Abstract
Primary malignant aortic tumors are rare and aggressive. Most cases are diagnosed at advanced stages or during autopsies with a median overall survival of 8 months from diagnosis. We present the case of a 59-year-old male with angiosarcoma involving all segments of the thoracic aorta and a large floating thrombus causing acute mesenteric ischemia, which was treated successfully with embolectomy. Graft replacement of the aorta should be considered in cases of localized disease and when patients are fit for surgery. The best medical and surgical treatment remains unclear, and further studies are needed.
Keywords: Sarcoma, Angiosarcoma, Aorta, Aortic tumor, Intimal sarcoma
INTRODUCTION
Primary malignant aortic tumors (PMATs) are a rare and aggressive pathology with a high tendency for distal embolization and metastasis [1,2]. The most frequent location of growth is the thoracic aorta [3], and the most frequent histotype is angiosarcoma [4]. The diagnosis is made during autopsy or after surgical treatment for embolic or aneurysmal complications [2]. We report a case of angiosarcoma involving the ascending, arch, and descending aorta that manifested as acute mesenteric ischemia that was treated with embolectomy and conservative surgical therapy. The case report has been approved by ethical committee of Brotzu Hospital (approving no. Pg 198/2020). Informed consent was obtained from the patient for publication of the case report and the accompanying images.
CASE
A 59-year-old Caucasian male was admitted to the emergency department for acute abdominal pain, nausea, and diarrhea. The patient had hypertension, dyslipidemia, and diabetes. Clinical examination revealed tenderness in the lower abdomen, and the only significant laboratory finding was leukocytosis (19,500/mm3). Chest and abdominal computed tomography (CT) scan with contrast revealed a floating thrombus formation partially occluding the ascending, arch, and descending aorta (Fig. 1, 2). The superior mesenteric artery (SMA) was occluded at the origin, and the bowel appeared distended with air-fluid levels. The patient was transferred to our department of vascular surgery and underwent embolectomy of the SMA using a Fogarty catheter; a soft, whitish material was extracted and sent to pathology. Histopathologic reports revealed myofibroblastic sarcoma that was, as with the majority of intimal angiosarcomas, poorly differentiated. After embolectomy, SMA patency was restored and bowel function recovered completely.
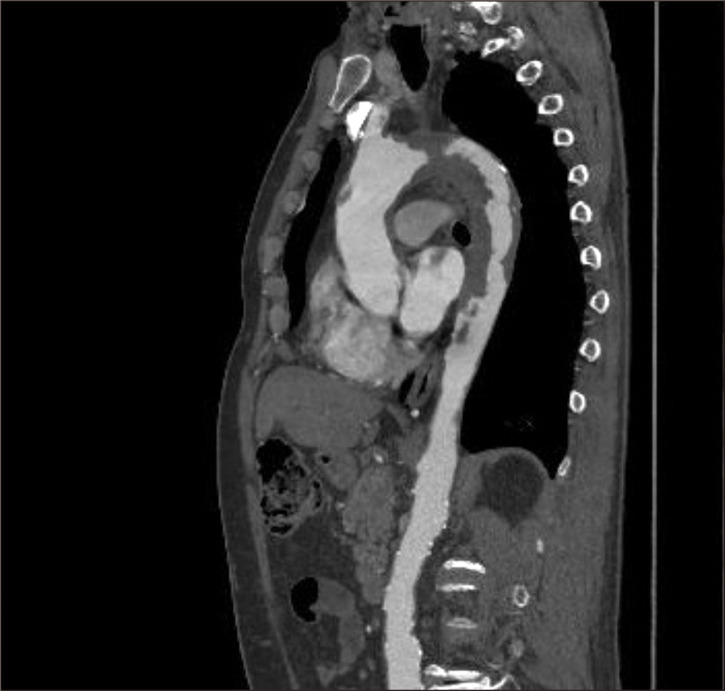
Fig. 1.
Computed tomography scan showed neoplastic invasion of the entire thoracic aorta.
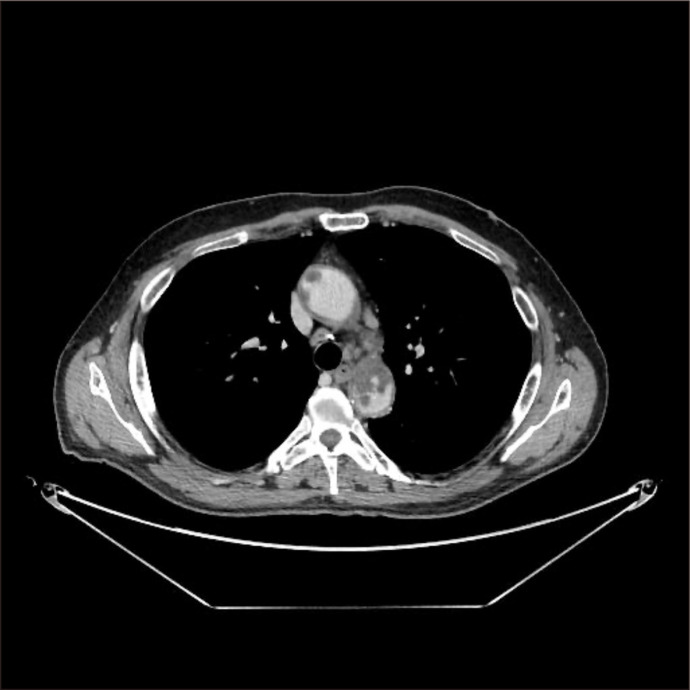
Fig. 2.
Computed tomography scan revealed an aortic wall mass with floating thrombus.
On the third postoperative day, the patient experienced sudden onset abdominal pain with diffuse abdominal distension. Laboratory examinations revealed a slight increase in lactate dehydrogenase (417 IU/L), alanine aminotransferase (155 IU/L), and aspartate aminotransferase (55 IU/L) levels. Chest and abdominal CT scans with contrast were performed immediately after reporting of symptoms and revealed partial thrombosis of the common hepatic artery and total occlusion of the splenic artery at its origin (Fig. 3). Positron emission tomography and chest magnetic resonance imaging (MRI) with contrast confirmed massive neoplastic invasion of the thoracic aorta without distant metastasis or lymphadenopathy (Fig. 4). Radiotherapy and chemotherapy were not recommended by oncologists.
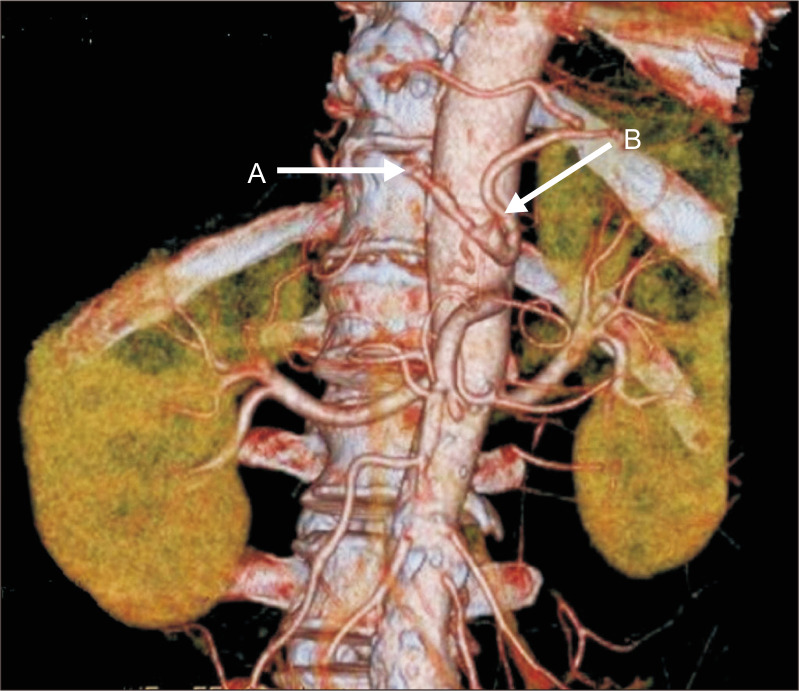
Fig. 3.
Computed tomography scan showed partial thrombosis of the common hepatic artery (arrow A) and total occlusion of the splenic artery (arrow B).
Fig. 4.
Magnetic resonance imaging showed neoplastic invasion of the aortic wall.
The patient was discharged on the tenth postoperative day in a stable general condition. He underwent anticoagulant therapy with low-molecular-weight heparin for one month. Follow-up CT after 2 months showed no evidence of disease progression, which was maintained seven months after surgery. While the patient died of cancer cachexia 8 months after diagnosis, an overall good quality of life was maintained after surgery.
DISCUSSION
PMAT is a rare disease that was first reported by Brodowski [5] in 1873. Currently, only 224 cases, including ours, have been described in literature. This disease is more frequent in men with a male to female ratio of 1.5:1, and the mean age of presentation is 60±11.9 years. The median overall survival from diagnosis is 8 (7-9) months, and the 1-, 3-, and 5-year survival rates are 26%, 7.6%, and 3.5%, respectively [6].
Aortic sarcomas present in one of two varieties: intimal and mural. The intimal variety is more frequent and often develops into intraluminal polyps or exhibits longitudinal extension resulting in aortic obstruction and distal embolization [7-9]. The mural form originates from the media or adventitia and generally grows outward to invade periaortic tissue [10]. While PMAT can involve all segments of the thoracoabdominal aorta, they are most commonly found in the abdominal aorta (42.6%), followed by the thoracic aorta (37.7%), thoracoabdominal aorta (12.1%), and the aortic arch (11.3%) [6]. Our case represents an aggressive and rare form of the tumor involving the ascending, arch, and descending aorta, with a large floating thrombus.
The clinical presentation is often characterized by nonspecific symptoms such as fever, asthenia, and claudication which can delay a timely and correct diagnosis [6,7]. Symptoms directly related to tumor growth, such as back pain or aneurysm rupture, usually occur at advanced stages of the disease [1].
In most cases, the diagnosis is made at the time of autopsy [1,2] or secondary to embolic events in the intra-abdominal organs, extremities, skin, or brain [2,6-9]. While rare, PMAT can cause hypertensive crisis secondary to renal artery embolization [10], as well as myocardial infarction due to cardiac involvement [7,11]. Overall, these tumors exhibit aggressive behavior with a high tendency for metastasis, particularly to the bone, lung, lymph nodes, and liver [1,2].
CT scan and MRI with contrast are both useful for diagnosis, although some authors prefer MRI due to superior soft tissue characterization and differentiation between enhancing tumors and frequently associated thrombi [2,7,11]. If a thrombus-like mass is revealed by imaging, an aortic angiosarcoma should be considered especially if the mass is located inside the aorta and exhibits a progressive course despite anticoagulation [12,13]. Sometimes PMAT can mimic an infected aneurysm [14]; thus, histopathological and immunohistochemical examinations are mandatory to arrive at a correct diagnosis [15,16]. The correct treatment for PMAT is still unclear, and evidence available in literature is scarce. A recent review noted that combined surgical and medical therapy showed the greatest benefit with a median survival rate of 12 months (range, 8-12 months) compared to 7 months if surgical treatment is used alone (range, 2-16 months) and 2 months if no treatment is given (range, 0.5-15 months) [6]. This has been confirmed by some authors who recommend surgical resection and chemotherapy as the standard care for angiosarcoma [2,11]. Radical surgical resection should be performed if the tumor is operable [10]. Complete surgical resection with negative margins is the goal of curative intent [11].
Our patient presented with a typical thromboembolic complication in the mesenteric area at the time of diagnosis. Despite the absence of metastasis, his disease was fairly advanced and had already invaded the entire thoracic aorta. These findings severely limited both our surgical and medical options as we are unable to substitute the entire thoracic aorta. Furthermore, chemotherapy and radiotherapy were not recommended by the oncology consultant at this stage of the disease. We performed urgent embolectomy and provided supportive care. In our case, the occlusion of the hepatic and splenic arteries did not reduce visceral blood flow as it was maintained by collateral reperfusion; therefore, we decided not to perform surgery and opted instead for conservative therapy. Given the highly malignant nature of angiosarcoma, palliative therapy to relieve symptoms should always be considered mainstream therapy [12]. We performed anticoagulation therapy to ensure that the patient does not suffer from another embolic event. A synergistic interplay between different specialists (vascular surgeon, oncologist, and internist) is essential to ensure the best clinical management [16]; the patient’s survival fell within the range reported in literature, good quality of life was maintained, and he eventually died of underlying disease.
In conclusion, malignant primitive tumors of the aorta are very rare; however, they present with aggressive behavior and a tendency to embolize and metastasize. Patients are usually diagnosed at an advanced stage of the disease with limited treatment options. Combined surgical and medical therapies may improve the overall survival. Surgical treatment should include tumor resection and graft replacement. Further studies are needed to assess optimal therapeutic options.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: GD, SC. Analysis and interpretation: GD, SC. Date collection: GD. Writing the article: GD, FU. Critical revision of the article: GD, SC, GG. Final approval of the article: GD, GG, ANG, MM, FS, FU, SC. Statistical analysis: none. Obtained funding: none. Overall responsibility: SC.
REFERENCES
- 1.Mohsen NA, Haber M, Urrutia VC, Nunes LW. Intimal sarcoma of the aorta. AJR Am J Roentgenol. 2000;175:1289–1290. doi: 10.2214/ajr.175.5.1751289. [DOI] [PubMed] [Google Scholar]
- 2.Rusthoven CG, Liu AK, Bui MM, Schefter TE, Elias AD, Lu X, et al. Sarcomas of the aorta: a systematic review and pooled analysis of published reports. Ann Vasc Surg. 2014;28:515–525. doi: 10.1016/j.avsg.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Chiche L, Mongrédien B, Brocheriou I, Kieffer E. Primary tumors of the thoracoabdominal aorta: surgical treatment of 5 patients and review of the literature. Ann Vasc Surg. 2003;17:354–364. doi: 10.1007/s10016-003-0018-x. [DOI] [PubMed] [Google Scholar]
- 4.Thalheimer A, Fein M, Geissinger E, Franke S. Intimal angiosarcoma of the aorta: report of a case and review of the literature. J Vasc Surg. 2004;40:548–553. doi: 10.1016/j.jvs.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Brodowski W. Primäres sarkom der aorta thoracica mit verbeitung des neugebildes in derunteren köerperhälfte [in German] Jahresb Leistung Geschl Med. 1873;8:243–245. [Google Scholar]
- 6.Vacirca A, Faggioli G, Pini R, Freyrie A, Indelicato G, Fenelli C, et al. Predictors of survival in malignant aortic tumors. J Vasc Surg. 2020;71:1771–1780. doi: 10.1016/j.jvs.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Muñoz A, Hitt R, Artiles V, López A, Hernández R, Cortés-Funes H, et al. Primary aortic sarcoma with widespread vascular embolic metastases. Eur J Intern Med. 2003;14:258–261. doi: 10.1016/S0953-6205(03)00068-2. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Kobayashi J, Matsuda H, Ishibashi-Ueda H. A primary angiosarcoma in the aorta. Interact Cardiovasc Thorac Surg. 2007;6:832–833. doi: 10.1510/icvts.2007.154575. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu M, Hashimoto T, Hanioka K, Itoh T, Hirose T. A rare autopsy case of epithelioid angiosarcoma arising in the aorta in a patient with massive multi-organ embolization. Pathol Int. 2018;68:574–576. doi: 10.1111/pin.12690. [DOI] [PubMed] [Google Scholar]
- 10.Dedeilias P, Koletsis E, Nenekidis I, Chatziioannou A, Tsipas P, Dimaka K, et al. Intimal aortic sarcoma mimicking ruptured thoracoabdominal type IV aneurysm. A rare case report and review of the literature. J Cardiothorac Surg. 2011;6:162. doi: 10.1186/1749-8090-6-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatima J, Duncan AA, Maleszewski JJ, Kalra M, Oderich GS, Gloviczki P, et al. Primary angiosarcoma of the aorta, great vessels, and the heart. J Vasc Surg. 2013;57:756–764. doi: 10.1016/j.jvs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Koshima R, Hashimoto M, Doi H, Mitsube K. Surgical therapy for angiosarcoma of the aorta: a case report. Ann Vasc Dis. 2019;12:225–227. doi: 10.3400/avd.cr.18-00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Squizzato F, Grego F. Primary aortic angiosarcoma presenting as progressive thrombosis of the thoracic aorta. Circ Cardiovasc Imaging. 2021;14:e010854. doi: 10.1161/CIRCIMAGING.120.010854. [DOI] [PubMed] [Google Scholar]
- 14.Park WK, Park KL, Cho YS, Han A, Ahn S, Min SK. Intravascular epithelioid angiosarcoma in the abdominal aorta mimicking an infected aneurysm. Vasc Specialist Int. 2019;35:232–236. doi: 10.5758/vsi.2019.35.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanem N, Riede U, Uhrmeister P, Weigang E, Altehoefer C. Epithelioid angiosarcoma of the aorta. Vasa. 2002;31:269–273. doi: 10.1024/0301-1526.31.4.269. [DOI] [PubMed] [Google Scholar]
- 16.Campana F, Nardin M, Coppini A, Muiesan ML. Case report of a sub-occluding thrombus in thoracic aorta: what is the origin? Eur Heart J Case Rep. 2019;3:ytz114. doi: 10.1093/ehjcr/ytz114. [DOI] [PMC free article] [PubMed] [Google Scholar]