Abstract
Objectives:
To assess differences in cognitive outcomes and sleep in adult survivors of critical illness, managed with venovenous extracorporeal membrane oxygenation as compared to conventional mechanical ventilation only.
Design:
Retrospective cohort study linked with data from the COGnitive outcomes and WELLness study.
Setting:
A multisite study from five adult medical/surgical ICUs in Toronto.
Patients:
Thirty-three ICU survivors including adult patients who received venovenous extracorporeal membrane oxygenation (n = 11) matched with patients who received mechanical ventilation only (n = 22) using specified covariates (e.g., age).
Interventions:
None.
Measurements and Main Results:
Baseline demographics and admission diagnoses were collected at enrollment. Cognitive outcome was evaluated using the Repeatable Battery for the Assessment of Neuropsychologic Status (global cognitive function) and Trail Making Test B (executive function), and sleep variables were estimated using actigraphy. Assessments occurred at 7 days post ICU discharge and again at 6- and 12-month follow-up. No statistically significant difference was seen between patients treated with or without venovenous extracorporeal membrane oxygenation in the mean daily Riker Sedation Agitation Score; however, patients in the venovenous extracorporeal membrane oxygenation group received greater amounts of fentanyl over their ICU stay as compared to patients receiving conventional mechanical ventilation only (p < 0.001). No significant differences were found in performance on either of the cognitive assessment tools, between survivors treated or not with venovenous extracorporeal membrane oxygenation at any of the time points assessed. Total sleep time estimated by actigraphy increased from approximately 6.5 hours in hospital to 7.5 hours at 6-month follow-up in all patients, regardless of treatment type. Total sleep time remained consistent in both groups from 6 to 12 months post ICU discharge.
Conclusions:
In this small retrospective case series, no significant differences were found in sleep or cognitive outcomes between extracorporeal life support and non–extracorporeal life support survivors. Further, in this hypothesis-generating study, differences in administered sedative doses during the ICU stay seen between the two groups did not impact 6- or 12-month cognitive performance or actigraphy-estimated sleep time.
Keywords: actigraphy, cognition, critical care outcomes, extracorporeal membrane oxygenation
Survivors of critical illness experience decrements in physical and cognitive function after critical illness, significantly impacting their quality of life (1–3). Extracorporeal life support (ECLS) is considered a live-saving measure for the most severe cases of acute respiratory failure (ARF) when conventional mechanical ventilation (CMV) cannot maintain adequate oxygenation or eliminate carbon dioxide safely. Although ECLS is expensive and resource intensive, remaining only available at expert centers (4), venovenous extracorporeal membrane oxygenation (VV-ECMO) treatment for ARF has increased dramatically (5). Although hospital survival is improving with VV-ECMO administration, patient-centered long-term outcomes after ECLS in general and VV-ECMO in particular remain poorly described.
The preservation of cognitive function after critical illness is an established priority for survivors (6). Two previous studies have investigated cognitive function after ECLS. In a study by Sylvestre et al (7), 22 patients with severe acute respiratory distress syndrome treated with VV-ECMO had similar cognitive and neuropsychologic outcomes at 2 years follow-up, as compared to 18 patients who were not treated with VV-ECMO. Another study found that 10 patients treated with ECLS had normal memory function based on population age-adjusted means at 9 years after discharge; global cognitive function could not be assessed in all patients as baseline formal education status was unknown (8). Existing evidence would suggest that specific characteristics of the patients who receive ECLS, technical specificities of the devices used, as well as physiologic changes that occur during ECLS, likely modify the risk of short- and long-term cognitive dysfunction. These variables include rapid and efficient changes in Paco2 and Pao2, differential use, and pharmacokinetics of sedative agents (e.g., higher circulating volume, increased clearance, sequestration by the circuit) (9, 10).Additionally, heightened risk of bleeding and cerebral complications in ECLS may increase the likelihood of short- and long-term cognitive impairment (11). Further, evidence would suggest that disrupted sleep, common in critical illness, is associated with poor health and cognitive impairment (12). To our knowledge, sleep has yet to be studied in an ECLS patient population.
Given the limited investigations into short-term cognitive outcomes in critically ill patients with ARF treated with VV-ECMO, we aimed to compare prevalence of cognitive impairment over the trajectory of recovery after ICU against similar patients treated with CMV only. We hypothesized that VV-ECMO does not worsen short- or long-term cognitive outcomes in patients with ARF.
METHODS
This small case series of adult patients was part of the COGnitive outcomes and WELLness (COGWELL) study, a multisite study of adult patients from five adult medical/surgical ICUs who received mechanical ventilation for at least 72 hours (13). COGWELL was approved by the local Research Ethics Board of each participating hospital: University Health Network Research Ethics Committee (13-6425-BE), Sunnybrook Health Centre Research Ethics Committee (365-2013), Mount Sinai Research Ethics Committee (14-0194-E), and St. Michael’s Hospital Research Ethics Committee (14-295). Exclusion criteria included the following: significant preadmission cognitive impairment, primary neurologic injury, known sleep disorder, anticipated death within 3 months of discharge, nonfluency in English, or unlikeliness to adhere with follow-up. Eleven patients in the dataset (n = 99 survivors) received ECLS.
Subjects
Patients who received ECLS were retrospectively matched with two patients who received CMV using available covariates: age (± 5 yr) and Pao2/Fio2 ratio at the time of intubation. Two patients died between hospital discharge and 12-month follow-up in the ECLS group, and three patients withdrew or were lost to follow-up over the course of the study. In the CMV group, two patients died, three withdrew, and six patients in total were lost to follow-up (Fig. 1).
Figure 1.
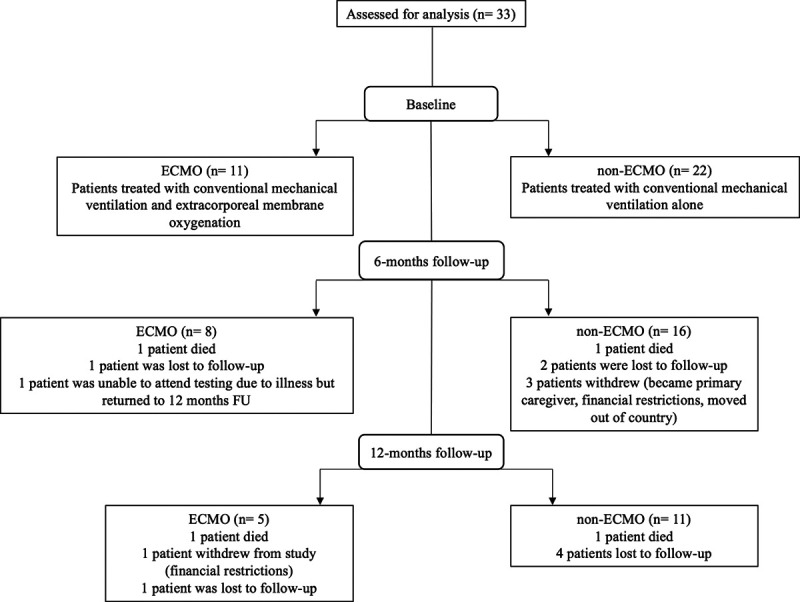
Consort diagram for patients included in analyses. ECMO = extracorporeal membrane oxygenation.
Study Protocol
At enrollment, baseline demographics and admission diagnosis were collected. Differences in Paco2 and Pao2 were assessed between intubation (and start of ECLS, if applicable) and 6 hours, as well as 24 hours after intubation. Daily minimum and maximum values of respiratory, blood, renal, and neurologic variables were collected for a maximum of 14 days. Total amounts of sedative or opioids administered were also collected for the same duration of ICU stay.
Cognitive and Sleep Measures
Outcome measures were assessed at three time points: 7 days after ICU discharge and again at 6 and 12 months after ICU discharge. During each visit, the Repeatable Battery for the Assessment of Neuropsychologic Status (RBANS) and Trail Making Test B (TMT B) were used to assess cognitive status. The RBANS is a brief but comprehensive and validated test battery for the evaluation of cognition that assesses a wide range of cognitive domains. The population age-adjusted mean (± sd) for the RBANS global cognition score and for individual domains is 100 ± 15 (on a scale ranging from 40 to 160, with lower scores indicating worse performance). Executive function (specifically, cognitive flexibility) and attention were assessed using the TMT B; age-, sex-, and education-adjusted mean T-score is 50 (range 0–100), with lower score indicating poor performance. Actigraphs were attached to each patient’s nondominant wrist starting at the time of each outcome assessment for approximately 10 days to assess sleep. Total sleep time (TST: mean time spent in epochs inferred as sleep by the Cole-Kripke algorithm per 24 hr period) and average activity during the most active 10-hour period were measured using the Actiwatch Plus, a wrist-watch-like microelectromechanical system–based accelerometer sampling at 32 Hz (Spectrum; Phillips Respironics, Murrysville, PA). Differences in clinical characteristics including sedative exposure, respiratory data, and sleep variables estimated by actigraphy were explored as possible confounders.
Statistics
Numbers are generally expressed as mean and sd. Group differences were assessed using Student t test and chi-square test. Analyses were performed on cognitive and actigraphy variables at each time point. All analyses were performed using R (Foundation for Statistical Computing, Vienna, Austria, 2019). p values of less than 0.05 were considered to be of statistical significance.
RESULTS
Demographic and ventilation data are displayed in Table 1. ECLS patients (n = 11) tended to have a longer average ICU length of stay as compared to the CMV group (23 vs 13 d; p = 0.06). There were however no statistically significant differences in baseline characteristics. It is noted that all ECLS patients were admitted with respiratory failure, whereas admission diagnoses for CMV varied between patients (p = 0.03). Mean minimum mean arterial pressure was found to be marginally lower in the ECLS group than CMV group over course of ICU stay (66.4 vs 71.8; p < 0.001), and mean maximum glycemic control was higher in the ECLS group than CMV group (11.2 vs 10.2; p = 0.008). Patients in the ECLS group received higher mean amounts of fentanyl as compared to the CMV group (1,790 vs 944 µg; p < 0.001). No significant differences were found in the remaining variables.
TABLE 1.
Demographic and Ventilation Data
| Demographic and Baseline Characteristics | ECMO (n = 11) | Non-ECMO (n = 22) | p | |
|---|---|---|---|---|
| Age, mean (sd) | 47 (11) | 52 (16) | 0.9 | |
| Male sex, n (%) | 7 (65) | 12 (55) | 0.75 | |
| Education level, n (%) | ||||
| Less than grade 12 | 0 (0) | 1 (5) | — | |
| High school | 5 (63) | 8 (37) | — | |
| College or university | 3 (37) | 10 (46) | — | |
| Postgraduate studies | 0 (0) | 1 (5) | — | |
| Admission diagnosis, n (%) | ||||
| Respiratory | 11 (100) | 9 (40) | 0.03 | |
| Sepsis | 0 (0) | 3 (14) | — | |
| Neurologic | 0 (0) | 3 (14) | — | |
| Gastrointestinal | 0 (0) | 4 (18) | — | |
| Other medical diseases | 0 (0) | 2 (9) | — | |
| Vascular/cardiovascular | 0 (0) | 1 (5) | — | |
| Postoperative admission, n (%) | 7 (64) | 9 (40) | 0.24 | |
| Acute Physiology And Chronic Health Evaluation III, mean (sd) | 57 (17) | 64 (28) | 0.53 | |
| Days in ICU, mean (sd) | 23 (15) | 13 (9) | 0.06 | |
| Mean arterial pressure, mean (sd) | ||||
| Maximum | 96 (8) | 99 (14) | 0.10 | |
| Minimum | 66 (3) | 72 (12) | < 0.001 | |
| Glycemic control, mean (sd) | ||||
| Maximum | 11 (3) | 10 (4) | 0.008 | |
| Minimum | 7 (1) | 7 (2) | 0.08 | |
| Days of continuous sedation, mean (sd) | 8 (6) | 6 (5) | 0.34 | |
| Sedative medications, mean (sd) | ||||
| Lorazepam/midazolam (mg) | 29 (91) | 20 (93) | 0.56 | |
| Propofol (mg) | 798 (1,763) | 554 (583) | 0.13 | |
| Fentanyl (µg) | 1,790 (2,293) | 944 (1,636) | < 0.001 | |
| Dexmedetomidine (mg) | 0.5 (3.4) | 1.5 (6.4) | 0.05 | |
| Maximum SAS, mean (sd) | 3.5 (0.4) | 3.7 (0.5) | 0.46 | |
| Minimum SAS, mean (sd) | 2.5 (0.5) | 2.6 (0.4) | 0.51 | |
| Ventilation data, mean (sd) | ||||
| ΔPco2a—6 hr ICU | 6.7 (4.2) | 7.9 (7.3) | 0.57 | |
| ΔPo2b—6 hr ICU | 70.9 (57.4) | 73.4 (80.5) | 0.92 | |
| ΔPco2—24 hr ICU | 8.5 (7.3) | 10 (9.9) | 0.62 | |
| ΔPo2—24 hr ICU | 84.9 (78.3) | 81.6 (81) | 0.91 | |
| P/Fc | 162 (108.2) | 246.7 (162) | 0.88 | |
| ΔPco2—6 hr ECMO | 8.1 (5.1) | — | — | |
| ΔPo2—6 hr ECMO | 53.9 (57.1) | — | — | |
| P/F—ECMO start | 148 (102.8) | — | — | |
| ECMO data, mean (sd) | Minimum | Maximum | ||
| Flow (L/min) | 2.8 (1.3) | 4.0 (1.3) | — | — |
| Gas sweep (L/min) | 3.0 (1.2) | 3.4 (1.3) | — | — |
| Fraction of sweep gas O2 | 0.96 (0.1) | 0.98 (0.2) | — | — |
| Days on ECMO, median (range) | 9 (3–30) | — | — | |
| Pre-ECMO days of mechanical ventilation, median (range) | 1.5 (0–10) | — | — | |
ECMO = extracorporeal membrane oxygenation, P/F = Pao2/Fio2, SAS = Riker Sedation-Agitation Scale.
Dash indicates not available/applicable.
aChange in Paco2 (difference in Paco2 from ICU admission or ECMO start to 6 ± 2 and 24 ± 4 hr later).
bChange in Pao2 (difference in Pao2 from ICU admission or ECMO start to 6 ± 2and 24 ± 4 hr later).
cP/F (taken after intubation).
Sleep and cognition data are displayed in Table 2. At 7 days after ICU discharge, half of ECLS survivors had cognitive impairment as compared to two thirds of non-ECLS patients. At 6 months post discharge, a smaller proportion of ECLS survivors (14%) had significant cognitive impairment than non-ECLS survivors (28%). At 12 months, no surviving ECLS patients had significant cognitive impairment, whereas 19% of survivors in the CMV group did. Both ECLS and CMV patients saw an increase in TST from 6.6 hours after ICU discharge to 7.7 hours at 12 months follow-up. No significant group differences in sleep variables were seen at any assessments.
TABLE 2.
Cognitive and Sleep Outcomes
| In-Hospital | 6 mo | 12 mo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | ECMO, n = 11 | Non-ECMO, n = 22 | p | ECMO, n = 8 | Non-ECMO, n = 16 | p | ECMO, n = 5 | Non-ECMO, n = 11 | p |
| Richard Campbell Sleep Questionnaire, mean (sd) | 47 (28.84) | 38 (30.14) | 0.51 | — | — | — | — | — | — |
| Total sleep time, hr, mean (sd) | 6.7 (1.81) | 6.3 (4.12) | 0.89 | 8.75 (1.3) | 7.61 (1.89) | 0.64 | 7.62 (1.27) | 8.47 (2.59) | 0.49 |
| Most active 10 hr period, mean (sd) | 9,398 (4,691) | 8,463 (5,011) | 0.67 | 16,033 (5,387) | 17,676 (6,379) | 0.56 | 21,871 (7,836) | 20,448 (10,010) | 0.76 |
| Pittsburgh Sleep Quality Index, mean (sd) | 2.38 (0.74) | 2.67 (0.93) | 0.56 | 1.71 (0.49) | 2.27 (0.99) | 0.10 | 2 (0) | 2.2 (1) | 0.59 |
| RBANS, mean (sd) | 83.8 (7.6) | 80 (16.46) | 0.44 | 94.9 (14.3) | 90.1 (18.3) | 0.52 | 98.2 (5.4) | 90.2 (16) | 0.17 |
| Immediate memory | 82.5 (9.0) | 74.5 (21.1) | 0.18 | 91.7 (20.7) | 84.6 (16.2) | 0.44 | 96.8 (6.0) | 85.4 (15.1) | 0.06 |
| Visuospatial | 95.1 (14.4) | 84.7 (29.3) | 0.23 | 94.1 (7.8) | 100.4 (18.9) | 0.30 | 99.6 (14.2) | 100.8 (21.2) | 0.90 |
| Language | 90.3 (9.8) | 86.8 (19.1) | 0.54 | 98.7 (14.5) | 89.4 (9.3) | 0.15 | 94.8 (4.2) | 88.1 (10.5) | 0.10 |
| Attention | 87.4 (15.8) | 79 (26.2) | 0.32 | 99.6 (8.7) | 97.4 (22) | 0.74 | 103.2 (13.3) | 100.37 (20.1) | 0.75 |
| Delayed memory | 84.5 (15.8) | 78.4 (19.9) | 0.41 | 96.57 (16.5) | 89.47 (21.3) | 0.41 | 96.57 (5.9) | 93.36 (18.6) | 0.23 |
| RBANS, n (%) impaireda | 4 (50) | 12 (55) | 0.66 | 1 (14) | 6 (28) | 0.20 | 0 (0) | 4 (19) | 0.04 |
| Telephone interview for cognitive status, mean (sd) | 31.81 (3.4) | 31.58 (3.9) | 0.88 | 33.86 (2.5) | 34 (3.4) | 0.91 | 34.17 (2.6) | 34.7 (4.2) | 0.75 |
| Trails B, s, mean (sd) | 107.7 (29.5) | 179.3 (128.4) | 0.05 | 75.57 (23.1) | 95.74 (43) | 0.18 | 57.8 (16.1) | 73.8 (35.7) | 0.27 |
| Beck’s Depression Inventory-II, mean (sd) | 13.3 (12.8) | 14.3 (8.9) | 0.88 | 5.7 (4.4) | 8 (7.7) | 0.45 | 8.5 (4.2) | 10 (3.4) | 0.55 |
ECMO = extracorporeal membrane oxygenation, RBANS = Repeatable Battery for the Assessment of Neuropsychologic Status.
Dash indicates not available/applicable.
aRBANS score ≥ 1.5 sd below population mean on at least two RBANS domains or ≥ 2 sds below the population mean on any one domain.
DISCUSSION
We describe the presence of cognitive and sleep dysfunction in 33 patients who were ventilated in ICU, one-third of whom received ECLS. No significant differences were found in sleep or cognitive outcomes between ECLS and non-ECLS survivors. Similar to previous studies of general medical-surgical ICU survivors, significant cognitive impairment was seen in a young group of patients, primarily in the domains of memory and attention (1). The trajectory of cognitive recovery was similar to that of other cohort studies where the majority of cognitive gains occurred between hospital discharge and 6 months, whereas little gains were seen in testing between 6 and 12 months (1). Further, our sleep findings are similar to those reported by Delaney et al (14), where both medical and surgical patients admitted to the wards experienced a mean reduction of approximately 2 hours sleep (TST of approximately 6 hr) while in hospital.
Given the rapidly increasing utilization and availability of ECMO in critical care, the need to better elucidate potential short- and long-term outcomes is increasingly relevant. The results of the present study suggest that VV-ECMO does not worsen short- or long-term sleep or cognitive outcomes in survivors. Our results are similar to those found by Sylvestre et al (7), another small comparator study of VV-ECMO patients (n = 22) as compared to conventional mechanically ventilated patients. Our study however is novel as it follows patients from ICU discharge to 12-month follow-up, providing a description of trajectory as opposed to a single cross-sectional measurement. Although limited by sample size, it would seem that ECLS patients experienced less impairment overall or perhaps a more expeditious cognitive recovery after critical illness as compared to CMV patients. This finding may be secondary to differences in group composition; the ECLS group tended to be younger, have a lower severity of illness, and all had respiratory failure as admitting diagnosis. Although it was hypothesized that cognitive and sleep variables may have been influenced by differences in sedative use, no differences in administered sedative doses or measured sedation levels were observed between the two groups. The present data do not suggest that sedative drugs mediate the risk of cognitive or sleep impairments in ECLS recipients; however, findings are limited by small sample size and lack of a uniform sedation protocol used for all patients.
One of the strengths of the present study is the comprehensive in-person evaluation of cognitive function over a number of time points, a design that strengthens associations of causality. The comparative design of the present study is a further strength; few studies in this population include a control group. The study is, however, limited by the absence of randomization, although observational studies are often more feasible with critically ill patients. Further, the small sample size of this study was small and heterogenous, increasing the risk of type II error and limiting the strength of any conclusions. A large-scale prospective investigation is therefore necessary to validate and contextualize the present findings. Further, exploring how cognitive outcomes may be mediated by sleep and other physiologic factors (e.g., sedation clearance) in other patient populations may help better elucidate mechanisms of persistent morbidity in survivors.
In this small case series, patients in receipt of ECLS for acute respiratory failure experienced no significant differences in sleep or cognition at 6 or 12 months after critical illness when compared with non-ECLS ICU survivors. Further study is needed to determine the impact of ECLS on other outcomes for survivors of acute respiratory failure. In these patients, survival cannot be considered as the only criterion to assess the usefulness of ECLS, and gathering more data on long-term outcomes, such as cognition, is a research priority.
Footnotes
Supported, in part, by Physician Services Incorporated grant number 13-42-COGWELL.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013; 369:1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilcox ME, Herridge MS. Long-term outcomes in patients surviving acute respiratory distress syndrome. Semin Respir Crit Care Med. 2010; 31:55–65 [DOI] [PubMed] [Google Scholar]
- 3.Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: A systematic review. Ann Am Thorac Soc. 2017; 14:1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roch A, Hraiech S, Masson E, et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med. 2014; 40:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Extracorporeal Life Support Organization. ECLS Registry Report: International Summary [Internet] 2020Available at https://www.elso.org/Registry/Statistics/InternationalSummary.aspx. Accessed June 22, 2020
- 6.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002; 346:1061–1066 [DOI] [PubMed] [Google Scholar]
- 7.Sylvestre A, Adda M, Maltese F, et al. Long-term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients. Ann Intensive Care. 2019; 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Bahr V, Hultman J, Eksborg S, et al. Long-term survival in adults treated with extracorporeal membrane oxygenation for respiratory failure and sepsis. Crit Care Med. 2017; 45:164–170 [DOI] [PubMed] [Google Scholar]
- 9.DeGrado JR, Hohlfelder B, Ritchie BM, et al. Evaluation of sedatives, analgesics, and neuromuscular blocking agents in adults receiving extracorporeal membrane oxygenation. J Crit Care. 2017; 37:1–6 [DOI] [PubMed] [Google Scholar]
- 10.Shah AG, Peahota M, Thoma BN, et al. Medication complications in extracorporeal membrane oxygenation. Crit Care Clin. 2017; 33:897–920 [DOI] [PubMed] [Google Scholar]
- 11.Risnes I, Wagner K, Nome T, et al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2006; 81:1401–1406 [DOI] [PubMed] [Google Scholar]
- 12.Altman MT, Knauert MP, Murphy TE, et al. Association of intensive care unit delirium with sleep disturbance and functional disability after critical illness: An observational cohort study. Ann Intensive Care. 2018; 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox ME, Lim AS, McAndrews MP, et al. A study protocol for an observational cohort investigating COGnitive outcomes and WELLness in survivors of critical illness: The COGWELL study. BMJ Open. 2017; 7:e015600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaney LJ, Currie MJ, Huang HC, et al. “They can rest at home”: An observational study of patients’ quality of sleep in an Australian hospital. BMC Health Serv Res. 2018; 18:524. [DOI] [PMC free article] [PubMed] [Google Scholar]


