Graphical abstract
Keywords: COVID-19, SARS-CoV, Metabolism, Sex difference, Fatty acid
Abbreviations: ALT, Alanine aminotransferase; AP, Acute period (AP); APTT, Activated partial thromboplastin time; BCAAs, Branched‐chain amino acids; BP, Blood platelet; CA, Carbamide; COVID-19, Novel coronavirus disease 2019; CRP, C-reactive protein; DAA, Dehydroascorbic acid; DD, D-dimer; DP, Diastolic pressure; FIB, Fibrinogen; FP, Follow-up period; GPCs, Glycerophosphocholines; HGB, Hemoglobin; LY, Lymphocyte; NG, Neutrophilic granulocyte; NK, Natural killer; PCT, Procalcitonin; PLS-DA, Partial least squares-discriminant analysis; PLSR, Partial least squares regression; PT, Prothrombin time; PTC, Phosphatidylcholine; RDW, Red cell distribution width; RR, Respiratory rate; S1P, Sphingosine-1-phosphate; TBL, Total B lymphocyte; TTL, Total T lymphocyte; WBC, White blood cell
Highlights
-
•
Sex-specific metabolic changes in COVID-19 plasma during the recovery process.
-
•
Metabolic activities are increased in COVID-19 patients after the cure.
-
•
Women have shorter length of hospitalization of COVID-19 infection than men.
-
•
Plasma metabolic profiling may predict the duration from positive to negative in non-severe COVID-19 patients.
Abstract
Metabolic profiling in COVID-19 patients has been associated with disease severity, but there is no report on sex-specific metabolic changes in discharged survivors. Herein we used an integrated approach of LC-MS-and GC–MS-based untargeted metabolomics to analyze plasma metabolic characteristics in men and women with non-severe COVID-19 at both acute period and 30 days after discharge. The results demonstrate that metabolic alterations in plasma of COVID-19 patients during the recovery and rehabilitation process were presented in a sex specific manner. Overall, the levels of most metabolites were increased in COVID-19 patients after the cure relative to acute period. The major plasma metabolic changes were identified including fatty acids in men and glycerophosphocholines and carbohydrates in women. In addition, we found that women had shorter length of hospitalization than men and metabolic characteristics may contribute to predict the duration from positive to negative in non-severe COVID-19 patients. Collectively, this study shed light on sex-specific metabolic shifts in non-severe COVID-19 patients during the recovery process, suggesting a sex bias in prognostic and therapeutic evaluations based on metabolic profiling.
1. Introduction
The novel coronavirus disease 2019 (COVID-19) broke out in December 2019 due to SARS-CoV-2 and is rapidly spreading around the world. As of November 24, 2020, over 59 million subjects have been infected worldwide, causing over 1 million deaths. COVID-19 is initially characterized by inflammatory syndrome such as fever, fatigue, cough, and muscle pain [1]. Yet, no effective treatments are available to date except comprehensive support. Therefore, some patients will develop to severe symptoms including acute respiratory distress, septic shock, metabolic acidosis, and even organ failure,1 leading to increased mortality. Scientists and medical staffs around the world are still working to discover a cure for COVID-19.
At the beginning, most studies on COVID-19 focused on its epidemiological and clinical characteristics [2], [3], [4], [5]. Later, researchers started to be concerned about the diagnosis [6], [7], [8], [9] and treatment [10], [11], [12] of COVID-19. Omics technique as a systems biology approach attempts to dissect the pathophysiological complexities of human diseases [13] and has also been used to explore the pathogenesis and diagnostic markers of COVID-19. For example, using lipidomics and metabolomics, Song et al. revealed that GM3-enriched exosomes are positively associated with COVID-19 pathogenesis and a panel of 10 plasma metabolites can distinguish COVID-19 patients from healthy controls with an AUC value of 0.975 [14]. In addition, Shen et al. identified 93 proteins and 204 metabolites in serum that correlate with the severity of COVID-19 and uncovered metabolic and immune dysregulation in COVID-19 patients through an integrated method of proteomics and metabolomics [15]. These two studies focused on multi-omics characteristics during the onset and progression of COVID-19, but understanding metabolic alterations during the recovery and rehabilitation process is also of great importance [16]. However, so far information regarding this aspect is not available. Interestingly, the sex effect on COVID-19 is also drawing public attention, and male patients have more severe disease and higher mortality than female patients [17], [18], [19]. Takahashi et al. reported that sex differences in immune response may cause disparities in outcomes of COVID-19 between two genders, providing an important basis for developing sex-based strategies for the treatment and care of COVID-19 [20]. Thus, it is of great interest to elucidate sex-specific metabolic changes during recovery from COVID-19.
In this study, we retrospectively recruited a cohort of patients with non-severe COVID-19 including 20 male and 20 female at both acute period and 30 days after discharge. Plasma metabolic profiling was analyzed by using an integrated method of LC-MS-and GC–MS-based untargeted metabolomics. The aims of this study were (1) to identify sex-specific metabolic changes in discharged survivors, (2) to analyze correlations between metabolites and clinical parameters, and (3) to predict the duration from positive to negative in non-severe COVID-19 using metabolic profiling.
2. Materials and methods
2.1. Patients and sampling
We retrospectively recruited a total of 40 COVID-19 patients including 20 male and 20 female at the Second Affiliated Hospital of Wenzhou Medical University from February 23 to March 07, 2020. According to the Chinese government diagnosis and treatment guideline (Trial 5th version, NHCPRC, 2020), patients were diagnosed as COVID-19 when they met the following criteria: 1) fever, dry cough or respiratory symptoms; 2) positive for SARS-CoV-2 nucleic acid; 3) imaging manifestation of pneumonia. Moreover, these patients were scheduled for follow-up visit at 30 days after discharge. Demographic and clinical data of COVID-19 patients were obtained from electronic medical records as shown in Tables 1, S1 and S2. All blood samples were collected in the morning and transferred to the clinical laboratory using a biosafety transport box. Plasma samples were separated by centrifuging at 1,500 g for 15 min, collected into new tubes and immediately stored at −80 °C. This study was approved by the Ethical Committee of Wenzhou Medical University (ID: 2020–025), and written informed consents were acquired from all subjects after discharge. All procedures in this study were carried out according to the 2008 Helsinki Declaration and the clinical-ethical guidelines of the Second Affiliated Hospital of Wenzhou Medical University.
2.2. Sample preparation
All plasma samples were inactivated and sterilized at 56 °C for 30 min. An aliquot of 100 μL sample was added to a 1.5 mL centrifuge tube and mixed with 10 μL of L-2-chlorolphenylalanine (0.3 mg/mL) and LysoPC17:0 (0.01 mg/mL) prepared in methanol as internal standards, and the mixture was vortexed for 10 s. Then, 300 μL of ice-cold mixture of methanol and acetonitrile (v:v = 2:1) was added and vortexed for 1 min, ultrasonicated at 0 °C for 10 min, and stood at −20 °C for 30 min. The extract was centrifuged at 13,000 rpm for 15 min at 4 °C, and the supernatant was transferred into a new centrifuge tube and dried using a freeze concentration centrifugal dryer. The lyophilized powder was redissolved in 100 μL mixed solution of methanol and water (v:v = 1:4), vortexed for 30 s, and then placed at 4 °C for 2 min. Then, the mixture was centrifuged at 13,000 rpm for 5 min at 4 °C, and the supernatant was filtered through 0.22 μm microfilters and transferred to LC vials for LC-MS analysis. An aliquot of 200 μL supernatant was transferred to a glass sampling vial for vacuum dry at 25 °C, and mixed with 80 μL of methoxylamine hydrochloride prepared in pyridine (15 mg/mL). The mixture was vigorously vortexed for 2 min and incubated at 37 °C for 90 min. Subsequently, 80 μL of BSTFA (with 1% TMCS) and 20 μL of n-hexane was added, vortexed for 2 min and then derivatized at 70 °C for 60 min. The sample was placed at 25 °C for 30 min for GC–MS analysis. The quality control (QC) sample was prepared by mixing aliquots of all samples.
2.3. LC-MS-based untargeted metabolomics analysis
Plasma metabolic profiling was acquired by an ACQUITY UHPLC system (Waters Corporation Milford, USA) coupled with AB SCIEX Triple TOF 5600 System (AB SCIEX, Framingham, MA) under both ESI positive and negative ion modes. A Waters BEH C18 column (1.7 μm, 2.1 × 100 mm) was used and the mobile phase consisted of acetonitrile and water containing 0.1% formic acid. In addition, the following parameters were set: flow rate, 0.4 mL/min; column temperature, 45 °C; injection volume, 1 μL. Data acquisition was carried out in full scan mode from 70 to 1000 m/z and the IDA mode with m/z range of 25–1000 and collision energy of 30 eV. The QC samples were injected every 10 runs for evaluation of data repeatability.
The LC-MS raw data were analyzed by Progqenesis QI software (Waters Corporation, Milford, USA) with the following parameters: precusor tolerance, 5 ppm; fragment tolerance, 10 ppm; retention time (RT) tolerance, 0.02 min; noise elimination level, 10.00. Moreover, isotopic peaks were removed for data analysis and minimum intensity was set to 15% of base peak intensity. Metabolites were identified by using HMDB (https://hmdb.ca/), LIPID MAPS (https://lipidmaps.org/) and self-built databases according to RT-m/z pairs and tandem mass spectrometry (MS/MS) spectra. Then, the data matrix was outputted as the Excel file with three dimension datasets including m/z, peak RT and intensities for further analysis.
2.4. GC–MS-based untargeted metabolomics analysis
Metabolic profiling of the derivative sample was analyzed by using an Agilent 7890B gas chromatography system (Agilent Technologies Inc., CA, USA) equipped with a DB-5MS fused-silica capillary column (30 m × 0.25 mm × 0.25 μm) at a constant helium flow rate of 1 mL/min. The parameters of GC–MS condition were set as follows: injector temperature, 260 °C; injection volume, 1 μL; MS quadrupole temperature, 150 °C; ion source temperature, 230 °C; collision energy, 70 eV; mass range, 50–500 m/z. The QC samples were injected every 10 runs for data quality control.
The raw data of GC–MS analysis were converted to ABF format via the Analysis Base File Converter software, and then imported into the MS-DIAL software for peak detection, deconvolution, alignment, and filtering. Metabolites were identified in the basis of the LUG database (Untargeted database of GC–MS from Lumingbio). All internal standards and pseudo positive peaks were removed and then the data matrix with three dimension datasets including sample information, peak names and intensities was exported as the Excel file with for further analysis.
2.5. Statistical analysis
In the present study, we selected metabolite peaks with coefficients of variations (CV) < 15% across nine QC samples for further analysis. To examine changes in metabolic patterns between different groups, partial least squares-discriminant analysis (PLS-DA) was carried out using Pareto-scaled data in SIMCA 12.0 software (Umetrics, Umeå, Sweden). Moreover, partial least squares regression (PLSR) was used to predict the duration from positive to negative in non-severe COVID-19 patients based on clinical parameters or metabolomics data in SIMCA 12.0 software. In this study, we used 10-fold cross validation (10-CV) to evaluate the performances of PLS-DA and PLSR. The number of latent variables (LV) was set to 2 for both PLS-DA and PLSR. For PLS-DA, R2 and Q2 values were calculated to indicate goodness-of-fit and predictive capability, respectively. For PLSR, linear regression analysis was performed to assess the agreement between predicted and observed values. Important metabolites were identified by the variable importance in the projection (VIP) method, where metabolites with VIP values>1.0 were selected for further analysis. The difference in metabolite level between two groups was analyzed using paired-samples t-tests with the Bonferroni correction using SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA) and considered to be statistically significant when p < 0.05. The relationship between metabolic data and clinical parameters was analyzed by Spearman’s correlation in MATLAB (R2012a, the MathWorks, Inc., MA, USA) and illustrated as heatmaps.
3. Results
3.1. Metabolomics profiling in plasma of non-severe COVID-19 patients
We collected plasma samples from a cohort of patients with non-severe COVID-19 including 20 male and 20 female at both acute period and 30 days after discharge (Fig. 1a), and their detailed clinical characteristics are shown in Tables 1, S1 and S2. The results reveal that the main symptoms of COVID-19 included fever, dry cough, expectoration, weak and sore throat, and there were no significant differences between two genders (Table 1). Of note, after infecting with SARS-CoV-2, male might have a higher proportion of dry cough than female (60% vs. 30%, P = 0.111). In addition, we observed significantly higher levels of C-reactive protein (P = 0.018), hemoglobin (P < 0.001), alanine aminotransferase (P = 0.044), total bilirubin (P < 0.001), carbamide (P = 0.024), and serum creatinine (P = 0.005) in male patients with COVID-19 at acute period relative to female patients, as shown in Table S1. These results indicate that COVID-19-induced multi-organ injuries were more serious in men compared with women, leading to a higher risk of developing a severe case and even higher mortality in male patients [17], [18], [19]. Relative to women, however, men had significantly lower concentrations of Na (P = 0.005), Cl (P = 0.015) and P (P = 0.020) in plasma sample (Table S1). Interestingly, in the present study, women had a relatively shorter length of hospitalization than men (P = 0.090), although no significant differences in therapeutic regimens were carried out between two genders (Table 1). At 30 days after discharge, we found that total B lymphocyte (P < 0.001), total NK cell (P = 0.023) and the percentage of NK cell (P = 0.033) were significantly lower in women when compared with men (Table S2). Besides, several lung function parameters, including FEV1 (p < 0.001), FVC (P < 0.001), FEF25 (P < 0.001), FEF50 (P = 0.004) and FEF50 predicted (P = 0.017), were also significantly poorer in female patients (Table S2). These findings might be attributed to normal physiological differences between two genders.
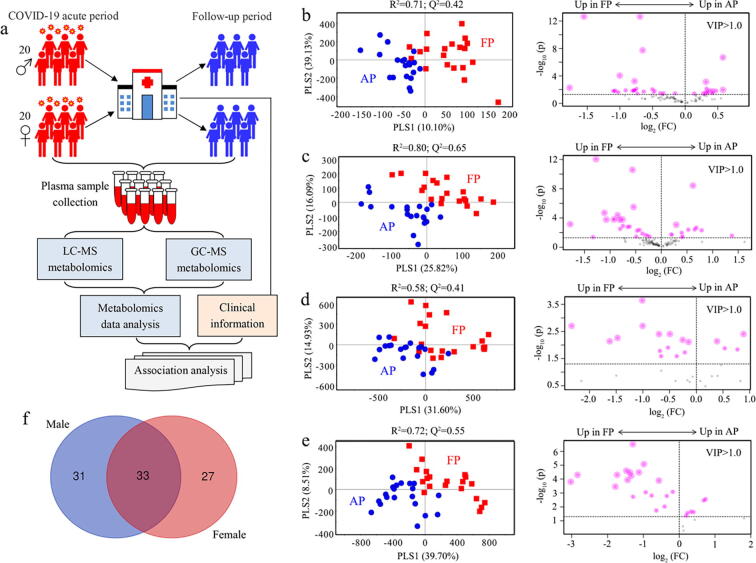
Fig. 1.
Plasma metabolomics analysis of COVID-19 patients. (a) Plasma samples were collected from patients with non-severe COVID-19 including 20 male and 20 female at both acute period and 30 days after discharge for both LC-MS-and GC–MS untargeted metabolomics analysis. The differences of metabolic phenotypes in plasma between COVID-19 patients at acute period (AP) and follow-up period (FP) analyzed by PLS-DA using LC-MS-based (b, men; c, women) and GC–MS-based (d, men; e, women) metabolic profiling. Subsequently, metabolites with VIP > 1.0 were selected and volcano plot analysis was used to identify important metabolites (p < 0.05) as highlighted in Fig. 1b-1e. (f) Venn diagram analysis based on the selected important metabolites, showing that there were 33 common metabolites in both male and female patients, and 31 and 27 metabolites were identified as unique metabolites in male and female patients, respectively.
Table 1.
Demographics and baseline characteristics of COVID-19 patients.
| Characteristics | Male (n = 20) | Female (n = 20) | P |
|---|---|---|---|
| Age (year) | 47.70 ± 14.41 | 48.90 ± 10.92 | 0.768 |
| BMI (kg/m2) | 24.89 ± 2.16 | 22.32 ± 3.36 | 0.007 |
| Heart rate | 81.60 ± 14.74 | 82.10 ± 12.19 | 0.908 |
| Respiration rate | 19.75 ± 0.91 | 21.20 ± 9.21 | 0.488 |
| Systolic blood pressure | 129.70 ± 15.55 | 132.10 ± 11.50 | 0.590 |
| Diastolic blood pressure | 80.90 ± 11.63 | 84.40 ± 10.32 | 0.321 |
| Symptoms (n, %) | |||
| Fever | 16 (80%) | 13 (65%) | 0.480 |
| Dry cough | 12 (60%) | 6 (30%) | 0.111 |
| Expectoration | 10 (50%) | 12 (60%) | 0.751 |
| Weak | 7 (35%) | 6 (30%) | 1.000 |
| Sore throat | 6 (30%) | 6 (30%) | 1.000 |
| Anorexia | 2 (10%) | 5 (25%) | 0.407 |
| Diarrhea | 5 (25%) | 3 (15%) | 0.695 |
| Nasal congestion | 2 (10%) | 4 (20%) | 0.661 |
| Myalgia | 3 (15%) | 4 (20%) | 1.000 |
| Nausea | 3 (15%) | 4 (20%) | 1.000 |
| Headache | 1 (5%) | 4 (20%) | 0.342 |
| Stomachache | 0 (0%) | 1 (5%) | 1.000 |
| Dspnea | 1 (5%) | 0 (0%) | 1.000 |
| Treatment (n, %) | |||
| Antiviral druga | 20 (100%) | 20 (100%) | 1.000 |
| Antibioticsb | 9 (45%) | 8 (40%) | 1.000 |
| Chinese medicine | 18 (90%) | 20 (100%) | 0.487 |
| Treatment time (day) | 24.00 ± 5.98 | 20.40 ± 7.05 | 0.090 |
Antiviral drugs mainly included arbidol hydrochloride granules, recombinant human interferon alfa-2b, lopinavir/ritonavir, indinavir and ribavirin; b Antibiotics mainly included methylprednisolone.
We used an integrated method of LC-MS and GC–MS untargeted metabolomics for global metabolic profiling of plasma sample. Our untargeted approach detected a total of 2,698 identified metabolite peaks based on m/z, RT and MS/MS fragments. Then, we selected metabolite peaks with coefficients of variations (CV) < 15% across nine QC samples and finally 2,022 metabolites were included for further analysis, mainly including lipids and lipid-like molecules (54%), organic acids and derivatives (7%), organoheterocyclic compounds (5%), organic oxygen compounds (3%), benzenoids (3%), as well as phenylpropanoids and polyketides (3%) (Figure S1).
3.2. Sex-specific metabolic changes in plasma of non-severe COVID-19 patients
To examine sex-specific metabolic changes in plasma of non-severe COVID-19 patients between acute period (AP) and follow-up period (FP), PLS-DA model was performed using metabolomics data in this study. The results demonstrate that clear separations were obtained between AP and FP in both male (Fig. 1b and 1d) and female (Fig. 1c and 1e) patients, suggesting that the metabolic phenotypes of non-severe COVID-19 patients were significantly altered after the cure. Subsequently, metabolites with VIP > 1.0 were selected and volcano plot analysis was used to identify important metabolites as highlighted in Fig. 1b-1e, where we found that the levels of most metabolites were significantly lower in AP relative to FP, indicating a hypometabolic state in COVID-19 patients. Venn diagram analysis shows that there were 33 common important metabolites in two genders, and 31 and 27 metabolites were identified as unique important metabolites in male and female patients, respectively (Fig. 1f).
We found that the levels of most metabolites were increased in plasma of both male and female patients with non-severe COVID-19 from AP to FP (Figure S2), indicating a higher metabolic activity after the cure. Shen et al. have reported that COVID-19 patients displayed a massive metabolic suppression relative to controls [15]. Thus, our data suggest that improving metabolic activity could be conducive to the recovery of COVID-19. However, sphingoid bases, such as C16 sphinganine and xestoaminol C, were significantly reduced in COVID-19 patients after healing. Sphingoid bases have been revealed to affect the resistance of rat renal and mesenteric vessels through G protein-coupled receptor [21] and exhibit cytotoxic activity [22]. A decreased level of tryptophan was observed in both serum [23] and plasma [14] of COVID-19 patients, but here we did not detect an increased tryptophan level after treatment. Oxoproline, an important mediator of oxidative stress [24], was reduced in COVID-19 patients after the cure. In addition, we also found decreased levels of glucose, 2-hydroxyphenethylamine, trans-cinnamic acid and 3-acrylamidopropyl trimethylammonium in COVID-19 patients from AP to FP (Figure S2).
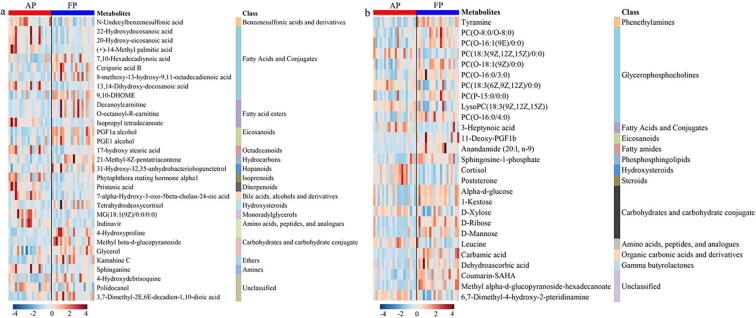
In male patients with non-severe COVID-19, several long-chain fatty acids (LCFAs), such as 22-hydroxydocosanoic acid, 20-hydroxy-eicosanoic acid, (+)-14-methyl palmitic acid and 13,14-dihydroxy-docosanoic acid, were significantly reduced after discharge, while 8-methoxy-13-hydroxy-9,11-octadecadienoic acid, 7,10-hexadecadiynoic acid, ceriporic acid B and 9,10-DHOME were raised (Fig. 2a). Increased LCFAs have been identified in serum of COVID-19 patients by Thomas et al. [23]. Therefore, we speculate that lowering LCFAs level may be helpful for treating COVID-19, but specific LCFAs need to be identified. Moreover, we found that two carnitines (decanoylcarnitine and O-octanoyl-R-carnitine) were elevated after treatment. Carnitines have been reported to suppress inflammation [25] and oxidative stress [26] and protect against lung injury [27]. Coronavirus infection can trigger ER stress and subsequent eicosanoid and cytokine storms, and then cause tissue injury [28]. Targeting eicosanoids could be a therapeutic strategy for COVID-19 [28]. In the present study, yet, the levels of eicosanoids such as PGF1a alcohol and PGE1 alcohol were observed to be significantly increased in male patients after treatment. Besides, we detected lower levels of 17-hydroxy stearic acid, phytophthora mating hormone alpha1, pristanic acid, 7-alpha-hydroxy-3-oxo-5beta-cholan-24-oic acid, MG(18:1(9Z)/0:0/0:0), indinavir and sphinganine as well as higher levels of 21-methyl-8Z-pentatriacontene, tetrahydrodeoxycortisol, 4-hydroxyproline, methyl beta-d-glucopyranoside, glycerol, kamahine C, 4-hydroxydebrisoquine and 3,7-dimethyl-2E,6E-decadien-1,10-dioic acid in COVID-19 patients after the cure (Fig. 2a).
Fig. 2.
Sex-specific metabolic changes in plasma of non-severe COVID-19 patients after discharge. Heatmap showing changes of important metabolites between COVID-19 patients at acute period (AP) and follow-up period (FP) in men (a) and women (b). Metabolites with VIP > 1.0 and p < 0.05 were selected as important metabolites.
Fig. 2b illustrates plasma metabolic changes in female patients with non-severe COVID-19 from AP to FP and overall a higher metabolic level was observed. Of these, glycerophosphocholines (GPCs) were detected as the main metabolites that significantly altered in female patients after the cure, as indicated by increased levels of PC(O-8:0/O-8:0), PC(O-16:1(9E)/0:0), PC(O-18:1(9Z)/0:0), PC(O-16:0/3:0), PC(P-15:0/0:0) and PC(O-16:0/4:0) as well as decreased levels of PC(18:3(9Z,12Z,15Z)/0:0), PC(18:3(6Z,9Z,12Z)/0:0) and LysoPC(18:3(9Z,12Z,15Z)). GPCs-related metabolites have been reported to modulate systemic oxidative stress and inflammation, but it should be noting that different structures may result in functional differences [29], [30], [31]. Thus, regulation of specific GPCs would be beneficial to treat COVID-19 especially in female patients. Cheudjeu suggested D-xylose as a possible therapeutic use for COVID-19 due to its anti-inflammatory and antiviral properties in lung infections [32]. However, our data show a reduced level of D-xylose in plasma of female COVID-19 patients after discharge. Interestingly, we found that the levels of alpha-d-glucose, 1-kestose, D-ribose and D-mannose were significantly increased after treatment. D-ribose has been reported to be an energy enhancer for maintaining mitochondrial functions [33], but it can also promote the production of advanced glycation end products (AGEs) and cause severe cytotoxicity [34]. Of note, D-mannose can alleviate immunopathology by increasing the proportion of regulatory T cells [35]; therefore, we suggest that D-mannose might have a potential therapeutic effect on COVID-19. Sphingosine-1-phosphate (S1P) as a key cell signaling molecule [36] was significantly increased in female patients after the cure, which is in agreement with the finding of Song et al. that the level of S1P was reduced in COVID-19 patients but increased after discharge [14]. Additionally, we detected higher levels of tyramine, 11-deoxy-PGF1b, anandamide (20:l, n-9), carbamic acid, dehydroascorbic acid, coumarin-SAHA and methyl alpha-d-glucopyranoside-hexadecanoate, and lower levels of 3-heptynoic acid, cortisol, poststerone, leucine and 6,7-dimethyl-4-hydroxy-2-pteridinamine in female COVID-19 patients after treatment (Fig. 2b).
3.3. Sex-specific associations between metabolic changes and clinical parameters
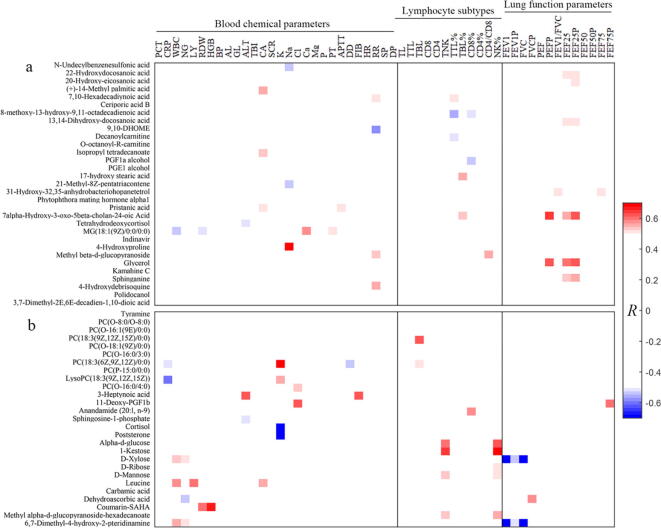
To assess the relationship between metabolic changes and clinical parameters, spearman correlation analysis was performed and only correlations with |R|>0.5 and P < 0.05 were highlighted in heatmaps (Fig. 3). In male patients with non-severe COVID-19, respiratory rate (RR) was found to be positively associated with methyl beta-d-glucopyranoside, 7,10-hexadecadiynoic acid and 4-hydroxydebrisoquine and negatively related with 9,10-DHOME (Fig. 3a). MG(18:1(9Z)/0:0/0:0) had positive correlations with prothrombin time (PT) and the level of Ca and negative correlations with red cell distribution width (RDW) and white blood cell (WBC), suggesting that a high level of MG(18:1(9Z)/0:0/0:0) may disrupt blood cell homeostasis and cause an adverse effect on COVID-19. Tetrahydrodeoxycortisol (THS) is derived from cortisol metabolism as a regulator of glucocorticoid action in liver and kidney [37], [38]. For this reason, a negative relationship between THS and alanine aminotransferase (ALT) indicates that glucocorticoid might be an effective drug for improving the liver and kidney functions in COVID-19 patients. In addition, we found that carbamide (CA) was positively correlated with (+)-14-methyl palmitic acid, isopropyl tetradecanoate and pristanic acid (Fig. 3a). A positive relationship was also obtained between activated partial thromboplastin time (APTT) and pristanic acid as well as between the level of Na and 4-hydroxyproline in male patients.
Fig. 3.
Correlation heatmap between plasma metabolites and clinical parameters. The relationships between metabolites and blood chemical parameters, lymphocyte subtypes and lung function parameters in male (a) and female (b) patients with non-severe COVID-19. Spearman correlation analysis was carried out to assess the associations between metabolic changes and clinical parameters, and only correlations with |R|>0.5 and p < 0.05 were highlighted in Fig. 3.
In female patients with non-severe COVID-19, we found that WBC and neutrophilic granulocyte (NG) were significantly and positively correlated with D-xylose and 6,7-dimethyl-4-hydroxy-2-pteridinamine (Fig. 3b). NG is the most highly abundant myeloid cell type in humans, accounting for 40% to 70% of total WBC, and plays an important role in inflammatory response [39]. In COVID-19 patients, granulocytic makers including NG were enriched and used to discriminate between mild and severe patients [40]. Of note, our results reveal that a higher level of dehydroascorbic acid (DAA) was linked with decreased NG in female patients, suggesting a protective effect of DAA on COVID-19. One possible explanation for this finding is that DAA can be metabolized to ascorbic acid, and thereby exerts a vitamin activity on neutrophils and inflammation [41], [42]. Lymphocyte (LY) as one of WBCs was positively correlated with leucine. The reduction in LY has been associated with the severity of COVID-19 [14], [15]. Here we suggest that increased leucine might be helpful to treat COVID-19 via LY activation [43]. In female patients, ALT was found to be positively and negatively correlated with 3-heptynoic acid and sphingosine-1-phosphate (S1P), respectively (Fig. 3b). Hence, a lower level of S1P in COVID-19 patients may indicate a higher ALT, suggesting an impaired liver and kidney functions in COVID-19 patients [14]. S1P has been revealed to promote the resolution of inflammation through activating macrophages [44], [45]. Glycerophospholipids are also involved in inflammatory response [46], [47]. Here we found that C-reactive protein (CRP) was negatively linked with PC(18:3(6Z,9Z,12Z)/0:0) and LysoPC(18:3(9Z,12Z,15Z)) (Fig. 3b). Moreover, PC(18:3(6Z,9Z,12Z)/0:0) was positively correlated with the level of K and negatively correlated with D-dimer (DD). Lower levels of PC(O-16:0/4:0) and 11-deoxy-PGF1b were associated with higher level of Cl (Fig. 3b). Coumarin-SAHA was positively related with hemoglobin (HGB) and red cell distribution width (RDW). Moreover, positive relationships were also obtained between leucine and CA as well as between 3-heptynoic acid and fibrinogen (FIB).
The associations between metabolites and lymphocyte subtypes after discharge were analyzed in male and female patients as shown in Fig. 3a and 3b, respectively. Ni et al. reported that a reduction of T lymphocytes was a common symptom in patients with severe COVID‐19 [48]. After treatment, CD4 + and CD8 + T lymphocytes were significantly increased, but no significant changes were obtained in B lymphocytes and natural killer (NK) cells [48]. In the present study, the percentage of total T lymphocyte (TTL%) shows a positive correlation with 7,10-hexadecadiynoic acid and negative correlations with decanoylcarnitine and 8-methoxy-13-hydroxy-9,11-octadecadienoic acid in male patients. However, the percentage of total B lymphocyte (TBL%) was positively linked with 17-hydroxy stearic acid and 7alpha-hydroxy-3-oxo-5beta-cholan-24-oic acid. Fatty acids have been reported to influence lymphocytes [49], [50] and inflammation [51], [52] through multiple mechanisms; therefore, regulation of specific fatty acid metabolism may improve COVID-19-induced disturbance of lymphocytes. Besides, CD8% was negatively related with 8-methoxy-13-hydroxy-9,11-octadecadienoic acid and PGF1a alcohol. A positive relationship was obtained between CD4/CD8 and methyl beta-d-glucopyranoside (Fig. 3a). NK cells play a key role in regulating the immune response in the body, and a significantly reduced number of NK cells were detected in COVID-19 patients [53], [54], [55]. In female patients, interestingly, carbohydrates including alpha-d-glucose, 1-kestose, D-ribose and D-mannose were positively correlated with TNK and NK% (Fig. 3b). Carbohydrates as T cell-activating antigens have been attracting great interest [56]. For example, carbohydrate consumption has been shown to increase the number and activity of NK cells [57], [58], [59]. TBL displayed positive correlations with glycerophospholipids, including PC(18:3(9Z,12Z,15Z)/0:0) and PC(18:3(6Z,9Z,12Z)/0:0). Moreover, a positive relationship was also found between CD8% and Anandamide (20:l, n-9) in female patients (Fig. 3b).
Lung injury is a typical characteristic in COVID-19 patients, so concerns have been raised about the evaluation of lung functions in discharged patients [60], [61]. In the present study, the correlation heatmap between metabolites and lung functions were analyzed in male (Fig. 3a) and female (Fig. 3b) patients after discharge. The results demonstrate that pulmonary functions were positively correlated with fatty acids, including 22-hydroxydocosanoic acid, 20-hydroxy-eicosanoic acid and 13,14-dihydroxy-docosanoic acid in male patients (Fig. 3a). Fatty acids have been shown to improve lung functions via the free fatty acid receptor FFA4 [62], [63]. Higher levels of glycerol and bile acid (7alpha-hydroxy-3-oxo-5beta-cholan-24-oic acid) were related with improved pulmonary functions of male patients (Fig. 3a). In female patients, however, lung function parameters were positively correlated with 11-deoxy-PGF1b and DAA, but negatively linked with D-xylose and 6,7-dimethyl-4-hydroxy-2-pteridinamine (Fig. 3b). We speculate that DAA can be converted to ascorbic acid, and thereby exhibits a protective effect on lung injury [64]. Of note, Cheudjeu suggested D-xylose as a possible therapeutic use for COVID-19 [32], but our results did not support this hypothesis.
3.4. Prediction of the duration from positive to negative in non-severe COVID-19 patients using clinical parameters and metabolic data
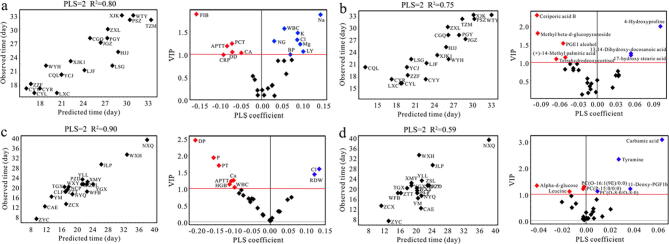
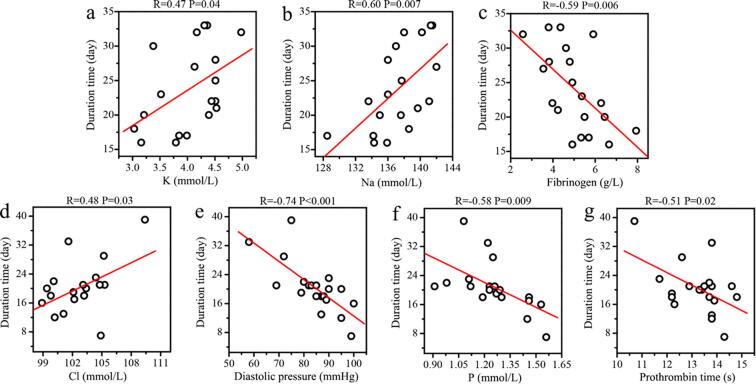
We then used PLSR to predict the duration from positive to negative in non-severe COVID-19 patients using clinical parameters and metabolic data (Fig. 4). A good agreement between predicted and observed values was obtained in both male (Fig. 4a, R2 = 0.80) and female (Fig. 2c, R2 = 0.90) patients from PLSR based on clinical parameters. The corresponding volcano plots identified a series of important clinical parameters, including Na, K, Mg, Cl, APTT, CRP, DD, WBC, LY, NG, FIB, blood platelet (BP), procalcitonin (PCT) and carbamide (CA) in male patients as well as P, Ca, Cl, APTT, WBC, RDW, diastolic pressure (DP), prothrombin time (PT) and hemoglobin (HGB) in female patients. Furthermore, regression analysis reveals that the concentrations of K (Fig. 5a, R = 0.47, P = 0.04) and Na (Fig. 5b, R = 0.60, P = 0.007) were significantly and positively correlated with the duration from positive to negative in male patients with non-severe COVID-19, while a negative association was obtained for FIB (Fig. 5c, R = -0.59, P = 0.006). Electrolyte imbalances were a common symptom in patients with COVID-19 by Lippi et al., who reported that K and Na were significantly lower in patients with severe COVID-19 [65]. However, our results indicate that non-severe COVID-19 patients with higher levels of K and Na may have a longer length of hospitalization in male patients. Of note, decreased FIB level can lead to hemorrhage. Although COVID-19 patients had been reported to show an increased level of FIB [66], higher FIB might reduce the duration from positive to negative in non-severe male patients. In female patients with non-severe COVID-19, however, we found that the duration from positive to negative was positively related with Cl (Fig. 5d, R = 0.48, P = 0.03) and negatively related with DP (Fig. 5e, R = -0.74, P < 0.001), P (Fig. 5f, R = -0.58, P = 0.009) and PT (Fig. 5g, R = -0.51, P = 0.02).
Fig. 4.
Prediction of the duration from positive to negative after COVID-19 infection. The correlation between predicted and observed duration from positive to negative in non-severe COVID-19 using PLSR based on clinical parameters (a, men; c, women) and metabolic profiling (b, men; d, women). The corresponding volcano plots showing a series of important clinical parameters and metabolites (VIP > 1.0).
Fig. 5.
Linear regression analysis between the duration from positive to negative and clinical parameters in non-severe COVID-19 patients. Correlations of the duration from positive to negative with (a) the level of K, (b) the level of Na and (c) fibrinogen in male patients, and (d) the level of Cl, (e) DP, (f) the level of P and (g) prothrombin time in female patients. The correlation was considered to be statistically significant when p < 0.05.
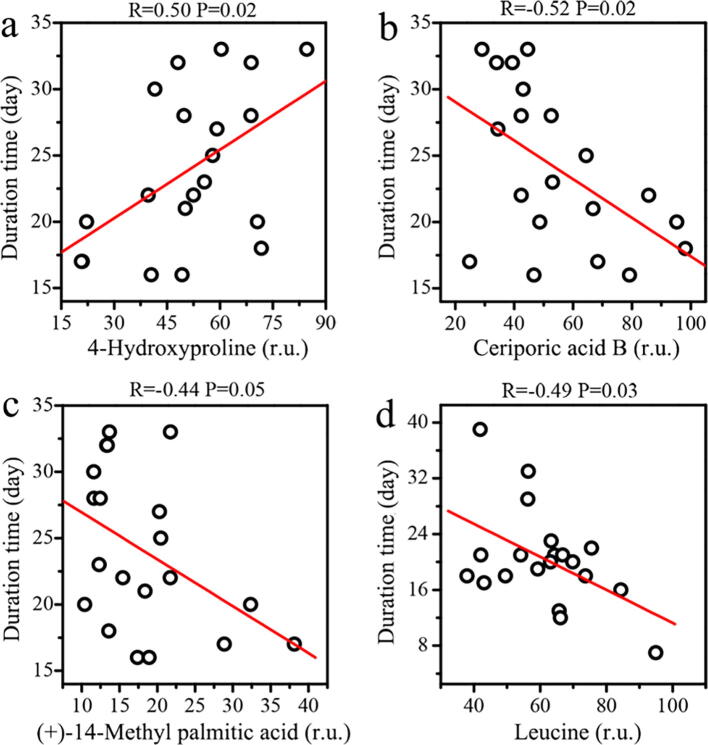
Based on metabolomics data, PLSR exhibited a better agreement between predicted and observed values in male patients relative to female patients (R2, 0.75 vs. 0.59). A series of contributive metabolites for correlations were identified, such as ceriporic acid B, 4-hydroxyproline, methyl beta-d-glucopyranoside, PGE1 alcohol, 13,14-dihydroxy-docosanoic acid, (+)-14-methyl palmitic acid, 17-hydroxy stearic acid and tetrahydrodeoxycortisol in male patients (Fig. 4b), as well as carbamic acid, tyramine, alpha-d-glucose, leucine, PC(O-16:1(9E)/0:0), PC(P-15:0/0:0), PC(O-8:0/O-8:0) and 11-deoxy-PGF1b in female patients (Fig. 4d). Subsequently, by regression analysis, we found that the duration from positive to negative in male patients with non-severe COVID-19 was positively correlated with 4-hydroxyproline (Fig. 6a, R = 0.50, P = 0.02) and negatively linked to fatty acids, including ceriporic acid B (Fig. 6b, R = -0.52, P = 0.02) and (+)-14-methyl palmitic acid (Fig. 6c, R = -0.44, P = 0.05). As 4-hydroxyproline is a biomarker of collagen catabolism, higher circulating level of 4-hydroxyproline may indicate more serious tissue and organ injuries in COVID-19 patients [54], [67]. Fatty acids have been shown to affect immune system [50]. Das suggested that administration of bioactive lipids could be beneficial to treat COVID-19 [68]. Moreover, in female patients, we found that high leucine level contributed to reduce the duration from positive to negative in non-severe COVID-19 patients (Fig. 6d, R = -0.49, P = 0.03). Leucine, a major component of branched‐chain amino acids (BCAAs), plays an important role in activating protein synthesis and suppressing protein degradation [69] and also regulating immune functions [70]. In addition, virus infection can cause a decrease in BCAAs levels and then reduce albumin synthesis [71], [72]. Therefore, leucine supplementation might be valuable for the treatment of non-severe COVID-19 especially in women.
Fig. 6.
Linear regression analysis between the duration from positive to negative and metabolites in non-severe COVID-19 patients. Correlations of the duration from positive to negative with (a) 4-hydroxyproline, (b) ceriporic acid B and (c) (+)-14-methyl palmitic acid in male patients, and (d) leucine in female patients. The correlation was considered to be statistically significant when p < 0.05.
4. Discussion
COVID-19 has been spreading rapidly around the world caused by SARS-CoV-2 in 2020, resulting in an unprecedented crisis in public health. Of note, men infected with SARS-CoV-2 have more severe disease and higher mortality than women due to sex differences in hormone [73], drug response [74] and immune system [20]. In the current study, we reveal that sex differences also exist for metabolic characteristics in plasma of non-severe COVID-19 patients during the recovery and rehabilitation process. In this process, metabolomics results demonstrate that the major metabolic alterations involved in fatty acids for men and glycerophosphocholines (GPCs) and carbohydrates for women. Many studies suggested a disrupted fatty acid metabolism after COVID-19 infection [14], [15], [23]. Moreover, fatty acids can affect lymphocytes and inflammation and thereby treat the viral infection [50], [52]. Recently, Toelzer et al. reported that the essential free fatty acid linoleic acid (LA) binding stabilized the locked structure of SARS-CoV-2 spike protein and suppressed viral replication [75]. GPCs are derived from the breakdown of phosphatidylcholine (PTC), a major component of cellular membranes. We speculate that this metabolic pathway would be active during organ injury and repair in COVID-19 patients [54], [67]. Additionally, carbohydrates as T cell-activating antigens have been reported to play a key role in immune system functions [76]. Our data show that carbohydrates such as alpha-d-glucose, 1-kestose, D-ribose and D-mannose were positively correlated with NK cells in female patients but not in male patients. Collectively, attention has to be paid to the sex-specific impact of SARS-CoV-2 on host metabolism.
Metabolomics is becoming as a capable tool to discover metabolic biomarkers for disease diagnosis as well as prognostic and therapeutic evaluations [77]. Using metabolomics and lipidomics, potential metabolite panels have been identified in both serum and plasma to distinguish COVID-19 and assess disease severity [14], [15]. Interestingly, here we proposed that metabolic profiling may contribute to predict the duration from positive to negative in non-severe COVID-19 patients, but men have a better predictive performance than women. Although this finding still needs to be further validated, prediction of the duration from positive to negative based on metabolic characteristics can be used to group patients for personalized treatment. Migaud et al. suggested that metabolomics phenotyping may predict antiviral drug efficacy in COVID-19 [78]. Metabolomics was also utilized to predict therapeutic efficacy such as chemotherapy [79], traditional Chinese medicine [80] and immunotherapy [81]. Therefore, we speculate that different metabolic environments in patients might influence drug efficacy and immune function [82], resulting in different durations from positive to negative in non-severe COVID-19 patients. Our results suggest a possible association between metabolic characteristics and the duration from positive to negative in non-severe COVID-19 patients, but sex differences in metabolic changes need to be considered.
5. Conclusion
In this study, we report an untargeted metabolomics analysis of plasma samples from both men and women with non-severe COVID-19 at acute period and after discharge. We reveal that the metabolic phenotypes of COVID-19 patients during the recovery and rehabilitation process were altered in a sex specific manner. In general, increased metabolic activities were detected in both male and female patients after discharge. We identified the major metabolic changes including fatty acids in men as well as GPCs and carbohydrates in women. Moreover, our results suggest that metabolic characteristics could be used to predict the duration from positive to negative in non-severe COVID-19 patients, but sex differences need to be paid attention. Several limitations and further works should be considered: (1) This study was based on a small Chinese cohort of COVID-19 patients in Wenzhou City of China, so we suggest that these findings still need to be validated from a larger longitudinal cohort and even from different racial and geographical cohorts; (2) Here we collected matched samples of male and female patients with non-severe COVID-19 at both acute period and after discharge, but a cohort of healthy men and women may need to be considered to examine sex-specific metabolic differences in the general population; (3) Metabolic data in the present study were not absolutely quantified, so more accurate quantification is required in clinical application.
6. Availability of data and materials
All data are present in the main text and the supplementary materials. LC-MS and GC–MS metabolomics data used in this study have been made publicly available in MetaboLights (ID: MTBLS2224). Additional data and materials related to this work can be requested from corresponding author.
CRediT authorship contribution statement
Hong Zheng: Methodology, Investigation, Formal analysis, Resources, Visualization, Writing - original draft, Writing - review & editing. Shengwei Jin: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing - review & editing. Ting Li: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Weiyang Ying: Investigation, Methodology, Resources, Writing - review & editing. Binyu Ying: Investigation, Methodology, Resources, Writing - review & editing. Dong Chen: Investigation, Methodology, Resources, Writing - review & editing. Jie Ning: Investigation, Methodology, Writing - review & editing. Chanfan Zheng: Investigation, Data curation, Writing - review & editing. Yuping Li: Investigation, Methodology, Resources, Writing - review & editing. Chen Li: Investigation, Methodology, Writi, ng - review & editing. Chengshui Chen: Investigation, Resources, Writing - review & editing. Xiaokun Li: Conceptualization, Supervision, Project administration, Funding acquisition, Writing - review & editing. Hongchang Gao: Conceptualization, Supervision, Project administration, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the Critical Care Group at Wenzhou Medical University for collecting plasma samples and all patients involved in this study. Additionally, we would like to give special thanks to all nurses and physicians in the world for confronting COVID-19 pandemic. This study was supported by the Key Research and Development Program of Zhejiang Province (No. 2020C03131 and No. 2019C03030), and the Ten-thousand Talents Program of Zhejiang Province (No. 2018R52052).
Authors' contributions
XKL, SWJ, YPL, CSC and HCG contributed to design and supervise experiments. TL, WYY, BYY and DC contributed to collect clinical plasma samples. TL, DC and CFZ contributed to collect clinical information. JN, CL and HZ contributed to sample preparation and metabolomics analysis. HZ and HCG contributed to data analysis, result discussion and interpretation. HZ and HCG contributed to write the manuscript. All authors have read, revised and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2021.03.039.
Contributor Information
Xiaokun Li, Email: xiaokunli@wmu.edu.cn.
Hongchang Gao, Email: gaohc27@wmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;2020(395):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. (COVID-19) in China: a report of 1014 cases. Radiology. 2019;2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 8.Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 9.Bastos M.L., Tavaziva G., Abidi S.K., Campbell J.R., Haraoui L.P., Johnston J.C. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X. Multitissue multiomics systems biology to dissect complex diseases. Trends Mol Med. 2020;26:718–728. doi: 10.1016/j.molmed.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J.W., Lam S.M., Fan X., Cao W.J., Wang S.Y., Tian H. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020;32(2):188–202. doi: 10.1016/j.cmet.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182(1):59–72. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nature Metab. 2020;2(7):572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein S.L., Dhakal S., Ursin R.L., Deshpande S., Sandberg K., Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLoS Pathog. 2020;16(6) doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Diff. 2020;11(1):1–13. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian J., Zhao L., Ye R.Z., Li X.J., Liu Y.L. Age-dependent gender differences of COVID-19 in mainland China: comparative study. Clin Infect Dis. 2020;ciaa683. doi: 10.1093/cid/ciaa683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;1–6 doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. Bischoff P. Czyborra Meyer zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner Br J Pharmacol. 130 8 2000 1878 1883 [DOI] [PMC free article] [PubMed]
- 22.Riley R.T., Voss K.A. Differential sensitivity of rat kidney and liver to fumonisin toxicity: organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicol Sci. 2006;92(1):335–345. doi: 10.1093/toxsci/kfj198. [DOI] [PubMed] [Google Scholar]
- 23.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O. COVID-19 infection results in alterations of the kynurenine pathway and fatty acid metabolism that correlate with IL-6 levels and renal status. medRxiv. 2020 doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Pol A., Gil A., Silljé H.H., Tromp J., Ovchinnikova E.S., Vreeswijk-Baudoin I. Accumulation of 5-oxoproline in myocardial dysfunction and the protective effects of OPLAH. Sci Transl Med. 2017;9(415):eaam8574. doi: 10.1126/scitranslmed.aam8574. [DOI] [PubMed] [Google Scholar]
- 25.Haghighatdoost F., Jabbari M., Hariri M. The effect of L-carnitine on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Eur J Clin Pharmacol. 2019;75:1037–1046. doi: 10.1007/s00228-019-02666-5. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed D., Youssef M.Y., Emam N.M. Oxidative stress in amiodarone-induced pulmonary toxicity in rats and the protective effect of L-carnitine and vitamin C. Man J Foren Med Clin Toxicol. 2020;28(1):43–53. [Google Scholar]
- 27.Tousson E., Hafez E., Zaki S., Gad A. P53, Bcl-2 and CD68 expression in response to amethopterin-induced lung injury and ameliorating role of l-carnitine. Biomed Pharmacotherapy. 2014;68(5):631–639. doi: 10.1016/j.biopha.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Hammock B.D., Wang W., Gilligan M.M., Panigrahy D. Eicosanoids: the overlooked storm in COVID-19? Am J Pathol. 2020;190:1782–1788. doi: 10.1016/j.ajpath.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan J.J., Jung J.S., Lee J.E., Lee J., Huh S.O., Kim H.S. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10(2):161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 30.Ojala P.J., Hirvonen T.E., Hermansson M., Somerharju P., Parkkinen J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J Leukocyte Biol. 2007;82(6):1501–1509. doi: 10.1189/jlb.0507292. [DOI] [PubMed] [Google Scholar]
- 31.Syme C., Pelletier S., Shin J., Abrahamowicz M., Leonard G., Perron M. Visceral fat-related systemic inflammation and the adolescent brain: a mediating role of circulating glycerophosphocholines. Int J Obes. 2019;43(6):1223–1230. doi: 10.1038/s41366-018-0202-2. [DOI] [PubMed] [Google Scholar]
- 32.Cheudjeu A. Correlation of D-xylose with severity and morbidity-related factors of COVID-19 and possible therapeutic use of D-xylose and antibiotics for COVID-19. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahoney D.E., Hiebert J.B., Thimmesch A., Pierce J.T., Vacek J.L., Clancy R.L. Understanding d-ribose and mitochondrial function. Adv Biosci Clin Med. 2018;6(1):1–5. doi: 10.7575/aiac.abcmed.v.6n.1p.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Yu L., Wang Y., Wei Y., Xu Y., He T. d-Ribose contributes to the glycation of serum protein. BBA-Mol Basis Dis. 2019;1865(9):2285–2292. doi: 10.1016/j.bbadis.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D., Chia C., Jiao X., Jin W., Kasagi S., Wu R. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med. 2017;23(9):1036–1045. doi: 10.1038/nm.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maceyka M., Harikumar K.B., Milstien S., Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sbardella E., Tomlinson J.W. The Hypothalamic-Pituitary-Adrenal Axis in Health and Disease. Springer; Cham: 2017. Cortisol Metabolism as a Regulator of the Tissue-Specific Glucocorticoid Action; pp. 271–301. [Google Scholar]
- 38.White P.C. Alterations of cortisol metabolism in human disorders. Horm Res Paediatrics. 2018;89(5):320–330. doi: 10.1159/000485508. [DOI] [PubMed] [Google Scholar]
- 39.Geering B., Stoeckle C., Conus S., Simon H.U. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34(8):398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Vitte J., Diallo A.B., Boumaza A., Lopez A., Michel M., Allardet-Servent J. A granulocytic signature identifies COVID-19 and its severity. J Infect Dis. 2020;222(12):1985–1996. doi: 10.1093/infdis/jiaa591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strohle A., Wolters M., Hahn A. Micronutrients at the interface between inflammation and infection ascorbic acid and calciferol. Part 1: general overview with a focus on ascorbic acid. Inflam Allergy-Drug. Targets. 2011;10(1):54–63. doi: 10.2174/187152811794352105. [DOI] [PubMed] [Google Scholar]
- 42.Bozonet S.M., Carr A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients. 2019;11(6):1363. doi: 10.3390/nu11061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ananieva E.A., Powell J.D., Hutson S.M. Leucine metabolism in T cell activation: mTOR signaling and beyond. Adv Nutr. 2016;7(4):798S–805S. doi: 10.3945/an.115.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weigert A., Weis N., Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology. 2009;214(9–10):748–760. doi: 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Joshi J.C., Joshi B., Rochford I., Rayees S., Akhter M.Z., Baweja S. SPHK2-generated S1P in CD11b+ macrophages blocks STING to suppress the inflammatory function of alveolar macrophages. Cell Rep. 2020;30(12):4096–4109. doi: 10.1016/j.celrep.2020.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz M., Jové M., Schlüter A., Casasnovas C., Villarroya F., Guilera C. Altered glycolipid and glycerophospholipid signaling drive inflammatory cascades in adrenomyeloneuropathy. Human Mol Genetics. 2015;24(24):6861–6876. doi: 10.1093/hmg/ddv375. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C., Wang Y., Wang F., Wang Z., Lu Y., Xu Y. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Sci Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-00341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni M., Tian F.B., Xiang D.D., Yu B. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J Med Virol. 2020;92:2600–2606. doi: 10.1002/jmv.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaqoob P., Calder P.C. Fatty acids and immune function: new insights into mechanisms. Br J Nutr. 2007;98(S1):S41–S45. doi: 10.1017/S0007114507832995. [DOI] [PubMed] [Google Scholar]
- 50.Hradilkova K., Maschmeyer P., Westendorf K., Schliemann H., Husak O., von Stuckrad A.S.L. Regulation of fatty acid oxidation by twist 1 in the metabolic adaptation of T helper lymphocytes to chronic inflammation. Arthritis Rheumatol. 2019;71(10):1756–1765. doi: 10.1002/art.40939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calder P.C. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668:S50–S58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 52.Namgaladze D., Brüne B. Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. BBA-Mol Cell Biol Lipids. 2016;1861(11):1796–1807. doi: 10.1016/j.bbalip.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D., Chen Y., Liu H., Jia Y., Li F., Wang W. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal Transduc Target Therapy. 2020;5:62. doi: 10.1038/s41392-020-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infec Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun L., Middleton D.R., Wantuch P.L., Ozdilek A., Avci F.Y. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology. 2016;26(10):1029–1040. doi: 10.1093/glycob/cww062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McFarlin B.K., Flynn M.G., Stewart L.K., Timmerman K.L. Carbohydrate intake during endurance exercise increases natural killer cell responsiveness to IL-2. J App Physiol. 2004;96(1):271–275. doi: 10.1152/japplphysiol.00585.2003. [DOI] [PubMed] [Google Scholar]
- 58.McFarlin B.K., Flynn M.G., Hampton T. Carbohydrate consumption during cycling increases in vitro NK cell responses to IL-2 and IFN-γ. Brain Behav Immun. 2007;21(2):202–208. doi: 10.1016/j.bbi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F., Yao M., Lin Z., Chen Y., Jiang H., Zeng M. The Effects of preoperative oral carbohydrate on frequency of T and NK cells in patients with cervical cancer treated using Neoadjuvant chemotherapy and surgery: A prospective cohort study. BioMed Res Int. 2020;2020:2101480. doi: 10.1155/2020/2101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mo X., Jian W., Su Z., Chen M., Peng H., Peng P. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olveira G., Olveira C., Acosta E., Espíldora F., Garrido-Sánchez L., García-Escobar E. Fatty acid supplementation improves respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Archivos de Bronconeumología (English Edition). 2010;46(2):70–77. doi: 10.1016/j.arbres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Tobin A.B., Prihandoko R., Kaur D., Wiegman C.H., Alvarez-Curto E., Donovan C. Pathophysiological regulation of lung function by the free fatty acid receptor FFA4. bioRxiv. 2020 doi: 10.1126/scitranslmed.aaw9009. [DOI] [PubMed] [Google Scholar]
- 64.Fisher B.J., Seropian I.M., Kraskauskas D., Thakkar J.N., Voelkel N.F., Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med. 2011;39(6):1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- 65.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Micco P., Russo V., Carannante N., Imparato M., Rodolfi S., Cardillo G. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J Clin Med. 2020;9(5):1371. doi: 10.3390/jcm9051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das U.N. Bioactive lipids in COVID-19-further evidence. Arch Med Res. 2020 doi: 10.1016/j.arcmed.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem Biophys Res Commun. 2004;313(2):417–422. doi: 10.1016/j.bbrc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 70.Kelly B., Pearce E.L. Amino assets: how amino acids support immunity. Cell Metab. 2020;32:154–175. doi: 10.1016/j.cmet.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Wada Y., Takeda Y., Kuwahata M. Potential role of amino acid/protein nutrition and exercise in serum albumin redox state. Nutrients. 2018;10(1):17. doi: 10.3390/nu10010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambade V. Biochemical rationale for hypoalbuminemia in COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Jerkic M., Slutsky A.S., Zhang H. Molecular mechanisms of sex bias differences in COVID-19 mortality. Crit Care. 2020;24(1):1–6. doi: 10.1186/s13054-020-03118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bischof E., Wolfe J., Klein S.L. Clinical trials for COVID-19 should include sex as a variable. J Clin Invest. 2020;130:3350–3352. doi: 10.1172/JCI139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toelzer C., Gupta K., Yadav S.K., Borucu U., Davidson A.D., Williamson M.K. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science. 2020;370(6517):725–730. doi: 10.1126/science.abd3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao H., Raines L.N., Huang S.C.C. Carbohydrate and amino acid metabolism as hallmarks for innate immune cell activation and function. Cells. 2020;9(3):562. doi: 10.3390/cells9030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacob M., Lopata A.L., Dasouki M., Abdel Rahman A.M. Metabolomics toward personalized medicine. Mass Spectr Rev. 2019;38(3):221–238. doi: 10.1002/mas.21548. [DOI] [PubMed] [Google Scholar]
- 78.Migaud M., Gandotra S., Chand H.S., Gillespie M.N., Thannickal V.J., Langley R.J. Metabolomics to predict antiviral drug efficacy in COVID-19. Am J Respir Cell Mol Biol. 2020;63(3):396–398. doi: 10.1165/rcmb.2020-0206LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian Y., Wang Z., Liu X., Duan J., Feng G., Yin Y. Prediction of chemotherapeutic efficacy in non–small cell lung cancer by serum metabolomic profiling. Clin Cancer Res. 2018;24(9):2100–2109. doi: 10.1158/1078-0432.CCR-17-2855. [DOI] [PubMed] [Google Scholar]
- 80.Zhang A.H., Sun H., Yan G.L., Han Y., Zhao Q.Q., Wang X.J. Chinmedomics: a powerful approach integrating metabolomics with serum pharmacochemistry to evaluate the efficacy of traditional Chinese medicine. Engineering. 2019;5(1):60–68. [Google Scholar]
- 81.Shi H.Y., Pan C., Ma T.T., Chen Y.L., Yan W.J., Liu J.G. Clinical efficacy evaluation of 1-year subcutaneous immunotherapy for Artemisia sieversiana pollen allergic rhinitis by serum metabolomics. Front Pharmacol. 2020;11:305. doi: 10.3389/fphar.2020.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J.H., Bhargava P., McCloskey D., Mao N., Palsson B.O., Collins J.J. Antibiotic-induced changes to the host metabolic environment inhibit drug efficacy and alter immune function. Cell Host Microbe. 2017;22(6):757–765. doi: 10.1016/j.chom.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are present in the main text and the supplementary materials. LC-MS and GC–MS metabolomics data used in this study have been made publicly available in MetaboLights (ID: MTBLS2224). Additional data and materials related to this work can be requested from corresponding author.