Abstract
Background:
We aimed to assess the efficacy of resistance exercise in rheumatoid arthritis (RA) in randomized controlled trials (RCTs).
Method:
PubMed, the Cochrane Library, and Embase were searched according to the index words to identify eligible RCTs, and relevant literature sources were also searched. The latest search was done in August 2019. Odds ratios (OR), mean difference (MD), and 95% confidence interval (95% CI) were used to analyze the main outcomes.
Result:
Seventeen RCTs were included in the meta-analysis with 512 patients in the resistance exercise group and 498 patients in the control group. The results showed that compared with the control group, resistance exercise significantly decreased disease activity score in 28 joints (DAS-28) scores (standard mean difference [SMD]: –0.69, 95% CI: –1.26 to –0.11), reduced erythrocyte sedimentation rate (ESR) (SMD: –0.86, 95% CI: –1.65 to –0.07), and shortened the time of 50 ft. walking (SMD: –0.64, 95% CI: –0.99 to –0.28). No significant difference was observed in visual analog scale (VAS) scores (SMD: –0.61, 95% CI: –1.49–0.27) and health assessment questionnaire (HAQ) scores (weighted mean difference: –0.10, 95% CI: –0.26–0.06).
Conclusion:
Resistance exercise showed reducing DAS-28 score, ESR score, and the time of 50 ft. walking in RA patients compared with the control group. However, high quality multicenter RCTs with larger sample sizes to confirm the conclusion.
Keywords: meta-analysis, randomized controlled trial, resistance exercise, rheumatoid arthritis
1. Introduction
Despite the remarkable impact of pharmaceutical interventions, physical therapy and exercise training remain an important part of rheumatoid arthritis (RA) management.[1,2] Moreover, given that cardiovascular events are an important issue in RA outcomes, improving cardiovascular risk[3] through aerobic exercise seems to be the most relevant ancillary therapy in RA management.[4] Indeed, aerobic exercises have been shown to improve cardiovascular fitness and the quality of life of patients, while reducing RA-associated disability and pain.[5] However, the use of resistance exercise therapy for RA patients is still controversial because its effects on cardiovascular risk are still a concern.[6] Although some studies have shown a statistically significant effect on RA disability,[7–9] other studies have suggested that this improvement is not statistically significant[10] or clinically relevant.[11] Similarly, discrepancies were observed between studies reporting a positive effect of exercise on functional capacity[11] versus others that did not find such a positive effect.[8,10] These disparities are likely due to sample size variations and the fact that most of the studies on resistance exercises only addressed changes in muscle strength. In fact, few studies have addressed the efficacy of resistance exercise-based therapy for RA patients with respect to pain, disease activity, functional capacity, quality of life, and structural damage; thus, the effects of this therapy remain unclear. Therefore, we conducted a systematic review of the literature to determine whether resistance exercise effectively improved the above-mentioned parameters of RA. Finally, we assessed whether this treatment addition is clinically relevant, and evaluated its dependence on exercise modalities and/or patient characteristics.
Based on these considerations, the aim of this study was to perform a meta-analysis of all available literature to obtain updated evidence on the efficacy of resistance exercise for women with RA.
2. Methods
2.1. Search strategy
The Cochrane Library, PubMed, and Embase were searched for all randomized control trials (RCTs) on the efficacy of resistance exercise in the treatment of RA. Other related articles and reference materials were also searched. The latest research was performed on August 2019. Two investigators carried out the literature search independently; a third investigator was involved when disagreement occurred.
2.2. Inclusion and exclusion criteria
A study was included if it was: a RCT; the research subjects were patients with RA and did not have other serious diseases; the intervention of the treatment group was resistance exercise, repetition training aimed to improve muscle strength, and rehabilitation exercise was initiated at the discretion of the rehabilitation specialist; the interventions of the control group were general nursing, non-aerobic exercise, or range of motion exercise; only articles published in English were included.
A study was excluded if it was: duplicate publication, or the content and result were the same; data had obvious mistake; case report, theoretical research, conference report, systematic review, meta-analysis, expert comment, or economic analysis; the outcomes were not what we need; postoperative rehabilitation training.
All the studies were screened by 2 reviewers independently to determine whether an article satisfied the inclusion and exclusion criteria, and discrepancies were resolved by a third reviewer.
2.3. Data extraction and quality assessment
The data were extracted from all the included studies and consisted of 2 parts: baseline information and primary outcomes. The first part was baseline information: author name, publication year, the interventions of the treatment group and the control group, sample size, treatment, main age, sex, and Jadad score. The second part was clinical outcomes: disease activity score in 28 joints (DAS-28), the erythrocyte sedimentation rate (ESR), visual analog scale (VAS) score, the health assessment questionnaire (HAQ), and 50 ft. walking test. The Jadad scoring checklist was used to appraise the quality of involved studies. We evaluated all the RCTs from the 5 items: statement of randomization; appropriateness of generating randomized sequence; use of double blind; description of double blinding method; detail of withdrawals and dropouts. Studies with a score of <3 represented a low-quality and high bias risks, studies gota score exceed 3 were indicated as high-quality trial. All the above process was done by 2 reviewers independently, and disagreements between the 2 reviewers were resolved by discussion until a consensus was reached.
2.4. Statistical analysis
All statistical analyses were performed using STATA 10.0 (StataCorp LLC, College Station, Texas). Chi-squared and I2 tests were used to test the heterogeneity of clinical trial results and decided the analysis model (the fixed-effects model or the random-effects model). When the Chi-squared test P-value was ≤.05 and I2 tests-value was >50%, it was defined as high heterogeneity and assessed by the random-effects model. When the Chi-squared test P-value was >.05 and I2 tests-value was ≤50%, it was defined as acceptable heterogeneity data and assessed by the fixed-effects model. Continuous variables were expressed as mean ± standard deviation and analyzed by mean difference (MD). Categorical data were presented as percentages and analyzed by relative risk (RR) or odds ratio (OR). MD along with 95% CI was used to analyze all the outcomes.
3. Results
3.1. Characteristics of the included studies
Totally 1157 articles were searched by the indexes, and 1095 articles were excluded by screening the title or abstract, leaving 62 articles for further evaluation. After obtaining and thoroughly reviewing the complete manuscripts, 45 articles did not meet the inclusion criteria because they were non-RCTs (8), they were theoretical or economic studies (14), they had no clinical outcomes (10), and the intervention was not qualified (13). At last, 17 RCTs[11–28] were involved in the meta-analysis with 512 patients in the resistance exercise group and 498 patients in the control group. The selection process is presented in Fig. 1.
Figure 1.
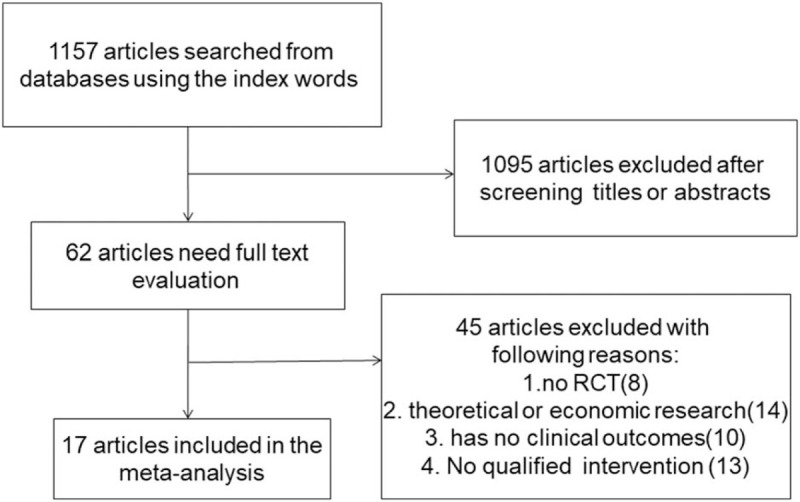
The flow diagram of the literature search and selection process.
The main characteristics of the included studies are summarized in Table 1. The basic information included treatment, country, age, and sex. The main Jadad score of the included studies was 3.83, and the main score was higher than 3, indicating high-quality of the 17 included RCTs.
Table 1.
The basic characteristics description of included studies.
| Duration of RA, y | Therapy | No. of patients | Age | Gender | ||||||||
| Study | Country | T | C | T | C | T | C | T | C | T | C | Jadad score |
| Joan Mcmeeken 1999 | USA | – | – | Quadriceps strength training, maximum load 70%, 4 times/wk, 40–80 min, 24 weeks | Routine nursing | 17 | 18 | 51.4 | 49.7 | 15F | 14F | 3 |
| C.H.M. van denEnde 2000 | Netherlands | 8 | 7 | Quadriceps strength training and shoulder strength training, maximum load 60%, 3 times/wk, 15 min, 4 weeks | ROM exercise | 34 | 30 | 62 | 58 | 20F | 20F | 5 |
| Hilary G. Flint-Wagner 2009 | USA | 11.2 ± 8.9 | 15.4 ± 10.8 | Leg and arm strength training, maximum load 90%, 3 times/wk, 75 min, 16 weeks | Routine nursing | 16 | 8 | 52.2 | 49 | – | – | 4 |
| L.M.Bearne 2002 | UK | - | - | Quadriceps strength training, maximum load 100%, 2 times/wk, 30–45 min, 5 weeks | Routine nursing | 47 | 46 | – | – | – | – | 5 |
| Amir I. Buljina 2001 | Bosnia and Herzegovina | 5.04 ± 4.80 | 5.23 ± 4.89 | Grip strength training, maximum load 85%, 7 times/wk, 20–30 min, 3 weeks | Routine nursing | 50 | 50 | 47.94 | 48.46 | 38F | 37F | 3 |
| A. Haè Kkinen 1997 | Fineland | - | - | Quadriceps strength training, maximum load 40–80%, 2–3 times/wk, 24 weeks | Routine nursing | 21 | 18 | 41.4 | 45.6 | – | – | 3 |
| Arja Hakkinen 2001 | Fineland | 10 | 8 | Lumbar, leg and arm strength training, maximum load 50–70%, 2 times/wk, 30–45 min, 104 weeks | ROM exercise | 31 | 31 | 49 | 49 | 18F | 20F | 4 |
| Andrew B. Lemmey 2009 | USA | 6.17 ± 6.33 | 10.42 ± 8.42 | Leg and arm strength training, maximum load 80%, 2 times/wk, 24 weeks | ROM exercise | 13 | 15 | 55.6 | 60.6 | 11F | 12F | 4 |
| C. BostrÎm 1998 | Sweden | 10.5 (0.3–27) | 7 (3–43) | Shoulder strength training, maximum load 30%, 3 times/wk, 40–60 min, 10 weeks | Anaerobic exercise | 20 | 17 | 56 | 59 | – | – | 3 |
| Usmary S. Siqueira 2017 a | USA | 7.7 ± 2.9 | 8.5 ± 4 | Land-based aerobic group, knee, hip and lower limbr strength training, maximum load 90%, 5–30 min, 3 times/wk, 16 weeks | Routine nursing | 33 | 34 | 54 | 53.2 | - | - | 5 |
| Usmary S. Siqueira 2017 b | USA | 9.2 ± 3.1 | 8.5 ± 4 | Water-based aerobic group, knee, hip and lower limbr strength training, maximum load 90%, 5–30 min, 3 times/wk, 16 weeks | Routine nursing | 33 | 34 | 55 | 53.2 | - | - | 5 |
| Jeong-Hun Shin 2015 | Korea | 10.3 ± 9.4 | 15.4 ± 8.0 | Arm and leg strength training (Tai Chi exercise), 1 times/wk, 60 min, 3 months | Routine nursing | 29 | 14 | 64 | 62.7 | – | – | 5 |
| Susan V.Baxter 2015 | New Zealand | 16 ± 10.9 | 11 ± 11.2 | Walking programme | Routine nursing | 11 | 22 | 66.6 | 59.4 | – | – | 3 |
| Laura Durcan 2014 | Ireland | - | - | Walking programme, 30–60 min/d | Routine nursing | 40 | 38 | 61 | 59 | 30F | 20F | 5 |
| Keegan Knittle 2013 | Netherlands | - | - | - | - | 38 | 40 | 60.7 | 64.7 | 30F | 22F | 3 |
| Victoria L. Manning 2014 | UK | 1.67 ± 1.50 | 1.67 ± 1.58 | - | - | 52 | 56 | 53 | 57 | 44F | 38F | 3 |
| Antonios Stavropoulos-Kalinoglou 2012 | UK | 5.5 (3.0–9.7) | 7.0 (5.0–10.0) | Strength training, 3 times/wk, 60 min, 3–6 months | Routine nursing | 18 | 18 | 55 | 52.8 | 14F | 14F | 3 |
| Andrew B. Lemme 2012 | UK | 6.85 ± 7.34 | 7.89 ± 7.59 | - | - | 9 | 9 | 55.7 | 59.4 | 8F | 6F | 3 |
3.2. DAS-28
Eight studies with 252 patients in the resistance exercise group and 241 patients in the control group reported DAS-28 changes. Based on the Chi-squared test P-value of = .000 < .05 and I2 test-value = 88.5% > 50%, we chose the random effects model to analyze changes in DAS-28. The pooled results showed that compared with the control group, resistance exercise significantly decreased DAS-28 score (standard mean difference [SMD]: –0.69, 95% CI: –1.26 to –0.11, Fig. 2).
Figure 2.
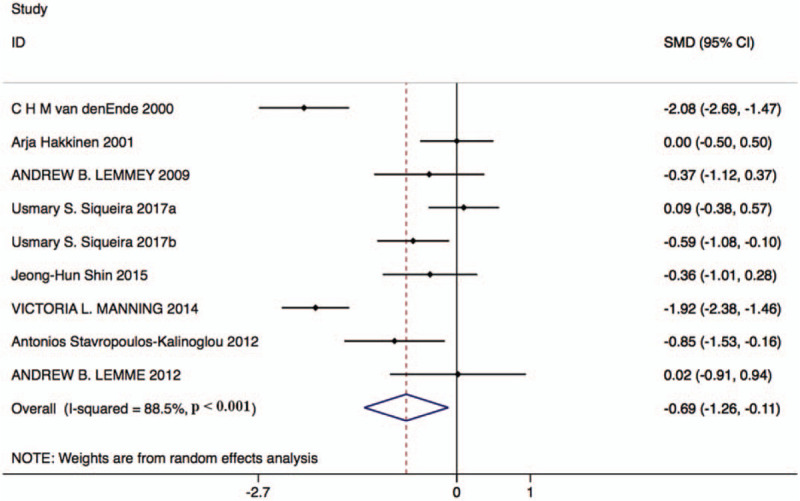
Forest plot of changes in DAS-28 in the resistance exercise group and the control group. DAS-28 = disease activity score in 28 joints.
3.3. ESR
Six studies with 178 patients in the resistance exercise group and 158 patients in the control group reported ESR changes. Based on the Chi-squared test P-value of = .000 < .05 and I2 tests-value = 90.7% > 50%, we chose the random effects model to analyze changes in ESR. The pooled results showed that the ESR score was significantly decreased by resistance exercise versus the control group (SMD: –0.86, 95% CI: –1.65 to –0.07, Fig. 3).
Figure 3.
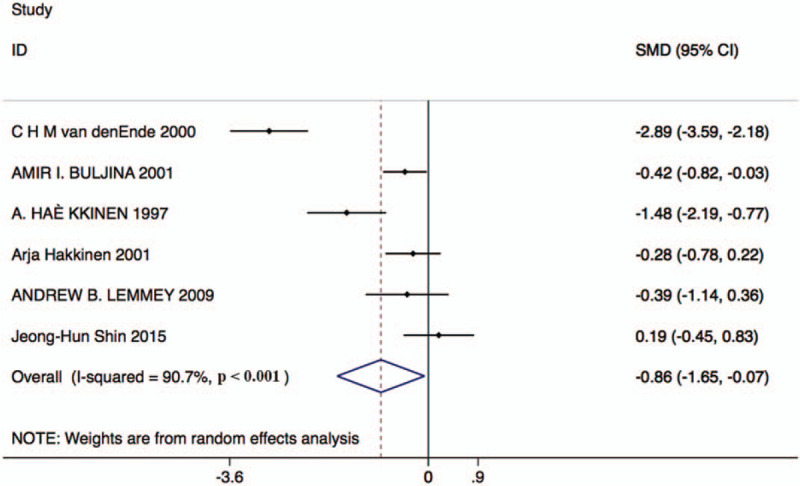
Forest plot of ESR in the resistance exercise group and the control group. ESR = erythrocyte sedimentation rate.
3.4. VAS score
Six studies with 159 patients in the resistance exercise group and 159 patients in the control group reported changes in VAS scores. Based on the Chi-squared test P-value of = .000 < .05 and I2 tests-value = 92.1% > 50%, we chose the random effects model to analyze changes in VAS scores. The pooled results showed no significant difference in VAS scores after the intervention between the 2 groups (SMD: –0.61, 95% CI: –1.49–0.27, Fig. 4).
Figure 4.
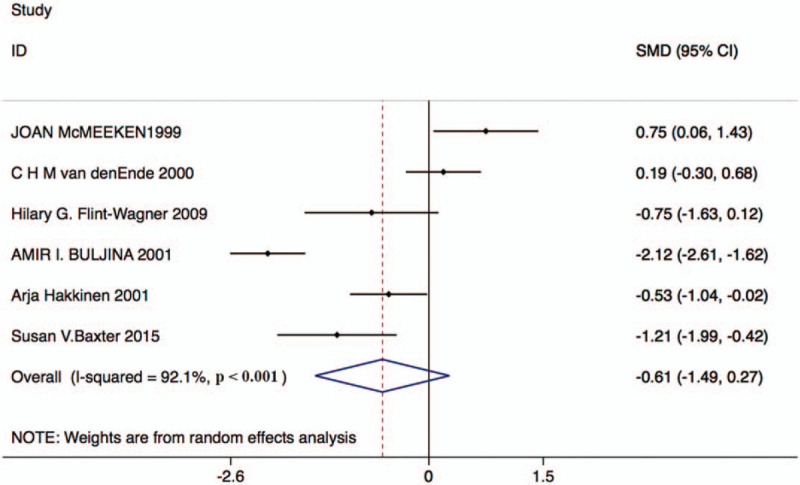
Forest plot of VAS score in the resistance exercise group and the control group. VAS = visual analog scale.
3.5. HAQ
Thirteen studies with 380 patients in the resistance exercise group and 365 patients in the control group reported HAQ changes. Based on the Chi-squared test P-value of = 0.000 < 0.05 and I2 tests-value = 84.8% > 50%, we chose the random effects model to analyze changes in HAQ. The pooled results showed no significant difference in HAQ score after the intervention between the 2 groups (weighted mean difference [WMD]: –0.10, 95% CI: –0.26–0.06, Fig. 5).
Figure 5.
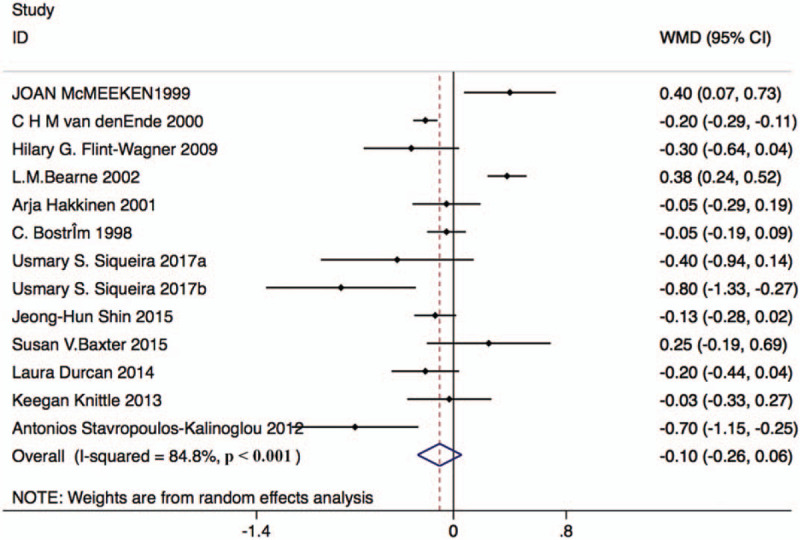
Forest plot of HAQ in the resistance exercise group and the control group. HAQ = health assessment questionnaire.
3.6. 50 ft. walking test
Four studies with 72 patients in the resistance exercise group and 62 patients in the control group reported changes in the 50 ft. walking test. Based on the Chi-square test P-value of = .392 > .05 and I2 tests-value = 0.0% < 50%, we chose the fixed effects model to analyze the 50 ft. walking test. The pooled results showed that compared with the control group, resistance exercise significantly decreased the time of 50 ft. walking (SMD: –0.64, 95% CI: –0.99 to –0.28, Fig. 6).
Figure 6.
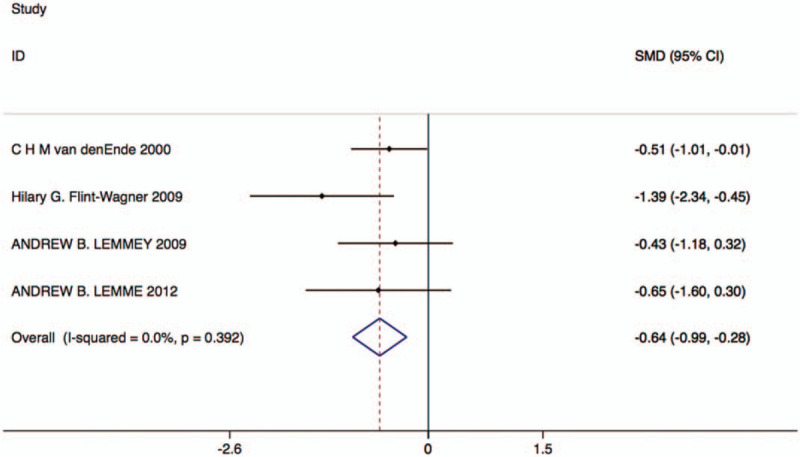
Forest plot of 50 ft. walking test in the resistance exercise group and the control group.
3.7. Quality assessment and potential bias
Based on the inclusion and exclusion criteria, 17 articles were included in the meta-analysis. Quality assessment and potential bias were assessed by funnel plot, Begg and Mazumdar rank test, and Egger test. The funnel plot for log WMD in HAQ of the included studies was notably symmetrical, suggesting no significant publication bias (Fig. 7). In addition, significant symmetry was detected by Begg and Mazumdar rank test (Z = 0.67, P = .502). However, the Egger test result showed no significant publication bias (P = .784).
Figure 7.
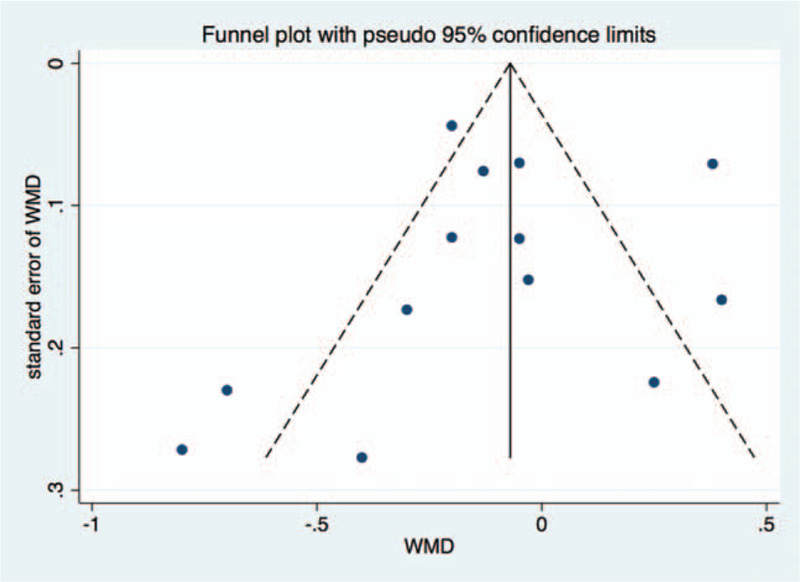
Funnel plot of studies included in the meta-analysis.
4. Discussion
In previous similar studies, Baillet et al[29] found that resistance exercises significantly improved isokinetic strength, isometric strength, grip strength, and HAQ. Exercise also had a positive effect on the 50-foot walking test and ESR. Withdrawals (RR = 0.95, 95% CI 0.61, 1.48) and adverse events (RR = 1.08, 95% CI 0.72, 1.63) were well balanced in both groups. Patient and exercise characteristics did not influence the results. Wang et al[30] include 13 studies, found that functional exercises could delay the development of the disease activity of RA patients (MD = –0.76; 95% CI: –1.13, –0.38), improve the joint function (MD = 0.36; 95% CI: –0.47, –0.24), alleviate the pain of joints (MD = –1.75; 95% CI: –1.98, –1.53), and reduce the duration of morning stiffness (MD = –17.65; 95% CI: –22.09, –13.21). Sieczkowska et al[31] included 29 studies, indicated that resistance training improves the general health-related quality of life (HR-QoL), the physical role functioning, physical functioning, social aspects, and body pain compared with control group.
In our study, we included 17 RCTs with the main Jadad score of 3.83. Compared with the general nursing or non-aerobic exercise, resistance exercise significantly decreased DAS-28 scores (SMD: –0.69, 95% CI: –1.26 to –0.11), reduced ESR (SMD: –0.86, 95% CI: –1.65 to –0.07), and shortened the time of 50 ft. walking (SMD: –0.64, 95% CI: –0.99 to –0.28). No significant difference was observed in VAS scores (SMD: –0.61, 95% CI: –1.49–0.27) and HAQ scores (WMD: –0.10, 95% CI: –0.26–0.06). The conclusion about ESR and the time of 50 ft. walking was consistented with the previous meta-analysis.
Some advantages of the present meta-analysis are as follows: this systemic review findings might be more convincing than any individual study among all included RCTs because the effect of resistance exercise in patients with RA was quantitatively determined using pooled large sample size; this meta-analysis provided evidence for the effects of resistance exercise; the strict inclusion and exclusion criterion were used to select eligible studies; all the data were analyzed by standard statistical analysis to make sure the results were accuracy.
However, there are some limitations should be attention in this analysis. The limitations are as follows: only randomized controlled trials were included; differences in the inclusion criteria and exclusion criteria for patients; different patients with previous disease and treatments were unavailable; most trials with low quality and low Jadad score were included in our study; the frequency, maximum load, and duration of resistance exercise were various; pooled date were used for analysis, and individual patients’ data were unavailable, so it limited us to make more comprehensive analysis.
Based on the available evidence, our meta-analysis demonstrated that compared with the control group, resistance exercise could significantly reduce DAS-28 scores, ESR scores, and the time of 50 ft. walking in patients with RA. Thus, further high-quality studies with lager sample sizes and longer follow-up duration are needed to confirm the results of our meta-analysis. In future study, researchers can explore the relationship between intensity and frequency of resistance exercise and outcomes of RA.
Author contributions
Conceptualization: Zhigang Wen, Yi Chai.
Data curation: Zhigang Wen, Yi Chai.
Formal analysis: Zhigang Wen, Yi Chai.
Methodology: Yi Chai.
Writing – original draft: Zhigang Wen.
Writing – review & editing: Yi Chai.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, MD = mean difference, OR = odds ratios, RA = rheumatoid arthritis, RCTs = randomized controlled trials.
How to cite this article: Wen Z, Chai Y. Effectiveness of resistance exercises in the treatment of rheumatoid arthritis: a meta-analysis. Medicine. 2021;100:13(e25019).
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and material: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests: There is no competing interest.
No funding was received for this study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Park J, Ernst E. Ayurvedic medicine for rheumatoid arthritis: a systematic review. Semin Arthritis Rheum 2005;34:705–13. [DOI] [PubMed] [Google Scholar]
- [2].Singh BB, Vinjamury SP, Der-Martirosian C, et al. Ayurvedic and collateral herbal treatments for hyperlipidemia: a systematic review of randomized controlled trials and quasi-experimental designs. Altern Ther Health Med 2007;13:22–8. [PubMed] [Google Scholar]
- [3].Gossec L, Pavy S, Pham T, et al. Nonpharmacological treatments in early rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine 2006;73:396–402. [DOI] [PubMed] [Google Scholar]
- [4].Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–31. [DOI] [PubMed] [Google Scholar]
- [5].Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology (Oxford) 2008;47:239–48. [DOI] [PubMed] [Google Scholar]
- [6].Baillet A, Zeboulon N, Gossec L, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2010;62:984–92. [DOI] [PubMed] [Google Scholar]
- [7].Hakkinen A, Hakkinen K, Hannonen P. Effects of strength training on neuromuscular function and disease activity in patients with recent-onset inflammatory arthritis. Scand J Rheumatol 1994;23:237–42. [DOI] [PubMed] [Google Scholar]
- [8].Komatireddy GR, Leitch RW, Cella K, et al. Efficacy of low load resistive muscle training in patients with rheumatoid arthritis functional class II and III. J Rheumatol 1997;24:1531–9. [PubMed] [Google Scholar]
- [9].Metsios GS, Stavropoulos-Kalinoglou A, Kitas GD. The role of exercise in the management of rheumatoid arthritis. Expert Rev Clin Immunol 2015;11:1121–30. [DOI] [PubMed] [Google Scholar]
- [10].Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. London; 2009. [PubMed] [Google Scholar]
- [11].McMeeken J, Stillman B, Story I, et al. The effects of knee extensor and flexor muscle training on the timed-up-and-go test in individuals with rheumatoid arthritis. Physiother Res Int 1999;4:55–67. [DOI] [PubMed] [Google Scholar]
- [12].van den Ende CH, Breedveld FC, le Cessie S, et al. Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2000;59:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flint-Wagner HG, Lisse J, Lohman TG, et al. Assessment of a sixteen-week training program on strength, pain, and function in rheumatoid arthritis patients. J Clin Rheumatol 2009;15:165–71. [DOI] [PubMed] [Google Scholar]
- [14].Bearne LM, Scott DL, Hurley MV. Exercise can reverse quadriceps sensorimotor dysfunction that is associated with rheumatoid arthritis without exacerbating disease activity. Rheumatology (Oxford) 2002;41:157–66. [DOI] [PubMed] [Google Scholar]
- [15].Buljina AI, Taljanovic MS, Avdic DM, et al. Physical and exercise therapy for treatment of the rheumatoid hand. Arthritis Rheum 2001;45:392–7. [DOI] [PubMed] [Google Scholar]
- [16].Hakkinen A, Malkia E, Hakkinen K, et al. Effects of detraining subsequent to strength training on neuromuscular function in patients with inflammatory arthritis. Br J Rheumatol 1997;36:1075–81. [DOI] [PubMed] [Google Scholar]
- [17].Hakkinen A, Sokka T, Kotaniemi A, et al. A randomized two-year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis Rheum 2001;44:515–22. [DOI] [PubMed] [Google Scholar]
- [18].Lemmey AB, Marcora SM, Chester K, et al. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1726–34. [DOI] [PubMed] [Google Scholar]
- [19].Bostrom C, Harms-Ringdahl K, Karreskog H, et al. Effects of static and dynamic shoulder rotator exercises in women with rheumatoid arthritis: a randomised comparison of impairment, disability, handicap, and health. Scand J Rheumatol 1998;27:281–90. [DOI] [PubMed] [Google Scholar]
- [20].Siqueira US, Orsini Valente LG, de Mello MT, et al. Effectiveness of aquatic exercises in women with rheumatoid arthritis: a randomized, controlled, 16-week intervention-The HydRA Trial. Am J Phys Med Rehabil 2017;96:167–75. [DOI] [PubMed] [Google Scholar]
- [21].Shin JH, Lee Y, Kim SG, et al. The beneficial effects of Tai Chi exercise on endothelial function and arterial stiffness in elderly women with rheumatoid arthritis. Arthritis Res Ther 2015;17:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baxter SV, Hale LA, Stebbings S, et al. Walking is a feasible physical activity for people with rheumatoid arthritis: a feasibility randomized controlled trial. Musculoskeletal Care 2016;14:47–56. [DOI] [PubMed] [Google Scholar]
- [23].Knittle K, De Gucht V, Hurkmans E, et al. Explaining physical activity maintenance after a theory-based intervention among patients with rheumatoid arthritis: process evaluation of a randomized controlled trial. Arthritis Care Res (Hoboken) 2016;68:203–10. [DOI] [PubMed] [Google Scholar]
- [24].Durcan L, Wilson F, Cunnane G. The effect of exercise on sleep and fatigue in rheumatoid arthritis: a randomized controlled study. J Rheumatol 2014;41:1966–73. [DOI] [PubMed] [Google Scholar]
- [25].Knittle K, De Gucht V, Hurkmans E, et al. Targeting motivation and self-regulation to increase physical activity among patients with rheumatoid arthritis: a randomised controlled trial. Clin Rheumatol 2015;34:231–8. [DOI] [PubMed] [Google Scholar]
- [26].Manning VL, Hurley MV, Scott DL, et al. Education, self-management, and upper extremity exercise training in people with rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res 2014;66:217–27. [DOI] [PubMed] [Google Scholar]
- [27].Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819–25. [DOI] [PubMed] [Google Scholar]
- [28].Lemmey AB, Williams SL, Marcora SM, et al. Are the benefits of a high-intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3-year followup study. Arthritis Care Res (Hoboken) 2012;64:71–5. [DOI] [PubMed] [Google Scholar]
- [29].Baillet A, Vaillant M, Guinot M, et al. Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2012;51:519–27. [DOI] [PubMed] [Google Scholar]
- [30].Wang L, Gao C, Zhu D, et al. [Effect of functional exercises on patients with rheumatoid arthritis: a meta-analysis]. Beijing Da Xue Xue Bao Yi Xue Ban 2018;50:991–7. [PubMed] [Google Scholar]
- [31].Sieczkowska SM, Coimbra DR, Vilarino GT, et al. Effects of resistance training on the health-related quality of life of patients with rheumatic diseases: systematic review with meta-analysis and meta-regression. Semin Arthritis Rheum 2020;50:342–53. [DOI] [PubMed] [Google Scholar]


