Abstract
Hypertension is associated with chronic inflammation in the tissues and organs that are involved in the regulation of arterial pressure, such as kidneys and blood vessels. Periodontal disease affects systemic inflammatory markers, leading to endothelial dysfunction, atherosclerotic plaque instability, dyslipidaemia, and insulin resistance. These conditions can also cause an increase in the blood pressure. Nonsurgical periodontal therapies, such as scaling and root planning, can affect systemic markers of inflammation. We evaluated the effect of scaling and root planning on serum levels of inflammation biomarkers in hypertensive patients. The sample consisted of 19 hypertensive patients with Periodontitis. The patients underwent laboratory tests that included glycaemia, cholesterol, triglycerides and blood count. Blood pressure was measured before periodontal therapy, and the second blood pressure recording was obtained at the re-evaluation appointment. Quantification of peripheral blood cytokines was performed using the Milliplex Inflammation Human Cytokine kit (Interleukin 1-β, Interleukin-4, Interleukin-6, Interleukin-8, Interleukin-10, Interleukin-12 P70, Interleukin-17A, vascular endothelial growth factor and tumor necrosis factor-alpha). All cytokine levels decreased from the initial examination to reassessment. Cytokines that reflected a statistically significant difference included Interleukin-1β and endothelial vascular growth factor (P = .04 and P = .004). Hypertensive patients with periodontitis undergoing non-surgical periodontal treatment exhibited a decrease in proinflammatory cytokine levels. Non-surgical periodontal treatment decreases the levels of systemic proinflammatory cytokines in controlled hypertensive patients.
Keywords: cytokines, hypertension, mediators of inflammation, periodontal debridement, periodontal disease, periodontal infection
1. Introduction
Hypertension is associated with chronic inflammation in the tissues and organs that are involved in the regulation of arterial pressure, such as kidneys and blood vessels. Vascular inflammation may contribute to the alteration of functions, such as endothelial resistance and stiffness.[1–4] Periodontal disease is a multifactor pathology, and its main aetiological factor is the accumulation of bacterial biofilm. This disease affects gingival tissue and subsequently destroys the insertion and support tissues of the teeth.[5] Periodontal disease severity affects systemic inflammatory markers, such as C-reactive protein (CRP), fibrinogen, tumor necrosis factor-alpha (TNF-α), and Interleukin (IL)-6, leading to endothelial dysfunction, atherosclerotic plaque instability, dyslipidaemia, and insulin resistance. These conditions can also cause an increase in the blood pressure and mortality risk in hypertension patients.[6–8] The elimination of the infectious and inflammatory load of the periodontal disease may be associated with improved endothelial function and could be accompanied by a decrease in inflammatory markers. Furthermore, periodontal therapy increases nitric oxide production, also leading to improved endothelial function.[9–11]
Considering this background, the following question arises: What is the effect of non-surgical periodontal therapy (root scaling and debridement) on serum levels of IL 1-β, IL-4, IL-6, IL-8, IL-10, IL-12 P70, IL-17A, vascular endothelial growth factor (VEGF), and TNF-α in patients with generalized periodontitis stage II, III, and IV and arterial hypertension? Thus, the aim of this study is to analyse whether non-surgical periodontal treatment will improve the systemic inflammatory response. These findings will be important for the interdisciplinary management of blood pressure control and the systemic inflammatory response in hypertensive patients.
2. Methods
2.1. Study design and setting
The present study was observational and evaluates the changes in systemic inflammatory biomarkers before and after non-surgical periodontal therapy. Nineteen patients over 30 years with periodontitis stage II, III, and IV, grade A and B and controlled arterial hypertension were included in this study. Patients diagnosed with hypertension and undergoing pharmacological treatment with a minimum of 6 teeth present in the oral cavity were classified as periodontitis stage II, III, and IV, grade A and B. Patients with diabetes, patients who smoke, patients undergoing antibiotic treatment in the last 3 months and patients who received periodontal treatment in the last 6 months were excluded. Patients with immunological diseases that may alter blood pressure, such as rheumatoid arthritis and systemic lupus erythematosus, were excluded from the study.
2.2. Ethical approval
The Investigation and Ethics Committee from the Odontology Faculty of the Pontificia Universidad Javeriana (CIEFOPUJ)-Bogotá, Colombia approved this study (Reference: 007/2015). The study was explained to the patients, who signed informed consent to participate in the study. We confirm that this study was conducted in accordance with the Helsinki Declaration of 1964, and its subsequent modifications. We confirm that all subjects gave their informed consent to participate in the study
2.3. Data collection
Periodontal examinations were performed by 2 calibrated evaluators who used a graduated manual North Carolina Probe. Biofilm control was measured using the O’Leary index. The periodontal examination was performed around 6 points in each tooth present in the mouth. The periodontal sulcus or pocket was measured from the gingival margin to the bottom of the pocket or sulcus. It was considered a pathological periodontal pocket when the measurement was 4 mm or greater. Likewise, the gingival margin measure was taken from the cement-enamel junction to the gingival margin. Using this probing depth and gingival margin data, the clinical attachment level was calculated and a periodontal diagnosis was made based on the Workshop of 2017 and Armitage 1999 classification.[12,13]
After the periodontal examination, laboratory exams were performed in San Ignacio Hospital (HUSI) during the first hour in the morning, and patients were fasting. Exams assessed blood count, glycaemia, triglycerides, High-density lipoprotein cholesterol (cHDL), Low-density lipoprotein cholesterol (cLDL) and serum levels of the inflammatory markers IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12 P70, IL-17A, VEGF, and TNF-α. After patients had breakfast and rested for 1 hour, blood pressure was measured by a professional from the Cardiology Department of HUSI.
All patients received oral hygiene instruction and non-surgical periodontal treatment (scaling and root planning) with ultrasonic piezoelectric and Gracey curettes in 1 session. After an average of 4 to 5 weeks after the periodontal treatment, a revaluation was performed in which the periodontal status of each patient was verified by taking the same reference values of the initial examination appointment. In the same session, blood count, glycaemia, cholesterol, triglycerides, cHDL, cLDL, and systemic inflammatory biomarkers were measured. Furthermore, blood pressure was also measured.
Three blood tubes were obtained as samples, including 2 dry tubes and 1 with an anticoagulant. The resulting sample from the anticoagulant tube with ethylenediaminetetraacetic acid (EDTA) was processed in the clinical laboratory of HUSI to assess blood count and glycaemic values. One of the dry tubes was used for total cholesterol, triglycerides, cHDL, and cLDL analysis. Gel to separate plasma and serum was placed in the other dry tube. The tube was centrifuged at 10,000 rpm for 10 minutes and frozen at −20°C in the HUSI until its use. These samples were used for the cytokines measurements, analysis at the end of the sample collection.
Serum cytokines were quantified using the Luminex system with the Milliplex Inflammation Human Cytokine kit. The test was performed on a plate where reagents and working standards were prepared following the manufacturer's instructions. The pearls were prepared for each cytokine to be evaluated and placed in a single container. Each vessel containing vortex pearls was stirred for 1 minute, and 60 μl of pearl reconstituting agent was placed for each cytokine. The standard, test buffer, and serum matrix were added to the background, standard and control wells, and the serum from the sample was added to the corresponding wells. The bottle with the premixed pearls was placed in the vortex, and 25 μl was added to each well. The bottle was sealed with adhesive paper and wrapped in aluminium foil. The sample was stirred at 700 rpm at 4°C for 16 hours. Then, 2 washes were performed with a magnetic plate, and detection antibodies were added and stirred for 1 hours at room temperature (RT). Then, streptavidin-phycoerytrina was added to each well, and the sample was sealed, covered with aluminium and stirred at RT for 30 minutes. Then, the washing procedure was repeated, and drive fluid was added. The microparticles (pearls) were resuspended in the stirrer for 5 minutes. All samples were assembled in duplicate. Finally, the plate was assessed using Magpix Luminex and analysed by Magpix Luminex 200 software. The equipment gave different values that we used to identify the total count of captured pearls, the average fluorescence intensity and the concentrations of the cytokines of interest. These values were determined according to the standard curve and expressed in pg/ml, and the values were adjusted to the dilution factor The Luminex kit assay sensitivity has the following ranges in pg/ml of Minimum Detectable Concentration (MinDC) and Minimum Detectable Concentration +2SD, as described below: IFN-γ: 0.8 and 1.1; IL-10: 1.1 and 1.6; IL-12 P70: 0.6 and 1.0; IL-17A: 0.7 and 1.2; IL-1β: 0.8 and 1.0; IL-4: 4.5 and 7.1; IL-6: 0 and 1.3; IL-8: 0.4 and 0.7; TNF-α: 0.7 and 1.1; VEGF: 26.3 and 47.9.
To establish blood pressure, measurements were obtained from both arms with an interval of at least 2 minutes. The patient had to meet the following requirements for adequate intake, sit with back supported and arms at heart level, blood pressure should be taken after 5 minutes of rest, the patient should not have smoked or consumed caffeine 30 minutes before the blood pressure reading, do not speak during the measurement and, support the discreetly strapped arm with the palm facing up, preferably on the right arm or the dominant arm. An electronic blood pressure monitor was used, the same instrument was used in all patients.
2.4. Statistic analysis
Demographic characteristics and the results of the periodontal evaluation and the systemic biomarkers of inflammation are reported as the means, medians, ranges, standard deviations and 95% confidence intervals. Comparisons between groups were performed using Student's t test or chi-square as appropriate. Here, P < .05 (2-tailed) was considered significant.
3. Results
The study included a total of 22 patients, of which 3 were excluded due to incomplete follow-up. Of the final sample, 52.6% were women (10 patients), and 47.3% were men (9 patients), with an average age of 57.6 years (SD 2.2)
Of the 19 patients included in the study, periodontal status was assessed according to the 2017 Workshop classification at the start of the study and at the time of re-evaluation. In total, 2 patients were diagnosed with Stage III Grade A Periodontitis, 10 with Stage III Grade Periodontitis B, 2 with Stage IV Grade A Periodontitis and 5 with Stage IV Grade B Periodontitis. Regarding the 1999 Armitage classification, at the beginning of treatment, 17 patients presented with generalized severe chronic periodontitis and 2 with generalized moderate chronic periodontitis. At the time of revaluation, 4 presented with generalized moderate chronic periodontitis, 8 generalized severe chronic periodontitis, 2 localized moderate chronic periodontitis and 5 presented localized severe chronic periodontitis.
A total of 464 teeth were evaluated, of which 265 had periodontal pockets at the initial evaluation. The pre-treatment range was 4 to 14 mm. At the month of the re-evaluation, the number of teeth for which 2 exodonces were performed in the hygienic phase was 462, and there were 175 teeth with periodontal pockets. The number of periodontal pockets in the re-evaluation ranged from 4 to 12 mm. When evaluating the measured clinical parameters at the initial evaluation the average number of teeth with periodontitis was 12.89, the average sulcus or pocket depth was 3.17 mm, the clinical attachment level was 3.38 mm, the bleeding on probing percentage was 58.43% and the plaque index was 41.36%, the following changes were observed at the time of the re-evaluation: the average number of teeth with periodontitis was 4.05, the average sulcus or pocket depth was 0.28 mm, the clinical attachment level was 0.23 mm, the bleeding on probing percentage was 18.67% and the plaque index was 12.55%. These findings indicate reductions in all the parameters evaluated (Table 1).
Table 1.
Clinical parameters pre-treatment and post-treatment.
| Clinical parameters | |||||
| Pre-treatment | Post-treatment | ||||
| Mean | SE | Mean | SE | P value | |
| Teeth with periodontitis | 12.89 | 1.13 | 8.84 | 1.11 | .00∗ |
| Probing Depth (mm) | 3.17 | 0.12 | 2.89 | 0.10 | .00∗ |
| CAL (mm) | 3.38 | 0.27 | 3.15 | 0.29 | .01∗ |
| BOP (%) | 58.43 | 4.38 | 39.75 | 3.20 | .0001∗∗ |
| BIOFILM (%) | 41.36 | 2.23 | 28.80 | 2.08 | .0004∗∗ |
BOP = bleeding on probing, CAL = clinical attachment level, SE = standard error.
Paired t test ≤.05.
Test of proportions ≤0.05.
The average blood pressure was 135.15/86.1 mmHg at the beginning of the study and 133.26/83.15 mmHg at the end of treatment, and the difference was not statistically significant (Table 2). Clinical laboratory serum tests revealed an increase of 0.26 mg/dl in glycaemia at the time of reassessment, triglycerides, cHDL and cLDL were reduced by 10.6, 16.2, 38.6, and 8.3 mg/dl, respectively. Statistically significant P values for the level of triglycerides and cHDL (Table 3).
Table 2.
Blood pressure pre-treatment and post-treatment.
| Blood pressure (mmHg) | |||||||
| Pre-treatment | Post-treatment | ||||||
| Mean | SE | Mean | SE | Mean difference | CI 95% | P value∗ | |
| Systolic | 135.15 | 3.69 | 133.26 | 2.47 | 1.89 | −5.89 | .61 |
| Diastolic | 86.10 | 1.80 | 83.15 | 1.14 | 2.94 | −0.45 | .08 |
CI 95% = 95% confidence interval, SE = standard error
Paired t test P ≤ .05.
Table 3.
Laboratory tests pre-treatment and post-treatment.
| Laboratory tests results (mg/dl) | |||||||
| Pre-treatment | Post-treatment | ||||||
| Mean | SE | Mean | SE | Mean difference | CI 95% | P value∗∗ | |
| Glycemia | 100.78 | 2.23 | 101.05 | 2.19 | −0.26 | −5.30 | .91 |
| Cholesterol | 201.05 | 8.28 | 190.45 | 8.34 | 10.6 | −0.51 | .06 |
| Triglycerides | 176.38 | 18.08 | 160.14 | 17.62 | 16.24 | 0.33 | .04 |
| HDL∗ | 83.71 | 10.53 | 45.05 | 2.70 | 38.65 | 17.27 | .001 |
| LDL† | 124.23 | 6.88 | 115.89 | 4.87 | 8.33 | −2.87 | .13 |
CI 95% = 95% confidence interval, SE = standard error.
High-density lipoprotein
Low-density lipoprotein.
Paired t test P ≤ .05.
Patient cytokine levels decreased from the initial examination to re-evaluation. The following initial values were observed: 0.53 pg/ml IL-1β, 2.98 pg/ml IL-4, 0.31 pg/ml IL-6, 9.17 pg/ml IL-8, 1.15 pg/ml IL-10, 1.82 pg/ml IL-12 P70, 3.8 pg/ml IL-17A, 15.84 pg/ml TNF-α, and 152.10 pg/ml VEGF. The following values were obtained after periodontal treatment: 0.30 pg/ml IL-1β, 1.23 pg/ml IL-4, 0.24 pg/ml IL-6, 7.79 pg/ml IL-8, 0.86 pg/ml IL-10, 0.94 pg/ml IL-12 P70, 3.19 pg/ml IL-17A, 14.29 pg/ml TNF-α, and 101.55 pg/ml VEGF. IL-1β and VEGF (P = .04 and P = .004) exhibited statistically significant differences when evaluated with the Wilcoxon test (Table 4; Fig. 1).
Table 4.
Cytokines levels pre-treatment and post-treatment.
| Cytokines levels (pg/ml) | |||||||
| Pre-treatment | Post-treatment | ||||||
| Mean | SE | Mean | SE | Mean difference | CI 95% | P value∗ | |
| IL-1β | 0.53 | 0.14 | 0.30 | 0.07 | 0.22 | −0.09 | .04 |
| IL-4 | 2.98 | 1.62 | 1.23 | 1.03 | 1.75 | −0.78 | .27 |
| IL-6 | 0.31 | 0.18 | 0.24 | 0.13 | 0.07 | −0.05 | .30 |
| IL-8 | 9.17 | 1.05 | 7.79 | 0.62 | 1.37 | −0.74 | .22 |
| IL-10 | 1.15 | 0.43 | 0.86 | 0.26 | 0.29 | −0.50 | .42 |
| IL-12 P70 | 1.82 | 1.08 | 0.94 | 0.48 | 0.87 | −0.49 | .36 |
| IL-17A | 3.8 | 1.08 | 3.19 | 0.69 | 0.60 | −0.47 | .74 |
| TNF-α | 15.84 | 1.06 | 14.29 | 1.05 | 1.54 | −0.38 | .18 |
| VEGF | 152.10 | 28.70 | 101.55 | 18.36 | 50.55 | 16.56 | .004 |
CI 95% = 95% confidence interval, IL = interleukin, SE = standard error, TNF-α = tumor necrosis factor-alpha, VEGF = vascular endothelial growth factor.
Wilcoxon signed-rank test P ≤ .05.
Figure 1.
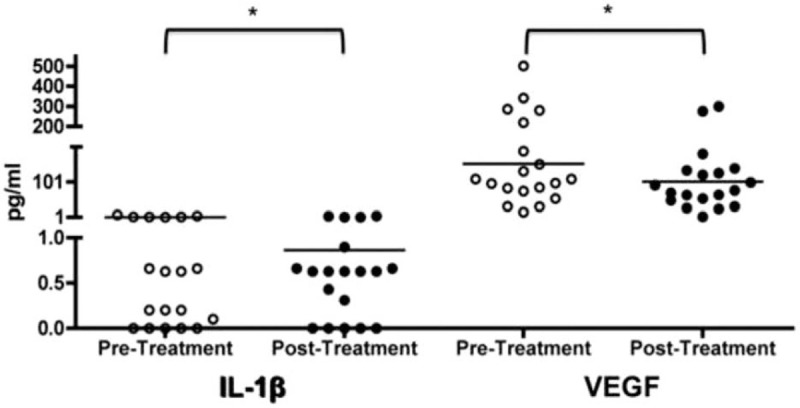
Patient cytokine levels decreased from the initial examination to re-evaluation. Interleukin 1β (IL-1β) and vascular endothelial growth factor (VEGF) expression in initial examination (pre-treatment) and re-evaluation (post-treatment) in 19 patients with different periodontitis status and diagnosed with hypertension. The concentrations obtained were expressed in pg/ml and comparisons between groups were performed using Wilcoxon test.
A cut-off point for each cytokine was established based on reference values reported in the literature. Of the articles evaluated, there is no definitive cut-off point for cytokines since they can be detected by different techniques such as ELISPOT, Flow Cytometry, ELISA, and Luminex. This cut-off point is described in each study in a particular way, for example, it is obtained from the mean plus two (2) standard deviations, and a range is determined. Each study establishes it independently depending on the technique used, the selected groups, and the biological sample analyzed. On the other hand, each laboratory obtains its cut-off point or reference value. We obtained these values from the Mayo Clinic (Bogotá), a recognized clinic in Colombia.[14–17] IL-1β, IL-6, IL-8, and IL-10 initial and final treatment values were normal based on cut-off points. At the initial evaluation, only 2 patients presented normal TNF-α values, and 17 presented increased values. However, in the final evaluation, 3 patients presented normal values, and 16 values increased based on the cut-off point (P = .018∗). (Table 5).
Table 5.
Cut-off point of cytokines levels pre-treatment and post-treatment.
| Cut-off point of cytokines levels | ||||
| Cut-off point (pg/ml) | Pre-treatment | Post-treatment | P value | |
| IL-10 | ≤9.1 | 19 | 19 | Not calculable |
| >9.1 | 0 | 0 | ||
| IL-1β | ≤5 | 19 | 19 | Not calculable |
| >5 | 0 | 0 | ||
| IL-6 | ≤3.4 | 18 | 19 | Not calculable |
| >3.4 | 1 | 0 | ||
| IL-8 | ≤50 | 19 | 19 | Not calculable |
| >50 | 0 | 0 | ||
| TNF-α | ≤8.1 | 2 | 3 | .018∗ |
| >8,1 | 17 | 16 | ||
Fisher's exact test ≤.05.
4. Discussion
Scientific literature has described the relationship between hypertension and periodontal disease due to the inflammatory response that underlies these 2 pathologies. Periodontal therapy could decrease the levels of inflammation biomarkers and positively impact the development of hypertension.
Beyond the periodontal diagnosis, this study found that when performing basic periodontal treatment, average periodontal clinical parameters, such as the level of clinical insertion (from 3.38 to 3.15), the rate of bleeding (from 58.43% to 39.75%) and the biofilm index (from 41.36%. to 28.80%), improved with statistically significant differences for all the analysed clinical parameters. These findings were expected and are consistent with that reported by Aristizábal et al.[18] Al Bush et al published the effects of surgical and non-surgical periodontal treatment on vascular markers in hypertensive patients. Both the surgical group and the non-surgical group presented statistically significant decreases in periodontal clinical parameters. These results are consistent with our study; however, in this investigation, non-surgical periodontal therapy was performed in all the patients in the study.[19]
Regarding blood pressure before and after treatment, a slight decrease in systolic and diastolic blood pressure was evidenced from an average of 135.15/86.1 to an average of 133.26/83.15 mmHg, respectively. This finding is consistent with the study by Pietropaoli D. and colleagues in 2014, demonstrating that the mean systolic blood pressure was approximately 2.3 to 3 mmHg increased in the presence of periodontitis among hypertensive adults undergoing treatment (P < .0001). These results further indicate that good periodontal health is associated with a better profile of systolic blood pressure during antihypertensive therapy.[20] The slight change (which was not significant for systolic and diastolic pressure) in blood pressure before and after treatment can be attributed to the sample of patients who were all on medical antihypertensive treatment in the present study. However, we consider that even a difference of 1.89 (systolic pressure) and 2.94 (diastolic pressure) mmHg represents an improvement in the systemic condition of hypertensive patients. Evidence of the change in blood pressure after periodontal treatment in the literature is controversial. In 2013, D’Aiuto et al reported limited evidence on the effects of periodontal therapy on changes in blood pressure. These findings support our results where a slight improvement was also evident although the patients were hypertensive controlled.[21]
In their systematic review, Muñoz-Aguilera et al found a prevalence of hypertension in patients with periodontal disease versus those without disease. It was concluded that periodontitis could be associated with an increased risk of hypertension in a linear way. The findings highlight the potential to improve cardiovascular disease outcomes by improving oral health in the general population.[22]
When the systemic inflammatory response was analysed through cytokine levels, a decrease after periodontal treatment was evident in all the interleukins analysed. Statistically significant differences were noted for IL-1β and VEGF cytokines (P = .04 and P = .004), reflecting the decrease in the local bacterial load. In this sense, the literature has also analysed systemic inflammatory cytokines after periodontal treatment. Montenegro et al evaluated patients with stable coronary disease and periodontitis. They found that non-surgical periodontal therapy leads to lower levels of CRP, IL-6, and IL-8 in cardiovascular patients.[23]
In 2019, a systematic review by D’Isidoro et al analysed the influence of non-surgical periodontal treatment on inflammation biomarkers, reporting that periodontal treatment contributed to the resolution of oral inflammation and could positively influence the levels of biomarkers of systemic inflammation.[24] Similarly, Roca-Millan et al also found a decrease in TNF-α but not IL-1-α after non-surgical instrumentation and oral hygiene instruction.[25]
Higashi et al, Vidal et al and Piconi et al, analysed the effect of periodontal therapy on plasma levels of biomarkers of inflammation. Levels of biomarkers of inflammation decreased after periodontal treatment.[26–28] These results are consistent with those found in our study given that cytokine levels decreased after periodontal treatment. The novelty of this study is that we analysed a greater number of cytokines, 1 month after the treatment and the patients were hypertensive controlled.
Another relevant finding in our study was the statistically significant decrease in VEGF from 152.10 to 101.55 with a P value of .004. The increase in VEGF is related to arterial hypertension. In 2017, Touyz et al analysed recent advances in hypertension and cardiovascular toxicity upon inhibition of VEGF. They emphasized that this biomarker is associated with a higher incidence of cardiovascular pathologies, including hypertension, ischaemic heart disease, heart failure, QT prolongation, and thromboembolism. The magnitude of hypertension induced by this biomarker is significant, and almost all trials report an increase in blood pressure (BP) greater than 150/100 mmHg. The development of hypertension depends on the dose of VEGF.[29]
We consider that the significant reduction of VEGF in addition to IL-1β is an important predictor in the improvement of systemic inflammation in patients with arterial hypertension. Together with the improvement of other risk factors, these effects contribute to improved disease control.
In addition, the importance of periodontal therapy must be taken into account, since an improvement in local periodontal inflammation can decrease systemic interleukin levels and help control one of the risk factors of arterial hypertension, namely, systemic inflammation. Therefore, interdisciplinary management together with periodontal treatment can improve the systemic condition of hypertensive patients by controlling risk factors, such as periodontal infection.
5. Conclusions
According to the study performed here and taking into account its limitations, it can be concluded that non-surgical periodontal treatment decreases the levels of systemic proinflammatory cytokines in hypertensive patients. Periodontal treatment improved blood pressure levels and clinical parameters, such as probing depth, clinical insertion level, bleeding and biofilm rate.
These findings are relevant for the interdisciplinary management of hypertensive patients in the control of risk factors for their disease. Today more than ever, more studies of the levels of inflammatory biomarkers after periodontal treatment are needed with a longer follow-up time; however, the results obtained as predictive parameters in subsequent studies should be taken into account and could have a positive impact on public health.
Acknowledgments
We thank at Clinic Laboratory – Hospital Universitario San Ignacio and Purificación y Análisis PAF for his advice and accompaniment in quantification and analysis of cytokines by Luminex Technology. Thanks also to Javesalud who collaborated with us in the collection of patients.
Author contributions
Conceptualization: Francina Maria Escobar Arregocés, María José Sáenz Martinez, Federico José Hernández Meza, Nelly S Roa M, Juliana Velosa-Porras, Catalina Latorre Uriza.
Data curation: Mariella Del Hierro Rada.
Formal analysis: Juliana Velosa-Porras.
Funding acquisition: Francina Maria Escobar Arregoces.
Investigation: Francina Maria Escobar Arregoces, Mariella Del Hierro Rada, María José Sáenz Martinez, Federico José Hernández Meza, Catalina Latorre Uriza.
Methodology: Francina Maria Escobar Arregoces, Juliana Velosa-Porras.
Project administration: Francina Maria Escobar Arregoces, Catalina Latorre Uriza.
Resources: Francina Maria Escobar Arregoces.
Supervision: Catalina Latorre Uriza.
Validation: Nelly S Roa M.
Visualization: Juliana Velosa-Porras.
Writing – original draft: Francina Maria Escobar Arregoces, Mariella Del Hierro Rada, María José Sáenz Martinez, Federico José Hernández Meza, Nelly S Roa M, Juliana Velosa-Porras.
Writing – review & editing: Francina Maria Escobar Arregoces, Catalina Latorre Uriza.
Footnotes
Abbreviations: BOP = bleeding on probing, CAL = clinical attachment level, cHDL = high-density lipoprotein, cLDL = low-density lipoprotein, CRP = C-reactive protein, EDTA = ethylenediaminetetraacetic acid, HUSI = San Ignacio Hospital, IL = interleukin, RT= room temperature, TNF-α = tumor necrosis factor-alpha, VEGF = vascular endothelial growth factor.
How to cite this article: Escobar Arregocés FM, Del Hierro Rada M, Sáenz Martinez MJ, Hernández Meza FJ, Roa NS, Velosa-Porras J, Latorre Uriza C. Systemic inflammatory response to non-surgical treatment in hypertensive patients with periodontal infection. Medicine. 2021;100:13(e24951).
This research was conducted with resources from the Vice-Rectorate of Research at Pontificia Universidad Javeriana (ID: 00007258).
No conflict of commercial interest is present.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Hear J 2019;40:3459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American Heart Association Rask. Hypertension 2018;71:1269–324. [DOI] [PubMed] [Google Scholar]
- [3].Manfredi Carabetti JA. Endotelio, inflamación e hipertensión arterial. Rev Urug Cardiol 2012;27:413–7. [Google Scholar]
- [4].Calle CM, Ángel MP, Duque A. Enfermedad periodontal y su relación con las enfermedades cardiovasculares. Rev CES Odont 2012;25:82–91. [Google Scholar]
- [5].Contreras A, Ramirez J. Relación entre periodontitis y enfermedad cardiovascular. Rev Clin Periodoncia Implantol Rehabil Oral 2009;2:91–7. [Google Scholar]
- [6].Lang NP, Lindhe J. Clinical Periodontology and Implant Dentistry. 5a edBuenos Aires: Médica Panamericana; 2009. [Google Scholar]
- [7].Zhou SY, Duan XQ, Hu R, et al. Effect of non-surgical periodontal therapy on serum levels of TNF-a, IL-6 and C-reactive protein in periodontitis subjects with stable coronary heart disease. Chin J Dent Res 2013;16:145–51. [PubMed] [Google Scholar]
- [8].Elter J, Hinderliter A, Offenbacher S, et al. The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J 2006;151:47.e1–6. [DOI] [PubMed] [Google Scholar]
- [9].Orlandi M, Suvan J, Petrie A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis 2014;236:39–46. [DOI] [PubMed] [Google Scholar]
- [10].Martin-Cabezas R, Seelam N, Petit C, et al. Association between periodontitis and arterial hypertension: a systematic review and meta-analysis. Am Heart J 2016;180:98–112. [DOI] [PubMed] [Google Scholar]
- [11].Ramich T, Asendorf A, Nickles K, et al. Inflammatory serum markers up to 5 years after comprehensive periodontal therapy of aggressive and chronic periodontitis. Clin Oral Invest 2018;22:3079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J Periodontol 2018;89: Suppl 1: S1–8. [DOI] [PubMed] [Google Scholar]
- [13].Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1–6. [DOI] [PubMed] [Google Scholar]
- [14].Jason J, Archibald LK, Nwanyanwu OC, et al. Comparison of serum and cell-specific cytokines in humans. Clin Diagn Lab Immunol 2001;8:1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miranda TS, Figueiredo NF, Figueiredo LC, et al. Cytokine profiles of healthy and diseased sites in individuals with periodontitis. Arch Oral Biol 2020;120:104957. [DOI] [PubMed] [Google Scholar]
- [16].Günther A, Becker M, Göpfert J, et al. Comparison of bead-based fluorescence versus planar electrochemiluminescence multiplex immunoassays for measuring cytokines in human plasma. Front Immunol 2020;11:572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Usenko TS, Nikolaev MA, Miliukhina IV, et al. Plasma cytokine profile in synucleinophaties with dementia. J Clin Neurosci 2020;78:323–6. [DOI] [PubMed] [Google Scholar]
- [18].Aristizábal PA, Gómez MP, Escobar F, et al. Asociación entre enfermedad periodontal y disfunción endotelial. Revisión sistemática de la literatura. Univ Odontol 2013;32:147–60. [Google Scholar]
- [19].Al Bush MM, Razan KK, Raed AD. Effect of surgical and non-surgical periodontal debridement on vascular thrombotic markers in hypertensives. J Indian Soc Periodontol 2013;17:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pietropaoli D, Pinto R, Ferri C, et al. Poor oral health and blood pressure control among US hypertensive adults results from the National Health and Nutrition Examination Survey 2009 to 2014. Hypertension 2018;72:1365–73. [DOI] [PubMed] [Google Scholar]
- [21].D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol 2013;40: Suppl 14: S85–105. [DOI] [PubMed] [Google Scholar]
- [22].Muñoz-Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res 2019;116:28–39. [DOI] [PubMed] [Google Scholar]
- [23].Montenegro M, Ribeiro I, Kampits C, et al. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. J Clin Periodontol 2019;46:321–31. [DOI] [PubMed] [Google Scholar]
- [24].D’Isidoro O, Perrotti V, Lai Hui W, et al. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: a literature review. Am J Dent 2019;32:191–200. [PubMed] [Google Scholar]
- [25].Roca-Millan E, González-Navarro B, Sabater-Recolons MM, et al. Periodontal treatment on patients with cardiovascular disease: systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal 2018;23:e681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Higashi Y, Goto C, Hidaka T, et al. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis 2009;206:604–10. [DOI] [PubMed] [Google Scholar]
- [27].Vidal F, Figueredo CMS, Cordovil IFR. Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. J Clin Periodontol 2009;80:786–91. [DOI] [PubMed] [Google Scholar]
- [28].Piconi S, Trabattoni D, Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J 2009;23:1196–204. [DOI] [PubMed] [Google Scholar]
- [29].Touyz RM, Lang NN, Herrmann J, et al. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension 2017;70:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


