Abstract
Approximately 4% of pregnant patients with coronavirus disease 2019 require intensive care unit admission. Given the practical implications of advanced ventilatory and circulatory support techniques, urgent or emergent delivery for nonreassuring fetal status frequently presents a logistical impossibility. This article proposes a protocol for obstetrical management of patients in these situations, emphasizing coordinated preparation among obstetrical, anesthesiology, and intensivist teams for planned preterm delivery at gestational ages when neonatal outcomes are likely to be favorable.
A 25-year-old G2P1001 at 30+2/7 weeks’ gestation is admitted to the intensive care unit (ICU) because of coronavirus disease 2019 (COVID-19) pneumonia with hypoxemia. Although she remains hemodynamically stable, attempts at noninvasive ventilation and prone positioning led to minimal improvements in maternal oxygenation. At this time, the fetal heart rate tracing begins to demonstrate repetitive late decelerations with minimal variability. What is the most appropriate obstetrical course of action?
Decisions regarding the delivery timing of critically-ill pregnant patients with COVID-19 present a challenging obstetrical juxtaposition: balancing a consultative role on the critical care team with respect to obstetrical concerns while simultaneously assuming the primary responsibility for evaluation and management of the fetal status. Inevitably, the role of fetal monitoring and specific indications for delivery arise whenever a pregnant patient at a viable gestational age is admitted to the ICU. This article intends to review the contemporary evidence regarding the pregnant COVID-19 patient requiring intensive care owing to a requirement for escalating oxygen therapy and proposes explicit guidance regarding delivery timing.
Introduction
Approximately 13% of pregnant patients who contract COVID-19 present or progress to a severe disease status, with 4% of those requiring ICU admission (Table ).1, 2, 3, 4 The Centers for Disease Control and Prevention has suggested that pregnancy confers a higher risk for requiring either mechanical ventilation or extracorporeal membrane oxygenation (ECMO); overall, mortality appears to be increased, and limited contemporary data have suggested a higher rate of intrauterine fetal demise.5 , 6 Fortunately, the majority of pregnant patients with COVID-19 infection experience mild symptomatology and do not require hospitalization, and many of these patients do not come to the attention of healthcare providers beyond reporting positive test results, confirmation of a stable clinical status, and recommendation for self-quarantine.3 However, for patients who do require supplemental O2, mechanical ventilation, or ECMO, there is growing recognition that recovery and eventual extubation (and/or decannulation) is commonly a very protracted process.7 Thus, the obstetrician may be confronted with a situation in which delivery either may be performed electively in a controlled manner before escalation (or expected escalation) of therapy or cannot be permitted until maternal cardiopulmonary stability has been reached because of inherent practical limitations.
Table.
Criteria for severe or critical coronavirus disease 2019 cases
| Parameter | NIH1 | Wu2 |
|---|---|---|
| Pulmonary | Severe | Severe |
| Respiratory rate >30/min | Respiratory rate >30/min | |
| SpO2 <94% on room air | SpO2 <93% on room air | |
| PaO2 or FiO2 <300 | PaO2 or FiO2 <300 | |
| Lung infiltrates >50% | Lung infiltrates >50% | |
| Critical | Critical | |
| Respiratory failure (mechanical ventilation) | Respiratory failure (mechanical ventilation) | |
| Circulatory | Septic shock | Shock |
| Other | Multiorgan dysfunction | Multiorgan dysfunction |
FiO2, fraction of inspired O2; NIH, National Institutes of Health; PaO2, partial pressure of O2; SpO2, O2 saturation.
Rose. Delivery timing with coronavirus disease 2019 pneumonia. Am J Obstet Gynecol MFM 2021.
Existing Evidence
Maternal
Most of the contemporary literature regarding obstetrical COVID-19 cases falls into the realm of either epidemiologic reports describing maternal and neonatal outcomes or case reports describing management in a variety of clinical circumstances.8 , 9 Preliminary data from China suggest that preterm delivery by cesarean delivery was almost 3-fold more common among patients with COVID-19, although specific distinction between indicated or spontaneous subcategories was not reported.10 A subsequent meta-analysis has shown an overall preterm delivery rate of approximately 29%, whereas another group found a cesarean delivery rate of 89%.11 , 12 Early data from the United States demonstrated a lower but overall increased (15%) incidence of preterm delivery with a concomitant increase in cesarean delivery rate (38%), whereas more recent reviews focusing exclusively on the critically-ill population have found an 88% rate of preterm delivery with 94% of these occurring by cesarean delivery.6 , 13 An expert consensus panel has recommended delivery for patients with severe disease status at ≥32 to 34 weeks’ gestation owing to the anticipated high likelihood of intact neonatal survival.14 , 15
Neonatal
Outcomes of neonates born to mothers with COVID-19 are primarily dependent on the gestational age at birth.16 Although isolated reports have described rare individual cases of vertical transmission, the overall incidence of postnatal infection is reported to fall in the range of 3% to 5%; given the relatively recent onset of the pandemic, long-term data are not yet available.17, 18, 19 Most neonatal complications arise because of iatrogenic prematurity instead of the infection itself.
The Case for Indicated Preterm Delivery
Pregnant patients admitted to the ICU constitute a relatively unique population in that both the maternal and fetal status must simultaneously be taken into account. Guidelines for management are primarily derived from clinical experience—not clinical trials—and tend to focus on treatment of the maternal condition, with only general recommendations regarding fetal assessment and indications for delivery.20 With respect to COVID-19 pneumonia, the principles of care are most similar to those of patients with acute respiratory distress syndrome with modifications in the medication regimens, target laboratory and physiological parameters, and maternal positioning.21 Decisions regarding the frequency of fetal monitoring and/or delivery are governed by the maternal status and gestational age, with the fetal cardiotocograph proposed to represent an additional “vital sign” to both guide and determine the efficacy of therapy.22
Criteria for refractory maternal hypoxemia generally include a partial pressure of O2 (PaO2) of <60 mm Hg while receiving a fraction of inspired O2 (FIO2) of 1.0, however, on a clinical basis, this definition can be expanded to include severe hypoxemia (PaO2 to FIO2 ratio of <150) unresponsive to incremental increases in positive end expiratory pressure (PEEP), recruitment of lung volumes, prone positioning, and/or deep sedation with chemical paralysis.23 Advanced therapies such as pulmonary vasodilator medications or veno-venous (V-V) ECMO are often considered in these situations, however, complications of hemorrhage requiring transfusion (57%–67%) and infection (58%) are frequent with ECMO, and the requirement for continuous anticoagulation therapy introduces a further complicating factor in the event of an unplanned delivery.24, 25, 26
When a pregnant patient with COVID-19 pneumonia is admitted to the ICU, parameters for urgent delivery should be established and a contingency plan communicated to all involved teams. Often there are substantial practical impediments involved in rapidly moving a critically-ill patient from the ICU to a delivery suite or operating room, particularly if located in geographically separate areas of a hospital; consequently, the ability to respond to a nonreassuring fetal heart rate pattern by performing an “emergent” cesarean delivery in the conventional obstetrical manner may become a logistical impossibility. Moreover, the actual process of transporting a patient with COVID-19 leads to exposure of additional personnel to a risk of infection. Proceeding with delivery in the ICU presents an alternative solution, however, this also may present attendant difficulties owing to the unfamiliarity of the surgical staff to an unconventional setting that can affect their ability to expediently and efficiently manage intraoperative complications (ie, postpartum hemorrhage). At the primary author's institution, this option would generally be reserved for cases involving perimortem or resuscitative cesarean deliveries.
It should be emphasized that admission to the ICU for COVID-19 pneumonia does not constitute an intrinsic indication for delivery per se. For clinically stable patients at gestational ages of 23 or 24 to 32 weeks’ gestation, continuation of the pregnancy with intermittent monitoring of the fetal heart rate represents the preferred approach. If possible, the patient or responsible family members should be engaged to ascertain their preferences for delivery and neonatal resuscitation; a neonatology consultation may be advantageous for counseling. If refractory maternal hypoxemia leads to a nonreassuring fetal status, maternal interventions should be trialed for a defined interval, balancing the risk of potential neonatal acidosis with that of an (indicated) iatrogenic premature delivery.
To preclude such a scenario, given the anticipated favorable neonatal outcomes, the authors would suggest that in pregnant patients requiring advanced oxygen delivery modalities (mechanical ventilation with PEEP of ≥10 cm H2O or V-V ECMO), elective delivery should be considered at gestational ages of 32+0/7 to 33+6/7 weeks, particularly if a course of antenatal corticosteroids has been completed. If a patient stabilizes with escalated O2 therapy, delivery should be considered once a course of antenatal corticosteroids has been administered; however, if maternal oxygenation does not improve with escalating support, expedient delivery—irrespective of completion of an antenatal corticosteroid course—is recommended. This would be similarly applicable to patients at gestational ages of ≥34+0/7 weeks; although a course of antenatal corticosteroids may be administered if gestational age is 34plus;0/7-36plus;6/7 weeks, delivery should be considered once the maternal status is optimized.
The method of delivery can be individualized based on the maternal clinical status, fetal condition, and obstetrical history. Successful induction of labor for mechanically-ventilated patients with COVID-19 has been described, however, a nonreassuring fetal status has been reported intrapartum in 34% of pregnancies in an early systematic review. Consequently, for coordination of the necessary resources and personnel availability, a cesarean delivery may represent the most pragmatic option.27 , 28 Interestingly, a single case report described marked pulmonary improvement following a cesarean delivery in a patient with COVID-19 at 30+5/7 weeks’ gestation, suggesting that mechanical relief of the reduction in functional residual capacity and residual volume imposed by the gravid uterus may confer a therapeutic benefit; this may also be true with concurrent maternal respiratory conditions such as asthma and/or obesity, which alter lung volumes.29 Postpartum improvement in hepatic disease has also been reported.30 However, objective maternal benefit following delivery remains largely conjectural at this time, and is likely to remain so secondary to the inability to conduct clinical trials in this specific population.
The primary risks of elective preterm delivery include iatrogenic prematurity in the neonate and subjecting a critically-ill, hypoxemic patient to further labor and/or surgical stress in the context of an uncertain prognosis. As noted, inherently, most deliveries in these circumstances are by cesarean delivery, and at the current time, there is no evidence that the route of delivery adversely influences the maternal prognosis.
Recommendations
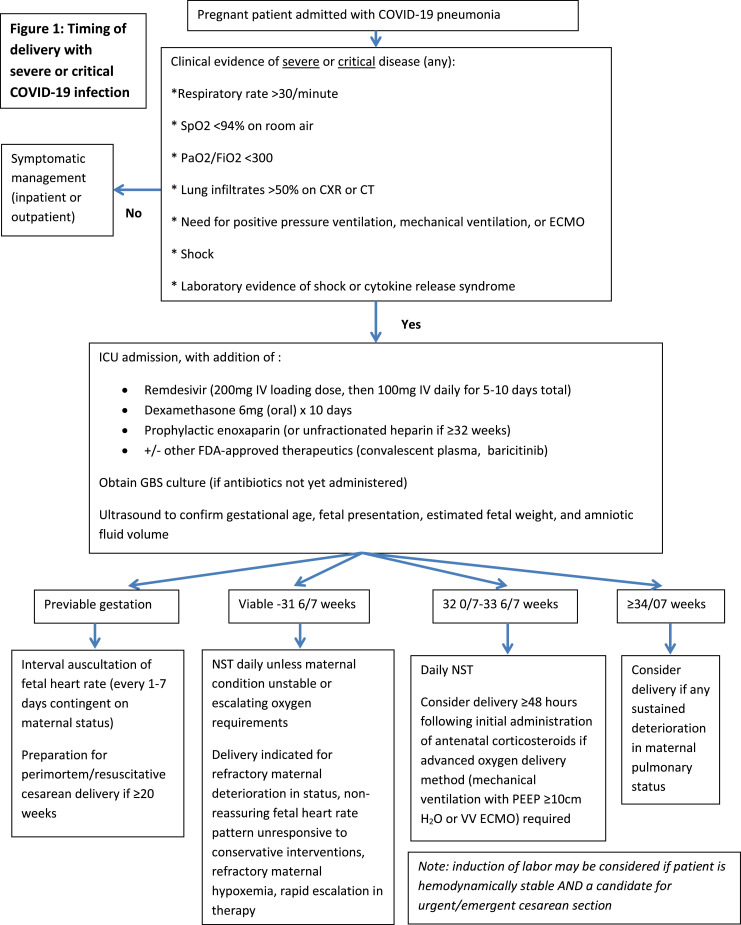
Accordingly, based on the authors’ combined institutional experiences, we propose the following general guidelines for pregnant patients requiring ICU admission for COVID-19 pneumonia (Figure ):
Figure.
Timing of delivery in severe or critical COVID-19 cases
This flowchart diagram denotes a guideline for the management and delivery of COVID-19 patients requiring intensive care unit admission based on gestational age.
COVID-19, coronavirus disease 2019; CXR, chest x-ray; ECMO, extracorporeal membrane oxygenation; FDA, US Food and Drug Administrator; FiO2, fraction of inspired O2; IV, intravenous; NST, non-stress test; PaO2, partial pressure of O2; PEEP, positive end expiratory pressure; SpO2, O2 saturation; V-V, veno-venous.
Rose. Delivery timing with coronavirus disease 2019 pneumonia. Am J Obstet Gynecol MFM 2021.
At time of ICU admission:
-
•
Obtain group B streptococcal culture if antibiotics have not been started previously.
-
•
Start on prophylactic anticoagulation therapy with unfractionated heparin.31
-
•
Administer remdesivir, dexamethasone, and/or other FDA-approved therapeutics according to institutional protocols; if dexamethasone is elected, a combination protocol of 48 hours of dexamethasone (6 mg intramuscular every 12 hours over 4 doses) followed by methylprednisolone (32 mg daily in single or divided doses) to complete a 10-day course would limit fetal corticosteroid exposure.32
-
•
Perform an obstetrical ultrasound to confirm fetal viability and gestational age in previable cases; evaluate gestational age, fetal presentation, estimated fetal weight, and amniotic fluid volume in viable cases.
If gestational age is <23+0/7 to 24+0/7 weeks (previable):
-
•
Perform interval auscultation of the fetal heart rate (every 1 to 7 days, contingent on provider preference), with a repeat assessment if the maternal clinical status deteriorates.
-
•
Delivery at these previable gestational ages would only be indicated in the event of maternal cardiopulmonary arrest at a gestational age of ≥20 weeks (resuscitative cesarean delivery).
If gestational age is between 23+0/7 to 24+0/7 and 31+6/7 weeks:
-
•
Administer an initial course of antenatal corticosteroids; a repeat course may be given if ≥7 days have elapsed and delivery in the next 7 days is deemed probable.33
-
•
Perform daily fetal assessments in the setting of a stable maternal clinical status and O2 requirement; convert to continuous fetal monitoring if the maternal condition becomes unstable.
-
•
Complete surgical consent form for cesarean delivery before administration of sedation (if feasible).
-
•
Brief multidisciplinary meeting (“huddle”) between ICU staff, obstetrics, anesthesiology, and neonatology to devise a strategy for management in the event of a nonreassuring fetal status.
-
•Delivery is suggested for:
-
○Nonreassuring fetal status in the setting of refractory hypoxemia despite optimization of all aspects of conventional and/or advanced therapies.
-
○
Note that interventions to improve fetal status may be attempted briefly, recognizing that a delay in proceeding with delivery could result in neonatal acidosis.
-
•If delivery is planned:
-
○Discontinue anticoagulation therapy 12 to 24 hours in advance.
- ○
-
○Cognizant that the fetal status is inherently reflective of that of the mother, consider obtaining matched maternal arterial and fetal umbilical artery blood gas values at the time of delivery for comparative purposes.35
-
○
If the gestational age is between 32+0/7 and 33+6/7 weeks:
-
•
Administer a course of antenatal corticosteroids.
-
•
Perform daily fetal assessments in the setting of a stable maternal clinical status and O2 requirements; convert to continuous fetal monitoring if the maternal condition begins to deteriorate further (ie, increasing PEEP or FiO2 to maintain PaO2 at >70 mm Hg or O2 saturation at >95%); it should be recognized that both the PEEP and FiO2 needs can fluctuate throughout the COVID-19 disease process, and the increases in PEEP and FiO2 in this context are intended to reflect persistent increases without substantial improvement in maternal oxygenation.
-
•
Complete a surgical consent form for cesarean delivery before administration of sedation (if feasible).
-
•
Brief multidisciplinary meeting (“huddle”) among ICU staff, obstetrics, anesthesiology, and neonatology to devise a strategy for management in the event of a nonreassuring fetal status.
-
•Delivery should be considered for:
-
○Nonreassuring fetal status in the setting of refractory maternal hypoxemia independent of corticosteroid completion.
-
○Requirement for mechanical ventilation with PEEP >10 cm H2O or V-V ECMO, particularly if a course of antenatal corticosteroids has been completed.
-
○
-
•If delivery is planned:
-
○Discontinue anticoagulation therapy 12 to 24 hours in advance.
-
○Cognizant that the fetal status is inherently reflective of that of the mother, consider obtaining matched maternal arterial and fetal umbilical artery blood gas values at the time of delivery for comparative purposes.35
-
○
If gestational age is ≥34+0/7 weeks:
-
•
Continuous fetal monitoring.
-
•
Complete surgical consent form for cesarean delivery before administration of sedation (if feasible).
-
•
A course of antenatal corticosteroids may be administered if gestational age is 34+0/7-36+6/7 weeks, but should not delay indicated preterm delivery.36
-
•
Brief multidisciplinary meeting (“huddle”) among ICU staff, obstetrics, anesthesiology, and neonatology with consideration of planned delivery following maternal stabilization.
-
•Delivery should be considered for:
-
○Any sustained deterioration in the maternal pulmonary status.
-
○
-
•Before delivery:
-
○Discontinue anticoagulation therapy 12 to 24 hours in advance.
-
○Cognizant that the fetal status is inherently reflective of that of the mother, consider obtaining matched maternal arterial and fetal umbilical artery blood gas values at the time of delivery for comparative purposes.35
-
○
As the COVID-19 epidemic continues to besiege intensive care units nationwide, the authors recognize that this protocol is based primarily on the limited existing data and collective personal experience. The fundamental intent is to provide an initial blueprint for individual institutions to modify based on specific facilities, personnel, and circumstances and is anticipated to evolve with the introduction of new therapies.
Footnotes
The authors report no conflict of interest.
Supplementary material associated with this article can be found in the online version, at doi:https://doi.org/10.1016/j.ajogmf.2021.100373.
Appendix. Supplementary materials
References
- 1.National Institutes of Health . 2020. COVID-19 treatment guidelines. Special Considerations in pregnancy.https://www.covid19treatmentguidelines.nih.gov/special-populations/pregnancy/ Available at. [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huntley BJF, Huntley ES, Di Mascio D, Chen T, Berghella V, Chauhan SP. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020;136:303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 5.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. Health care centers, March 1-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1355–1359. doi: 10.15585/mmwr.mm6938e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustafa AK, Alexander PJ, Joshi DJ, et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. 2020 doi: 10.1001/jamasurg.2020.3950. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Garcia C, Montaner-Ramon A, Hernandez-Perez S, et al. Severe COVID-19 during pregnancy and the subsequent premature delivery. Pediatr Neonatol. 2021;62:113–114. doi: 10.1016/j.pedneo.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takayama W, Endo A, Yoshii J, et al. Severe COVID-19 pneumonia in a 30-year-old woman in the 36th week of pregnancy treated with postpartum extracorporeal membrane oxygenation. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang R, Mei H, Zheng T, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18:330. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yee J, Kim W, Han JM, et al. Clinical manifestations and perinatal outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Sci Rep. 2020;10:18126. doi: 10.1038/s41598-020-75096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamseddine RS, Wahbeh F, Chervenak F, Salomon LJ, Ahmed B, Rafii A. Pregnancy and neonatal outcomes in SARS-CoV-2 infection: a systematic review. J Pregnancy. 2020;2020 doi: 10.1155/2020/4592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D, Yang H, Cao Y, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. 2020;149:130–136. doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berghella V, Hughes B. Coronavirus disease 2019 (COVID-19): pregnancy issues and antenatal care. In: Post TW, ed. UpToDate. Waltham, MA. Accessed February 24, 2021.
- 16.Flaherman VJ, Afshar Y, Boscardin J, et al. Infant outcomes following maternal infection with SARS-CoV-2: first report from the PRIORITY study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenizia C, Biasin M, Cetin I, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11:5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker KF, O'Donoghue K, Grace N, et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG. 2020;127:1324–1336. doi: 10.1111/1471-0528.16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crozier TME. General care of the pregnant patient in the intensive care unit. Semin Respir Crit Care Med. 2017;38:208–217. doi: 10.1055/s-0037-1600905. [DOI] [PubMed] [Google Scholar]
- 21.Oxford-Horrey C, Savage M, Prabhu M, et al. Putting it all together: clinical considerations in the care of critically ill obstetric patients with COVID-19. Am J Perinatol. 2020;37:1044–1051. doi: 10.1055/s-0040-1713121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubey J, Zork N, Sheen JJ. Inpatient obstetric management of COVID-19. Semin Perinatol. 2020;44 doi: 10.1016/j.semperi.2020.151280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 24.Pacheco LD, Saade GR, Hankins GDV. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42:21–25. doi: 10.1053/j.semperi.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151:1154–1160. doi: 10.1016/j.jtcvs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med. 2011;37:648–654. doi: 10.1007/s00134-011-2138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slayton-Milam S, Sheffels S, Chan D, Alkinj B. Induction of labor in an intubated patient with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020;136:962–964. doi: 10.1097/AOG.0000000000004044. [DOI] [PubMed] [Google Scholar]
- 28.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliva M, Hsu K, Alsamarai S, Chavez V, Ferrara L. Clinical improvement of severe COVID-19 pneumonia in a pregnant patient after caesarean delivery. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronnje L, Länsberg JK, Vikhareva O, Hansson SR, Herbst A, Zaigham M. Complicated COVID-19 in pregnancy: a case report with severe liver and coagulation dysfunction promptly improved by delivery. BMC Pregnancy Childbirth. 2020;20:511. doi: 10.1186/s12884-020-03172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1–17. doi: 10.1097/AOG.0000000000002706. [DOI] [PubMed] [Google Scholar]
- 32.Saad AF, Chappell L, Saade GR, Pacheco LD. Corticosteroids in the management of pregnant patients with coronavirus disease (COVID-19) Obstet Gynecol. 2020;136:823–826. doi: 10.1097/AOG.0000000000004103. [DOI] [PubMed] [Google Scholar]
- 33.Committee on Obstetric Practice Committee Opinion No. 713: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102–e109. doi: 10.1097/AOG.0000000000002237. [DOI] [PubMed] [Google Scholar]
- 34.Boelig RC, Manuck T, Oliver EA, et al. Labor and delivery guidance for COVID-19. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simhan HN. 2017. Umbilical cord blood acid-base analysis at delivery. UpToDate.https://www.uptodate.com/contents/umbilical-cord-blood-acid-base-analysis-at-delivery Available at: [Google Scholar]
- 36.American College of Obstetricians and Gynecologists. COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. The American College of Obstetricians and Gynecologists Novel Coronavirus 2019 (COVID-19). 2020. Available at: https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed May 15, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.