Abstract
It has been reported that some male breast cancer patients may refuse the recommended surgery, but the incidence rate in the United States is not clear. The purpose of this study was to identify the incidence, trends, risk factors, and eventual survival outcomes associated with the rejection of such cancer-directed surgery.
We collected data on 5860 patients with male breast cancer (MBC) from the Surveillance, Epidemiology, and End Results database, including 50 patients refusing surgery as recommended. Kaplan–Meier survival analysis and Cox proportional hazard regression were used to identify the effects of refusing surgery on cancer-specific survival (CSS) and overall survival (OS). The association between acceptance or rejection of surgery and mortality were estimated by nested Cox proportional hazards regression models with adjustment for age, race, clinical characteristics, and radiation.
Of the 5860 patients identified, 50 (0.9%) refused surgery. Old age (≥65: hazard ratio [HR]: 3.056, 95% confidence interval [CI]: 1.738–5.374, P < .0001), higher AJCC stage (III: HR: 3.283, 95% CI: 2.134–5.050, P < .0001, IV: HR: 14.237, 95% CI: 8.367–24.226, P < .0001), progesterone receptor status (negative: HR: 1.633, 95% CI: 1.007–2.648, P = .047) were considered risk factors. Compared with the surgery group, the refusal group was associated with a poorer prognosis in both OS and CSS (χ2 = 94.81, P < .001, χ2 = 140.4, P < .001). Moreover, significant differences were also observed in OS and CSS among 1:3 matched groups (P = .0002, P < .001).
Compared with the patients undergoing surgery, the patients who refused the cancer-directed surgery had poor prognosis in the total survival period, particularly in stage II and III. The survival benefit for undergoing surgery remained even after adjustment, which indicates the importance of surgical treatment before an advanced stage for male breast cancer patients.
Keywords: male breast cancer; refusal; the Surveillance, Epidemiology, and End Results Program; surgery; survival
1. Introduction
Male breast cancer accounts for about 1% of all breast cancers. Although rare, its incidence has increased steadily.[1] An estimated 2670 cases of MBC were reported during 2019 in the US, 230 cases in 2017 in Canada, 140 cases in 2014 in Australia and 149 from 2012 to 2016 in Nordic Countries.[2] Although great progress has been made in screening, diagnosis and treatment strategies of female breast cancer in recent years, we know little about the best treatment for male patients. Due to the lack of prospective data and guidelines for male breast cancer, most of the treatments recommended by clinicians are inferred from the data of female patients.[3] While MBC and female breast cancer have some common characteristics, there are significant differences in prognostic factors, epidemiological factors and biological behavior between them.[4] For male breast cancer, the main treatment mode is axillary lymph node dissection for clinically node-positive patients or sentinel lymph node surgery based on sentinel lymph node pathology.[5,6] These invasive operations often have a considerable impact on the quality of life of patients, which damaged their psychosocial function even after breast reconstruction surgery.[7] As a result, some patients may refuse the recommended surgery. A study has shown that for patients with breast cancer, rejection of recommended surgery can affect their prognosis and survival.[8] However for male patients, the research of this area still needs to be further explored.
In this study, we used the Surveillance, Epidemiology, and End Results (SEER) registered database to determine the rate, time-related trends, and risk factors associated with refusal of male breast cancer-directed surgery. Importantly, we estimated the impact of refusal of cancer-directed surgery refusal on eventual survival in men.
2. Methods
2.1. Data collection
The population-based data for this study were extracted from the SEER database established by the National Cancer Institute. Since SEER is a publicly available database with anonymized data, no ethical review was required. We obtained male patients who had histologically confirmed invasive breast cancer diagnosis from 1975 to 2016 from the SEER database. Only patients in whom it was specified that either surgery was performed or was recommended by physicians, but not performed due to patients’ refusal, were included in the study. Patients were excluded for the following reasons: surgery was contraindicated because of the presence of other comorbid conditions, surgery was recommended but not performed because of the patient's death before surgery, and surgery was recommended but not performed with unspecified reasons.
We extracted multiple variables from the selected object of study. Demographic characteristics consisted of age at diagnosis (<50, 50–64, or ≥65 years), race (white, black, or other), year of diagnosis (1975–1989, 1990–2004, 2005–2016) and marital status (single, married, separated/widowed/ divorced). Pathological characteristics included Histology (ductal, papillary, others), AJCC stage, tumor grade, hormone receptor status. Treatment characteristics included surgery (yes or no) and radiation (yes or no). The primary endpoints of this study were breast cancer-specific survival (CSS) and overall survival (OS).
2.2. Statistical analysis
Clinicopathological features were compared between the operation group and the refusal group using the χ2 test or Fisher exact test as appropriate. To assess the prognostic factors associated with the rejection of surgery, we built a logistic regression model. We used the univariate and multivariate analysis to compare the CSS and OS, and the Kaplan–Meier method was performed to generate the survival curves in different AJCC stage. OS refers to the interval from breast cancer diagnosis until death due to all causes (including breast cancer) or last follow-up. CSS was measured from the date of diagnosis to either the date of breast cancer death or the date of the last contact. In order to evaluate the effects of different clinicopathological features and overall, 3-year, and 5-year mortality associated with refusing surgery, we used Cox proportional hazard regression analysis to estimate the hazard ratio (HR) and 95% confidence interval (CI). We calculated relative attenuation in HR by including additional sets of covariates in the nested models: (HRR-HRROF) divided by (HRR-1), in which HRR was the less adjusted HR for mortality comparing patients who refusing surgery with undergoing surgery, and HRROF was the HR with additional adjustment. We performed a 1:3 case-matched analysis based on rejecting surgery or not and matching for the AJCC stage and age, utilizing the propensity score matching method to control for confounding variables. MBC patients in stage II and III were used to establish a nomogram. Some patients (n = 1392) with unknown status were excluded. The eligible patients were randomly divided into a training set (n = 799) and a validation set (n = 763). Univariate and multivariate Cox regression analyses were used to identify the prognostic value of the factors. The independent factors were used to build the nomogram for the OS by using the rms package in R version 3.6.1. The nomogram was internally validated in the training set and externally validated in the validation set. The model performance for predicting the survival outcomes was evaluated by calculating the Harrell concordance index (C-index)[9] and the calibration plot. The C-index shows relatively good discriminative ability between 0.71 and 0.90, while the C-index >0.90 shows better accuracy. In a perfectly calibrated model, the predictions should fall at a diagonal 45° line in the calibration plot.
All statistical analyses and charts of survival probabilities were performed using the SPSS 22.0 (IBM Corporation, Armonk, NY), Stata/SE version 12 (Stata Corp., College Station, TX), R statistical software (R Core Development Team, Vienna, Austria), and GraphPad Prism version 6 (GraphPad Software Inc., La Jolla, CA). All the statistical tests were two-sided, and statistical significance was defined as P value <.05.
3. Results
3.1. Patient demographics
Overall, 5860 patients with male breast cancer were enrolled, including 5810 (99.1%) underwent cancer-directed surgery, and 50 (0.9%) patients refused to undergo surgery despite it being recommended. Their characteristics were analyzed, and the results are summarized in Table 1. There were significant differences in clinical characteristics, including age, histology, AJCC stage, and radiation. Most patients were diagnosed at ≧50 years old (87.6%). Most patients were married (70.0%), and separated/divorced/widowed patients also comprised a considerable proportion of this cohort (16.0%). Most patients were diagnosed as having AJCC stage II disease (44.9%), followed by stage I disease (31.3%) and stage III disease (19.0%), and a small minority had stage IV disease (4.8%). Although radiation therapy, either adjuvant or neoadjuvant, was administered in 26% of all patients, only 10.0% of patients who refused surgery received it (Table 1).
Table 1.
Characteristics of the patient cohort.
| Total (%) | Patients underwent surgery | Patients refused surgery | ||
| Variable | 5860 (100.0) | 5810 (99.1) | 50 (0.9) | P value |
| Age | .003∗ | |||
| <50 | 725 (12.4) | 722 (12.4) | 3 (6.0) | |
| 50–64 | 2077 (35.4) | 2066 (35.6) | 11 (22.0) | |
| ≥65 | 3058 (52.2) | 3022 (52.0) | 36 (72.0) | |
| Race | .064 | |||
| White | 4747 (81.5) | 4714 (81.6) | 33 (66.0) | |
| Black | 764 (13.1) | 750 (13.0) | 14 (28.0) | |
| Other | 313 (5.4) | 310 (5.4) | 3 (6.0) | |
| Year of diagnosis | .259 | |||
| 1975–1989 | 701 (12.0) | 697 (12.0) | 4 (9.0) | |
| 1990–2004 | 1826 (31.2) | 1812 (31.2) | 14 (28.0) | |
| 2005–2016 | 3333 (56.9) | 3301 (56.8) | 42 (64.0) | |
| Histology | .006∗,# | |||
| Ductal | 4711 (80.4) | 4680 (80.6) | 31 (62.0) | |
| Papillary | 168 (2.9) | 168 (2.9) | 0 (0) | |
| Others | 981 (16.7) | 962 (16.6) | 19 (38.0) | |
| Marital status | .067 | |||
| Single | 779 (14.0) | 769 (13.9) | 10 (21.7) | |
| Married | 3904 (70.0) | 3889 (70.3) | 15 (32.6) | |
| Separated/Widowed/ Divorced | 893 (16.0) | 872 (15.8) | 21 (45.7) | |
| AJCC stage | <.0001∗,# | |||
| I | 1449 (31.3) | 1449 (31.5) | 0 (0) | |
| II | 2077 (44.9) | 2065 (44.9) | 12 (40.0) | |
| III | 877 (19.0) | 868 (18.9) | 9 (30.0) | |
| IV | 223 (4.8) | 214 (4.7) | 9 (30.0) | |
| Unknown | 1234 (−) | 1214 (−) | 20 (−) | |
| Grade | .627# | |||
| I | 596 (12.1) | 595 (12.1) | 1 (4.4) | |
| II | 2476 (50.2) | 2460 (50.1) | 16 (69.6) | |
| III | 1796 (36.4) | 1790 (36.4) | 6 (26.1) | |
| IV | 68 (1.4) | 68 (1.4) | 0 (0) | |
| Unknown | 924 (−) | 897 (−) | 27 (−) | |
| ER status | .920 | |||
| Positive | 4337 (95.4) | 4314 (95.4) | 23 (95.8) | |
| Negative | 209 (4.6) | 208 (4.6) | 1 (4.2) | |
| Unknown | 1314 (−) | 1288 (−) | 26 (−) | |
| PR status | .907 | |||
| Positive | 3839 (86.1) | 3819 (86.1) | 20 (87.0) | |
| Negative | 619 (13.9) | 616 (13.9) | 3 (13.0) | |
| Unknown | 1402 (−) | 1375 (−) | 27 (−) | |
| Her2 status | .384 | |||
| Positive | 243 (12.6) | 240 (12.5) | 3 (20.0) | |
| Negative | 1690 (87.4) | 1678 (87.5) | 12 (80.0) | |
| Unknown | 3927 (−) | 3892 (−) | 35 (−) | |
| Radiation | <.0001∗ | |||
| No | 4335 (74.0) | 4290 (73.8) | 45 (90.0) | |
| Yes | 1525 (26.0) | 1520 (26.2) | 5 (10.0) |
represent the P value <.05.
represent that the P value is performed by Fishe exact test.
Table 2 summarizes the univariate and multivariate logistic regression analyses. Refusal of surgery was independently associated with race, histology, marital status, AJCC stage and the refusal of radiation.
Table 2.
Predictors of Refused surgery assessed by univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
| Age | ||||
| <50 | reference | reference | ||
| 50–64 | 1.281 (0.356–4.606) | .704 | 1.297 (0.257–6.537) | .753 |
| ≥65 | 2.867 (0.880–9.336) | .080 | 2.606 (0.563–12.066) | .221 |
| Race | ||||
| White | reference | reference | ||
| Black | 2.667 (1.420–5.006) | .002∗ | 3.230 (1.380–7.560) | .007∗ |
| Other | 1.382 (0.442–4.533) | .593 | 2.838 (0.618–13.031) | .180 |
| Histology | ||||
| Ductal | reference | reference | ||
| Papillary | – | – | – | – |
| Others | 2.982 (1.677–5.300) | <.0001∗ | 1.838 (0.762–4.433) | .176 |
| Marital status | ||||
| Single | reference | reference | ||
| Married | 0.540 (0.253–1.154) | .112 | 0.306 (0.108–0.868) | .026∗ |
| Separated/Widowed/Divorced | 0.160 (0.082–0.312) | <.0001∗ | 1.119 (0.406–3.088) | .828 |
| AJCC stage | ||||
| IV | reference | reference | ||
| III | 0.247 (0.097–0.629) | .003∗ | 0.218 (0.077–0.621) | .004∗ |
| II | 0.138 (0.058–0.332) | <.0001∗ | 0.143 (0.055–0.370) | <.0001∗ |
| I | – | – | – | – |
| Radiation | ||||
| No | reference | reference | ||
| Yes | 0.314 (0.124–0.791) | .014∗ | 0.213 (0.063–0.725) | .013∗ |
represent the P value <.05.
3.2. Impact of refusal of cancer-directed surgery on OS and CSS of MBC patients
In univariate analyses, age (P < .0001), race (P < .0001), histology (papillary, P = .032), AJCC stage (II, III stage, P < .0001), grade (P < .0001), marital status (P < .0001), PR status (P < .0001), HER2 status (P = .048), and surgery (P < .0001) were also significantly associated with OS in MBC patients (Table 3). In multivariate Cox regression analysis of these factors, the refusal group were found to have a risk for cancer-specific mortality (HR: 1.143, 95% CI: 0.378–3.456, P = .813). Age, marital status, AJCC stage, and PR status were validated as independent prognostic factors as well.
Table 3.
Univariate and multivariate analysis of overall survival (OS).
| Univariate analysis | Multivariate analysis | |||
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | ||||
| <50 | reference | reference | ||
| 50–64 | 1.466 (1.265–1.700) | <.0001∗ | 1.144 (0.632–2.069) | .657 |
| ≥65 | 3.184 (2.769–3.661) | <.0001∗ | 3.056 (1.738–5.374) | <.0001∗ |
| Race | ||||
| White | reference | reference | ||
| Black | 1.235 (1.107–1.378) | <.0001∗ | 1.260 (0.868–1.829) | .225 |
| Other | 0.740 (0.610–0.899) | .002∗ | 0.837 (0.406–1.729) | .632 |
| Histology | ||||
| Ductal | reference | reference | ||
| Papillary | 0.772 (0.610–0.978) | .032∗ | 0.432 (0.106–1.756) | .241 |
| Others | 1.020 (0.926–1.123) | .691 | 0.930 (0.591–1.462) | .753 |
| Marital status | ||||
| Single | reference | reference | ||
| Married | 0.825 (0.735–0.925) | .001∗ | 0.556 (0.386–0.799) | .002∗ |
| Separated/Widowed/Divorced | 1.469 (1.284–1.682) | <.0001∗ | 1.071 (0.691–1.659) | .759 |
| AJCC stage | ||||
| I | reference | reference | ||
| II | 1.647 (1.467–1.849) | <.0001∗ | 1.387 (0.935–2.056) | .104 |
| III | 2.844 (2.503–3.231) | <.0001∗ | 3.283 (2.134–5.050) | <.0001∗ |
| IV | 8.727 (7.352–10.359) | <.0001∗ | 14.237 (8.367–24.226) | <.0001∗ |
| Grade | ||||
| I | reference | reference | ||
| II | 1.381 (1.180–1.617) | <.0001∗ | 0.864 (0.515–1.451) | .582 |
| III | 1.982 (1.692–2.322) | <.0001∗ | 1.367 (0.813–2.298) | .238 |
| IV | 2.472 (1.830–3.341) | .113 | NI | NI |
| ER status | ||||
| Positive | reference | reference | ||
| Negative | 1.215 (0.994–1.484) | .058 | 2.707 (1.343–5.455) | .005∗ |
| PR status | ||||
| Positive | reference | reference | ||
| Negative | 1.290 (1.138–1.462) | <.0001∗ | 1.633 (1.007–2.648) | .047∗ |
| Her2 status | ||||
| Positive | reference | reference | ||
| Negative | 0.708 (0.504–0.997) | .048∗ | 0.844 (0.575–1.239) | .386 |
| Radiation | ||||
| No | reference | reference | ||
| Yes | 1.069 (0.980–1.166) | .134 | 0.628 (0.454–0.871) | .005∗ |
| Surgery | ||||
| Refused | reference | reference | ||
| Performed | 0.222 (0.163–0.320) | <.0001∗ | 1.143 (0.378–3.456) | .813 |
represent the P value <.05.
Univariate and multivariate analysis were also used to evaluate effect of refusal of cancer-directed surgery on CSS of MBC patients (Table 4). In univariate analysis, age (≥65 years, P = .044), race (P < .0001), histology (papillary, P < .0001), AJCC stage (P < .0001), grade (P < .0001), PR status (P < .0001), HER2 status (P = .032), radiation (P < .0001) and surgery (P < .0001) were also associated with CSS (Table 4). Radiotherapy is an important confounding factor of MBC. In univariate analysis, it presents as a risk factor for CSS (HR: 1.446, 95% CI: 1.281–1.632, P < .0001). However, in multivariate analysis, it shows the opposite effect (HR: 0.609, 95% CI: 0.391–0.950, P = .029). In the multivariate analysis, the effect of rejection of cancer-directed surgery on CSS was not significant when other prognostic factors were added (HR: 0.833, 95% CI: 0.242–2.869, P = .772).
Table 4.
Univariate and multivariate analysis of cancer-specific survival (CSS).
| Univariate analysis | Multivariate analysis | |||
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | ||||
| <50 | reference | reference | ||
| 50–64 | 1.135 (0.953–1.350) | .155 | 0.695 (0.350–1.380) | .298 |
| ≥65 | 1.192 (1.005–1.415) | .044∗ | 1.752 (0.910–3.372) | .093 |
| Race | ||||
| White | reference | reference | ||
| Black | 1.775 (1.536–2.050) | <.0001∗ | 1.505 (0.910–2.489) | .111 |
| Other | 0.705 (0.519–0.957) | .025∗ | 0.599 (0.184–1.958) | .397 |
| Histology | ||||
| Ductal | reference | reference | ||
| Papillary | 0.291 (0.165–0.515) | <.0001∗ | – | .952 |
| Others | 0.961 (0.830–1.113) | .598 | 0.839 (0.426–1.653) | .612 |
| Marital status | ||||
| Single | reference | reference | ||
| Married | 0.755 (0.640–0.892) | .001∗ | 0.502 (0.306–0.826) | .007∗ |
| Separated/Widowed/Divorced | 1.285 (1.054–1.566) | .013∗ | 1.100 (0.600–2.017) | .757 |
| AJCC stage | ||||
| I | reference | reference | ||
| II | 2.488 (2.006–3.087) | <.0001∗ | 1.874 (0.939–3.743) | .075 |
| III | 6.460 (5.194–8.035) | <.0001∗ | 6.601 (3.228–13.500) | <.0001∗ |
| IV | 29.153 (22.859–37.181) | <.0001∗ | 42.960 (19.558–94.363) | <.0001∗ |
| Grade | ||||
| I | reference | reference | ||
| II | 2.172 (1.603–2.942) | <.0001∗ | 1.303 (0.499–3.406) | .589 |
| III | 4.199 (3.110–5.668) | <.0001∗ | 2.583 (1.016–6.566) | .046∗ |
| IV | 6.191 (3.966–9.664) | <.0001∗ | NI | NI |
| ER status | ||||
| Positive | reference | reference | ||
| Negative | 1.759 (1.361–2.273) | .058 | 4.734 (2.118–10.579) | <.0001∗ |
| PR status | ||||
| Positive | reference | reference | ||
| Negative | 1.835 (1.552–2.169) | <.0001∗ | 2.402 (1.280–4.506) | .006∗ |
| Her2 status | ||||
| Positive | reference | reference | ||
| Negative | 0.611 (0.390–0.958) | .032∗ | 0.962 (0.565–1.638) | .886 |
| Radiation | ||||
| No | reference | reference | ||
| Yes | 1.446 (1.281–1.632) | <.0001∗ | 0.609 (0.391–0.950) | .029∗ |
| Surgery | ||||
| Refused | reference | reference | ||
| Performed | 0.142 (0.098–0.206) | <.0001∗ | 0.833 (0.242–2.869) | .772 |
represent the P value <.05.
During the follow-up period, cancer-specific death occurred in 29 patients in the refusal group and 1190 patients in the surgery group, whose cancer-specific mortality rates per 1000 person-years were 2208.178 (95% CI: 143.739–301.507) and 30.739 (95% CI: 29.030–32.549), respectively (Table 5). In addition, during the follow-up period, all-cause death occurred in 41 patients in the refusal group and 2692 patients in the surgery group. The all-cause mortality rates per 1000 person-years were 282.528 (95% CI: 205.579–388.280) for the refusal group and 69.752 (95% CI: 67.153–72.452) for the surgery group (Table 5).
Table 5.
Cancer-specific mortality and all-cause mortality of male breast cancer stratified by different factors.
| Cancer-specific mortality | Cancer-specific mortality | All-cause mortality | All-cause mortality | |||||
| No. | % | 1000 person-years | 95% CI | No. | % | 1000 person-years | 95% CI | |
| Age | ||||||||
| <50 | 174 | 24.0 | 26.367 | 22.727–30.591 | 232 | 32.0 | 35.156 | 30.911–39.984 |
| 50–64 | 476 | 22.9 | 31.137 | 28.448–34.080 | 758 | 36.5 | 49.582 | 46.157–53.260 |
| ≥65 | 569 | 18.6 | 33.551 | 30.878–36.457 | 1743 | 57.0 | 103.606 | 98.823–108.620 |
| Race | ||||||||
| White | 944 | 19.9 | 29.358 | 27.531–31.305 | 2246 | 47.3 | 70.004 | 67.152–72.978 |
| Black | 230 | 30.1 | 52.686 | 46.260–60.006 | 375 | 49.1 | 86.108 | 77.777–95.332 |
| Other | 43 | 13.7 | 20.549 | 15.186–7.806 | 107 | 34.2 | 51.863 | 42.872–62.738 |
| Histology | ||||||||
| Ductal | 990 | 21.0 | 32.582 | 30.605–34.686 | 2154 | 45.7 | 70.909 | 67.964–73.982 |
| Papillary | 12 | 7.1 | 9.361 | 5.317–16.484 | 71 | 42.3 | 55.390 | 43.894–69.895 |
| Others | 217 | 22.1 | 30.132 | 26.311–34.506 | 508 | 51.8 | 71.508 | 65.484–78.087 |
| Marital status | ||||||||
| Single | 170 | 21.8 | 36.678 | 31.517–42.685 | 348 | 44.7 | 75.333 | 67.768–83.742 |
| Married | 769 | 19.7 | 27.814 | 25.904–29.865 | 1732 | 44.4 | 62.885 | 59.979–65.932 |
| Separated/Widowed/Divorced | 233 | 26.1 | 47.397 | 41.639–3.951 | 539 | 60.4 | 109.904 | 100.942–119.661 |
| AJCC stage | ||||||||
| I | 111 | 7.7 | 9.905 | 8.224–11.931 | 437 | 30.2 | 38.997 | 35.507–42.830 |
| II | 326 | 15.7 | 24.176 | 21.678–26.961 | 842 | 40.5 | 62.647 | 58.544–67.038 |
| III | 304 | 34.7 | 62.170 | 55.539–69.592 | 520 | 59.3 | 106.018 | 97.246–115.581 |
| IV | 172 | 77.1 | 251.103 | 215.164–293.047 | 197 | 88.3 | 283.856 | 245.473–∼328.242 |
| Grade | ||||||||
| I | 47 | 7.9 | 10.957 | 8.207–14.628 | 187 | 31.4 | 43.827 | 37.930–50.640 |
| II | 371 | 15.0 | 24.109 | 21.762–26.710 | 927 | 37.4 | 60.602 | 56.810–64.648 |
| III | 469 | 26.1 | 46.280 | 42.246–50.698 | 869 | 48.4 | 85.747 | 80.191–91.688 |
| IV | 33 | 48.5 | 61.580 | 43.307–87.562 | 55 | 80.9 | 105.282 | 80.433∼137.808 |
| ER status | ||||||||
| Positive | 718 | 16.6 | 28.552 | 26.534–30.724 | 1604 | 37.0 | 63.534 | 60.488–66.734 |
| Negative | 64 | 30.6 | 46.855 | 36.456–60.220 | 102 | 48.8 | 76.043 | 62.447∼92.599 |
| PR status | ||||||||
| Positive | 583 | 15.2 | 26.549 | 24.477–28.796 | 1265 | 35.6 | 61.856 | 58.650–65.237 |
| Negative | 180 | 29.1 | 47.630 | 41.088–55.213 | 299 | 48.3 | 79.293 | 70.714–88.912 |
| Her2 status | ||||||||
| Positive | 23 | 9.5 | 36.236 | 24.080–54.530 | 39 | 16.0 | 61.444 | 44.893–84.098 |
| Negative | 110 | 6.5 | 22.235 | 18.430–26.827 | 215 | 12.7 | 43.247 | 37.800–49.478 |
| Radiation | ||||||||
| No | 837 | 19.3 | 27.976 | 26.125–29.958 | 2058 | 47.5 | 69.189 | 66.243–72.267 |
| Yes | 382 | 25.0 | 42.368 | 38.326– 46.838 | 675 | 44.3 | 74.755 | 69.319–80.617 |
| Surgery | ||||||||
| Refused | 29 | 58.0 | 208.178 | 143.739–301.507 | 41 | 82.0 | 282.528 | 205.579–388.280 |
| Performed | 1190 | 20.5 | 30.739 | 29.030– 32.549 | 2692 | 46.3 | 69.752 | 67.153–72.452 |
3.3. Survival analyses
Median survival was 114.89 months in the surgery group, compared with 22.23 months in the refusal group (Table 6). For patients in stage II, the median survival time of the refusal group was significantly shorter than that of the surgery group. And this trend is also evident in patients in stage III or IV (Table 6).
Table 6.
Median survival time.
| Therapy | Overall (months) | Stage I | Stage II | Stage III | Stage IV |
| Refused surgery | 22.23 | – | 28.40 | 25.00 | 15.00 |
| Underwent surgery | 114.89 | 187.85 | 124.80 | 75.49 | 30.02 |
As shown in Kaplan–Meier plots, OS was better in the group that had undergone surgery than in the refusal group (χ2 = 94.81, P < .001, Fig. 1A). We also analyzed the breast cancer-specific survival and slightly significant differences were observed (χ2 = 140.4, P < .001, Fig. 2A). Subgroup analyses by stage identified that the refusal group compared with the operation group had significantly worse OS and CSS in both Stage II and III, but not in Stage I (Fig. 1B–1D, Fig. 2B–2D).
Figure 1.
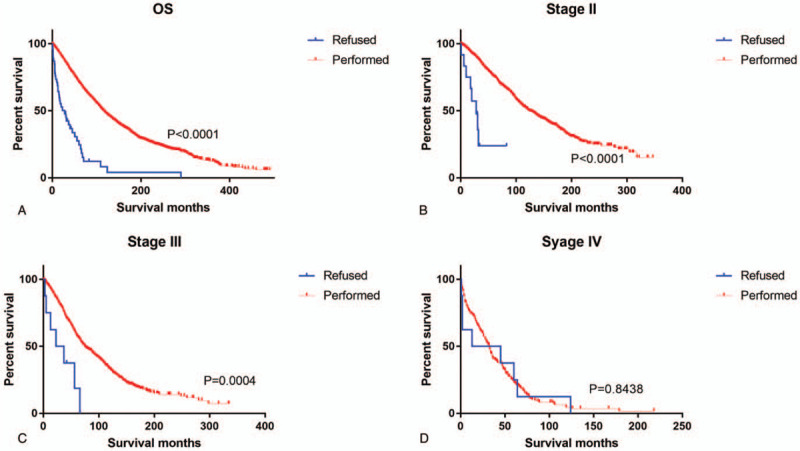
Kaplan–Meier curves depicting overall survival of patients who underwent cancer-directed surgery and those who refused it: (A) All Stages; (B–D) AJCC Stages II-IV.
Figure 2.
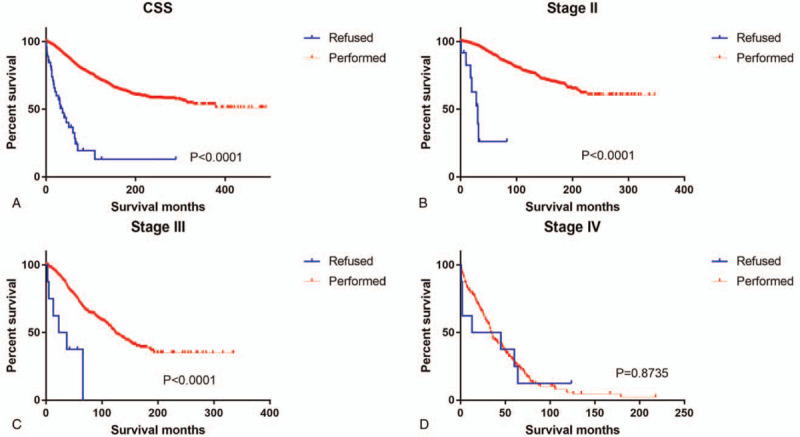
Kaplan–Meier curves depicting cancer-specific survival of patients who underwent cancer-directed surgery and those who refused it: (A) All Stages; (B–D) AJCC Stages II-IV.
For the entire cohort, time-dependent coefficient analyses showed constant associations between the refusal of the recommended surgery and total mortality over time (unadjusted HR: 4.507, 95% CI: 3.308–6.140, P for time-varying effect <.0001). Age-adjusted HR for total mortality among male compared with female patients (model 1) was 4.441 (95% CI: 3.259–6.052). Further adjustments for clinical factors (model 2) resulted in78.6% relative attenuation of the HR (adjusted HR: 1.735, 95% CI: 0.685–4.389). Combined adjustment for both clinical and treatment factors (model 6) resulted in 74.5% relative attenuation (adjusted HR: 1.879, 95% CI: 0.743–4.752), and adjustment for all 5 factor groups (model 7) resulted in 86.1% relative attenuation (adjusted HR: 1.479, 95% CI: 0.525–4.170). Similar patterns were observed for 3-year (adjusted HR: 1.179, 95% CI: 0.483–2.878) and 5-year (adjusted HR: 0.743, 95% CI: 0.305–1.811) mortality analyses (Table 7).
Table 7.
Factors in surgery-based disparity in mortality among patients with male breast cancer.
| Model | Total mortality, refused vs underwent, HR (95% CI) | Excess mortality associated with additional adjusted covariates, % | 3-y mortality, refused vs underwent, HR (95% CI) | Excess mortality associated with additional adjusted covariates, % | 5-y mortality, refused vs underwent, HR (95% CI) | Excess mortality associated with additional adjusted covariates, % |
| Unadjusted model | 4.507 (3.308–6.140) | NA | 3.269 (2.340–4.568) | NA | 2.917 (2.143–3.971) | NA |
| Model 1 | 4.441 (3.259–6.052) | NA | 3.098 (2.217–4.330) | NA | 2.775 (2.038–3.778) | NA |
| Model 2 | 1.735 (0.685–4.389) | 78.6 | 1.471 (0.652–3.321) | 77.6 | 0.959 (0.426–2.160) | 102.3 |
| Model 3 | 4.467 (3.278–6.087) | – | 3.158 (2.259–4.414) | – | 2.804 (2.059–3.817) | – |
| Model 4 | 4.397 (3.226–5.995) | 1.3 | 3.028 (2.166–4.233) | 3.3 | 2.712 (1.991–3.693) | 3.5 |
| Model 5 | 4.307 (3.123–5.940) | 3.9 | 3.051 (2.150–4.328) | 2.2 | 2.669 (1.929–3.693) | 6.0 |
| Model 6 | 1.879 (0.743–4.752) | 74.5 | 1.475 (0.653–3.329) | 77.4 | 0.953 (0.423–2.148) | 102.7 |
| Model 7 | 1.479 (0.525–4.170) | 86.1 | 1.179 (0.483–2.878) | 91.5 | 0.743 (0.305–1.811) | 114.5 |
Time-dependent coefficient analyses showed that the associations of refusal of cancer-directed surgery with total mortality were constant over time for the entire cohort (P < .0001), and results from a Cox proportional hazards regression model are reported in this table.
Model 1 Adjusted for age.
Model 2 Adjusted for age and clinical factors (grade, histology, AJCC stage, ER, PR, Her2 status).
Model 3 Adjusted for age and treatment factors (radiation).
Model 4 Adjusted for age and race.
Model 5 Adjusted for age and marital status.
Model 6 Adjusted for age, clinical factors, and treatment factors.
Model 7 Adjusted for age, race, clinical and treatment factors, marital status.
(HRR-HRROF)/(HRR-1) HRR was the HR for model 1, and HRROF was the HR with additional adjustment.
3.4. Survival analysis in matched groups
Considering that the sample size was quite different and there were some differences of population baseline characteristics between these 2 groups, to ensure that the outcomes were not based on the differences of the patient quantity of the groups, we performed a 1:3 (refusing: undergoing recommended surgery) matched case control analysis using the propensity score matching method. We finally focused on a group of 116 patients, including 30 patients refusing surgery and 86 counterparts who had undergone surgery (see Table S1, Supplemental Content, which shows characteristics of male patients with breast cancer by Refused or Underwent recommended surgery, in 1:3 matched groups). Table S1 shows similar results to Table 1.
We also used the Kaplan–Meier to investigate the effects of refusing cancer-directed surgery on OS and CSS in the matched group. We also found that the refusal group was associated with a poorer prognosis in both OS and CSS [χ2 = 22.07, P = .0002, χ2 = 13.78, P < .0001, see Figure S1, Supplemental Content which shows Kaplan–Meier survival curves of 1:3 matched group for CSS and OS in Refused vs Underwent recommended surgery in male breast cancer (MBC)], particularly in stage II and III (see Figure S2A-B and Figure S3A-B, Supplemental Content, shows Kaplan–Meier survival curves of 1:3 matched group for CSS and OS in Refused vs Underwent recommended surgery in MBC in stage II and III), similar to the total group.
3.5. Independent prognostic factors and construction of the nomogram
Table S2 (Supplemental Content) shows univariate and multivariate analyses of potential predictors for the OS in MBC patients with stage II and III. Age at diagnosis, race, marital status, AJCC stage, grade, Her2, radiation, and surgery were significantly associated as risk factors for the OS in the univariate analysis. Multivariate analysis identified the same results (Table S2). The independent factors were used to build the nomogram for 3-, and 5-year OS (Fig. 3).
Figure 3.
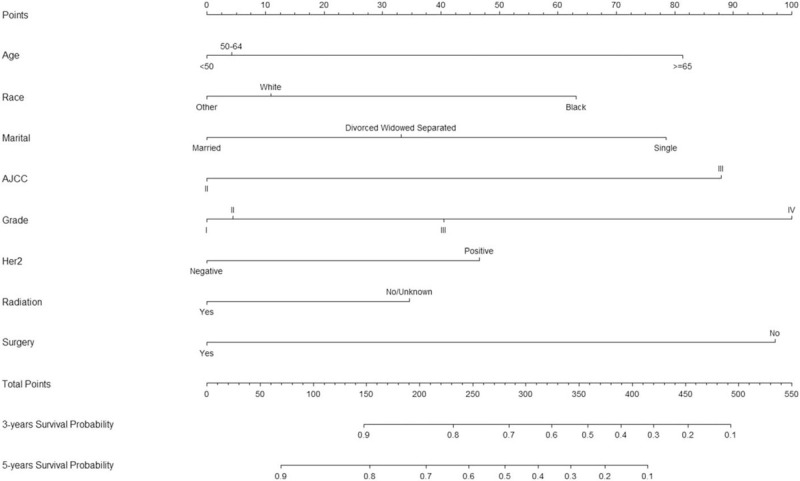
Nomogram to Predict 3-, and 5-year OS of Male Stage II and III Breast Cancer Patients. Notes: Vertical line between each variable and points scale can be drawn to acquire points of each variable. Predicted survival rate was calculated according to the total points by drawing a vertical line from total points scale to overall survival scale.
3.6. Calibration and validation of the nomogram
The C-index of OS in training and validation cohorts were 0.711 (95% CI: 0.680–0.742) and 0.710 (95% CI: 0.675–0.745), respectively. The calibration plots showed satisfactory agreement in the training and validation cohort between the nomogram-predicted survival probability and the actual survival probability (see Figure S4, Supplemental Content, Calibration plots in the training and validation cohorts for 3-year and 5-year OS).
4. Discussion
Due to the delay of diagnosis, and the lack of social male-specific information, the mortality rate of male breast cancer is on the rise. However, since the incidence of MBC is significantly lower than that of female breast cancer, reducing its incidence and prevention measures have not attracted the same attention. However, in some cases, mirror therapy may not be the best option.[4] Given that the optimized therapeutic approaches must be used in both sexes, the question remains whether MBC patients should undergo cancer-directed surgery.
As far as we know, this is one of the first studies to address the impact of refusing cancer-directed surgery in men with breast cancer. In this large-scale registration based study, a 4-fold increase in cancer cases among men in 2005 to 2016 compared to 1975 to 1989, which is consistent with previous reports that the incidence of MBC is increasing year by year.[10] The increase in MBC found in our study may partly be due to increases in the number of cases registered by SEER within the 41 years time frame of our study. The proportion of patients refusing surgery is an upward trend, with 64.0% of patients refusing surgery during 2005 to 2016 versus 28.0% during 1990 to 2014. This observation is similar to the recently reported trend in the whole population of patients with breast cancer.[8] This finding could be attributed to several factors, such as economic fluctuations that may impair access to care, but also to growing mistrust of the medical community and pursuit of alternative treatments.[8]
Perhaps not surprisingly, there was a significant difference between patients who refused surgery and patients who underwent surgery, which was characterized by older age at the time of diagnosis, more advanced diseases, and a smaller proportion of receiving radiotherapy. Similar conclusions that the older patients were more likely to refuse recommended surgery have been drawn from the previous studies.[11] Multiple studies have shown that older women with invasive breast cancer are less actively treated than the younger.[12] Also, some studies have indicated that socio-economic factors have an independent impact on treatment choice, and the increase of income deprivation can predict the non-surgical treatment of 70 to 85 years old patients.[13] Although the reasons behind this association are not completely clear, this phenomenon may be attributed to the more habitual concept of death, the low estimate of expected survival rate, as well as the fear and coping capacity of complications. Interestingly, surgery was not predictive of survival for the women diagnosed with invasive breast cancer aged ≥80 years.[14] But for elderly patients diagnosed with HR negative advanced breast cancer, not candidates for standard therapies, mastectomy should be recommended as palliative therapy.[15] Nevertheless, given the greater risk of death from non-cancer causes and the powerful fear of loss of functionality, it is important to ask whether the outcomes end-points used in conventional cancer studies are as relevant for the older population.[16] One study has shown that chemotherapy is a key prognostic factor for glioma, which may be beneficial to survival.[17,18] Whether chemotherapy has an effect on the prognosis of those who refuse surgery needs further study.
Single patients were significantly more likely to forego surgery compared to the married. Studies with similar results have discussed the possibility of a lack of support system available to help the patient with the anxiety of undergoing a process as arduous as surgery.[19] In addition, this finding is consistent with recent research on the psychological needs of breast cancer patients, which indicates that patients desire psychosocial support after diagnosis.[20] However, a Japanese study shows that a high degree of distress does not necessarily lead those patients to seek psychosocial support services.[21] In our study, blacks are more likely to reject surgery and are associated with poor prognosis. Compared with white, members of ethnic minorities in the United States are more likely to be in medically underserved and impoverished areas with limited access to health care. In addition, poor people with inadequate or less likely access to cancer screening are more likely to be diagnosed with advanced cancer than others.
The role of surgery in MBC is still controversial, with differing outcomes between limited randomized trials and retrospective studies. Our study showed higher overall and cancer specific survival in the surgical group through multivariate analysis and mortality 1000 person-years. In general, the mortality rate in the refusal group was higher than that in the operation group. Even after adjusting for age, race, clinical, and therapeutic characteristics, these differences still exist. In this study, we found that adjustment for age, race, clinical, and treatment factors attenuated HRs associated with refusal of surgery for total mortality by 86.1%, which may support our hypothesis. A research performed in 2016 demonstrated that patients who received partial or total mastectomy experienced a reduced risk of death of approximately 81% compared with patients who did not receive surgery.[22] However, results on stage-specific survival have been inconsistent. As expected, patients with lower AJCC stage had better outcomes with surgery in multivariate analysis. We have successfully constructed the nomogram to predict the 3-year and 5-year OS of MBC with stage II and III, which was confirmed by the favorable discrimination and calibration in both training and validation cohort. Moreover, nomograms have been validated with a superior predictive capacity than the classic TNM staging classification in certain types of malignancies.[23] The proposed nomogram further confirms our results. Although metastatic breast cancer is still considered an incurable disease, the survival rate of metastatic breast cancer has increased in the past decades.[24] However, we did not find that refusal of surgery had a significant poor effect on the prognosis of patients in stage IV patients with MBC. In patients with stage IV breast cancer, some studies have reported that primary tumor removal could improve the outcomes,[25–27] but others have suggested that surgery provides no evidence of improvement for overall survival.[28] Although there is no difference in survival in some study, locoregional control can be improved with surgery.[29] Another SEER based study revealed that local surgery for patients with bone-only metastasis offers a significant survival advantage over non-operative management, whereas the opposite effect is observed among simultaneous liver and lung metastasis patients.[30] Whether the survival benefits of surgery vary according to the mode of metastasis in male breast cancer remains to be further verified.
Breast conserving therapy (BCT) is getting popular for MBC treatment recently, although modified radical mastectomy (MRM) is the surgical gold standard of MBC (approximately 70% of all cases).[31] According to the literature on the treatment of MBC, MRM is generally preferred to BCT.[32] However, previous researches indicated that the OS after mastectomy was not inferior to lumpectomy.[33,34] Meanwhile, the present data support the superiority or BCT with postoperative radiotherapy over mastectomy without radiotherapy.[35] Hartmann-Johnsen et al found that there is a survival benefit of BCT compared with mastectomy in stage T1N1M0, but no other early stages of breast cancer.[36] In addition, mutant alleles or structural variations in genes are presumed to be important, whereas variants that drive cancers are not unique.[37] So divergent variants in the same gene could produce tumors with different characteristics and prognosis.[38] For the association between type of surgery and survival may vary depending on cancer stage, hormone receptor status and divergent variants in gene, this aspect in the field of male breast cancer still needs an advanced research.
This study had some limitations. First, such databases may be associated with miscoding and missing information. Due to the absence of information on chemotherapy, targeted therapy, and Ki-67 status in the SEER database, their effects on survival could not be evaluated. And the database does not provide any information regarding family history of breast cancers, nor any information on genetic testing results. Also, SEER does not provide information on comorbidities. The presence of comorbidities may negatively affect survival and may also be a reason for these patients to refuse surgery. Second, this study is a non-randomized study and the sample size is relatively small, so intrinsic defects exist. Finally, although we included marital status, other socioeconomic variables might affect accessibility and compliance to care that we could not account for in our analyses.
Despite these potential limitations, this study demonstrated that refusal of cancer-directed surgery is a risk factor for survival in MBC patients. Compared with the patients undergoing surgery, the patients who refused the cancer-directed surgery had poor prognosis in the total survival period, particularly in stage II and III. The survival benefit for undergoing surgery remained even after adjustment, which indicates the importance of surgical treatment before advanced stage for MBC patients.
Author contributions
Conceptualization: Sichao Chen.
Data curation: Shipei Wang, Sichao Chen.
Formal analysis: Shipei Wang, Yihui Huang, Wen Zeng.
Funding acquisition: Di Hu, Ling Zhou.
Investigation: Wei Wei, Min Wang.
Methodology: Wei Zhou, Min Wang.
Project administration: Yihui Huang, Di Hu, Wei Zhou, Chao Zhang.
Resources: Wen Zeng, Zeming Liu.
Software: Shipei Wang, Di Hu.
Supervision: Ling Zhou, Haifeng Feng, Liang Guo.
Validation: Danyang Chen, Haifeng Feng.
Visualization: Danyang Chen, Chao Zhang.
Writing – original draft: Shipei Wang, Zeming Liu.
Writing – review & editing: Zeming Liu, Liang Guo.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BCT = breast conserving therapy, CI = confidence interval, CSS = cancer specific survival, HR = hazard ratio, MBC = male breast cancer, MRM = modified radical mastectomy, OS = overall survival, SEER = the Surveillance, Epidemiology, and End Results Program.
How to cite this article: Wang S, Chen S, Huang Y, Hu D, Zeng W, Zhou L, Zhou W, Chen D, Feng H, Wei W, Zhang C, Liu Z, Wang M, Guo L. Refusal of cancer-directed surgery in male breast cancer. Medicine. 2021;100:13(e25116).
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental digital content is available for this article.
References
- [1].Liu L, Chi YY, Wang AA, et al. Marital status and survival of patients with hormone receptor-positive male breast cancer: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Med Sci Monit 2018;24:3425–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Younas A, Sundus A, Inayat S. Transitional experience of men with breast cancer from diagnosis to survivorship: an integrative review. Eur J Oncol Nurs 2019;42:141–52. [DOI] [PubMed] [Google Scholar]
- [3].Leon-Ferre RA, Giridhar KV, Hieken TJ, et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer Metastasis Rev 2018;37:599–614. [DOI] [PubMed] [Google Scholar]
- [4].Gucalp A, Traina TA, Eisner JR, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat 2019;173:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol 2018;29:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gargiulo P, Pensabene M, Milano M, et al. Long-term survival and BRCA status in male breast cancer: a retrospective single-center analysis. BMC Cancer 2016;16:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gold M, Dunn LB, Phoenix B, et al. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur J Oncol Nurs 2016;20:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gaitanidis A, Alevizakos M, Tsalikidis C, et al. Refusal of cancer-directed surgery by breast cancer patients: risk factors and survival outcomes. Clin Breast Cancer 2018;18:e469–76. [DOI] [PubMed] [Google Scholar]
- [9].Xiong Y, Cao H, Zhang Y, et al. Nomogram-predicted survival of breast cancer brain metastasis: a SEER-based population study. World Neurosurg 2019;128:e823–34. [DOI] [PubMed] [Google Scholar]
- [10].Liu N, Johnson KJ, Ma CX. Male breast cancer: an updated surveillance, epidemiology, and end results data analysis. Clin Breast Cancer 2018;18:e997–1002. [DOI] [PubMed] [Google Scholar]
- [11].Restrepo DJ, Sisti A, Boczar D, et al. Characteristics of breast cancer patients who refuse surgery. Anticancer Res 2019;39:4941–5. [DOI] [PubMed] [Google Scholar]
- [12].de Glas N, Bastiaannet E, de Boer A, et al. Improved survival of older patients with advanced breast cancer due to an increase in systemic treatments: a population-based study. Breast Cancer Res Treat 2019;178:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Richards P, Ward S, Morgan J, et al. The use of surgery in the treatment of ER+ early stage breast cancer in England: Variation by time, age and patient characteristics. Eur J Surg Oncol 2016;42:489–96. [DOI] [PubMed] [Google Scholar]
- [14].Ferrigni E, Bergom C, Yin Z, et al. Breast cancer in women aged 80 years or older: an analysis of treatment patterns and disease outcomes. Clin Breast Cancer 2019;19:157–64. [DOI] [PubMed] [Google Scholar]
- [15].Pan H, Zhang K, Wang M, et al. Palliative local surgery for locally advanced breast cancer depending on hormone receptor status in elderly patients. Clin Breast Cancer 2019;19:e247–60. [DOI] [PubMed] [Google Scholar]
- [16].Exman P, Burstein HJ. How old is too old? Breast cancer treatment in octogenarians. Ann Surg Oncol 2018;25:1458–60. [DOI] [PubMed] [Google Scholar]
- [17].Deng Y, Zhou L, Li N, et al. Impact of four lncRNA polymorphisms (rs2151280, rs7763881, rs1136410, and rs3787016) on glioma risk and prognosis: a case-control study. Mol Carcinog 2019;58:2218–29. [DOI] [PubMed] [Google Scholar]
- [18].Zhou L, Dong S, Deng Y, et al. GOLGA7 rs11337, a polymorphism at the MicroRNA binding site, is associated with glioma prognosis. Mol Ther Nucleic Acids 2019;18:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Adekolujo OS, Tadisina S, Koduru U, et al. Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. Am J Mens Health 2017;11:1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Neamtiu L, Deandrea S, Pylkkanen L, et al. Psycho-oncological support for breast cancer patients: a brief overview of breast cancer services certification schemes and national health policies in Europe. Breast 2016;29:178–80. [DOI] [PubMed] [Google Scholar]
- [21].Matsui T, Tanimukai H. The use of psychosocial support services among Japanese breast cancer survivors. Jpn J Clin Oncol 2017;47:743–8. [DOI] [PubMed] [Google Scholar]
- [22].Leone JP, Zwenger AO, Iturbe J, et al. Prognostic factors in male breast cancer: a population-based study. Breast Cancer Res Treat 2016;156:539–48. [DOI] [PubMed] [Google Scholar]
- [23].Cao J, Yuan P, Wang L, et al. Clinical nomogram for predicting survival of esophageal cancer patients after esophagectomy. Sci Rep 2016;6:26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [25].Thomas A, Khan SA, Chrischilles EA, et al. Initial surgery and survival in stage IV breast cancer in the United States, 1988-2011. JAMA Surg 2016;151:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Quinn EM, Kealy R, O’Meara S, et al. Is there a role for locoregional surgery in stage IV breast cancer? Breast 2015;24:32–7. [DOI] [PubMed] [Google Scholar]
- [27].Teshome M. Role of operative management in stage IV breast cancer. Surg Clin North Am 2018;98:859–68. [DOI] [PubMed] [Google Scholar]
- [28].Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380–8. [DOI] [PubMed] [Google Scholar]
- [29].Weiss A, Menen RS, Lin HY, et al. Factors associated with improved outcomes for metastatic inflammatory breast cancer patients. Breast Cancer Res Treat 2018;169:615–23. [DOI] [PubMed] [Google Scholar]
- [30].Wang K, Shi Y, Li ZY, et al. Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: a real-world observational study. Eur J Surg Oncol 2019;45:1364–72. [DOI] [PubMed] [Google Scholar]
- [31].Zaenger D, Rabatic BM, Dasher B, et al. Is breast conserving therapy a safe modality for early-stage male breast cancer? Clin Breast Cancer 2016;16:101–4. [DOI] [PubMed] [Google Scholar]
- [32].Fogh S, Kachnic LA, Goldberg SI, et al. Localized therapy for male breast cancer: functional advantages with comparable outcomes using breast conservation. Clin Breast Cancer 2013;13:344–9. [DOI] [PubMed] [Google Scholar]
- [33].Landercasper J, Ramirez LD, Borgert AJ, et al. A reappraisal of the comparative effectiveness of lumpectomy vs mastectomy on breast cancer survival: a propensity score-matched update from the National Cancer Data Base (NCDB). Clin Breast Cancer 2019;19:e481–93. [DOI] [PubMed] [Google Scholar]
- [34].Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (</ = 40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery vs mastectomy. Breast 2015;24:175–81. [DOI] [PubMed] [Google Scholar]
- [35].de Boniface J, Frisell J, Bergkvist L, et al. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg 2018;105:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hartmann-Johnsen OJ, Karesen R, Schlichting E, et al. Better survival after breast-conserving therapy compared to mastectomy when axillary node status is positive in early-stage breast cancer: a registry-based follow-up study of 6387 Norwegian women participating in screening, primarily operated between 1998 and 2009. World J Surg Oncol 2017;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deng Y, Zhou L, Yao J, et al. Associations of lncRNA H19 polymorphisms at MicroRNA binding sites with glioma susceptibility and prognosis. Mol Ther Nucleic Acids 2020;20:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].DeWeerdt S. The genomics of brain cancer. Nature 2018;561:S54–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


