Abstract
To evaluate the effect of astragaloside IV for hepatic fibrosis.
The multiple databases like Pubmed, Embase, Cochrane databases, and China National Knowledge database were used to search for the relevant studies, and full-text articles involved in the evaluation on effect of astragaloside IV for hepatic fibrosis. Review Manager 5.2 was adopted to estimate the effects of the results among selected articles. Forest plots, sensitivity analysis and bias analysis for the articles included were also conducted.
Finally, 7 eligible studies were eventually satisfied the included criteria. Alanine aminotransferase (ALT) in model was higher than astragaloside group (mean difference [MD] = −58.01, 95% confidential interval (CI) [−93.97, −22.05], P = .002; I2 = 99%). The meta-analysis suggested that aspartate aminotransferase (AST) in model group was more than that in astragaloside group (MD = −39.94, 95% CI [−129.38, 49.50], P = .38; I2 = 100%). Model group had higher α - smooth muscle actin (α-SMA) than astragaloside group (MD was −1.13, P of heterogeneity <.0001, I2 = 94%, Z = 5.18, P of over effect <.0001). Transforming growth factor β (TGF-β) in model group was higher than that in astragaloside group (MD was −0.55, P of heterogeneity <.00001, I2 = 97%, Z = 2.54, P of over effect = .01). Limited publication bias was observed in this study.
Astragaloside IV is a potential clinical drug for the treatment of liver fibrosis considering liver function and hepatic fibrosis related protein factor in experimental rats are improved.
Keywords: astragaloside IV, hepatic fibrosis, meta-analysis, rat
1. Introduction
Liver fibrosis is a scarring process caused by viral hepatitis, alcoholic liver disease and other chronic liver diseases. It is characterized by an increase in the production of extracellular matrix (ECM), especially collagen in liver. Hepatic stellate cells (HSCs) secrete a large number of ECM components, which play an important role in the pathogenesis of liver fibrosis.[1,2] The activation of HSCs is controlled by a variety of cytokines, growth factors and other molecules (such as reactive oxygen species). Transforming growth factor β1 (TGF-β1) is the most important cell factor in HSC activation molecules, which plays an important role in HSC activation and proliferation.[3,4] Activated HSC is a proliferating cell that secretes extracellular matrix protein, including type I collagen and α - smooth muscle actin (α-SMA). Activated HSC is the key cell type of liver injury.[5]
According to the principle of western medicine, the therapeutic methods often have limited efficacy and risk of adverse reactions. Therefore, it is very attractive to treat liver diseases with plant-derived compounds. These compounds can be obtained and do not need to be painstakingly synthesized. Traditional Chinese medicine has potential applications in the treatment of chronic diseases.[6] Astragalus is a genus of legumes and astragaloside IV is the main active component extracted from astragalus root. Astragaloside IV has anti-inflammatory, anti-aging, anti-oxidation, anti myocardial injury, and no obvious toxicity or side effects, which has been widely confirmed. Astragaloside IV has a delayed effect on the formation and improvement of liver fibrosis similar to the structure of Col I treatment.[7]
The anti fibrosis mechanism of astragaloside IV may be related to its down-regulation of TGF-β1, thus inhibiting the activation of HSCs. Some studies[8,9] have shown that astragalus saponins can significantly inhibit the proliferation of bile duct, reduce the content of Hyp in liver tissue, significantly reduce the expression of α-SMA, TGF-β1, and MCP-1, and significantly increase the expression of Smad7 protein. These results suggest that Astragalus saponins may inhibit the activation of TGF-β signal pathway by increasing Smad7 expression, and then inhibit the activation and recruitment of fibroblasts.
In recent years, the effects of astragaloside IV has been noted, but the detailed role of astragaloside IV in hepatic fibrosis has not been fully understood. Here, we conduct a meta-analysis to analyze the effects of astragaloside IV on hepatic fibrosis.
2. Methods
2.1. Literature search strategy
We searched articles published between January 2000 and March 2020 for astragaloside IV in hepatic fibrosis. Searchable databases include PubMed, EmBase, Cochrane database, and China National Knowledge database, and use the following keywords:
-
1.
hepatic fibrosis OR liver fibrosis;
-
2.
astragaloside IV.
In the strategy, all these words are combined using the boolean operator “and”. There are no restrictions on the publication language in the literature search. In order to maximize the specificity and sensitivity of the search, the author should also check the reference list of the searched research to seek other relevant research that was not found through the search strategy.
2.2. Study selection
We used the following inclusion criteria for our research:
-
1.
A study with case-control design;
-
2.
A study to establish animal model with hepatic fibrosis;
-
3.
Containing data in different year;
-
4.
Available in full text.
If the research meets one of the following conditions, it is excluded:
-
1.
overlapping data or overlapping review articles;
-
2.
Patients have other serious diseases, like other liver disease; and
-
3.
Patients received a combination of other treatments.
2.3. Data extraction and quality assessment
Two commentators independently scanned the full text of the manuscript and extracted the following data from each eligible study: first author's name, subjects, country of origin, year of publication, sample size in different groups, study period of each article. Cochrane risk of bias assessment tool is used to evaluate the methodological quality of the study.
2.4. Statistical analysis
Review Manager (version 5.2, Cochrane Collaboration, 2011) was used to assess the impact of results in selected reports. For continuous results, the mean difference is calculated by the average difference. Heterogeneity in research was evaluated by I2 Statistics, a quantitative measure of inconsistency in research. Twenty five percentage to 50% of the studies with I2 are considered as low heterogeneity, 50% to 75% of the studies with I2 are considered as medium heterogeneity, and 75% of the studies with I2 > 75% are considered as high heterogeneity. If I2 > 50%, the potential sources of heterogeneity are examined by sensitivity analysis, which omits 1 study in each round and investigates the impact of a single study on portfolio estimation. In addition, when heterogeneity is observed, the random effect model is used, and when it does not exist, the fixed effect model is used. Funnel charts are used to check for potential publication bias.
3. Results
3.1. Search process
The electronic search ended with 614 articles. After careful reading, 91 papers have reached the preliminary standard. In the further screening, 84 articles were excluded because of improper research type and insufficient data and article type. Finally, 7 papers are selected for analysis. Figure 1 is a flowchart of identification, inclusion, and exclusion, reflecting the search process and the reason for exclusion.
Figure 1.

Flow diagram of the study selection.
3.2. Characteristics of included studies
Detailed characteristics of the included studies were presented in Table 1. All these studies were published from 2005 to 2020. The sample size ranged from 17 to 35. Totally 93 patients were in astragaloside group, and 77 patients were in model group.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Year | Language | Country | Subjects | Groups | n | Years of onset |
| Guo[10] | 2018 | English | China | The LX-2 cell line | Astragaloside | 16 | January 2016 to January 2017 |
| Model | 18 | ||||||
| Liu[11] | 2009 | English | China | Male Wistar rats | Astragaloside | 10 | January 2006 to October 2008 |
| Model | 10 | ||||||
| Mu[12] | 2015 | English | China | Male Sprague–Dawley (SD) rats | Astragaloside | 10 | January 2013 to December 2014 |
| Model | 10 | ||||||
| Mu[13] | 2017 | Chinese | China | Male Wistar rats | Astragaloside | 18 | October 2012to October 2013 |
| Model | 6 | ||||||
| Wang[14] | 2017 | English | China | Male Wistar rats | Astragaloside | 20 | January 2014 to June 2015 |
| Model | 15 | ||||||
| Yuan[15] | 2018 | English | China | Te rat HSC-T6 cell line | Astragaloside | 10 | January 2016 to January 2017 |
| Model | 10 | ||||||
| Zhao[16] | 2020 | English | China | Male Wistar rats | Astragaloside | 9 | January 2017 to December 2018 |
| Model | 8 |
3.3. Results of quality assessment
The Cochrane risk of bias assessment tool was used to evaluate the risk of patient selection problems in 7 trials. Only 1 study showed the problem of selection bias. In view of the bias summary, there is limited problem in selection bias, no attrition bias and other bias. In general, there is only 1 trial with bias risk, and other 6 trials have no risk (Figs. 2 and 3).
Figure 2.
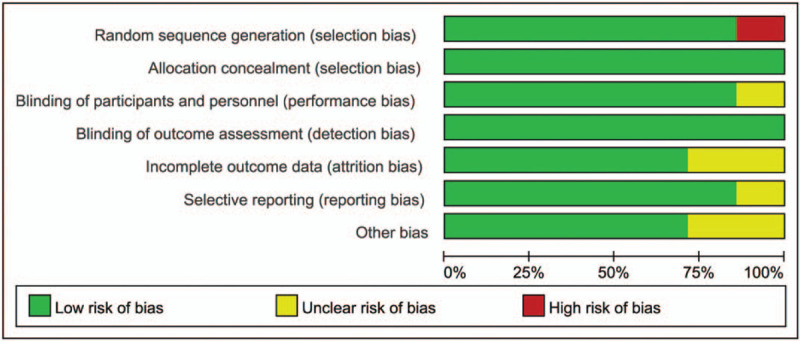
Assessment of the quality of the included studies: low risk of bias (green hexagons), unclear risk of bias (yellow hexagons), and high risk of bias (red hexagons).
Figure 3.

Quality assessment of included studies.
3.4. Results of meta-analysis
-
1.
Meta-analysis about alanine aminotransferase (ALT) between astragaloside IV and model.
Six included studies involve in ALT between astragaloside IV and model. The ALT between astragaloside IV and model was shown in Figure 4. The result suggested that there was significant difference of ALT and that in model was higher than astragaloside group (mean difference [MD] = −58.01, 95% confidential interval (CI) [−93.97, −22.05], P = .002; I2 = 99%).
-
2.
Meta-analysis about aspartate aminotransferase (AST) between astragaloside IV and model.
Five included studies were involved in AST between astragaloside IV and model. As shown in the forest plot (Fig. 5). The result of meta-analysis showed that there was significant difference between astragaloside IV and model about AST, and model group was more than that in astragaloside group (MD = −39.94, 95%CI [−129.38, 49.50], P = .38; I2 = 100%).
-
3.
Meta-analysis about α-SMA between astragaloside IV and model.
In the analysis, 5 articles were included. The results of heterogeneity test showed that random effect model wad was needed to analyze the data (P of heterogeneity <.0001, I2 = 94%, Z = 5.18, P of over effect <.0001). The overall effect of α-SMA was significant and the MD was −1.13, which showed that model group had higher α-SMA than astragaloside group (Fig. 6).
-
4.
Meta-analysis about TGF-β between astragaloside IV and model.
In the analysis of TGF-β between astragaloside IV and model, 6 articles were included. The results of heterogeneity test showed that random effect model wad was needed to analyze the data (P of heterogeneity <.00001, I2 = 97%, Z = 2.54, P of over effect = .01). The overall effect of TGF-β was significant, the overall MD was −0.55 and model group was higher than that in astragaloside group (Fig. 7).
Figure 4.

Forest plots of ALT between astragaloside IV and model.
Figure 5.

Forest plots of AST between astragaloside IV and model.
Figure 6.

Forest plots of α-SMA between astragaloside IV and model.
Figure 7.
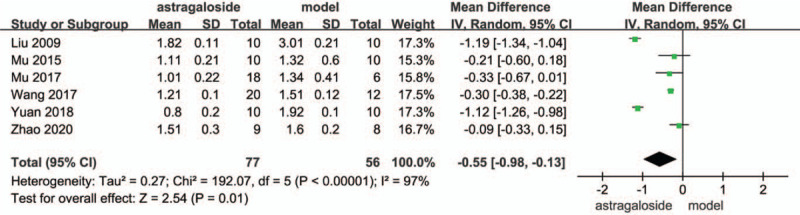
Forest plots of TGF-β between astragaloside IV and model.
3.5. Results of sensitivity analysis and publication bias
According to meta-analysis, the heterogeneity of ALT and hepatic fibrosis was high (I2 = 99%). As shown in Figure 5 the heterogeneity of ALT and hepatic fibrosis might be attributed to the different results of each study. When the article of Mu in 2015 was excluded, I2 changed to 100% (Fig. 8). This indicated that the result in this article was robust.
Figure 8.

Sensitivity analysis forest plots of ALT between astragaloside IV and model.
A funnel plot for ALT in different groups was performed and 6 studies were included in the plot. To some extent, the result indicated that there existed some publication bias since the symmetrical characteristic of the funnel plot is not good (Fig. 9). The result of Begg test suggested that no significant evidence of potential publication bias existed (Z = 1.33, P = .123) and Egger's test also suggested that no significant evidence of potential publication bias existed (t = 1.25, P = .223).
Figure 9.

Funnel plot of publication bias.
4. Discussion
The results showed that ALT in model group was higher than that in astragaloside group, which indicated that liver function in model group was worse than astragaloside group. Qu[17] state that liver fibrosis is characterized by excessive formation of extracellular matrix and deposition of matrix, which can form connective tissue in the liver. This process leads to the destruction of liver structure and the loss of liver function. The increase of ALT was associated with liver fibrosis. In the analysis of AST, the indicator in model group was also more than that in astragaloside group. The content of AST in normal serum is low, but when the corresponding cells are damaged, the permeability of cell membrane is increased, and AST in cytoplasm is released into the blood, so the serum concentration can be increased.[18,9] The normal value of transglutaminase was 0–40 units/L. when transglutaminase increased significantly, it suggested extensive damage of liver parenchyma. Obviously, astragaloside IV alleviate damage of liver fibrosis.
In the analyais of α-SMA and TGF-β, model group was higher than astragaloside group. Cheng[19] reported that TGF-β superfamily is a kind of multifunctional polypeptide, including TGF - β, bone morphogenetic protein, inhibin and activin. TGF-β is closely related to cirrhosis. At the same time, in vitro current experiment confirmed that HSC can be activated by TGFβ-1, and AST can inhibit the expression of α-SMA in HSC, inhibit cell activation and promoting cell apoptosis. The serum TGF-β level and liver tissue protein level increased with the development of liver fibrosis, but astragaloside IV decreased significantly after treatment. Astragaloside can inhibit the activation of HSC and promote apoptosis.[17,20] In addition, astragaloside can also regulate the expression of MMP-2 / tissue inhibitor of metalloproteinases-2 (TIMP-2), promote the synthesis and metabolism of collagen, which is conducive to the degradation of ECM. It also regulates lipid peroxidation and downregulates the messager Ribonucleic Acid (mRNA) expression of TGFβ-1, P-Smad 2, P-Smad 3, and upregulates the mRNA expression of Smad 7 in TGFβ-1/Smad signaling pathway.[21–24]
There are some limitations in this study. Firstly, more indicators evaluating other related gene or biochemical index should be included, and this could be conducted in the future. Secondly, with more researches on human beings published, a further research could be conducted in the next research.
In conclusion, this study shows that astragaloside IV is a potential clinical drug for the treatment of liver fibrosis considering liver function and hepatic fibrosis related protein factor are improved in experimental rats.
Author contributions
Conceptualization: Zhongying Han.
Data curation: Zhongying Han.
Investigation: Junfeng Zhu.
Methodology: Junfeng Zhu.
Software: Zheng Han.
Writing – original draft: Zheng Han.
Footnotes
Abbreviations: α-SMA = α - smooth muscle actin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidential interval, ECM = extracellular matrix, HSCs = hepatic stellate cells, MD = mean difference, mRNA = messager ribonucleic acid, TGF-β = transforming growth factor β, TIMP-2 = tissue inhibitor of metalloproteinases-2.
How to cite this article: Han Z, Zhu J, Han Z. Evaluation of astragaloside IV in hepatic fibrosis: a meta-analysis. Medicine. 2021;100:13(e25105).
Since this article is a meta-analysis, the data are collect from published articles and it is not necessary to provide ethical approval.
The authors have no funding and conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Tan Y. Traditional Chinese medicine Bao Gan Ning increase phosphorylation of CREB in liver fibrosis in vivo and in vitro. J Ethnopharmacol 2006;105:0–75. [DOI] [PubMed] [Google Scholar]
- [2].Liu C, Ping L, Hu Y, et al. Progress of clinical and basic research on Liver Fibrosis with Traditional Chinese Medicine. World Science and Technology-Modernization of Traditional Chinese Medicine and Materia Medica 2007. [Google Scholar]
- [3].Lu B, Cheng Nan. Anti-fibrotic Effect of Chinese Medicine, Ezhu, on CCl4-induced liver fibrosis mouse model and its probable molecular mechanisms. Biological Sci 2004;55:25–30. [Google Scholar]
- [4].Fan C, Feng Y, Ning W, et al. Effectiveness of Chinese herbal medicine in treating liver fibrosis: a systematic review and meta-analysis of randomized controlled trials. Chin Med 2012;34:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yongping MU, Liu PS. Hospital, Therapeutic ideas and approaches in liver fibrosis guided by combination of traditional Chinese medicine and modern medicine. Shanghai Med Pharmaceut J 2016;24:25–31. [Google Scholar]
- [6].Liu Y. Study on the relationship between IL-28B gene polymorphism and traditional Chinese medicine syndrome type and liver fibrosis in patients with chronic hepatitis C. Chin J Integrated Traditi Western Med Liver Dis 2015;35:90–104. [Google Scholar]
- [7].Yang CL, DO. Gastroenterology, effect of traditional Chinese medicine combined with Western medicine in treating chronic hepatitis B liver fibrosis and early liver cirrhosis. China Foreign Med Treatment 2018;43:58–64. [Google Scholar]
- [8].Yuewen, Gong. Identifying the targets for treatment of liver fibrosis and hepatocellular carcinoma from both Western medicine and Chinese medicine. Chin J Integrative Med 2012;67:115–26. [DOI] [PubMed] [Google Scholar]
- [9].Guo X. A research progress on Micro RNAs in liver fibrosis and traditional Chinese medicine. Modernization Traditi Chin Med Materia Medica-World Science Technol 2016;58:251–9. [Google Scholar]
- [10].Guo T. A combination of astragaloside I, levistilide A and calycosin exerts anti-liver fibrosis effects in vitro and in vivo. Acta Pharmacologica Sinica 2018;39:1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu H. Protective effects of astragaloside IV on porcine-serum-induced hepatic fibrosis in rats and in vitro effects on hepatic stellate cells. J Ethnopharmacol 2009;122:502–8. [DOI] [PubMed] [Google Scholar]
- [12].Yongping M. Astragaloside prevents BDL-induced liver fibrosis through inhibition of notch signaling activation. J Ethnopharmacol 2015;169:200–9. [DOI] [PubMed] [Google Scholar]
- [13].Yongping M. Mechanism of astragaloside prevents cholestatic liver fibrosis through inhibition of notch signaling activation. Chin J Hepatol 2017;25:575–82. [DOI] [PubMed] [Google Scholar]
- [14].Wang Z. Astragaloside alleviates hepatic fibrosis function via PAR2 signaling pathway in diabetic rats. Cell Physiol Biochem 2017;41:1156–66. [DOI] [PubMed] [Google Scholar]
- [15].Yuan X. Astragaloside inhibits hepatic fibrosis by modulation of TGF-(1/smad signaling pathway. Evidence-Based Complement Alternat Med 2018;2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao X. Astragaloside IV synergizes with ferulic acid to alleviate hepatic fibrosis in bile duct-ligated cirrhotic rats. Digest Dis Sci 2020;69:285–91. [DOI] [PubMed] [Google Scholar]
- [17].Qu F. Role of collagen metabolism in liver fibrosis and research progress in traditional Chinese medicine on the regulation of collagen metabolism. China Pharmacist 2017;55:381–9. [Google Scholar]
- [18].Yongping MU, Liu PS. Hospital, Therapeutic ideas and approaches in liver fibrosis guided by combination of traditional Chinese medicine and modern medicine. Shanghai Med Pharmaceutical J 2016;72:125–8. [Google Scholar]
- [19].2009;Cheng L, Ping L. Chinese medicine BioMed central review effect of Fuzheng Huayu formula and its actions against liver fibrosis. 58:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jian-Hua XU, Bin XU, Deng YQ. Clinical observation on treatment of liver fibrosis with combine traditional Chinese and western medicine. China J Traditi Chin Med Pharmacy 2015;85:468–72. [Google Scholar]
- [21].Liu C, Zhao Z, Jing L. Advances in the understanding and treatment of liver fibrosis in traditional Chinese medicine. J Clin Hepatol 2019;89:321–5. [Google Scholar]
- [22].Hao LR. A systematic review of traditional Chinese medicine for liver fibrosis of chronic hepatitis virus. Proceeding Clin Med 2011;91:210–5. [Google Scholar]
- [23].Rizk Fatma H, Sarhan Naglaa I, Soliman Nema A, et al. Heat shock protein 47 as indispensible participant in liver fibrosis: possible protective effect of lactoferrin. IUBMB Life 2018;70:795–805. [DOI] [PubMed] [Google Scholar]
- [24].Soliman H, Ziada Dina, Salama M, et al. Predictors for fibrosis regression in chronic HCV patients after the treatment with DAAS: results of a real-world cohort study. Endocr Metab Immune Disord Drug Targets 2020;20:104–11. [DOI] [PubMed] [Google Scholar]


