Abstract
Maternal immunisation is a public health strategy that aims to provide protection against certain infections to both mother and her foetus or newborn child. Vaccination of pregnant women induces vaccine-specific antibodies that lead to the subsequent transfer of these antibodies across the placenta or through breastfeeding to the offspring. At present, vaccinations in pregnancy are limited to pertussis, tetanus, diphtheria, polio, and the seasonal Influenza vaccine. Recently, some countries have incorporated routine antenatal vaccinations in their national immunisation programmes. Future vaccines targeted at pregnant women such as respiratory syncytial virus (RSV) and Group B streptococcus (GBS) are under development. The recently approved Covid-19 vaccines have no safety data for use in pregnancy at present, but have been considered in the UK in extremely vulnerable pregnant women or pregnant frontline health and social care workers.
In this article, we review the evidence supporting maternal immunisation and discuss the uptake of vaccines in pregnant women, challenges of recording the data on vaccine coverage, and consider reasons behind the present levels of uptake and strategies for future improvements.
Keywords: Immunisation, Maternal immunisation, Pertussis, Influenza, Vaccination uptake, Covid-19
Introduction
Maternal infections are estimated to contribute approximately 10–50% of stillbirths, in addition to 25% of neonatal deaths. Several of these adverse outcomes are thought to be vaccine-preventable [1]. Immunisation during pregnancy is a relatively new strategy where delivery of vaccines in the second or third trimester to pregnant women provides protection to the foetus, and subsequently, to the newborn by way of trans-placental transfer of the maternal antibodies. The transport of immunoglobulin G (IgG) across the placenta starts at about 17 weeks of gestation and increases with advancing gestation with higher foetal IgG levels than maternal serum levels seen at 40 weeks of gestation. The trans-placental transport of vaccine-specific antibodies can be affected by factors including placental abnormalities, total IgG concentration in maternal blood, the type of vaccine, and the interval between vaccination and delivery [2].
The potential role of maternal immunisation in protecting newborn infants has been made evident by maternal tetanus vaccination contributing to the lower incidence rates of neonatal tetanus [3]. Since the launch of the Maternal and Neonatal Tetanus Elimination (MNTE) initiative by the WHO, substantial progress has been made with the number of developing countries yet to obtain MNTE status down from 59 countries in 2000 down to only 12 countries as of July 2019 [4].
The WHO has recommended influenza vaccination during influenza season for all pregnant women since 2005 [5]; however, most European countries introduced seasonal influenza vaccination for pregnant women only after the H1N1/09 influenza pandemic [6].
During the last decade, an increasing number of countries have included antenatal immunisations in their national vaccination programmes. As mentioned earlier, tetanus vaccination in pregnancy has been used for years in most low- and middle-income countries. More recently, pertussis and influenza vaccination programmes in pregnancy have been recommended in a number of high-income countries along with some low- and middle-income countries [7] (see Fig. 1 ).
Fig. 1.
Countries with recommendations for immunisation against Pertussis in pregnancy by official authorities [7].
In particular, in the United Kingdom, the antenatal immunisation programme is presently limited to pertussis – combined with tetanus, diphtheria, polio (Tdap), and seasonal influenza vaccine. Similarly, in the US, the Centers for Disease Control and Prevention (CDC) recommends two vaccines directly for administration in pregnancy: the influenza vaccine and the Tdap (tetanus, diphtheria and pertussis) vaccine. Four other vaccines may also be considered in pregnancy depending on additional risk factors: Hepatitis A and B, pneumococcal, and meningococcal vaccines [8].
Pertussis
Pertussis is a highly infectious, vaccine-preventable acute respiratory illness also known as ‘whooping cough’ owing to its characteristic cough. Bordetella pertussis is the main organism responsible for acute illness with some cases also caused by Bordetella parapertussis [9]. Bordetella is an aerobic, Gram-negative coccobacilli [10] with overall 9 species identified to date [9].
B. pertussis, first isolated in 1906 by Bordet and Gengou, is a highly infectious bacterium responsible for the childhood infection, whooping cough. It is exclusive to human hosts and there is no evidence of an animal or environmental reservoir. The organism is transmitted mainly by respiratory droplets leading to the colonisation of ciliated respiratory epithelia in the trachea and bronchi [10].
The typical presentation of pertussis is seen in unvaccinated children and is a 3-stage illness: catarrhal, paroxysmal, and convalescent [11]. The onset of disease is gradual with symptoms similar to a mild upper respiratory tract infection; a worsening cough and the beginning of the paroxysmal stage that then follows other stages leading to the disease [9].
Globally, pertussis is ranked among the 10 leading causes of childhood mortality [12]. In 2018, there were more than 151,000 cases of pertussis, globally [13]. The illness course may become complicated by severe respiratory failure and pulmonary hypertension, and is associated with high mortality rates in newborn and young children [14]. Pertussis–associated encephalopathy is rare, occurring in 0.5–1% of all cases, and the diagnosis is suggested by seizures with pertussis infection, having excluded other differential diagnoses. It may be the result of the direct neurologic actions of toxins, effects of hypoxia, haemorrhages, vascular occlusions and latent virus infection [15].
Prevention
Routine vaccination of children and adolescents is the most effective preventive strategy. The 3-dose primary series diphtheria-tetanus-pertussis (DTP3) vaccines decrease the risk of severe pertussis in infancy. Although there are many different schedules in use worldwide, the WHO recommends the first dose to be administered as early as 6 weeks of age, with subsequent doses given 4–8 weeks apart [13]. The incidence of pertussis is highest in infants who are too young to have completed their primary vaccinations, who are at the highest risk of developing life-threatening complications [16]
Maternal vaccination
Vaccinating women in the third trimester of pregnancy offers the opportunity to provide early protection to infants through transplacental transfer of maternal antibodies [17]. A number of studies have demonstrated the efficient transfer of pertussis antibodies across the placenta [18,19]. Maternal pertussis vaccination has been introduced in a number of countries to minimise morbidity and mortality associated with pertussis in infants who are too young to be vaccinated.
In 2011–12, the UK saw the largest pertussis outbreak for over a decade, with 14 deaths in 2012 in infants born before the start of the pregnancy vaccination campaign who were too young to be vaccinated themselves [17,20]. This led the UK's Joint Committee on Vaccination and Immunisation to recommend the introduction of a temporary vaccination programme targeting pregnant women at 28–38 weeks' gestation, the aim is to protect children against pertussis before they reach their first routine immunisation. This programme began on October 1, 2012 with Repevax, a combined low-dose diphtheria, acellular pertussis, and inactivated poliomyelitis vaccine (dTAP/IPV).
An observational cohort study carried out by Donegan et al. evaluated the safety of pertussis vaccination in pregnancy. Their results concluded that there was no evidence of an increased risk of stillbirth during the 14 days immediately after vaccination (incidence rate ratio 0.69, 95% CI 0.23 to 1.62) or later in pregnancy (0.85, 95% CI 0.44 to 1.61) compared with historical national rates. Additionally, the researchers found no evidence of an increased risk of maternal or neonatal death, preeclampsia or eclampsia, haemorrhage, foetal distress, uterine rupture, placenta or vasa praevia, Caesarean section, low birth weight, or neonatal renal failure [∗[17], ∗[20]].
In 2014, an observational study by Amirthalingam et al. found the pertussis vaccination in pregnancy programme to be effective. Livebirth occurred in 26,684 women included in the Clinical Practice Research Datalink between October 1, 2012 and September 3, 2013; the average vaccine coverage before delivery based on this cohort was 64%. Vaccine effectiveness based on 82 confirmed cases in infants born from October 1, 2012, and younger than 3 months at onset was 91% (95% CI 84 to 95). Vaccine effectiveness was 90% (95% CI 82 to 95) when the analysis was restricted to cases in children younger than two months [21].
In 2013, the Centers for Disease Control and Prevention (CDC) in the United States recommended that pregnant women should receive tetanus, diphtheria, and acellular pertussis (Tdap) vaccine in every pregnancy during weeks 27 through 36 of gestation [16].
Munoz et al. evaluated the safety and immunogenicity of tetanus toxoid and reduced diphtheria toxoid acellular pertussis (TDaP) vaccine during pregnancy and its effect on infant responses to diphtheria and tetanus toxoids acellular pertussis (DTaP). In their study of 33 pregnant women who received TDaP between 30 and 32 weeks, the authors found no TDaP- associated serious adverse events in women or infants, and growth and development were similar in the treatment and placebo groups. They concluded that TDaP immunisation in the third trimester of pregnancy was well-tolerated and immunogenic, and infants of vaccinated women had significantly higher concentrations of antibodies to all vaccine antigens from birth till the initiation of immunisation with DTaP at age two months. Maternal immunisation with TDaP did not result in substantial or persistent interference of infant antibody responses to immunisation with DTaP [22].
The optimal timing of maternal immunisation against pertussis was investigated by a prospective observational study. The authors compared the effect of second trimester vs. third trimester tetanus-diphtheria-acellular pertussis (Tdap) immunisation in pregnant women who delivered at term. Geometric mean concentrations (GMCs) of cord blood antibodies to recombinant pertussis toxin (PT) and filamentous hemagglutinin (FHA) were assessed by an enzyme-linked immunosorbent assay. This study concluded that early second trimester maternal Tdap immunisation significantly increased neonatal antibodies [23].
In the UK, the Royal College of Obstetricians and Gynaecologists recommends offering the pertussis vaccine (Boostrix IPV in the UK) from the 20th week of pregnancy or soon after the anomaly scan [24]. Boostrix IPV also covers polio, diphtheria, and tetanus in pregnant women.
Uptake of pertussis vaccination
As discussed earlier, the antenatal pertussis vaccination programme aims to minimise morbidity, hospitalisation, and mortality in newborns through intrauterine transfer of maternal antibodies, until infants receive the first dose of the infant vaccination programme at 8 weeks of age [25]. The incidence of pertussis in infants <3 months of age has declined overall from 234 per 100,000 in 2012 to 52 per 100,000 in 2019 with a small, expected cyclical rise in numbers [26].
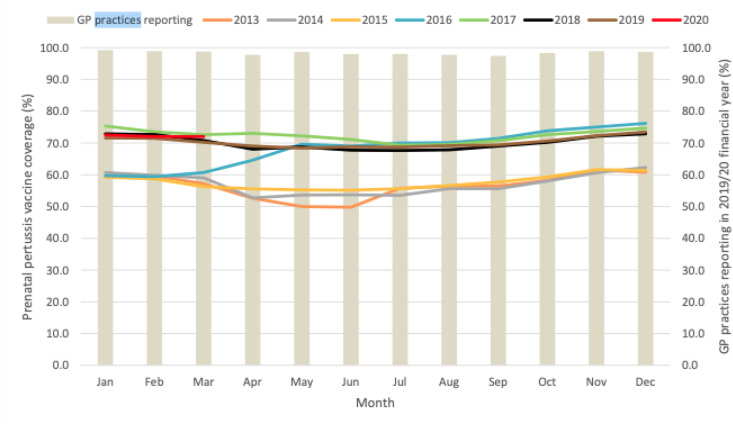
In the United Kingdom, monthly pertussis vaccine coverage in pregnant women ranged from 72.5% in January 2020 to 72.0% in March 2020 with an average coverage for the quarter at 72.2%. The average annual vaccine coverage for April 2019 to March 2020 was 70.5% [26] (see Fig. 2 ).
Fig. 2.
Monthly pertussis vaccination coverage (%) in pregnant women: England, 2013–2020 [26].
Influenza
Each year, an influenza vaccine is available to protect children and vulnerable adults from flu and its complications namely, pneumonia. Those thought to be high-risk include anyone above 65 years, pregnant women, children, adults with an underlying chronic illness and immunosuppressed patients. In England, influenza vaccination in pregnancy has been offered since 2010 [27].
The World Health Organisation (WHO) makes recommendations each year on which flu virus strains to include in the vaccine. This is usually either quadrivalent (protective against 4 virus strains – commonly 2 influenza A strains and 2 influenza B strains) or trivalent (protective against 3 strains) [Table 1 ]. It is imperative to stress that the WHO considers the cohort of pregnant women as the highest priority for receiving the seasonal influenza vaccine [Box 1 ] [28].
Table 1.
The recommended composition of influenza vaccine for use in 2020–2021 (northern hemisphere influenza season) by the WHO.
| Quadrivalent influenza vaccines |
Trivalent influenza vaccines |
||
|---|---|---|---|
| Egg-based vaccines | Cell- or recombinant-based Vaccines | Egg-based Vaccines | Cell- or recombinant-based Vaccines |
|
|
|
|
Box 1. WHO recommendations [20].
The WHO recommends seasonal influenza vaccination for
- a.Highest priority
- • Pregnant women
- b.Priority (in no particular order)
- • Children aged 6–59 months
- • Elderly
- • Individuals with specific chronic conditions
- • Healthcare workers
Alt-text: Box 1
Influenza in pregnancy
Influenza viruses are a group of RNA viruses that belong to the family Orthomyxoviridae and are classified into type A, B, and C on the basis of their core proteins. Droplets or respiratory secretions of the infected individuals primarily transmit the virus. Influenza infection occurs globally with an annual attack rate estimated at 5–10% among adults and 20–30% among children [29].
Although influenza infection typically results in a self-limiting illness, life-threatening complications can often be seen in high-risk groups which may lead to pneumonia, Intensive Care Unit (ICU) admissions, respiratory failure, sepsis, and death.
Pregnant women are among the at-risk groups feared to suffer from morbidity and mortality as a consequence of influenza infection. In the UK, 1-in-11 maternal deaths between 2009 and 2012 was caused by influenza [30].
Women at all stages of pregnancy, irrespective of the presence of pre-existing health conditions, are at an increased risk of serious respiratory illness and hospital admissions during influenza season [31]. In women hospitalised with influenza, an adverse outcome of pregnancy has been reported compared with the general population. Of note, preterm births of up to 59% have been reported in this cohort [32].
Influenza virus infection in pregnancy
The WHO has recommended maternal immunisation with inactivated influenza vaccine since 2005. A randomised study investigated the efficacy of maternal influenza immunisation. The results supported significant clinical effectiveness, with a reduction of 63% in laboratory-proven influenza illness in infants up to 6 months of age and reductions of 29% and 36% in the rates of respiratory illness with fever in infants and mothers, respectively [33].
Safety of flu vaccination in pregnancy
It remains an objective to address the concerns regarding the safety of influenza immunisation in pregnancy to improve uptake. In 2011, the WHO requested the Global Advisory Committee on Vaccine Safety (GACVS) to review the safety evidence of immunisation in pregnancy. This literature review concluded that there is robust safety evidence for multiple inactivated nonadjuvanted influenza vaccine preparations with no safety concerns, supporting WHO recommendation on influenza vaccination in pregnancy [34].
In the US, a study was conducted on the review of reports from the Vaccine Adverse Effect Reporting System (VAERS) in pregnant women who received trivalent inactivated influenza vaccine between 1990 and 2009 or live-attenuated vaccine from 2003 to 2009. This review did not find any unexpected or unusual adverse events in pregnancy or until the foetal outcome. Spontaneous miscarriage was the most reported outcome but even during the season with the highest rate reported for miscarriage in women following influenza vaccination (5.5 per 1 million vaccinations for the 2008–2009 season), this reported rate was lower than the published rate for miscarriage in the general population. The live-attenuated influenza vaccine is not indicated in pregnancy; however, this review did not identify any reports of adverse unexpected outcome where pregnant women received this inadvertently [35].
A recent systemic review has confirmed the safety of influenza vaccination in pregnancy. In particular, vaccination was not associated with preterm birth (PTB), low birthweight (LBW), small for gestational age (SGA), miscarriage, stillbirth, or congenital abnormalities. The authors also found high-quality evidence that inactivated influenza vaccine in pregnancy is associated with a lower incidence of low birthweight. Moderate quality data was also found on the association of maternal influenza vaccination and reduction in preterm births. The adjusted odds ratio for PTB was 0.87 (0.78–0.96), for LBW 0.82 (0.76–0.89), congenital abnormality 1.03 (0.99–1.07), SGA 0.99 (0.94–1.04), and stillbirth 0.84 (0.65–1.08) [36].
Uptake of influenza vaccine in pregnancy
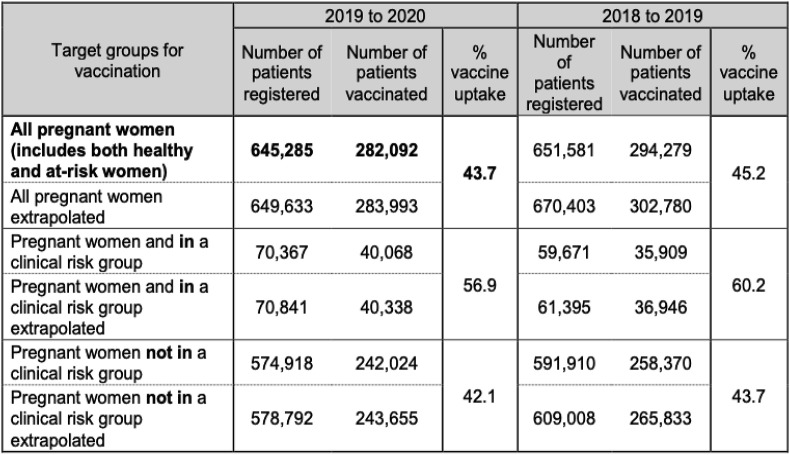
In England, the uptake of influenza vaccine in pregnant women (who were registered with a GP) from September 1, 2018 to February 28, 2019 was 45.2%, a reduction compared to 47.0% the previous year [37]. A further reduction to 43.7% of vaccination rates has been seen in this cohort in the 2019–2020 season [38] – Fig. 3 .
Fig. 3.
Observed and extrapolated estimate number of pregnant women registered and who received an influenza vaccine during the 2019 to 2020 season in England.
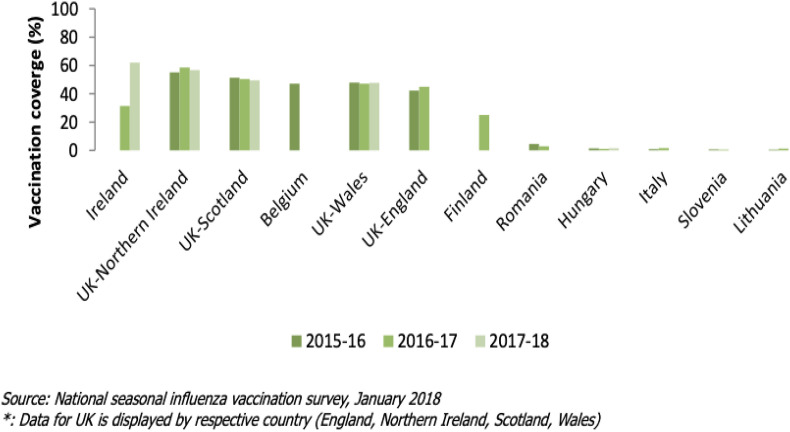
The European Centre for Disease Prevention and Control (ECDC) collects, shares, and disseminates information on national immunisation programmes and provides guidance for the improvement of immunisation systems in EU/EEA member states. Influenza vaccine coverage rates in pregnant women have been compared by the ECDC across 9 countries (Fig. 4 ) between 2015 and 2018. The remaining 19 out of 28 member states, where influenza vaccine is recommended in pregnancy reported that vaccine uptake in this cohort was not monitored [39] (see Fig. 4).
Fig. 4.
Seasonal influenza vaccination coverage rates for pregnant women in nine EU/EEA Member states, during the influenza seasons (2015–2016; 2016–2017; 2017–2018) [39].
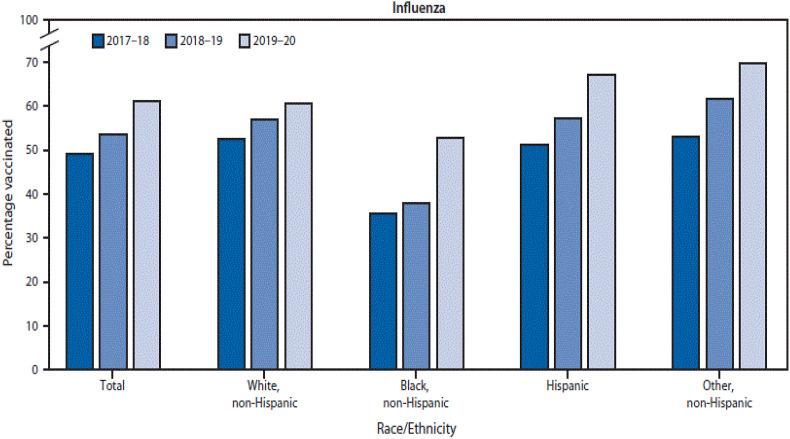
In the US, the Centre for Disease Control and Prevention has reported that 61.2% of pregnant women received influenza vaccination from 2019 to 2020. Although uptake remains suboptimal, the US did see an increased rate of vaccination in the pregnant cohort. Of note, influenza vaccine uptake amongst African-American and Hispanic women has increased in recent years (see Fig. 5 ) although disparities amongst ethnic groups still exist. The data for this report were collected through an internet panel survey and the authors have recognised the limitations including self-report of vaccination status and the associated recall bias [40].
Fig. 5.
Influenza vaccination coverage among pregnant women by race/ethnicity - Internet panel survey, United States, 2017-18 through 2019-20 influenza seasons [40].
Uptake of maternal influenza immunisation was reported in a tertiary referral maternity hospital in Dublin, Ireland. This was a quantitative study of postnatal women and out of the responders, 55.1% were vaccinated against influenza. Those of lower socio-economic status were less likely to be vaccinated (adjusted odds ratio [aOR] 0.29, 95% CI 0.09 to 0.89). Vaccination in a previous pregnancy (aOR 5.2, 95% CI 1.69 to 15.62) and information from a healthcare professional were strongly associated with vaccination (aOR 12.8, 95% CI = 2.65 to 62.5) [41]. This study identified that women from a higher socio-economic background, those with a university degree and those who attended as a private or semiprivate patient were more likely to be vaccinated against influenza in pregnancy.
Recording vaccine uptake data
Data collection on influenza vaccine uptake in England takes place through monthly (from October to February) and automated weekly surveys from all General Practice (GP) surgeries. The weekly survey is automated and acts as a ‘real time’ system for frontline staff to monitor the influenza vaccination programme in their local area.
Owing to the availability of influenza vaccination in settings other than general practice, for example, antenatal clinics and pharmacies, and the lag time required for GP records to be updated, the number of pregnant women vaccinated may be underestimated. To report influenza vaccine uptake more accurately, primary care practices should review the records for those patients who were pregnant but then ceased to be pregnant before the start of influenza vaccine season (1st of September in the United Kingdom) to ensure that these patients are not included and called for vaccination (unless they are in other high-risk groups). It is also imperative for GP surgeries to review their patient database and identify women who were not pregnant at the start of flu season, but who have subsequently become pregnant during winter months. Surgeries should also liaise with community midwives to ensure accurate and timely recording updates of pregnant women vaccinated outside general practice.
Factors influencing the uptake of vaccines in pregnancy
Understanding healthcare providers' approach in promoting maternal vaccinations and pregnant women's knowledge, attitudes and concerns are paramount in improving uptake. A recent multicentre questionnaire study from the UK investigated the acceptability of antenatal vaccination among patients and the healthcare providers' level of confidence in vaccine recommendations. This study found that the most commonly cited reason for declining antenatal vaccination was concerns regarding possible side effects for the baby and doubts regarding efficacy and necessity of immunisation. This study also found that women of ethnic minority backgrounds were significantly less likely to accept vaccination than Caucasian women. ‘White British’ women were significantly more likely to accept influenza (85% vs. 61%, OR 3.25, 95% CI 1.67–6.32) and pertussis (96% vs. 83%, OR 4.83, 95% CI 1.77–13.19) vaccination compared with those from all other ethnic groups. Another interesting finding was that a significant proportion of healthcare providers did not feel confident giving vaccination advice to pregnant women [42]. Immunisation rates are thought to be higher when a healthcare provider can recommend, offer, and administer the vaccine at the same visit as opposed to making a recommendation and referring the patient elsewhere to receive the vaccine.
In addition to ethnicity, it has also been reported that antenatal vaccination uptake is affected by other factors such as parity and deprivation [43]. McAuslane et al. reported that pertussis vaccine uptake was significantly lower in women with parity greater than three (48.1%; 95% CI 37.0–59.4) than in those with parity between one to three (63.0%; 95% CI 58.3–67.5). The same study also found that vaccine uptake was lowest in the most deprived quintile at 52.1% (95% CI 40.2–63.9) when compared with the least deprived quintile at 69.0% (95% CI 60.3–76.5), but this difference was not statistically significant [43].
Wilcox et al. [44] have also reported on the portrayal of antenatal pertussis and influenza vaccination in media articles; the majority of which positively portrayed vaccination in pregnancy (97%). Positive articles tended to focus on the benefits of protection for the child with regard to pertussis vaccination and maternal protection regarding influenza vaccination. The portrayal of pertussis vaccine as primarily benefitting the unborn child may have an influence on its higher uptake compared with influenza vaccination.
Vaccination uptake in pregnancy – how to improve uptake?
It is clear that the uptake of immunisation in pregnant women is still variable and suboptimal; improvements are necessary to reduce morbidity and mortality in both women and their offspring. The following recommendations could play a role in improving uptake:
-
•
The use of vaccination reminders in healthcare systems and patient information leaflets [45].
-
•
Healthcare professionals informing all pregnant women about the benefits of immunisations in pregnancy for them and their unborn child [45].
-
•
Making vaccination easily accessible and ideally provided at the same visit to avoid women having to make a separate appointment for the administration of the vaccine.
-
•
Exploring women's concerns with regard to vaccine safety and efficacy, and the ability of healthcare providers to discuss safety data and benefits for both patients and their unborn child.
-
•
Provision of vaccination during antenatal appointments by midwives or obstetricians rather than limiting provision to the primary care setting [46].
-
•
Supporting midwives with annual vaccination updates to enhance knowledge and confidence in recommending and administrating immunisations to pregnant women [47].
-
•
Use of posters promoting maternal immunisation in antenatal clinics, general practitioner waiting rooms, and childcare facilities [46].
COVID-19
A recent national cohort study from the UK has described the incidence and outcome of hospitalised pregnant women with symptomatic or asymptomatic Covid-19 infection compared with non-infected pregnant women. Compared with pregnant women without Covid-19, hospitalised pregnant women with symptomatic Covid-19 were more likely to be admitted to intensive care (aOR 57.67, 95% CI 7.80–26.70) but the absolute risk of poor outcomes was low. Iatrogenic preterm births were more common in women with symptomatic Covid-19 (aOR 11.43, 95% CI 5.07–25.75) [48].
Pregnant women were excluded from early Covid-19 vaccine research, leading to the lack of safety data. However, in the UK, the Joint Committee on Vaccination and Immunisation (JCVI) now advises that if a pregnant woman meets the definition of being extremely vulnerable to Covid-19, then she should discuss the option of the vaccine with her obstetrician. Some of the most likely relevant cohorts of women are those with solid organ transplants, homozygous sickle cell disease, chronic kidney disease or receiving dialysis, severe respiratory illness including cystic fibrosis/severe asthma, those with heart disease, or taking immunosuppressant medication. Furthermore, pregnant frontline healthcare or social care workers can also discuss the option of vaccination. The discussion should include lack of safety data of the approved vaccines for use in pregnancy, balanced against acknowledgment that other non-live vaccines used in pregnancy are not associated with risk [49].
Summary
-
•
Pregnancy and the immediate postnatal period are associated with a higher risk of morbidity and mortality secondary to infectious disease in both women and their babies. Some of these infections are vaccine preventable.
-
•
Maternal immunisation is a relatively new public health strategy to protect women and their babies. Transplacental transfer of maternal immunoglobulin G provides protection for foetuses and newborns who are too young to receive their primary course of vaccinations.
-
•
Routine antenatal vaccination has been adopted by some countries in recent years and is presently limited to pertussis, tetanus, diphtheria, inactivated polio, and seasonal influenza vaccine.
-
•
Despite recommendations by the WHO and healthcare providers, vaccine uptake still remains low, highlighting the need for continued education and promotion of benefits and safety profile along with patient education at every opportunity regarding the severity of vaccine preventable infections and potential complications for both women and their babies.
-
•
Healthcare providers should receive regular training and have up-to-date knowledge of maternal immunisations to counsel women and promote vaccinations in pregnancy with confidence.
-
•
Studies have identified a disparity in the uptake of maternal vaccinations in lower socioeconomic groups and ethnic minorities, highlighting the need to target education at these at-risk groups.
-
•
Further observational studies are required to identify other implementations that could improve vaccination uptake to more desirable levels.
Practice points.
-
•
The potential role of maternal immunisation in protecting newborn infants, has been made evident by maternal tetanus vaccination contributing to the lower incidence rates of neonatal tetanus.
-
•
The introduction of the pertussis vaccination programme in pregnancy has proved to be safe and effective in preventing morbidity and mortality in newborn infants.
-
•
Pertussis vaccine can be administered in pregnancy from 20 weeks' gestation or straight after the anomaly scan.
-
•
Women at all stages of pregnancy, irrespective of the presence of pre-existing health conditions, are at an increased risk of serious respiratory illnesses and hospital admissions during influenza season.
-
•
The WHO has recommended maternal immunisation with inactivated influenza vaccine since 2005.
-
•
Despite robust safety evidence of the influenza vaccine in pregnancy, uptake still remains suboptimal.
-
•
In the UK, it is now advised that certain clinically vulnerable cohorts of pregnant women and pregnant frontline workers should have discussions with regard to the Covid-19 vaccine with their doctor. Discussions should cover the lack of safety evidence in pregnancy versus its benefits.
Research agenda.
-
•
Observational studies of public health strategies to improve maternal vaccination uptake.
-
•
Research on the efficacy and safety of Covid-19 vaccines in pregnancy.
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
- 1.World health organisation . 2020. Maternal immunization and antenatal care service delivery situation analysis project.https://www.who.int/maternal_child_adolescent/topics/maternal/immunization-antenatal-care-delivery/en/ Online. Available from: [Google Scholar]
- 2.Englund J.A. The influence of maternal immunization on infant immune responses. J Comp Pathol. 2007 Jul;137(Suppl 1):S16–S19. doi: 10.1016/j.jcpa.2007.04.006. Epub 2007 Jun 5. PMID: 17553516. [DOI] [PubMed] [Google Scholar]
- 3.Thwaites C.L., Beeching N.J., Newton C.R. Maternal and neonatal tetanus. Lancet. 2015;385:362–370. doi: 10.1016/S0140-6736(14)60236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World health organisation . 2020. Maternal and neonatal tetanus elimination initiative.https://www.who.int/immunization/diseases/MNTE_initiative/en/index1.html online. Available from: [Google Scholar]
- 5.World health organisation . 2020. Influenza vaccines: WHO position paper.https://www.who.int/wer/2005/wer8033.pdf?ua=1 online. Available from: [Google Scholar]
- 6.Blanchard-Rohner G., Eberhardt C. Review of maternal immunisation during pregnancy: focus on pertussis and influenza. Swiss Med Wkly. 2017 Oct 27;147:w14526. doi: 10.4414/smw.2017.14526. PMID: 29120017. [DOI] [PubMed] [Google Scholar]
- Abu-Raya B., Maertens K., Edwards K.M., Omer S.B., Englund J.A., Flanagan K.L., et al. Global perspectives on immunization during pregnancy and priorities for future research and development: an international consensus statement. Front Immunol. 2020;11:1282. doi: 10.3389/fimmu.2020.01282. Published 2020 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swamy G.K., Heine R.P. Vaccinations for pregnant women. Obstet Gynecol. 2015;125(1):212–226. doi: 10.1097/AOG.0000000000000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieves D., Heininger U. Bordetella pertussis. Microbiolspec. 2016;4(3) doi: 10.1128/microbiolspec.EI10-0008-2015. [DOI] [PubMed] [Google Scholar]
- 10.Mattoo S., Foreman-Wykert A.K., Cotter P.A., Miller J.F. Mechanisms of bordetellapathogenesis. Front Biosci. 2001;6:E168–E186. doi: 10.2741/mattoo. [DOI] [PubMed] [Google Scholar]
- 11.Cherry J.D., Heininger U. In: Feigin and Cherry’sTextbook of pediatric infectious diseases. seventh ed. Cherry J.D., Harrison G.J., Kaplan S.L., Steinbach W.J., Hotez P.J., editors. Elsevier Saunders; Philadelphia, PA: 2014. Pertussis and other Bordetella infec- tions; pp. 1616–1639. [Google Scholar]
- 12.World health organisation . 2020. Pertussis.https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis/en/ online. Available from: [Google Scholar]
- 13.World health organisation . 2020. Pertussis.https://www.who.int/health-topics/pertussis online. Available from: [Google Scholar]
- 14.Paddock C.D., Sanden G.N., Cherry J.D., Gal A.A., Langston C., Tatti K.M., et al. Pathology and pathogenesis of FatalBordetella pertussisInfection in infants. Clin Infect Dis. 2008;47(3):328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 15.Rocha G., Soares P., Soares H., Pissarra S., Guimaraes H. Pertussis in the newborn: certainties and uncer- tainties in 2014. Paediatr Respir Rev. 2015;16:112–118. doi: 10.1016/j.prrv.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (Cdc) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131–135. [PMC free article] [PubMed] [Google Scholar]
- Amirthalingam G. Vol. 98. 2013. Strategies to control pertussis in infants Archives of Disease in Childhood; pp. 552–555. [DOI] [PubMed] [Google Scholar]
- Gall S.A., Myers J., Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204:334. doi: 10.1016/j.ajog.2010.11.024. e1–5. [DOI] [PubMed] [Google Scholar]
- 19.Leuridan E., Hens N., Peeters N., De Witte L., Van de Meeren O., Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J. 2011;30:608–610. doi: 10.1097/INF.0b013e3182093814. [DOI] [PubMed] [Google Scholar]
- Donegan K., King B., Bryan P. Vol. 349. 2014. Safety of pertussis vaccination in pregnant women in UK: observational study BMJ. g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirthalingam G., Andrews N., Campbell H., Ribeiro S., Kara E., Donegan K., et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014 doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- Munoz F.M., Bond N.H., Maccato M., Pinell P., Hammill H.A., Swamy G.K., et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. J Am Med Assoc. 2014;311:1760–1769. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberhardt C.S., Blanchard-Rohner G., Lemaitre B., Boukrid M., Combescure C., Othenin-Girard V., et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62:829–836. doi: 10.1093/cid/ciw027. an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joint committee on vaccination and immunisation . 2020. Pertussis (whooping cough) vaccination now offered from 20 weeks of pregnancy.https://www.rcog.org.uk/en/news/rcog-statement-pertussis-whooping-cough-vaccination-now-offered-from-20-weeks-of-pregnancy online. Available from: [Google Scholar]
- 25.Public health england . 2020. Complete routine immunisation schedule.https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule online. Available from: [Google Scholar]
- 26.Pertussis vaccination programme for pregnant women update: vaccine coverage in England, january to March 2020: Engl Health Protect Rep Volume 14 Number 10.
- 27.Public Health England . 2013. Immunisation against infectious disease (the green book)https://www.gov.uk/government/publications/influenza-the-green-book-chapter-19 Available from: [Google Scholar]
- 28.World health organisation . 2020. Recommended composition of influenza virus vaccines for use in the 2020 - 2021 northern hemisphere influenza season.https://www.who.int/influenza/vaccines/virus/recommendations/2020-21_north/en/ online. Available from: [Google Scholar]
- 29.World health organisation . 2020. Influenza.https://www.who.int/biologicals/vaccines/influenza/en/ online. Available from: [Google Scholar]
- 30.On behalf of MBRRACEUK . In: Knight M., Kenyon S., Brocklehurst P., Neilson J., Shakespeare J., Kurinczuk J.J., editors. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014. Saving lives, improving mothers' care - lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009–12. [Google Scholar]
- 31.Dodds L., McNeil S.A., Fell D.B., Allen V.M., Coombs A., Scott J., et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ (Can Med Assoc J) 2007;176:463–468. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer W.J., van Noortwijk A.G.A., Bruines H.W., Wensing A.M.J. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94:797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E., et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 34.Keller-Stanislawski B., Englund J.A., Kang G., Mangtani P., Neuzil K., Nohynek H., et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32:7057–7064. doi: 10.1016/j.vaccine.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 35.Moro P., Broder K., Zheteyeva Y., Walton K., Rohan P., Sutherland A., et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol. 2011;204(2):146. doi: 10.1016/j.ajog.2010.08.050. e1–146.e7. [DOI] [PubMed] [Google Scholar]
- 36.Giles M.L., Krishnaswamy S., Macartney K., Cheng A. The safety of inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccines Immunother. 2019;15(3):687–699. doi: 10.1080/21645515.2018.1540807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/804889/Seasonal_influenza_vaccine_uptake_in_GP_patients_1819.pdf
- 38.Seasonal influenza vaccine uptake in GP patients: winter season 2019 to 2020. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/912099/Annual-Report_SeasonalFlu-Vaccine_GPs_2019-20_FINAL_amended.pdf
- 39.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2018. Seasonal influenza vaccination and antiviral use in EU/EEA Member States – overview of vaccine recommendations for 2017–2018 and vaccination coverage rates for 2015–2016 and 2016–2017 influenza seasons. [Google Scholar]
- 40.Razzaghi H., Kahn K.E., Black C.L., Lindley M.C., Jatlaoui T.C., Fiebelkorn A.P., et al. Infleunza and Tdap vaccination coverage among pregnant women – United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391–1397. doi: 10.15585/mmwr.mm6939a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., McEntee E., Drew R., O’Reilly F., O’Carroll A., O’Shea A., et al. Influenza vaccination in pregnancy: vaccine uptake, maternal and healthcare providers’ knowledge and attitudes. A quantitative study. BJGP Open. 2018;2(3) doi: 10.3399/bjgpopen18X101599. bjgpopen18X101599. Published 2018 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilcox C.R., Calvert A., Metz J., Kilich E., MacLeod R., Beadon K., et al. Determinants of influenza and pertussis vaccination uptake in pregnancy: a multicenter questionnaire study of pregnant women and healthcare professionals. Pediatr Infect Dis J. 2019;38(6):625–630. doi: 10.1097/INF.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 43.McAuslane H., Utsi L., Wensley A., Coole L. Inequalities in maternal pertussis vaccination uptake: a cross-sectional survey of maternity units. J Publ Health. March 2018;40(Issue 1):121–128. doi: 10.1093/pubmed/fdx032. [DOI] [PubMed] [Google Scholar]
- 44.Wilcox C.R., Bottrell K., Paterson P., Schulz W.S., Vandrevala T., Larson H.J., et al. Influenza and pertussis vaccination in pregnancy: portrayal in online media articles and perceptions of pregnant women and healthcare professionals. Vaccine. 2018 doi: 10.1016/j.vaccine.2018.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong V.W.Y., Lok K.Y.W., Tarrant M. Interventions to increase the uptake of seasonal influenza vaccination among pregnant women: a systematic review. Vaccine. 2016;34:20–32. doi: 10.1016/j.vaccine.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Bisset K.A., Paterson P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: a systematic review. Vaccine. 2018;36(20):2751–2759. doi: 10.1016/j.vaccine.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Green D., Labriola G., Smeaton L., Falconer M. Prevention of neonatal whopping cough in England: the essential role of the midwife. Br J Midwifery. 2017;25(4):224–228. [Google Scholar]
- 48.Vousden N., Bunch K., Morris E., Simpson N., Gale C., O'Brian P., et al. The incidence, characteristics and outcomes of pregnant women hospitalised with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) MedRxiv. 2021 doi: 10.1101/2021.01.04.21249195. 01.04.21249195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updated advice on COVID-19 vaccination in pregnancy and women who are breastfeeding. Royal College of Obstetricians & Gynaecologists; 2021. https://www.rcog.org.uk/en/news/updated-advice-on-covid-19-vaccination-in-pregnancy-and-women-who-are-breastfeeding/ Internet. [cited 22 February 2021]. Available from. [Google Scholar]