figure 1.
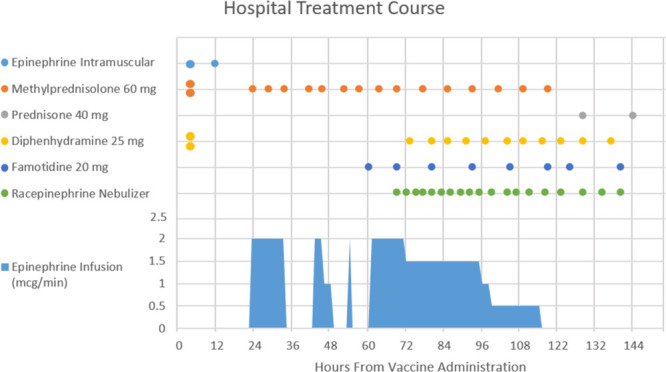
Hospital treatment course.
DISCUSSION
BNT162b2 is a two-dose vaccine regimen (30 μg per dose, administered 21 d apart) that was shown in preliminary trials to be safe and 95% effective against severe COVID-19. The rates of anaphylaxis in the placebo and vaccines groups in the phase III study were not statistically different; however, individuals with a history of anaphylaxis were excluded from the trials (4). In general, anaphylactic reactions to vaccines are rare, approximately one per one million doses (5). A report from the Center for Disease Control (CDC), released on January 6, 2021, stated that of the first 1.8 million recipients of the BNT162b2 (December 14–23, 2020), there were 4,393 reports of adverse events (0.2%). Of those cases, 21 were determined to be anaphylaxis, with an estimated rate of 11.1 per 1 million doses given. Although this rate is 10 times higher than other vaccines, it is still significantly lower than the rates of anaphylaxis associated with other common allergens. Of the reported anaphylaxis cases, 17 patients had a documented history of allergies, seven of which were anaphylaxis. The average onset of allergic symptoms from vaccine receipt was approximately 13 minutes (86% occurred within 30 min of vaccination) (6). The National Institute of Allergy and Infectious Diseases in collaboration with the Food and Drug Administration (FDA) is currently organizing a study to analyze these severe allergic responses.
Our patient had a level 1 Brighton Collaboration case definition score for anaphylaxis, as she met two of the three major criteria in the fields of dermatologic/mucosal (hives/generalized erythema) and respiratory (bilateral wheezing and tongue edema). The case was reported to Pfizer and to the FDA (report 208614) and was thought to be secondary to a component of the vaccine called polyethylene glycol (PEG), which is used as a vehicle for messenger RNA. Both the Pfizer/BioNTech and Moderna messenger RNA COVID-19 vaccines contain PEG-2000 (4, 7). PEG products of various molecular weights are commonly found in cosmetic, food, and pharmaceutical preparations (8). The process of PEGylation, attaching repeating units of PEG to a polypeptide drug, improves the shortcomings of many polypeptide drugs, which includes a short circulating half-life, rapid metabolism by the kidneys, and destruction by proteolytic enzymes. This technology has been incorporated in various drug formulations for treatment of hepatitis C, rheumatoid arthritis, and many cancers (9). PEG has previously been implicated as a cause of anaphylaxis in other medication administrations, and two cases of protracted anaphylaxis with PEG have previously been described (10–14). The half-life of PEG is difficult to predict as it is impacted by its molecular weight, route of administration, and drug cleavage. As the molecular weight increases, the half-life PEG is similarly prolonged (15). We hypothesize the protracted nature of our patient’s anaphylaxis that may have been in part due to the long half-life of PEG and it is unclear if the intramuscular route of administration may have further compounded this due to depot effect. The exact mechanism of PEG-induced anaphylaxis is not clearly understood but is thought to involve complement pathways (8). Complement activation-related pseudoallergy has also been described with administration of PEG and other lipid nanoparticles; interestingly, this reaction is more likely with lower doses of lipid (16).
Our patient’s most severe previous allergic reaction was to the rabies vaccine; however, neither Imovax nor RabAvert shares excipients with BNT162b2 and both notably lack PEG (17, 18). Similarly, there are no fish or iodine products in BNT162b2 (18). Our patient had previously received PEG as a bowel prep for colonoscopy without adverse event; however, it is important to note that laxative preparations generally contain PEG-3350 or PEG-4000, rather than PEG-2000, which is found in BNT162b2. According to available case reports, it appears patients may tolerate some PEG products and not others. Risk of allergic reaction depends on both molecular weight and the amount of PEG exposure (19). As such, testing and confirmation PEG allergy can be exceedingly difficult. In patients with numerous, seemingly unrelated allergies, it is reasonable to suspect PEG or other excipient-related allergies (19). Our patient has numerous recorded allergies without a clear relationship and has been evaluated for mast cell activation disorder, indicating a complex and perhaps incompletely understood allergy history. According to the American College of Allergy, Asthma and Immunology, the messenger RNA COVID-19 vaccines should be avoided in individuals with a history of anaphylaxis to PEG or other vaccines. They recommend an allergist to assist in the evaluation of patients with multiple complex allergies or unclear allergy history. Our patient, a nurse working in New York City performing COVID-19 tests, did consult her allergist and family physician who both determined that the risk of contracting COVID-19 was higher than the risk of any allergic reaction to the vaccine. The likely factors that led to this decision include the lack of shared ingredients between BNT162b2 and her prior allergens, tolerance to all other vaccines, and her high-risk profession.
Our patient received a significant quantity of corticosteroids for protracted anaphylaxis, including premedication with IV dexamethasone and diphenhydramine. The use of premedication to prevent allergic reactions in general is not well supported (20). Due to their slow onset of action (4–6 hr), corticosteroids are not effective in the acute management of anaphylaxis. There has been proposed a theoretical benefit to the prevention of biphasic reactions due to its anti-inflammatory properties; however, there is no definitive evidence to back this (21). Similarly, from a Cochrane review from 2010, there is no high-quality evidence to either support or refute the use of glucocorticoids for the treatment of anaphylaxis (22). Although administration of steroids did not prevent recurrence of anaphylaxis in our patient, whether it contributed to reduction of severity in recurring symptoms, such as airway edema, cannot be excluded. Generally, a single dose of steroids may be administered without significant harm, but in the setting of vaccine administration, it may be prudent to limit overuse of steroids and maximize potential effectiveness of the vaccine. Several studies comparing the use of long-term steroids in patients with asthma or chronic obstructive pulmonary disease suggest that, based on immunologic markers, there is appropriate response to vaccines (23, 24), but it is unclear if this translates to patients who have received high doses or prolonged courses of steroids.
This case highlights the need for complete assessment of allergic history prior to messenger RNA COVID-19 vaccine administration. The CDC currently recommends that all messenger RNA COVID-19 vaccines be administered in healthcare settings with monitoring for up to 30 minutes for those with a history of immediate allergic reaction to another vaccine or those with a history of anaphylaxis from any cause and up to 15 minutes for all other persons (25). Based on the protracted course our patient experienced, if anaphylaxis occurs, it may be prudent to monitor for an extended period of time and/or provide access and education on intramuscular epinephrine administration in case of recurrence.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Tsai SC, Lu CC, Bau DT, et al. Approaches towards fighting the COVID-19 pandemic (review). Int J Mol Med. 2021; 47:3–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tregoning JS, Brown ES, Cheeseman H, et al. Vaccines for COVID-19. Clin Exp Immunol. 2020; 202:162–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostoff RN, Briggs MB, Porter AL, et al. [Comment] COVID-19 vaccine safety. Int J Mol Med. 2020; 46:1599–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383:2603–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021; 384:643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimabukuro T: Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2020; 70:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Eng J Med. 2020; 383:1920–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Hwang SH, Park JS, et al. Anaphylaxis to polyethylene glycol (Colyte®) in a patient with diverticulitis. J Korean Med Sci. 2016; 31:1662–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003; 2:214–221 [DOI] [PubMed] [Google Scholar]
- 10.Sellaturay P, Nasser S, Ewan P. Polyethylene glycol–induced systemic allergic reactions (anaphylaxis). J Allergy Clin Immunol Pract. 2020; 9:2198–2213 [DOI] [PubMed] [Google Scholar]
- 11.Fertal SA, Bradeen HA, Friesen E, et al. Time course and management of protracted anaphylaxis due to PEG-asparaginase. J Pediatric Hematol/Oncol. 2020; 43:1–41 [DOI] [PubMed] [Google Scholar]
- 12.Sakatani A, Doi Y, Matsuda T, et al. Protracted anaphylaxis developed after peginterferon α-2a administration for chronic hepatitis C. World J Gastroenterol. 2015; 21:2826–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin Exp Allergy. 2016; 46:907–922 [DOI] [PubMed] [Google Scholar]
- 14.Stone CA, Jr, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: More common than we have recognized. J Allergy Clin Immunol Pract. 2019; 7:1533–1540.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santi DV, Schneider EL, Reid R, et al. Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates. Proc Natl Acad Sci U S A. 2012; 109:6211–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamed M, Abu Lila AS, Shimizu T, et al. PEGylated liposomes: Immunological responses. Sci Technol Adv Mater. 2019; 20:710–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccine and Immune Globulin Availability. Centers for Disease Control. 2020aAvailable at: https://www.cdc.gov/rabies/resources/availability.html. Accessed February 9, 2021
- 18.Pfizer-BioNTech COVID-19 Vaccine Information | CDC. Centers for Disease Control. 2020Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html. Accessed January 2, 2021
- 19.Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis). J Allergy Clin Immunol Pract. 2021; 9:670–675 [DOI] [PubMed] [Google Scholar]
- 20.Davenport MS, Cohan RH. The evidence for and against corticosteroid prophylaxis in at-risk patients. Radiol Clin North Am. 2017; 55:413–421 [DOI] [PubMed] [Google Scholar]
- 21.Campbell RL, Li JT, Nicklas RA, et al. ; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: A practice parameter. Ann Allergy Asthma Immunol. 2014; 113:599–608 [DOI] [PubMed] [Google Scholar]
- 22.Choo KJ, Simons E, Sheikh A. Glucocorticoids for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2010; 65:1205–1211 [DOI] [PubMed] [Google Scholar]
- 23.Laratta CR, Williams K, Vethanayagam D, et al. A case series evaluating the serological response of adult asthma patients to the 23-valent pneumococcal polysaccharide vaccine. Allergy Asthma Clin Immunol. 2017; 13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steentoft J, Konradsen HB, Hilskov J, et al. Response to pneumococcal vaccine in chronic obstructive lung disease–the effect of ongoing, systemic steroid treatment. Vaccine. 2006; 24:1408–1412 [DOI] [PubMed] [Google Scholar]
- 25.Management of Anaphylaxis at COVID-19 Vaccination Sites | CDC. Centers for Disease Control. 2021Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html. Accessed February 24, 2021


