Abstract
Objective:
To demonstrate how formative evaluation methods can be used to plan for implementation of evidence-based psychosocial screening in pediatric oncology.
Methods:
Multidisciplinary pediatric oncology professionals participated in focus groups to adapt the distress thermometer for electronic administration and develop health systems processes to promote psychosocial screening in the pediatric oncology outpatient clinic setting. Seven 1‐hour focus groups were conducted using a structured guide based on the reach, efficacy, adoption, implementation, and maintenance framework and transcribed verbatim. Two independent raters coded transcripts using a quasi‐deductive approach with high inter‐coder reliability (Cohen kappa >0.80).
Results:
Participants’ (N=44) responses were used to identify overarching topics related to the adoption, implementation, and maintenance of e-screening including: barriers to meeting families’ psychosocial needs, identification of champions, suggestions to adapt the proposed e-screening program, perceived barriers to e-screening, and potential impact of carrying out e-screening. Following review of qualitative data, we employed specific implementation strategies to promote adoption, implementation, and maintenance of an e-screening program.
Conclusions:
Perceived barriers to implementation of psychosocial screening remain substantial, yet enthusiasm for using EHR technology to help meet patient needs through regular assessment was evident among pediatric oncology professionals. Electronic administration of screening and integration of results into the EHR in real time were identified as critical needs to overcome barriers to e-screening. Formative evaluation including qualitative data from stakeholders can be used to tailor implementation strategies to successfully support the adoption, implementation, and maintenance of e-screening programs in pediatric oncology.
Keywords: Electronic Health Records, Pediatrics, Process Assessment, Health Care, Psychooncology, Screening
1. Introduction
Children and adolescents with cancer are at increased risk for a wide range of psychosocial difficulties that can negatively impact quality of life and health outcomes (1–8). These issues do not end when treatment is complete, and it is estimated that 20–30% of pediatric cancer survivors experience difficulties with coping or psychosocial distress (5–9). The psychosocial challenges that accompany childhood cancer are not limited to the patient, but affect the child’s parents/caregivers as well. Parents of children with cancer frequently encounter difficulties with anxiety, depression, post-traumatic stress, social isolation, and lack of financial resources (10–16). Given these risks, routine psychosocial screening of patients and family members was established as an evidence-based standard for pediatric oncology psychosocial care in 2015 (3). Psychosocial screening in pediatric oncology has also been recommended by the Institute of Medicine, American Cancer Society, and the National Comprehensive Cancer Network (3), yet results from a 2016 survey of pediatric oncology programs (N=144) indicated that only 25% of oncology programs had implemented an ongoing approach for psychosocial assessment at every clinic visit (18).
Barriers to implementing systematic psychosocial screening programs in pediatric oncology include challenges at the institutional, provider/staff, and patient/parent/caregiver levels. Many institutions have limited psychosocial resources available; thus allocating provider or staff time to conduct or review psychosocial screening is an impediment in the organizational context (18–21). Timely reporting and communication of screening results to relevant teams within an institution can also be difficult (18,20). Institutional technical support for psychosocial screening is necessary to overcome barriers to secure data storage of screening results and integration of psychosocial screening into patients’ electronic health records (EHR) (19.20). Electronic screening (e‐screening) efforts can be employed to overcome institutional barriers associated with paper administration (21–23), yet digital solutions can be hindered when organizations do not adopt formalized plans for screening or by e‐screening plans that do not fit within existing clinical workflows at the institution (21). Beliefs that psychosocial needs are not relevant to oncology treatment or fears that screening will uncover more needs than can be met by staff are among the barriers to screening noted by providers and staff (18,19). Additionally, staff may disagree about who should be responsible for screening (e.g., nurses, social workers, psychologists) and be concerned about increased workload stemming from screening (18,20). Achieving consistency in screening can also be challenging when staff hold differing opinions about which screening tool should be used and how often it should be administered (19). Clinicians have many factors to attend to during a patients’ medical appointments, and thus may forget to review or respond to screening results (21). Although there is evidence showing families find psychosocial screening acceptable in the context of pediatric oncology care (20, 23, 24), providers and staff often believe that families will find screening to be stigmatizing and be hesitant to share sensitive psychosocial information (19). Additionally, at the family level, limited English language proficiency, literacy limitations, and time to complete measures can all be barriers to screening (18–20).
In adult oncology settings, implementation strategies have been used to successfully overcome barriers to psychosocial screening and shift institutional cultures to become more supportive of screening programs (25). Formative qualitative inquiry using the reach, efficacy, adoption, implementation, and maintenance (RE‐AIM) framework can be useful in planning for and developing strategies to promote implementation of new clinical interventions (26). Thus, we sought to conduct a formative evaluation guided by RE‐AIM prior to implementing electronic psychosocial screening (e‐screening) in the pediatric setting. The RE‐AIM framework posits that the impact of an intervention is a function of five systems‐based and social ecological factors: RE‐AIM (27, 28). We conducted iterative focus groups with clinical staff to inform adaption of the pediatric distress thermometer (DT) for electronic administration and develop strategies to promote the adoption, implementation, and maintenance of psychosocial screening within a large pediatric oncology outpatient clinic practice. The objective of this article is to demonstrate how formative research methods can be used to plan for implementation of evidence‐based psychosocial screening in pediatric oncology.
Methods
2.1. Study design and setting
This formative evaluation study involved focus groups with providers across the spectrum of pediatric cancer care. Key informant interviews are established methods of measurement in implementation science (27, 29). The interview guide (Supplemental Table A) was structured around the adoption, implementation, and maintenance components of the RE-AIM framework (26–28). The study was conducted at the Aflac Cancer and Blood Disorders Center at Children’s Healthcare of Atlanta, Egleston and Scottish Rite campuses. This is a large, hospital-based pediatric hematology/oncology center (i.e., >16,000 pediatric oncology outpatient visits each year) in the southeastern region of the United States. The study was approved by the Institutional Review Board at Emory University (IRB00098758).
2.2. Screening tool
We sought to develop an e-screening platform using digital versions of the pediatric Distress Thermometer (DT) adapted by Patel et al. (2011) to assess: 1.) parent proxy-report of patient distress among pediatric oncology patients ≤17 years old, 2.) adolescent and young adult (AYA) self-report of distress among oncology patients ≥13 years old, and 3.) parent self-report of distress among parents of oncology patients ≤17 years old (30). The DT has been recommended by the National Comprehensive Cancer Network (31), repeatedly validated (32), and widely used around the world (33) to quickly screen for psychosocial distress within adult oncology settings. The DT includes a unidimensional rating of distress ranging from 0 (no distress) to 10 (extreme distress), as well as a brief problem checklist to identify emotional, practical, family, spiritual, and physical causes of the reported distress (31). The DT has been adapted and validated to assess distress among pediatric cancer patients and parents (11, 30, 34, 35). Within the pediatric oncology setting, ratings on the DT have been used to classify patient distress as mild (0–4), moderate (5–7), or severe (8–10)(30). We chose to use the pediatric DT as our e‐screening tool because we needed a measure that could be completed quickly in outpatient clinic in conjunction with physical review of systems (ROS). We also needed a validated measurement tool that offered AYA self‐report in addition to parent proxy and parent self‐reports of psychosocial distress.
2.3. Data collection
Focus group participants were sampled from pediatric oncology staff located across two outpatient clinic locations using a combination of purposeful sampling strategies common to implementation science research (36). We first used a criterion-i strategy and invited all psychosocial staff to participate in two focus groups conducted in October and November 2018. Results from these groups were reviewed and followed by a snowball sampling strategy where we asked the physician medical director of each of our disease based teams (i.e., leukemia/lymphoma, survivorship, neuro-oncology, bone marrow transplant, and solid tumor) to identify medical providers/staff for recruitment. Focus groups with medical staff were conducted between April and June 2018. Participants provided verbal consent (with a waiver of written consent to protect their anonymity) prior to participating in the focus groups. Participants also provided self-report information about their demographics (i.e. age, sex, race, and ethnicity), job title, years working under current title, and years working at the pediatric hospital. During each focus group, participants reviewed printed copies of the parent-proxy, AYA self-report, and parent self-report versions of the pediatric Distress Thermometer, adapted by Patel et al., and a proposed procedural workflow for administration of the electronic DT and review of e-screening scores (30). Focus group moderators (Sean N Halpin, Shade Owolabi, and Jordan Gilleland Marchak) had expertise in qualitative interviewing and followed a semi-structured interview guide. The interview guide included specific questions about the Distress Thermometer (n= 8), implementation of the distress e-screener (n= 5), how it would impact workflow (n= 5), and a closing question. All interviews were audio-recorded and transcribed verbatim.
2.4. Data analysis
We used NVivo 12.3 for data management and analysis (QSR International Pty Ltd, London). The study team developed a codebook with definitions based on the interview guide. A quasi-deductive approach was applied, emphasizing both deductive and inductive approaches to analysis (37). Initial deductive coding was completed by two researchers (Sean N. Halpin and Shadé Owolabi) with experience in qualitative data analysis. Deductive codes were directly based on questions from the interview guide (Supplemental Table A) related to adoption (i.e., understand why organizations and staff participate in an intervention), implementation (i.e., understand how the intervention can be delivered consistently and how it needs to be adapted), and maintenance (i.e., understand program sustainability and the reasons why organizations decide to continue or discontinue interventions) (26). The coding comparison tool within NVivo was used to ensure inter-rater reliability with a kappa coefficient of at least 0.8 for all coded text. Initial analysis resulted in all codes meeting or exceeding the 0.8 inter-rater reliability cut-off, thus there was no need for reconciliation. Next, an inductive approach was applied to identify subordinate themes within the data. For example, the deductive code called ‘suggestions’ had inductive codes associated with it which addressed how e-screening is administered, changes to the screener, and documentation and communication of scores. Finally, data were sorted into a coding matrix to further understand any interrelation between identified themes (Supplemental Table B).
2. Results
3.1. Participants
Seven focus groups were conducted across two outpatient clinic sites including N=44 multidisciplinary faculty and staff. At total of 46 clinicians/staff were approached to participate. No staff actively refused to participate; however n=2 staff were unable to attend the scheduled focus groups due to unexpected clinical duties. Groups were approximately one-hour long (M=52 min, SD=10.8 min) and comprised of psychosocial staff members serving at each site (n=15 and n=14), as well as medical staff from disease-based teams across both sites including leukemia/lymphoma (n=3), neuro-oncology (n=5), survivorship (n=3), bone marrow transplant (n=2), and solid tumor (n=2). Participant demographic and occupational characteristics are outlined in Table 1.
Table 1.
Participant demographics
| Characteristics | Total Participants (n= 44) |
|---|---|
| Mean age (years) | 38.8 (±10.84) |
| Female, n (%) | 43 (97.7%) |
| Race/Ethnicity, n (%) | |
| White, non-Hispanic | 39 (88.6%) |
| Black or African American | 3 (6.8%) |
| Asian | 1 (2.3%) |
| White, Hispanic | 1 (2.3%) |
| Job Title | |
| Social Worker | 12 (27.3%) |
| Nurse | 7 (15.9%) |
| Nurse Practitioner/Physician Assistant | 6 (13.6%) |
| Child Life Specialist | 5 (11.4%) |
| Psychologist | 5 (11.4%) |
| Teacher | 5 (11.4%) |
| Physician | 2 (4.5%) |
| Chaplain | 1 (2.3%) |
| Music Therapist | 1 (2.3%) |
| Years performing in current title, (mean, SD) | 8.79 (±9.39) |
| Years working at institution, (mean, SD) | 7.15 (±8.51) |
3.2. Qualitative results and tailored strategies
Overarching topics and subordinate themes related to the adoption, implementation, and maintenance of e-screening were identified (Table 2). Subordinate themes were nested within overarching topics (Table 2) and are described in detail below. Overarching topics and subordinate themes were organized into a matrix to illustrate representation across focus groups (Supplemental Table B). Following review of our qualitative data, we employed specific implementation strategies as outlined by Powell et al. to address identified barriers to adoption, implementation, and maintenance of an e-screening program in outpatient pediatric oncology (38).
Table 2.
Qualitative themes and tailored strategies to promote adoption, implementation, and maintenance of e-screening
| RE-AIM Component Qualitative topics / Subordinate themes & example quotes |
Tailored strategy |
|---|---|
| Adoption | |
| Barriers to meeting family needs | |
| Staffing barriers: “I feel like they’re [social workers] spread so thin though, just the per patient volumes and I mean we’ve had so many new patients. Just trying to keep up with their current patients and then the new ones as well… I think they do the best they can. They do an amazing job, but it’s just a lot for one person. It seems like they could use extra bodies, for sure.” | Changing the record systems |
| Family barriers: “I think it’s nice to know they would get to do it [e-screening] themselves versus having to talk with a nurse or something about it. I feel like they’re more likely to be honest, like if they’re filling it out themselves, versus us just asking them questions outright.” | Promote adaptability |
| Identifying champions | |
| “All of us [psychosocial staff]. We’ll have opinions about it for sure.” “I think it’s probably gonna be the nurses. I think mostly—just ‘cause they’re the frontline when patients come back.” |
Identify and prepare champions Build a coalition |
| Implementation | |
| Suggestions for e-screening | |
| Administration considerations: “I think that the front desk actually has a copy of the review of systems in Spanish. I feel like I never see it. I feel like they usually just give the English one and then we end up just doing it all by mouth with an interpreter” | Promote adaptability |
| Changes to Distress Thermometer: “I think ‘concerns’ would be a more neutral, less negative connotation.” | Promote adaptability |
| Documentation and communication: “Or it could pop up on—as like a box in Epic [EHR]. The question how you communicate it is gonna be important because it’s not—we’re not always on—at least I know I am not always on my email. It can’t be email. It can’t be email | Remind clinicians |
| Barriers to e-screening | |
| Logistical concerns: “Patients have very limited time, and trying to rush patients out of the room because the room needs to be cleaned and so you can get ready for the patient. It’s constant.” |
Develop educational materials Conduct ongoing training |
| Equipment and technology: “We’ve had iPads go missing pretty frequently.” “We’ll have to figure out how they’re cleaned after each parent, patient uses them.” |
Change physical structure and equipment |
| Maintenance | |
| Impact of e-Screening on Patient care, Job performance, & Clinic flow | |
| “Honestly, if it gives us more insight, they’re more willing to answer here than they are to tell us, it would impact [care] greatly.” “This might be a way for them [social work and psychology] to kind of be able to go into the room prepared and kind of know what they need.” “The risk is that you give them [families] this opportunity and then we can’t meet the need.” “If it adds time on, I think it’s something that probably needed to be taken care of anyways. So it’s necessary to be added into in the flow and incorporated into the flow.” |
Mandate change Change the record systems Revise professional roles |
3.3. Planning for adoption
3.3.1. Barriers to meeting family needs.
Participants identified both staffing barriers and family barriers to meeting psychosocial needs. Participants across four groups voiced appreciation for the clinical care psychosocial staff provide, while also discussing concerns about high caseloads and not having enough staff to meet all of the needs families experience throughout the course of treatment. Clinicians in three focus groups raised concerns about families being unwilling to engage in mental health services offered by psychosocial staff and how lack of supports negatively impacted coping during treatment.
3.3.2. Identifying champions.
Participants in all focus groups made recommendations for potential champions of e-screening. Nurses were most often proposed as champions (N=5) for e-screening because of their important role in outpatient oncology care. Securing buy-in from psychosocial staff was also deemed essential for adoption of e-screening. Front office staff and advanced practice providers (APP) were also mentioned as important potential supporters of e-screening.
3.3.3. Strategies to promote adoption.
Through integration with the EHR, psychosocial staff were hopeful about the opportunity to generate data to use to leverage for additional staff resources. Changes to the EHR were made to allow the division to track proportions of patients reporting distress and for psychosocial staff to record their encounters with patients using discrete, quantifiable data (Changes to record systems). By adapting the DT for electronic administration, staff believed families would be more likely to feel open and comfortable reporting their psychosocial concerns as opposed to verbal or paper-and-pencil administration (Promote adaptability). A multidisciplinary task-force of champions was developed that included nurses, social workers, psychologists, advanced practice providers, and physicians (Identify and prepare champions). In addition to champions, fostering relationships with leaders from hospital administration, information technology, medicine, nursing, psychology, and social work, were fundamental to obtaining formal commitments to support and adopt e-screening at our institution (Build a coalition).
3.4. Planning for implementation
3.4.1. Suggestions for e-screening.
Participants offered insights into how electronic distress screening could be administered, specific changes that could be made to adapt the DT to improve acceptability and utility in pediatric oncology, and how the scores should be documented and communicated.
Regarding Administration considerations, almost all groups (N=6) offered considerations about what administration accommodations would need to be made in order to collect reliable answers. Many of these conversations raised patient privacy concerns. Language and literacy issues were discussed as important considerations. Given the demographic make-up of the sites’ patient population, staff agreed having both an English and Spanish version of the screener would be necessary. For families whose primary language was neither English nor Spanish, coordination with interpretation services would be necessary. For families with literacy issues, participants suggested having an option for someone on staff to help read the instrument, as necessary.
All focus groups (N=7) discussed specific recommended Changes to the Distress Thermometer instruments. Recommendations for changing the instrument included using non-judgmental terminology to normalize the screening process for families. Participants also suggested refinement or addition of items on the checklist to improve face validity, usability, and inclusion of additional categories to the checklist (i.e., concerns about taking medications, needle sticks/procedures, bullying, social media, and sexuality for AYA patients). There was also concern about potential confusion around blank checklist, and whether the blank list reflected that the patient was not experiencing any problems or if it was a result of a patient not completing the DT. To address this concern a participant suggested, “I think one of the things for the electronic version would be to add a box that just says no concerns.”
Participants across four focus groups were also concerned about Documentation and communication of scores between staff. Groups discussed integration into the patients’ electronic health record (EHR) as essential for timely review of results and recommended use of alerts to ensure staff were made aware of families reporting high distress.
3.4.2. Barriers to e-screening.
Barriers to electronic distress screening included Logistical concerns and potential challenges with Equipment and technology. Logistical concerns were raised in three focus groups. These concerns ranged from questioning who will complete the screener if a non-parent attends the appointment, distractions competing for parent’s attention, and how often the screener should be completed. The strain screening might have on the allocation of limited resources (e.g., time and clinic rooms) for the units, was especially concerning. Participants across three focus groups also expressed concerns about the equipment including whether it would be stolen and if there would be enough tablets to accommodate the number of patients seen in clinic during peak times. Participants in the bone marrow transplant focus group raised the issue of infection control related to tablet use. Concerns were also mentioned related to the reliability of the Wi-Fi connection in clinics needed to transmit results entered on the tablets.
3.4.3. Strategies to promote implementation.
Promoting adaptability of e-screening was integral to implementation. As suggested by stakeholders, we adapted the language used on the DT to normalize psychosocial screening to families and capture additional items deemed valuable to the care of pediatric oncology patients. We also adapted English and Spanish versions of the pediatric DT for electronic administration. We sought to support clinicians by integrating screening results into the patients’ EHR in real-time and developed best practice alert functionality within the EHR to remind clinicians to review and respond to screening results indicating a patient was in severe distress (DT scores ≥8) and should be seen emergently by a psychologist (patient high distress) or social worker (parent high distress) before leaving clinic.
In order to better coordinate the logistics of administering and responding to e-screening, we developed educational materials including a clinical algorithm outlining the screening process (Figure 2) and discipline specific tip sheets outlining the responsibilities of nurses, physicians, psychologists, registration staff, and social workers with step-by-step instructions for the e-screening clinical and EHR workflows. To help alleviate concerns raised about equipment, we purchased tablets with geolocation software and sanitizing equipment (Change physical structure and equipment).
Figure 2.
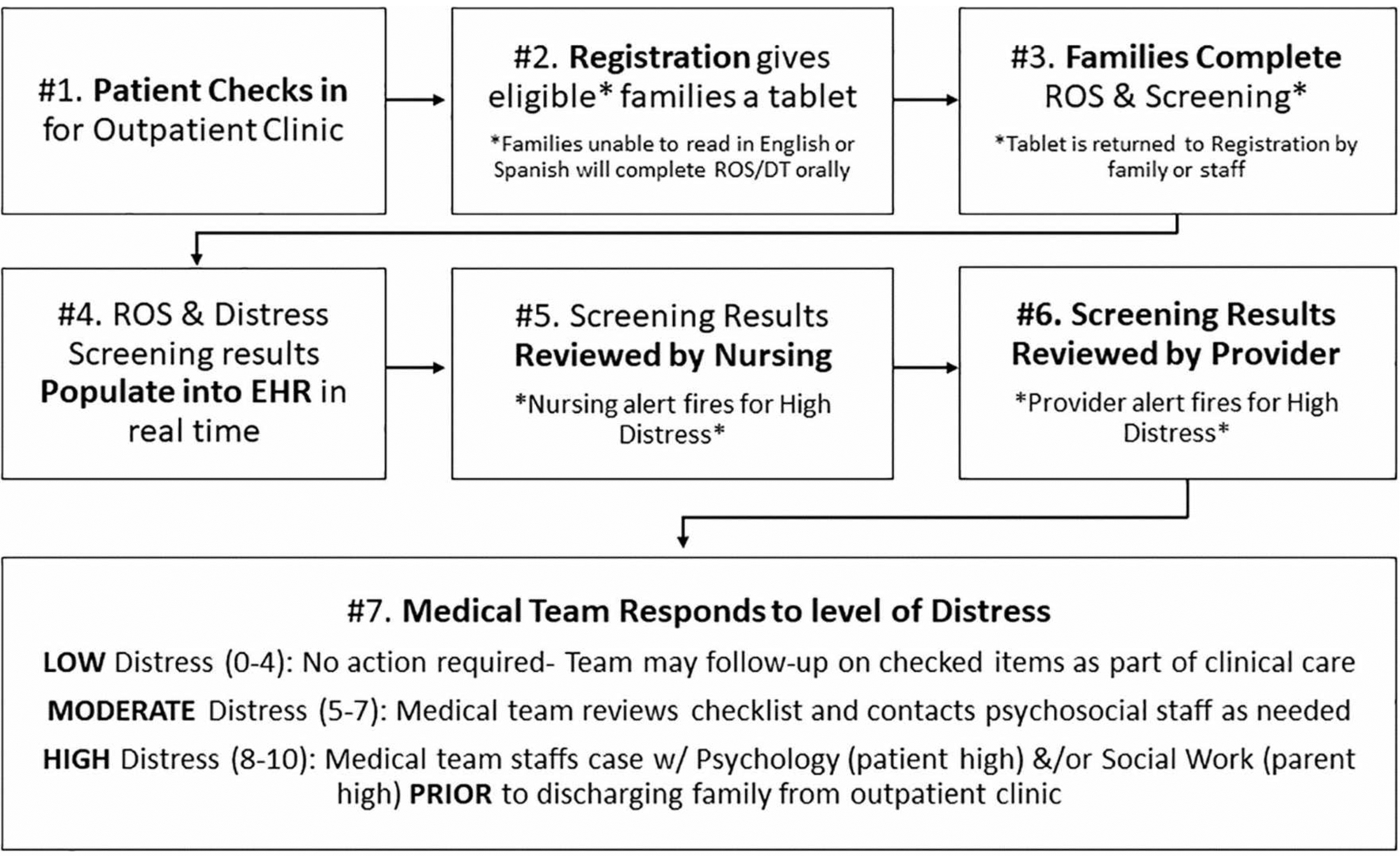
Clinical algorithm for e-screening process in outpatient pediatric oncology
Notes: ROS= Review of Systems. EHR= Electronic Health Record
3.5. Planning for Maintenance
3.5.1. Impact of e-screening.
Participants were asked how they expected electronic distress screening would impact Patient care, their personal Job performance, and how it would fit into the Clinic flow. Electronic distress screening was discussed as a method for opening lines of communication to patients who have unmet and unrecognized needs across all seven focus groups. Screening was discussed as a potential conversation starter between patients and staff, as well as a tool to focus the consultation with psychosocial staff members. Screening results could help identify important topics that might uncover important concerns previously unknown to staff and identify families who may “slip through the cracks.” Five focus groups discussed the impact of e-screening on their own and other staff members’ job performance. Participants felt there were both positive and negative implications to electronic distress screening on job performance. In particular, they felt that having more information about patient needs would make their job easier by helping connect patients to the care they need. Yet participants were also concerned about the potential for increasing their workloads and fear they would not be able to adequately address identified needs. Participants also voiced optimism that e-screening could help provide data to justify requests for increased psychosocial staff members. Discussion of clinic flow occurred in all seven focus groups and focused on the attention and time needed to address emergency situations identified by the electronic distress screening. Some participants voiced unique needs pertaining the workflows in specific clinics, with another participant reflecting on the need to communicate with patients about screening results prior to initiating sedation in sedation clinics.
3.5.2. Strategies to promote maintenance.
Strategies to change the infrastructure and culture around e-screening at our institution have been key to promoting maintenance in the pediatric oncology outpatient setting. Given that staff have limited time available during busy clinics, we tailored the physical symptoms checklist of the DT to mirror our medical review of systems (ROS) items allowing for electronic patient reporting. Thus, we were able to remove ROS administration tasks from nursing workflows freeing up bandwidth for nurses to perform their roles in the e-screening process without adding extra time (Revise professional roles). Leveraging the coalitions with physician and nursing leaders, we were able to mandate change and keep e-screening as a clinical care priority after the implementation research study concluded. By changing the record systems at our institution to allow patients to complete psychosocial screening integrated within their EHR, e-screening is programmed to be automatically administered every 7 days at outpatient clinic check-in, and thus can be universally offered at each clinic visit on a weekly basis. With these strategies in place, we have been able to continue running our e-screening program for one year after our study funding ended.
3. Discussion
Our formative evaluation sought to incorporate stakeholder feedback to promote the adoption, implementation, and maintenance of psychosocial screening practices in pediatric oncology. Overall, stakeholders expressed both enthusiasm and concerns about implementing e-screening in our outpatient clinics. Integration of results into the EHR in real time was identified as a critical need to overcome institutional barriers to adoption, implementation, and maintenance of e-screening. Participants agreed that e-screening would improve patient care and likely impact personal job performance, both positively and negatively. They recognized that registration, nursing, psychology, and social work would need to collaborate daily as co-champions for implementation to succeed. For example, registration staff must provide families with sanitized tablets at clinic check-in in order to complete e-screening. Nurses must review screening results in the EHR and place appropriate referrals for family supports as needed. Nurses also initiate urgent consults to psychology or social work who are available to respond with in-clinic supports and safety assessment for families in severe distress (DT scores ≥8). Developing and fostering a network of co-champions took over a year and required weekly group meetings in the weeks leading up to the launch of e-screening in our clinics.
Similar to other studies of barriers to psychosocial screening in pediatric oncology, our participants reported concerns about staffing limitations, lack of time, and fears about potential stigma to families (18–21). Additional barriers related to electronic administration of screening tools in pediatric oncology were more unique to our study and included logistical, equipment, and technological concerns; particularly with concern to timely integration into the electronic health record (EHR). We also heard participants’ perceptions of potential benefits to e-screening which likely impacted adoption, implementation, and maintenance. Participants noted that e-screening could facilitate conversation between clinical staff and patients, allow for proactive/focused discussions of patient needs, normalize patient concerns, identify families who may “slip through the cracks”, and provide data to justify staffing requests. Although we did not formally include cost as a measured implementation outcome in our evaluation, accessing new funding from research grants and donors to offset the initial costs to the hospital for purchasing tablets, contracting to build architecture within the EHR to integrate e-screening, and partial salary support for staff involved in leading efforts were also critical to program success.
5. Study limitations and future directions
Through studying the process of creating and implementing an e-screening program, we have been able to learn and document valuable lessons that can be adapted to develop psychosocial screening programs at other pediatric oncology institutions. However, there are limitations to the study which may impact the generalizability of the findings beyond our institution. We employed purposive sampling for the focus groups with the medical teams and the opinions shared may not be representative of all pediatric oncology professionals. Participants were comprised of stakeholders nominated by their program directors, and it is possible participants were chosen by directors because of their psychological mindedness which could have positively biased participant responses. Additionally, the majority of participants identified as female and non-Hispanic white. Although this reflects the workforce at our pediatric institution, this may not be reflective of the workforce demographics in other geographical regions. Future research should consider employing stratified sampling to capture a more diverse and generalizable group of participants. Additionally, future qualitative studies may assess variations in viewpoints between providers caring for subgroup of patients to explore contextual factors unique to each subgroup of patients. To complement the pre-implementation qualitative research presented in this paper, we are currently analyzing post-implementation quantitative data to characterize the reach and implementation fidelity of our e-screening program across a 10-month observation period using data abstracted from the EHR. Although several studies have found comparable validity between digital and paper screening tools (39–41), we are seeking to validate our adapted versions of the pediatric DT, as we have made slight modifications to wording and layout of the instrument from the original paper‐and‐pencil pediatric DT. Lastly, we have an ongoing project to assess the benefit, value, and ease of completing e‐screening among parents/caregivers and AYA patients.
4. Clinical implications
Routine screening for psychosocial difficulties can facilitate appropriate referrals for support services within the oncology setting and is helpful in improving mental health outcomes among vulnerable families (22, 42, 43). Longitudinal psychosocial screening can help identify families at risk for emotional and social problems and ensure they receive appropriate psychosocial supports throughout the pediatric cancer continuum from diagnosis to survivorship (2). Adult oncology setting have been able to use implementation strategies to successfully overcome barriers to psychosocial screening (26), and similarly, our clinical program has benefited from a formative evaluation including tailored strategies. Our e-screening program launched in September 2018 and was able to administer, document, and communicate the results of over 16,000 parent-proxy, AYA self-report, and parent-self report Distress Thermometers during the program’s first 10 months. Given that only 25% of pediatric institutions have implemented ongoing, standardized psychosocial screening (17), our approach to successfully leverage EHR technology and implementation strategies to provide psychosocial screening could be readily disseminated and used to help other pediatric oncology programs build systematic, evidence-based psychosocial screening programs.
5. Conclusions
Perceived barriers to implementation of psychosocial screening remain substantial, yet enthusiasm for using EHR technology to help meet patient needs through regular assessment was evident among pediatric oncology professionals. Electronic administration of screening and integration of results into the EHR in real time were identified as critical needs to overcome barriers to e-screening. Formative evaluation including qualitative data from stakeholders can be used to tailor implementation strategies to successfully support the adoption, implementation, and maintenance of e-screening programs in pediatric oncology.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported by a St. Baldrick’s Foundation, Inc. Supportive Care Grant (PI: JGM) and the Intervention Development, Dissemination and Implementation Developing shared resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement: Authors have no conflicts of interest to disclose.
Data Availability Statement: Research data are not shared due to privacy concerns for our stakeholder participants.
Contributor Information
Jordan Gilleland Marchak, Emory University School of Medicine and Aflac Cancer and Blood Disorders Center, 2015 Uppergate Drive, Atlanta, GA, 30322.
Sean N. Halpin, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322.
Cam Escoffery, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322.
Shadé Owolabi, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322.
Ann C. Mertens, Emory University School of Medicine and Aflac Cancer and Blood Disorders Center, 2015 Uppergate Drive, Atlanta, GA, 30322.
Karen Wasilewski Masker, Emory University School of Medicine and Aflac Cancer and Blood Disorders Center, 2015 Uppergate Drive, Atlanta, GA, 30322.
References
- 1.Kazak AE, Barakat LP, Ditaranto S, Biros D, Hwang WT, Beele D, et al. Screening for Psychosocial Risk at Pediatric Cancer Diagnosis: The Psychosocial Assessment Tool. J Pediatr Hematol Oncol. 2011;33(4):289. [DOI] [PubMed] [Google Scholar]
- 2.Kazak AE, Rourke MT, Alderfer MA, Pai A, Reilly AF, Meadows AT. Evidence-based assessment, intervention and psychosocial care in pediatric oncology: A blueprint for comprehensive services across treatment. J Pediatr Psychol. 2007;32(9):1099–110. [DOI] [PubMed] [Google Scholar]
- 3.Kazak AE, Abrams AN, Banks J, Christofferson J, DiDonato S, Grootenhuis MA, et al. Psychosocial Assessment as a Standard of Care in Pediatric Cancer. Pediatr Blood Cancer. 2015;62(S5):S426–S59. [DOI] [PubMed] [Google Scholar]
- 4.Wakefield CE, McLoone J, Goodenough B, Lenthen K, Cairns DR, Cohn RJ. The psychosocial impact of completing childhood cancer treatment: a systematic review of the literature. J Pediatr Psychol. 2010;35(3):262‐274. [DOI] [PubMed] [Google Scholar]
- 5.Kahalley LS, Wilson SJ, Tyc VL, et al. Are the psychological needs of adolescent survivors of pediatric cancer adequately identified and treated? Psychooncology. 2013;22(2):447‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianinazzi ME, Rueegg CS, Wengenroth L, et al. Adolescent survivors of childhood cancer: are they vulnerable for psychological distress? Psychooncology. 2013;22(9):2051‐2058. [DOI] [PubMed] [Google Scholar]
- 7.Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007; 25(24):3649‐3656. [DOI] [PubMed] [Google Scholar]
- 8.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol. 2005;30(1):9‐27. [DOI] [PubMed] [Google Scholar]
- 9.Mertens A, Gilleland‐Marchak J. Mental health status of adolescent cancer survivors. Clin Oncol Adolesc Young Adults. 2015;5:87‐95. [Google Scholar]
- 10.Phipps S, Dunavant M, Lensing S, Rai SN. Psychosocial predictors of distress in parents of children undergoing stem cell or bone marrow transplantation. J Pediatr Psychol. 2005;30(2):139‐153. [DOI] [PubMed] [Google Scholar]
- 11.Haverman L, van Oers HA, Limperg PF, et al. Development and validation of the Distress Thermometer for parents of a chronically ill child. J Pediatr. 2013;163(4):1140‐6. e2. [DOI] [PubMed] [Google Scholar]
- 12.Barakat LP, Kazak AE, Meadows AT, Casey R, Meeske K, Stuber ML. Families surviving childhood cancer: a comparison of posttraumatic stress symptoms with families of healthy children. J Pediatr Psychol. 1997;22(6):843‐859. [DOI] [PubMed] [Google Scholar]
- 13.Bona K, Dussel V, Orellana L, et al. Economic impact of advanced pediatric cancer on families. J Pain Symptom Manag. 2014;47(3):594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creswell PD, Wisk LE, Litzelman K, Allchin A, Witt WP. Parental depressive symptoms and childhood cancer: the importance of financial difficulties. Support Care Cancer. 2014;22(2):503‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakefield CE, McLoone JK, Butow P, Lenthen K, Cohn RJ. Parental adjustment to the completion of their child’s cancer treatment. Pediatr Blood Cancer. 2011;56(4):524‐531. [DOI] [PubMed] [Google Scholar]
- 16.Kearney JA, Salley CG, Muriel AC. Standards of psychosocial care for parents of children with cancer. Pediatr Blood Cancer. 2015;62(Suppl 5):S632‐S683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scialla MA, Canter KS, Chen FF, et al. Delivery of care consistent with the psychosocial standards in pediatric cancer: current practices in the United States. Pediatr Blood Cancer. 2018;65(3):e26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazak AE, Barakat LP, Askins MA, et al. Provider perspectives on the implementation of psychosocial risk screening in pediatric cancer. J Pediatr Psychol. 2017;42(6):700‐710. [DOI] [PubMed] [Google Scholar]
- 19.Barrera M, Alexander S, Shama W, Mills D, Desjardins L, Hancock K. Perceived benefits of and barriers to psychosocial risk screening in pediatric oncology by health care providers. Pediatr Blood Cancer. 2018;65(12):e27429. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy MC, Wakefield CE, DeGraves S, Bowden M, Eyles D, Williams LK. Feasibility of clinical psychosocial screening in pediatric oncology: implementing the PAT2.0. J Psychosoc Oncol. 2016;34-(5):363‐375. [DOI] [PubMed] [Google Scholar]
- 21.Schepers SA, Sint Nicolaas SM, Haverman L, et al. Real‐world implementation of electronic patient‐reported outcomes in outpatient pediatric cancer care. Psychooncology. 2017;26(7):951‐959. [DOI] [PubMed] [Google Scholar]
- 22.Engelen V, Detmar S, Koopman H, et al. Reporting health‐related quality of life scores to physicians during routine follow‐up visits of pediatric oncology patients: is it effective? Pediatr Blood Cancer. 2012;58(5):766‐774. [DOI] [PubMed] [Google Scholar]
- 23.Schepers SA, Engelen VE, Haverman L, et al. Patient reported outcomes in pediatric oncology practice: suggestions for future usage by parents and pediatric oncologists. Pediatr Blood Cancer. 2014;61(9):1707‐1710. [DOI] [PubMed] [Google Scholar]
- 24.Pierce L, Hocking MC, Schwartz LA, Alderfer MA, Kazak AE, Barakat LP. Caregiver distress and patient health‐related quality of life: psychosocial screening during pediatric cancer treatment. Psychooncology. 2017;26(10):1555‐1561. [DOI] [PubMed] [Google Scholar]
- 25.Loscalzo M, Clark KL, Holland J. Successful strategies for implementing biopsychosocial screening. Psychooncology. 2011;20(5):455‐462. [DOI] [PubMed] [Google Scholar]
- 26.Holtrop JS, Rabin BA, Glasgow RE. Qualitative approaches to use of the RE‐AIM framework: rationale and methods. BMC Health Serv Res. 2018;18(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasgow RE, Harden SM, Gaglio B, et al. RE‐AIM planning and evaluation framework: adapting to new science and practice with a 20‐year review. FrontPublic Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE‐AIM framework. Am J Public Health. 1999;89(9):1322‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis CC, Proctor E, Brownson RC. Measurement Issues in Dissemination and Implementation Research. In: Brownson RC, Colditz GA, Proctor EK, eds. Dissemination and Implementation Research in Health: Translating Science to Practice. 2nd ed. New York, NY: Oxford University Press; 2017:229. [Google Scholar]
- 30.Patel SK, Mullins W, Turk A, Dekel N, Kinjo C, Sato JK. Distress screening, rater agreement, and services in pediatric oncology. Psychooncology. 2011;20(12):1324‐1333. [DOI] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network. Distress management. Clinical practice guidelines. J Natl Compr Cancer Netw. 2003;1(3):344. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell AJ. Short screening tools for cancer‐related distress: a review and diagnostic validity meta‐analysis. J Natl Compr Cancer Netw. 2010;8(4):487‐494. [DOI] [PubMed] [Google Scholar]
- 33.Donovan KA, Grassi L, McGinty HL, Jacobsen PB. Validation of the distress thermometer worldwide: state of the science. Psychooncology. 2014;23(3):241‐250. [DOI] [PubMed] [Google Scholar]
- 34.Wiener L, Battles H, Zadeh S, Widemann BC, Pao M. Validity, specificity, feasibility and acceptability of a brief pediatric distress thermometer in outpatient clinics. Psychooncology. 2017;26(4):461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiener L, Battles H, Bedoya SZ, Baldwin A, Widemann BC, Pao M. Identifying symptoms of distress in youth living with Neurofibromatosis Type 1 (NF1). J Genet Couns. 2018;27(1):115‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patton MQ. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. Thousand Oaks, CA: Sage Publications; 2014. [Google Scholar]
- 38.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cronly J, Duff AJ, Riekert KA, et al. Online versus paper‐based screening for depression and anxiety in adults with cystic fibrosis in Ireland: a cross‐sectional exploratory study. BMJ Open. 2018;8(1): e019305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson G, Kaldo‐Sandström V, Ström L, Strömgren T. Internet administration of the hospital anxiety and depression scale in a sample of tinnitus patients. J Psychosom Res. 2003;55(3):259‐262. [DOI] [PubMed] [Google Scholar]
- 41.Holländare F, Andersson G, Engström I. A comparison of psychometric properties between internet and paper versions of two depression instruments (BDI‐II and MADRS‐S) administered to clinic patients. J Med Internet Res. 2010;12(5):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alderfer MA, Mougianis I, Barakat LP, et al. Family psychosocial risk, distress, and service utilization n pediatric cancer. Cancer. 2009;115(S18):4339‐4349. [DOI] [PubMed] [Google Scholar]
- 43.Barrera M, Alexander S, Atenafu EG, et al. Psychosocial screening and mental health in pediatric cancer: a randomized controlled trial. Health Psychol. 2020; 39(5): 381‐ 390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


